Abstract
We evaluated the in vitro efficacy of weak acid hypochlorous solution (WAHS) against murine norovirus (MNV) by plaque assay and compared the efficacy with diluted NaOCl (Purelox) and 70% ethanol. WAHS was as effective as 70% ethanol and diluted Purelox for 0.5-min reactions. For 0.5-min reactions in the presence of mouse feces emulsion, the efficacy of WHAS and 1:600 diluted Purelox was decreased, reducing the virus titers by 2.3 and 2.6 log10, respectively, while 70% ethanol reduced the titer by more than 5 log10. However, WAHS showed more than 5 log10 reductions for the 5-min reaction even in the presence of feces emulsion. Since WAHS showed enough efficacy in inactivating MNV in vitro, we tried to eliminate MNV from MNV-infected mice by substituting WAHS for their drinking water. However, MNV was found to be positive in feces of mice drinking WAHS by an RT-nested PCR and plaque assay. To investigate whether hypochlorite-based disinfectants could prevent infection of a mouse with MNV, WAHS or 1:6,000 diluted Purelox was substituted for the drinking water of mice for 2 or 4 weeks, and then the mice were placed in a cage with an MNV-infected mouse. The supply of disinfectants was continued after cohabitation, but MNV was detected in the feces of all the mice at 1 week after cohabitation. In this study, we tried to eliminate and prevent MNV infection from mice by supplying hypochlorite-based disinfectants as an easy and low-cost method. Unfortunately, drinking disinfectants was ineffective, so it is important to keep the facility environment clean by use of effective disinfectants. Also, animals introduced into facilities should be tested as MNV free by quarantine and periodically confirmed as MNV free by microbiological monitoring.
Keywords: disinfectant, murine norovirus, plaque assay, RT-nested PCR, weak acid hypochlorous solution
Introduction
Noroviruses are nonenveloped, positive-sense RNA viruses that belong to the genus Norovirus in the family Caliciviridae [26]. Human noroviruses cause acute nonbacterial gastroenteritis worldwide and are transmitted through the fecal-oral route, usually by eating food or drinking liquids contaminated with noroviruses [22]. Noroviruses are divided into five major genogroups, that is, GI, which infects humans; GII, which infects humans and swine; GIII, which infects bovine; GIV, which infects humans and canines; and GV, which infects mice [22]. Murine norovirus (MNV) was first discovered in 2003 in laboratory mice that were deficient in signal transducer and activator of transcription 1 and recombination-activating gene 2 [13]. Surveillance of microbiological contamination of MNV shows that MNV is the most prevalent viral pathogen in laboratory animal facilities in the USA , Canada, and Australia [10, 18]. Also, in Japan, it was reported that MNV is one of the most prevalent pathogens in conventional mouse colonies [8, 15]. The effect of MNV infection on research using mice has not been revealed completely. Some reports showed MNV did not affect the results of research [8, 11, 13, 15, 19]. On the other hand, there are reports on clinical signs such as weight loss, gastric bloating, and diarrhea in STAT1-deficient mice infected with MNV, and a modest inflammatory response and increase in necrotic cells in the small intestine of MNV-infected immunocompetent mice [12]. In research that strictly requires a normal immune system, it is better to prevent contamination of animal facilities with MNV and to eliminate MNV from mice contaminated with MNV.
While human norovirus is unable to grow in vitro [6, 16], MNV can replicate in both cultured cells and mice [5, 24]. Therefore, MNV has been used to study proteolytic processing, environmental stability and inactivation [1,2,3, 23, 25]. Belliot et al. [2] previously examined the efficacy of some disinfectants, and showed that ethanol, povidone-iodine, and sodium hypochlorite were effective against MNV. In animal facilities, these disinfectants have been used to keep the environment of facilities clean. Recently, because of the high antiseptic efficacy, low cost, and safety for humans, weak acid hypochlorous solution (WAHS) is beginning to be used in animal facilities, hospitals, and food industries. WAHS is composed of sodium hypochlorite blended with hydrochloric acid in tap water, with the pH value adjusted to 6.0–6.4 and the residual chlorine concentration adjusted to about 60 ppm. The main effective form of chlorine in WAHS is hypochlorous acid (HOCl). The efficacy of WAHS against various microorganisms has been reported [21]. In this study, we first evaluated the virucidal effect of WAHS against MNV in vitro. The efficacies of several dilutions of sodium hypochlorite (NaOCl) and 70% ethanol in inactivating MNV were also tested for comparison with WAHS. Though the main effective form of chlorine in NaOCl is also HOCl, NaOCl corrodes metal due to its strong alkalinity and has disadvantages such as its irritant properties and strong odor [7]. Compared with NaOCl, WAHS is a suitable substitute for drinking water of animals. We attempted to eliminate MNV from mice experimentally infected with MNV and to prevent mice from becoming infected with MNV by substituting WAHS for their drinking water and compared the results with those obtained with NaOCl.
Materials and Methods
Mice
Female, specific-pathogen-free (SPF) Slc:ICR mice (6 weeks of age) were obtained from Japan SLC, Inc. (Hamamatsu, Japan) and used for experimental infection with MNV. The breeder’s health monitoring report indicated that these mice were free of the following intestinal microorganisms: Pseudomonas aeruginosa, Citrobacter rodentium, Salmonella spp., Corynebacterium kutscheri, Clostridium piliforme, pinworm, intestinal protozoa, Helicobacter hepaticus, H. bilis, and MNV. They were also negative for mouse hepatitis virus, Sendai virus, ectromelia virus, lymphocytic choriomeningitis virus, pneumonia virus of mice, mouse adenovirus, Mycoplasma pulmonis, Pasteurella pneumotropica, Cilia-associated respiratory bacillus, ectoparasites, and Dermatophytes. All the animals were nursed under barrier conditions and provided with commercial laboratory mouse chow and water ad libitum unless otherwise indicated. Animal experiments were peer-reviewed by the Animal Care and Use Committee of the NIID and approved by the director of the NIID in accordance with the guides for animal experiments performed at the NIID.
Cells and viruses
RAW264.7 cells were purchased from ATCC (Manassas, VA, USA) and maintained in high-glucose Dulbecco’s modified essential medium (DMEM) (Sigma-Aldrich Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) and 0.1 mg/ml kanamycin (DMEM-FBS). The S7 strain of MNV (MNV-S7), which was isolated from a conventional mouse in Japan, was used in this study. A monolayer of RAW264.7 cells grown in a tissue culture flask was infected with MNV-S7 at a multiplicity of infection (MOI) of 0.1 and incubated in DMEM-FBS for 3 h at 37°C in a 5% CO2 atmosphere. After the cultured medium was removed, the cells were incubated in new DMEM-FBS for 2 days. Then, the cells were subjected to freezing-thawing, and the cultured medium was centrifuged at 3,000 rpm for 5 min at 4°C. The supernatant was used as the virus stock throughout the study.
Plaque assay
RAW264.7 cells were seeded into 12-well plates at a density of 8.5 × 105 viable cells per well. After the culture medium was removed, 0.3 ml of 1:10 serially diluted (10−1 to 10−6) samples in DMEM-FBS were added to each well. Plates were incubated for 2 h at 37°C in a 5% CO2, and the inocula were removed. After washing with DMEM, the cells were overlaid with 1 ml of 1.5% Agar Noble (Becton, Dickinson and Co., Sparks, MD, USA) in Eagle’s Minimum Essential Medium (EMEM) (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% FBS, 2 mM L-glutamine, and 0.22% NaHCO3 per well. Plates were incubated for 2 days at 37°C in 5% CO2. To visualize plaques, the cells were overlaid with 1 ml of 1.5% Agar Noble in EMEM supplemented with 10% FBS, 2 mM L-glutamine, and 0.01% neutral red per well, followed by incubation for 4–5 h.
Evaluation of disinfectant efficacies against MNV in vitro
Three disinfectants were used to inactivate the MNV. Ethanol was purchased from Wako Pure Chemical Industries (Tokyo, Japan). Purelox (6% NaOCl) was purchased from Oyalox (Tokyo, Japan). Weak acid hypochlorous solution (WAHS) was produced using a Steri Revo HSP-SR-600 (HSP Corp., Okayama, Japan), which blended sodium hypochlorite with hydrochloric acid and adjusted the pH value to 6.0–6.4 and the residual chlorine concentration to about 60 ppm. Ethanol was diluted in distilled water to a final concentration of 70%, and Purelox was diluted 1:200 (over 200 ppm of residual chlorine concentration), 1:400 (150 to 200 ppm), 1:600 (100 ppm), 1:800 (70 ppm), and 1:1,000 (60 ppm) in distilled water. WAHS was used without dilution.
To investigate the influence of organic materials, fresh feces were collected from an ICR mouse that was maintained as a sentinel animal in routine microbiological monitoring in our facility and homogenized in 9 vol. (w/v) of PBS. After centrifugation at 3,000 rpm for 1 min, the supernatant was used as a feces emulsion.
Inactivation of MNV was performed by adding 0.9 ml of the disinfectant to 0.05 ml of virus stock solution (8.8 × 107 PFU/ml) and 0.05 ml of PBS. In the case of presence of the feces emulsion, the virus solution was mixed with 0.05 ml of mouse feces emulsion instead of PBS. Immediately after the disinfectant was added to the virus solution, the reaction mixtures were vortexed and incubated for 0.5, 1, or 5 min at room temperature. As inactivation control samples, virus solution was incubated for 5 min with PBS instead of disinfectant. To stop the reaction, 9 ml of DMEM-FBS was added to the reaction mixture. Then, each solution was serially diluted 1:10 in DMEM-FBS, and the virus titer was measured by the plaque assay as described above.
Attempt to eliminate MNV from infected mice by substituting WAHS for drinking water
We used 6 ICR mice divided into 2 groups. Mice were orally infected with 1 × 106 plaque forming units (PFU) of MNV in PBS. At 1 week post infection, the drinking water for 3 mice was changed to WAHS and then repeatedly exchanged with fresh WAHS every other day. At 1, 2, 3, and 4 weeks post infection, feces were collected from each mouse. At 5 weeks post infection, mice were sacrificed, and the duodenum, jejunum, cecum, rectum, and feces were collected and kept at −80°C until use. The presence of viable virus in each intestine and feces was investigated by RT-nested PCR, and the virus titer was determined by plaque assay.
Attempt to prevent MNV infection by substituting hypochlorite-based disinfectants for drinking water
The drinking water of mice was changed to WAHS or 1:6,000 diluted Purelox and repeatedly exchanged with fresh disinfectant every three or four days during the experiment. After 2 or 4 weeks of supplying the disinfectant in place of water, 3 mice were moved into the same cage as a mouse that had been orally infected with 1 × 106 PFU of MNV 1 week before cohabitation. At 1, 2, and 3 weeks post cohabitation, feces were collected from each mouse. The presence of viable virus in feces was investigated by RT-nested PCR.
Extraction of RNA and RT-nested PCR of mouse samples
Feces were homogenized in 9 volumes of DMEM-FBS with a zirconia bead (5 mm in diameter) at 3,000 rpm for 0.5 min by use of a bead cell disrupter (Micro Smash MS-100, TOMY Seiko, Tokyo, Japan). Each piece (20–50 mg) of duodenum, jejunum (small intestine 3–5 cm below stomach), cecum, and rectum was washed by mild vortexing in 0.5 ml of DMEM-FBS and homogenized in 0.5 ml of new DMEM-FBS with about 50 zirconia beads (1 mm in diameter) 5 times at 3,000 rpm for 0.5 min by use of a Micro Smash MS-100. The supernatant obtained by centrifugation at 3,000 ×g for 5 min at 4°C was diluted 1:100 (feces) or 1:5 (intestines) in DMEM-FBS. Each dilution was inoculated onto a monolayer of RAW264.7 cells in 12-well plates at a rate of 0.3 ml per well and incubated for 2 h at 37°C in 5% CO2. The inoculated dilution was removed, and the cells were washed two times with PBS. Virus RNA from monolayer cells was extracted by use of a QuickGene RNA cultured cell kit S (KURABO Industries, Osaka, Japan) and an automated extraction system (QuickGene-810, KURABO). cDNA was synthesized by using ReverTra Ace −α− (TOYOBO, Osaka, Japan) and Random Primer (TOYOBO) according to the manufacturer’s instructions.
The primer sequences used in PCR are shown in Table 1. The target regions to detect MNV-S7 were focused on the ORF1/ORF2 junction region and ORF2 region. The four primers for the two target regions were prepared, and then the primer set for the nested PCR assay to detect the ORF1/ORF2 junction region was selected. To help in design of these primers, the FastPCR freeware (http://primerdigital.com) was used, and the GenBank accession numbers of the MNV sequences were as follows: DQ223041, DQ223043, DQ223042, DQ911368, EU 004663, EU 004664, EU 004665, EU 004666, EU 004667, EU 004668, EU 004669, EU 004670, EU 004671, EU 004672, EU 004673, EU 004674, EU 004675, EU 004676, EU 04677, EU 004678, EU 004679, EU 004680, EU 004681, EU 004682, EU 004683, and AY228235. PCR reactions were performed by use of a QIAGEN Multiplex PCR Kit (QIAGEN, Hilden, Germany). In the first PCR, the cDNA transcripts (5 µl) were mixed with 1 µl each of 10 µM MNoVorf1&2-F5 and MNoVorf1&2-R2 primer, 10 µl of 2×QIAGEN Multiplex PCR Master Mix, and 3 µl of Nuclease-free water. The first PCR products (5 µl) and the primer set comprised of MNoVorf1&2-F6 and MNoVorf1&2-R3 were used in the second PCR. Amplification was performed according to the manufacturer’s instructions. The second PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide and visualized under UV light.
Table 1. Primers used to detect MNV by RT-nested PCR.
| Primer | Sequence (5’ to 3’) | Polarity1) | Positions2) | |
|---|---|---|---|---|
| 1st PCR | MNoVorf1&2-F5 | CGCTTYGGAACRATGGATGCTG | + | 5001 − 5022 |
| MNoVorf1&2-R2 | AGCCRGTRTACATGGCTGAG | − | 5340 − 5359 | |
| 2nd PCR | MNoVorf1&2-F6 | CGCAGGAACGCTCAGCAGTC | + | 5029 − 5048 |
| MNoVorf1&2-R3 | CRAGRTARGGGTTRAGYCCYG | − | 5312 − 5332 | |
1)+, sense; −, anti-sense. 2)Nucleotide positions correspond to those of the MNV S7 complete genome (AB435514).
To confirm that only viable MNV RNA was recovered by the method described above, 5 µl of MNV stock solution was inactivated by reaction with 45 µl of 1:200 diluted Purelox for 3 min and diluted 1:1,000 in DMEM-FBS. As a positive control, MNV was reacted with PBS for 3 min and diluted. The dilutions were inoculated onto a monolayer of RAW264.7 cells, and RNA was extracted in the same manner as above. Then, RT-nested PCR was performed by use of the RNA.
Virus titration of mouse samples
Virus titration was performed by use of the supernatants of intestine and feces homogenates prepared for RNA extraction as described above. DMEM-FBS (0.3 ml) including the supernatant corresponding to 5 mg, 0.5 mg, or 0.05 mg of intestine was added to each well. Then, virus titer was determined according to the method mentioned in the “Plaque assay” section. For virus titration of the supernatant of feces homogenate, 0.8 ml of serially diluted (10−2 to 10−4) supernatant was added to each well of a 6-well plate seeded with RAW264.7 cells at a density of 2.0 × 106 viable cells per well. Subsequent procedures were as mentioned in the “Plaque assay” section, except for use of 2 ml of 1.5% Agar Noble for the 1st and 2nd overlays.
Results
To investigate the effect of WAHS on MNV inactivation, we determined the virus titer of MNV after reaction with WAHS by plaque assay and compared the results with the other disinfectants commonly used. In addition, it is well known that HOCl oxidizes organic materials and rapidly loses its disinfection efficacy when it comes into contact with something that oxidizes easily, such as organic materials [7]. To investigate the influence of organic materials on inactivation of MNV by disinfectants, we also performed inactivation of MNV in the presence of emulsion of mouse feces. Table 2 shows the results in the absence and presence of feces emulsion. The virus titers after reaction with PBS (control samples) were 4.4 × 106 PFU/ml and 3.9 × 106 PFU/ml in the absence and the presence of feces emulsion, respectively. For 0.5-min reactions in the absence of feces emulsion, WAHS reduced the virus titer by more than 5 log10. Similar to the results of WAHS, 1:200 to 1:800 diluted Purelox showed more than 5 log10 reductions in the virus titer, and even 1:1,000 diluted Purelox reduced the virus titer by 3.4 log10 in the absence of feces emulsion in a 0.5-min reaction. In the absence of mouse feces emulsion, 70% ethanol reduced the virus titer by more than 5 log10 in a 0.5-min reaction. In the case of the presence of feces emulsion, the reduction titers decreased to 2.3 and 2.5 log10 for WAHS in 0.5- and 1-min reactions, respectively. However, WAHS showed more than a 5 log10 reduction in the virus titer in a 5-min reaction even in the presence of feces emulsion. These results suggested that the virucidal effect of WAHS against MNV was retained for several or more minutes even in the presence of organic materials. The effect of Purelox also dropped to a 2.6 and 1.0 log10 reduction at the 1:600 and 1:1,000 dilutions, respectively, in the presence of feces emulsion in a 0.5-min reaction. In addition, the effect of 1:1,000 diluted Purelox on MNV inactivation was much lower than that of WAHS containing a residual chlorine concentration of 60 ppm, which was equivalent to that of 1:1,000 diluted Purelox. In the presence of mouse feces emulsion, 70% ethanol reduced the virus titer by more than 5 log10 in 0.5-min reactions.
Table 2. Virucidal activities of WAHS, Purelox, and ethanol for MNV.
| Disinfectant (dilution ratio) |
pH | Residual chlorine concentration (ppm) |
Reaction Time (min) |
Reduction in titer (log10
PFU/ml)1) |
|
|---|---|---|---|---|---|
| Feces emulsion | |||||
| Absennce | Presence | ||||
| WAHS | 6.0–6.4 | 60 | 0.5 | >5 | 2.3 |
| 1 | >5 | 2.5 | |||
| 5 | >5 | >5 | |||
| Purelox (1:200) | 10.2 | >200 | 0.5 | >5 | >5 |
| 1 | >5 | >5 | |||
| 5 | >5 | ND | |||
| Purelox (1:400) | 9.7 | 150–200 | 0.5 | >5 | >5 |
| 1 | >5 | >5 | |||
| 5 | ND2) | ND | |||
| Purelox (1:600) | 9.5 | 100 | 0.5 | >5 | 2.6 |
| 1 | >5 | 3.8 | |||
| 5 | ND | >5 | |||
| Purelox (1:800) | 9.4 | 70 | 0.5 | >5 | 2.0 |
| 1 | >5 | 2.2 | |||
| 5 | ND | >5 | |||
| Purelox (1:1,000) | 9.3 | 60 | 0.5 | 3.4 | 1.0 |
| 1 | 3.8 | 1.4 | |||
| 5 | 4.5 | 2.2 | |||
| 70% Ethanol | 0.5 | >5 | >5 | ||
| 1 | >5 | ND | |||
| 5 | >5 | ND | |||
1)MNV titer was determined by plaque assay. 2)ND, not determined.
Because WAHS had the important advantage of being drinkable without the need for dilution and showed a virucidal effect on MNV inactivation even in the presence of organic materials, we tried to eliminate MNV from mice already infected with MNV by substituting WAHS for their drinking water. Mice were orally infected with MNV, and their drinking water was changed to WAHS at 1 week post infection. Table 3 shows the results of detection of MNV from feces by RT-nested PCR and the virus titer in feces determined by plaque assay. Throughout this study, RT-nested PCR was performed by use of RNA extracted from RAW264.7 cells that were inoculated with the supernatant of homogenate of samples. In addition, no MNV-specific product was detected in RT-nested PCR using RNA extracted from RAW264.7 cells that were inoculated with inactivated MNV (Fig. 1). Therefore, a positive result for an MNV-specific product in RT-nested PCR indicates the presence of infectious MNV in samples. In the RT-nested PCR results, MNV was detected at 1 week post infection, and then MNV was successively positive in feces of all the mice after the drinking water was changed to WAHS at 1 week post infection. The virus titers were also as high as those of the control, whose drinking water was not changed to WAHS, at 5 weeks post infection. On the other hand, the results for the sites of the intestine showed some efficacy of WAHS (Table 4). The results of RT-nested PCR for the intestines showed a little difference between WAHS-drinking mice and control mice; that is, MNV was not detected in the duodena of WAHS-drinking mice, while it was detected from 2/3 of control mice. In addition, MNV was detected in the jejunum of only 1/3 WAHS-drinking mice, while it was detected from all control mice. However, MNV was detected in the ceca of all the mice. The virus titers were undetectable in the duodena and jejuna, and the same level was found in the ceca of all the mice examined.
Table 3. Detection of MNV in feces of mice that drank WAHS1) post MNV infection.
| Drinking water | Weeks post infection |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| WAHS (n=3) | RT-nested PCR | 3/34) | 3/3 | 3/3 | 3/3 | 3/3 |
| MNV titer3) (log10 PFU/g) | 5.29 ± 0.53 | 3.79 ± 0.34 | 3.69 ± 1.10 | 3.89 ±1.01 | 3.74 ± 0.82 | |
| Control (n=3)2) | RT-nested PCR | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| MNV titer (log10 PFU/g) | 5.52 ± 0.54 | 3.51 ± 0.79 | 3.38 ± 0.57 | 3.77 ± 1.24 | 3.95 ± 0.85 | |
1)Drinking water was changed from 1 week post infection. 2)The drinking water of control mice was not changed to disinfectant. 3)MNV titer was determined by plaque assay. Each value represents the mean ± SD of 3 samples. For calculation of the mean ± SD, titers of samples that did not show any plaque were estimated as the detection limit, 3.0 log10. 4)Each value represents number of positive samples / number of samples examined.
Fig. 1.
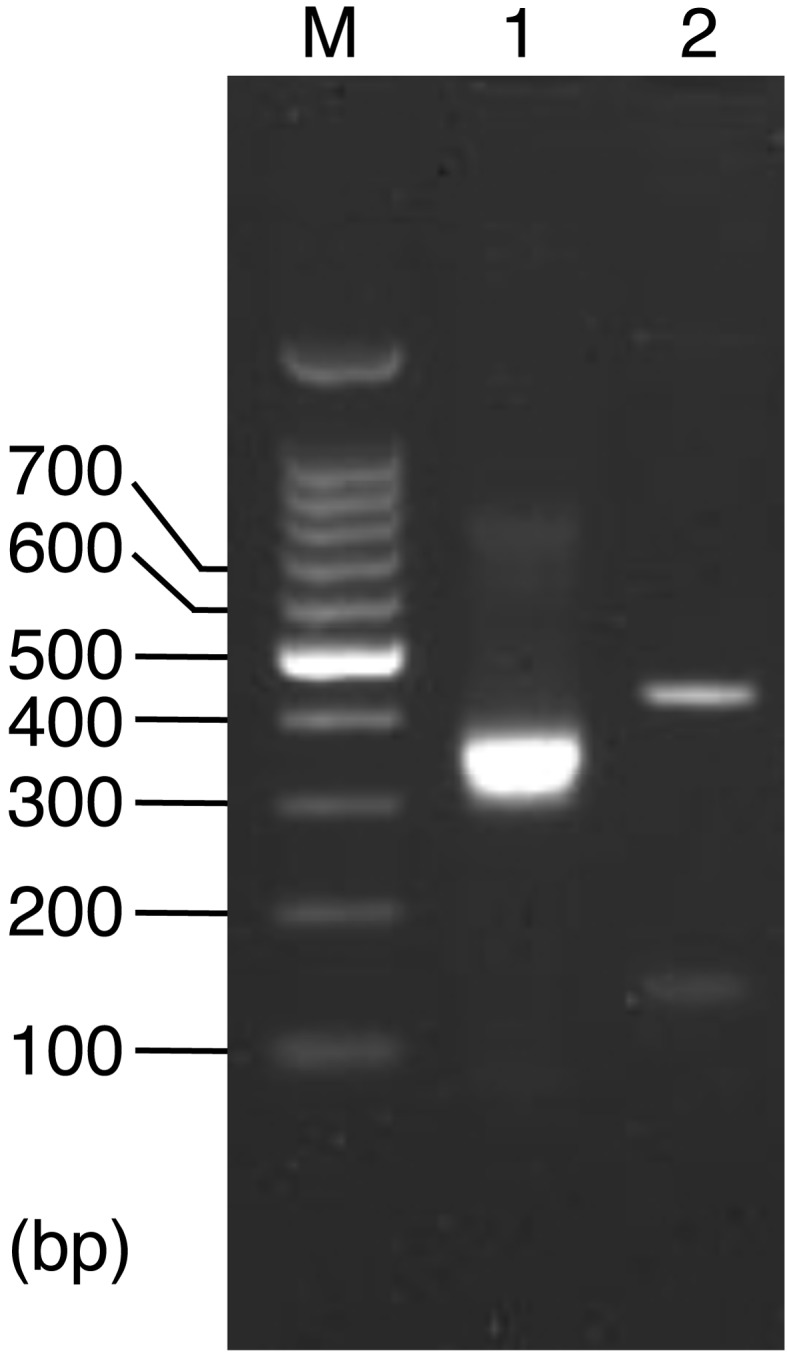
Agarose gel electrophoresis of RT-nested PCR products. Lane M, DNA marker with the sizes indicated; lane 1, MNV-specific RT-nested PCR product (304 bp) as a positive control; lane 2, RT-nested PCR product using RNA extracted from RAW264.7 cells that were inoculated with inactivated MNV.
Table 4. Detection of MNV in the intestines of mice that drank WAHS1) at 5 weeks post MNV infection.
| Drinking water | Intestine |
||||
|---|---|---|---|---|---|
| Duodenum | Jejunum | Cecum | Rectum | ||
| WAHS (n=3) | RT-nested PCR | 0/34) | 1/3 | 3/3 | 0/3 |
| MNV titer3) (log10 PFU/g) | <2.3 | <2.3 | 2.92 ± 0.56 | ND5) | |
| Control (n=3)2) | RT-nested PCR | 2/3 | 3/3 | 3/3 | 0/3 |
| MNV titer (log10 PFU/g) | <2.3 | <2.3 | 2.59 ± 0.76 | ND | |
1)Drinking water was changed from 1 week post infection. 2)The drinking water of control mice was not changed to disinfectant. 3)MNV titer was determined by plaque assay. Each value represents the mean ± SD of 3 samples. 4)Each value represents number of positive samples / number of samples examined. 5)ND, not determined.
Next, we evaluated the effect of WAHS and 1:6,000 diluted Purelox on prevention of MNV infection in the mouse. Mice drank the disinfectants for 2 or 4weeks and then were placed in the same cage as a mouse infected with MNV. Supply of mice with disinfectants in place of drinking water was continued for 3 weeks after cohabitation. However, MNV was detected from feces of all the mice by RT-nested PCR at 1, 2, and 3 weeks after cohabitation (Table 5).
Table 5. Detection of MNV in feces of disinfectant-drinking mice that cohabited with an MNV-infected mouse by RT-nested PCR.
| Drinking water | Weeks before cohabitation1) |
Weeks post cohabitation |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| WAHS (n=3) | 2 | 3/33) | 3/3 | 3/3 |
| Purelox (1:6,000) (n=3) | 2 | 3/3 | 3/3 | 3/3 |
| Control (n=3)2) | 2 | 3/3 | 3/3 | 3/3 |
| WAHS (n=3) | 4 | 3/3 | 3/3 | 3/3 |
| Purelox (1:6,000) (n=3) | 4 | 3/3 | 3/3 | 3/3 |
| Control (n=3) | 4 | 3/3 | 3/3 | 3/3 |
1)The drinking water of mice was changed to WAHS or 1:6,000 diluted Purelox 2 or 4 weeks before cohabitation with an MNV-infected mouse. 2)The drinking water of control mice was not changed to disinfectant. 3)Each value represents number of positive samples / number of samples examined.
Discussion
WAHS is composed of a blend of sodium hypochlorite with hydrochloric acid in tap water, with the pH value adjusted to 6.0–6.4 and the residual chlorine concentration adjusted to about 60 ppm. WAHS is reported to have microbiological effects on various microorganisms [21]. The main effective form of chlorine in WAHS is hypochlorous acid (HOCl), as is the case for NaOCl, which is commonly used for disinfection of microorganisms in animal facilities. Unfortunately, NaOCl has some disadvantages such as corrosion of metal, irritant properties, and strong odor [7]. Considering that WAHS has little corrosiveness, irritant properties, and odor, and does not need to be diluted before use, it might be more suitable than diluted Purelox at high concentrations for use in animal racks and animal rooms. Animal facilities also use 70% ethanol as a disinfectant. Ethanol is a good disinfectant but has the disadvantages of flammability and high cost.
We evaluated the virucidal effect of WAHS on MNV inactivation in vitro and compared the results with NaOCl and 70% ethanol. Because NaOCl (Purelox) is usually used after dilution, the virucidal effect of several dilutions of Purelox against MNV was evaluated. We also determined the influence of organic materials such as feces on the effect of disinfectants. In the absence of feces emulsion, WAHS showed as high efficacy as 70% ethanol and diluted Purelox at high concentration. However, the effect of WAHS dropped to 2.3 and 2.5 log10 in the 0.5- and 1-min reactions, respectively, in the presence of feces emulsion. The cause of efficacy reduction is considered to be that HOCl oxidizes organic materials in feces emulsion and rapidly loses its disinfection efficacy. Though 1:1,000 diluted Purelox could not completely inactivate MNV, other dilutions of Purelox with chlorine concentrations higher than 60 ppm showed more than 5 log10 reductions in the virus titer in the 0.5-min reaction in the absence of feces emulsion. However, in the presence of feces emulsion, the virucidal effect of Purelox diluted more than 1:600 was markedly down. The cause of the efficacy reduction of Purelox in the presence of feces emulsion is considered to be same as that of WAHS, because the main effective form of chlorine is HOCl in both WAHS and Purelox. On the other hand, WAHS showed more than 5 log10 reductions in the virus titer in the 5-min reaction even in the presence of feces emulsion, suggesting that the virucidal effect of WAHS on MNV was retained for several minutes or more even in the presence of organic materials. From these results, both hypochlorite-based disinfectants are thought to have definite efficacy with regard to inactivation of MNV in the presence of organic materials. As expected, both in the presence and absence of mouse feces emulsion, 70% ethanol showed more than 5 log10 reductions in the virus titer in the 0.5-min reaction as previously described [2]. These results indicated that the usual use of 70% ethanol is effective enough to inactivate MNV in animal facilities.
It was reported that chlorination (10–13 ppm) of drinking water greatly reduced the colonization of P. aeruginosa in the intestine of mice [20]. Another researcher showed that chlorinated drinking water, containing 6–8 ppm of available chlorine, cleared mice of infection with P. aeruginosa [9]. Another important advantage of WAHS is that it can be drank without dilution. Therefore, we tried to eliminate MNV from mice infected with MNV by substituting WAHS for their drinking water. MNV was not detected in the duodenum of WAHS-drinking mice 4 weeks after the mice started to drink WAHS. The detection rate of MNV in the jejunum of WAHS-drinking mice also became lower than that of control mice. However, MNV was detected in the cecum and successively excreted in feces of all the mice examined. These results suggested that the effect of WAHS on MNV was limited to the small intestine. MNV was reported to be excreted in the feces of mice on day 1 post oral inoculation [8], suggesting the rapid propagation of MNV in the mouse cecum. Therefore, even if WAHS reaches the cecum of the mouse and partly inactivates MNV, the rest of the MNV may rapidly propagate and be excreted in feces.
It is well known that HOCl, the main effective form of chlorine in WAHS, oxidizes organic materials and rapidly loses its disinfection efficacy. So, the HOCl in WAHS is possibly decreased by contact with organic materials in the stomach and intestine, and a sufficient volume of HOCl to inactivate MNV might be unable to come into contact with MNV in the mouse intestine.
Finally, we investigated whether hypochlorite-based disinfectants could prevent mice from getting infected with MNV. Mice drank WAHS or 1:6,000 diluted Purelox for 2 or 4 weeks and then were placed in a cage with a mouse infected with MNV. RT-nested PCR showed that MNV was excreted in feces of disinfectant-drinking mice as early as 1 week after cohabitation. These results showed that MNV infection was not prevented by drinking of hypochlorite-based disinfectants and suggested that the virucidal effects of these disinfectants were not retained for a long time in the mouse intestine.
Previously, it was reported that sentinel mice excreted MNV in feces after 2 days of cohabitation with a mouse infected with MNV [8], indicating that MNV rapidly spread to a mouse and propagated in its cecum. In addition, MNV survives in mouse feces stored at room temperature for 2 weeks [17] and is supposed to require only a small number of viral particles (less than 100 particles) to initiate infection like human norovirus [22]. The propagation velocity, environmental stability, and infectivity of MNV might cause the difficulty in preventing MNV infection by an intermittent inactivation effect.
Fostering was reported to be effective in preventing neonatal mice from becoming infected with MNV [4]. From the result that MNV was not detected in the ovaries and uteri of MNV-infected mice, embryo transfer and caesarean section are also suggested to be efficient means of eliminating MNV [8]. However, a method of eliminating MNV has not been established, and it is not easy to eradicate MNV from contaminated facilities by the test-and-removal method [14]. In this study, we tried to eliminate and prevent MNV infection in mice by supplying WAHS or diluted Purelox as drinking water as easy and low-cost methods. Unfortunately, drinking disinfectants was not effective, so it is important to keep the facility environment clean by use of effective disinfectants. Also, animals introduced into facilities should be tested as MNV free by quarantine and periodically confirmed as MNV free by microbiological monitoring using ELISA, IFA [15], and RT-PCR methods [10].
Acknowledgments
We thank Tomoichiro Oka (National Institute of Infectious Diseases) for providing primers for RT-nested PCR.
This work was supported by a Grant-in-Aid for Scientific Research (23650201).
References
- 1.Bae J., Schwab K.J.2008. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl. Environ. Microbiol. 74: 477–484. doi: 10.1128/AEM.02095-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belliot G., Lavaux A., Souihel D., Agnello D., Pothier P.2008. Use of murine norovirus as a surrogate to evaluate resistance of human norovirus to disinfectants. Appl. Environ. Microbiol. 74: 3315–3318. doi: 10.1128/AEM.02148-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon J.L., Papafragkou E., Park G.W., Osborne J., Jaykus L.A., Vinjé J.2006. Surrogates for the study of norovirus stability and inactivation in the environment: A comparison of murine norovirus and feline calicivirus. J. Food. Prot. 69: 2761–2765. [DOI] [PubMed] [Google Scholar]
- 4.Compton S.R.2008. Prevention of murine norovirus infection in neonatal mice by fostering. J. Am. Assoc. Lab. Anim. Sci. 47: 25–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Cox C., Cao S., Lu Y.2009. Enhanced detection and study of murine norovirus-1 using a more efficient microglial cell line. Virol. J. 6: 196–202. doi: 10.1186/1743-422X-6-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duizer E., Schwab K.J., Neill F.H., Atmar R.L., Koopmans M.P., Estes M.K.2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85: 79–87. doi: 10.1099/vir.0.19478-0 [DOI] [PubMed] [Google Scholar]
- 7.Fukuzaki S.2006. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol. Sci. 11: 147–157. doi: 10.4265/bio.11.147 [DOI] [PubMed] [Google Scholar]
- 8.Goto K., Hayashimoto N., Yasuda M., Ishida T., Kameda S., Takakura A., Itoh T.2009. Molecular detection of murine norovirus from experimentally and spontaneously infected mice. Exp. Anim. 58: 135–140. doi: 10.1538/expanim.58.135 [DOI] [PubMed] [Google Scholar]
- 9.Homberger F.R., Pataki Z., Thomann P.E.1993. Control of Pseudomonas aeruginosa infection in mice by chlorine treatment of drinking water. Lab. Anim. Sci. 43: 635–637. [PubMed] [Google Scholar]
- 10.Hsu C.C., Wobus C.E., Steffen E.K., Riley L.K., Livingston R.S.2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 12: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu C.C., Riley L.K., Wills H.M., Livingston R.S.2006. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 56: 247–251. [PubMed] [Google Scholar]
- 12.Kahan S.M., Liu G., Reinhard M.K., Hsu C.C., Livingston R.S., Karst S.M.2011. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology 421: 202–210. doi: 10.1016/j.virol.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karst S.M., Wobus C.E., Lay M., Davidson J., Virgin H.W., 4th2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299: 1575–1578. doi: 10.1126/science.1077905 [DOI] [PubMed] [Google Scholar]
- 14.Kastenmayer R.J., Perdue K.A., Elkins W.R.2008. Eradication of murine norovirus from a mouse barrier facility. J. Am. Assoc. Lab. Anim. Sci. 47: 26–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagawa Y., Tohya Y., Ike F., Kajita A., Park S.J., Ishii Y., Kyuwa S., Yoshikawa Y.2010. Indirect ELISA and indirect immunofluorescent antibody assay for detecting the antibody against murine norovirus S7 in mice. Exp. Anim. 59: 47–55. doi: 10.1538/expanim.59.47 [DOI] [PubMed] [Google Scholar]
- 16.Lay M.K., Atmar R.L., Guix S., Bharadwaj U., He H., Neill F.H., Sastry K.J., Yao Q., Estes M.K.2010. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology 406: 1–11. doi: 10.1016/j.virol.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manuel C.A., Hsu C.C., Riley L.K., Livingston R.S.2008. Soiled-bedding sentinel detection of murine norovirus 4. J. Am. Assoc. Lab. Anim. Sci. 47: 31–36. [PMC free article] [PubMed] [Google Scholar]
- 18.McInnes E.F., Rasmussen L., Fung P., Auld A.M., Alvarez L., Lawrence D.A., Quinn M.E., del Fierro G.M., Vassallo B.A., Stevenson R.2011. Prevalence of viral, bacterial and parasitological diseases in rats and mice used in research environments in Australasia over a 5-y period. Lab. Anim. (NY). 40: 341–350. doi: 10.1038/laban1111-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller B., Klemm U., Mas Marques A., Schreier E. 2007. Genetic diversity and recombination of murine noroviruses in immunocompromised mice. Arch. Virol. 152: 1709–1719. doi: 10.1007/s00705-007-0989-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council1991. Digestive System. pp. 85–163. In: Infectious Diseases of Mice and Rats, National Academy Press, Washington, D.C. [Google Scholar]
- 21.Ono T., Yamashita K., Murayama T., Sato T.2012. Microbicidal effect of weak Acid hypochlorous solution on various microorganisms. Biocontrol. Sci. 17: 129–133. doi: 10.4265/bio.17.129 [DOI] [PubMed] [Google Scholar]
- 22.Patel M.M., Hall A.J., Vinjé J., Parashar U.D.2009. Noroviruses: a comprehensive review. J. Clin. Virol. 44: 1–8. doi: 10.1016/j.jcv.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Sosnovtsev S.V., Belliot G., Chang K.O., Prikhodko V.G., Thackray L.B., Wobus C.E., Karst S.M., Virgin H.W., Green K.Y.2006. Cleavage map and proteolytic processing of the murine norovirus nonstructural polyprotein in infected cells. J. Virol. 80: 7816–7831. doi: 10.1128/JVI.00532-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wobus C.E., Karst S.M., Thackray L.B., Chang K.O., Sosnovtsev S.V., Belliot G., Krug A., Mackenzie J.M., Green K.Y., Virgin H.W.2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2: e432. doi: 10.1371/journal.pbio.0020432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wobus C.E., Thackray L.B., Virgin H.W., 4th2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 80: 5104–5112. doi: 10.1128/JVI.02346-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng D.P., Ando T., Fankhauser R.L., Beard R.S., Glass R.I., Monroe S.S.2006. Norovirus classification and proposed strain nomenclature. Virology 346: 312–323. doi: 10.1016/j.virol.2005.11.015 [DOI] [PubMed] [Google Scholar]


