Abstract
Adipose tissues are closely connected with the immune system. It has been suggested that metabolic syndromes such as type 2 diabetes, arteriosclerosis and liver steatosis can be attributed to adipose tissue inflammation characterized by macrophage infiltration. To understand a physiological and pathological role of natural killer T (NKT) cells on inflammation in adipose tissue, we characterized a subset of NKT cells in abdominal and subcutaneous adipose tissues in C57BL/6J mice fed normal or high-fat diets. NKT cells comprised a larger portion of lymphocytes in adipose tissues compared with the spleen and peripheral blood, with epididymal adipose tissue having the highest number of NKT cells. Furthermore, some NKT cells in adipose tissues expressed higher levels of CD69 and intracellular interferon-γ, whereas the Vβ repertoires of NKT cells in adipose tissues were similar to other cells. In obese mice fed a high-fat diet, adipose tissue inflammation had little effect on the Vβ repertoire of NKT cells in epididymal adipose tissues. We speculate that the NKT cells in adipose tissues may form an equivalent subset in other tissues and that these subsets are likely to participate in adipose tissue inflammation. Additionally, the high expression level of CD69 and intracellular IFN-γ raises the possibility that NKT cells in adipose tissue may be stimulated by some physiological mechanism.
Keywords: adipose tissue, diet induced obesity, NKT cell, Vβ repertoire
Introduction
The World Health Organization (WHO) recently estimated that more than one billion adults were overweight and 500 million were obese [34]. Numerous recent studies have shown that not only are metabolic syndromes such as type 2 diabetes, dyslipidemia, liver steatosis and arteriosclerosis caused by obesity, but also are cancers, strokes and other noncommunicable diseases [10].
Over the past 10 years, a large number of studies have established that chronic adipose tissue inflammation is closely associated with metabolic syndromes. Macrophages resident in adipose tissues activated by the innate immune response are thought to induce adipocyte death and lead to the secretion of several inflammatory mediators, resulting in inflammation in adipose tissues. Studies have shown that tumor necrosis factor α (TNF-α), IL-6, and IL-1β are produced in adipose tissues and the liver, and are exposed to inflammatory mediators. A long-term, low-dose exposure to these cytokines is connected to adipose tissue insulin resistance [7]. Other immune cell types, such as T helper cells, cytotoxic T cells, B cells, and mast cells, also cause adipose tissue inflammation [19, 24, 32, 33]. This makes it necessary to understand the role of each of the immune cells involved in adipose tissue inflammation, to develop new immunological therapies for metabolic syndrome. Although previous studies have reported a role for NKT cells in adipose tissue inflammation [17, 21, 27], this is controversial and little is known of the NKT cells resident in adipose tissue in lean mice.
NKT cells have the properties of NK and T cells. Unlike T cells, which recognize peptides, NKT cells are unique in recognizing glycolipids as antigens. These antigens are presented by the major histocompatibility complex (MHC) class 1-like molecule, CD1d [12]. Recent research suggests that NKT cells comprise various subsets. A major one of these, invariant NKT (iNKT) (also known type 1 NKT) cells, expresses an invariant T cell antigen receptor (TCR), the α-chain of which is formed by a variable region 14 α-chain and joint region 18 α-chain (Vα14Jα18) in mice and Vα24Jα18 in humans, and the variable β-chain (Vβ) of which has the following variants: Vβ8, Vβ7, and Vβ2. Moreover, NKT cells are classified into CD4-positive and CD4-negative groups [12].
NKT cells may be involved in both defense against microbial pathogens and the anti-tumor response; this protective effect may be associated with the production of T helper 1 (TH1) cytokines, including interferon-gamma (IFN-γ) [8]. Antitumor NKT cells resident in the liver are primarily CD4-negative [6]. Additionally, NKT cells are thought to be involved in the regulation of several autoimmune diseases including type 1 diabetes, bronchial asthma and experimental autoimmune encephalomyelitis [22, 25, 26, 27]. For example, production of TH2 cytokines, including IL-4 and IL-13, by NKT cells not only protects against experimental autoimmune encephalomyelitis by suppressing the TH1 response, but also induces airway hyperreactivity, a prime feature of the TH2 response, which promotes asthma [22, 27].
The immunological functions of NKT cells are thought to change within the subsets and according to environment [4, 29]. Therefore, identification of NKT cell subsets resident in adipose tissue may provide an important clue to the physiological and pathological roles of NKT cells. White adipose tissues are present in the abdominal cavity and subcutaneous areas, and are anatomically and physiologically distinct [15]. In this study, we evaluated the proportions of NKT, NK and T cells in abdominal and subcutaneous adipose tissue, the spleen and peripheral blood and identified a major subset of NKT cells resident within adipose tissue. Moreover, we show that NKT cells in adipose tissue were more active than those in the spleen and peripheral blood, and we investigated the TCR Vβ repertoire and the change in the main subset of NKT cells in obesity using diet-induced obese mice.
Materials and Methods
Animals
We used male C57BL/6J mice. Mice were housed under a 12-h light/12-h dark cycle and fed a normal CRF-1 diet (6% fat, Oriental Yeast Co., Ltd., Tokyo, Japan) or a high-fat diet (D12492, 60 Kcal% fat, Research Diets, New Brunswick, NJ, USA) ad libitum. For the diet-induced obese (DIO) mice, C57BL/6J mice were obtained at 7 weeks, acclimated for 1 week and then fed a high-fat diet from 8 until 20 weeks. Mice were maintained under an SPF environment, and all animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Tokyo, and performed in accordance with the guidelines for animal experiments.
Flow cytometry
We referred to adipose tissue protocols described by Brake et al. [2]. Epididymal adipose tissue and the spleen were removed from 20-week-old C57BL/6J male mice. Stromal vascular fraction (SVF) from adipose tissues was isolated by digesting adipose tissue with 2 mg/ml type 2 collagenase (Worthington Biochemical Corporation, Lakewood, NJ, USA), removing adipocytes. Splenocytes were isolated by physically dissociating spleens between the frosted ends of two glass slides. Red blood cells in SVF and splenocytes were removed with ACK lysing buffer.
The following monoclonal antibodies which were conjugated with biotin, FITC, PE, PE-Cy7, PerCP-Cy5.5, APC, APC-Cy7, and PE-Cy7 were purchased from eBioscience (San Diego, CA, USA), Miltenyi Biotec (Bergisch Gladbach, Germany) and BioLegend (San Diego, CA, USA): anti-CD3ε (145-2C11), anti-NK1.1 (PK136), anti-CD69 (H1.2F3) and anti-IFN-γ (XMG1.2) antibodies, and rat IgG isotype control (eBRG1). Purified anti-FcγRII/III (2.4G2) antibody for FcR blocking was purchased from Ancell (Bayport, MN, USA). Streptavidin PerCP-Cy5.5 was purchased from eBioscience.
For intracellular cytokines staining, SVF was cultured in RPMI-1640 (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS, and stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Life Technologies) and 500 ng/ml ionomycin (Life Technologies) for 5 h, and added to 10 µg/ml brefeldin A (eBioscience) at 2 h after stimulation at 37°C. Single-cell suspensions were incubated with anti-FcγRII/III antibody for 20 min, incubated with anti-cell surface marker mAbs for 30 min on ice and then washed. Subsequently, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature, and permeabilized by PBS with 0.5% saponin (Life technologies) and 0.5% BSA, and incubated with anti-cytokine mAbs overnight at 4°C. Data were acquired by flow cytometry with; FACSCaliber and FACSCanto II systems (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). The obtained data were analyzed with the Flowjo software (Tree Star, Inc., Ashland, OR, USA) and CellQuest software (Becton, Dickinson and Co.). SVF was gated according to cell size (FSC-H) and granularity (SSC-H) criteria as described elsewhere [5].
Histology
Mice were sacrificed by cervical dislocation. Epididymal adipose tissues were dissected, fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 4 µm. Sections were stained with hematoxylin and eosin.
Adipose tissue imaging
Three-dimensional adipose tissue imaging was performed with a confocal laser scanning microscope (LSM 510 META, Carl Zeiss, Oberkochen, Germany), and all images were scanned with <5 µm. Epididymal adipose tissue was removed from 20-week-old C57BL/6J mice and minced into small pieces <1 mm. The fat pieces were fixed with 4% paraformaldehyde for 45 min, permeabilized with 1% Triton-X 100 for 10 min and incubated with Hoechst 33342 (Life Technologies, Carlsbad, CA, USA), BODIPY FLC12 (Life Technologies), and purified anti-CD3ε (145-2C11) and anti-NK1.1 (PK136) antibodies. DyLight 649-conjugated anti-hamster IgG (BioLegend, San Diego, CA, USA) and Alexa 568-conjugated anti-mouse IgG (Life Technologies) antibodies were used as secondary antibodies.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed as reported previously [13]. Twenty-week-old lean and DIO mice were fasted for 16 h but had access to drinking water at all times. On the following day, intraperitoneal glucose or insulin tolerance tests were performed. Blood glucose concentration was determined using an Accu-Chek Active blood glucose monitor (Roche Diagnostics, Basel, Switzerland). Mice were administered 2 g/kg glucose intraperitoneally, and the blood glucose concentration was measured at 0, 15, 30, 60, 90, 120, 150, and 180 min post injection. For the intraperitoneal insulin tolerance test, mice were administered intraperitoneally 0.5 IU/kg of insulin, and the blood glucose concentration was determined at 0, 30, 45, 60, 75, and 90 min post injection.
Statistical analysis
All data were evaluated using a two-tailed Student’s t-test to determine statistical significance, unless otherwise specified. The two-tailed Student’s t-test was performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA). A value of P<0.05 was considered to indicate statistical significance.
Results
Correlation between adipose tissue weights
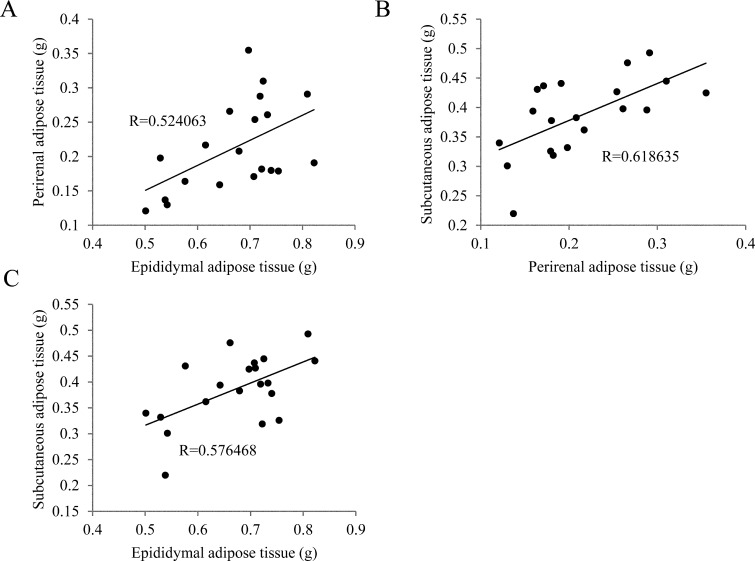
We assessed the relationship between body weight and adipose tissue weight in 20-week-old male mice. First, we extirpated epididymal and perirenal adipose tissue, and detached femoral subcutaneous adipose tissue from the femoral dermal, and then weighed the tissues. Body weight was not correlated with abdominal adipose tissue weights (data not shown). Both correlation coefficients (body weight vs. epididymal and perirenal adipose tissue) were <0.4 (data not shown). In contrast, adipose tissue weight was correlated with other adipose tissue weights, especially that of perirenal adipose tissue, which correlated highly with subcutaneous adipose tissue weight (Fig. 1). Subcutaneous and visceral adipose tissues are thought to have different metabolic functions, and these results suggested that the quantities of these adipose tissues were equal [15], whereas body weight did not provide an accurate indication of the quantity of adipose tissue in lean mice.
Fig. 1.
Correlations among adipose tissue weights. (A–C) Epididymal, perirenal and subcutaneous adipose tissues from 20-week-old mice. Epididymal adipose tissue weight was correlated with those of perirenal and subcutaneous adipose tissues. Perirenal adipose tissue weight was strongly correlated with that of subcutaneous adipose tissue (n=20).
Fraction of lymphocytes in adipose tissues, the spleen and peripheral blood
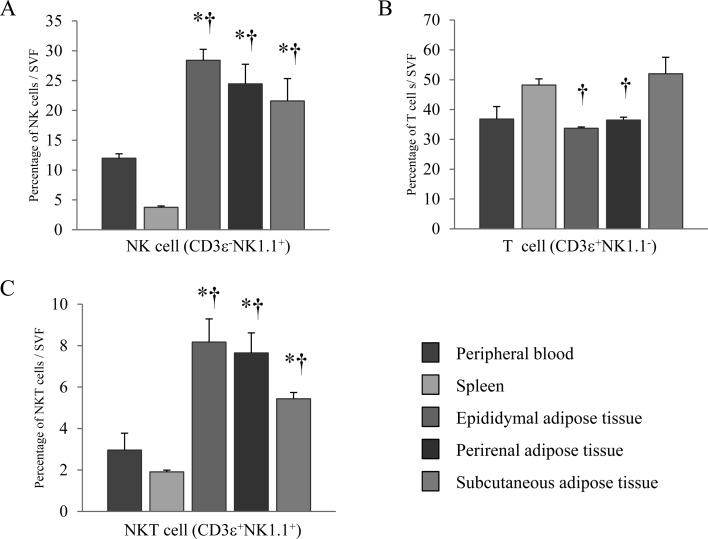
Both epididymal and subcutaneous adipose tissues contained many primitive lymphocytes, NK cells and NKT cells [5]. To confirm the fraction of lymphocytes present in adipose tissue, we enumerated NK cells (CD3ε−NK1.1+), T cells (CD3ε+NK1.1−), and NKT cells (CD3ε+NK1.1+). Their numbers were compared with those in the spleen (a major lymphoid tissue) and in peripheral blood. Adipose tissue consists primarily of adipocytes and stromal and vascular cells; we assessed only stromal and vascular cells by flow cytometry. The percentage of T cells present in epididymal and perirenal adipose tissue was equal to that in peripheral blood, but significantly less than in the spleen. However, the percentage of T cells in subcutaneous adipose tissue was equal to that in the spleen but slightly more than in peripheral blood (Fig. 2B). The proportions of NK and NKT cells in adipose tissues were higher than those in the spleen and peripheral blood (Fig. 2A and 2C). These results suggest that both NK and NKT cells were found more often in adipose tissue than in other tissues. In addition, these findings suggest that NKT cells play a pivotal role in the physiology of adipose tissue.
Fig. 2.
Lymphocytes fractions in adipose tissues, the spleen and peripheral blood. (A) NK cells (CD3ε−NK1.1+), (B) T cells (CD3ε+NK1.1−), and (C) NKT cells (CD3ε+NK1.1+) in SVFs from the spleen, peripheral blood and epididymal, perirenal and subcutaneous adipose tissues were evaluated by flow cytometry. SVFs derived from adipose tissues were obtained by removal of adipocytes from 20-week-old mice. Data represent means ± SEM (n=5).*P<0.05 vs. peripheral blood, †P<0.05 vs. spleen.
Expression of cell-surface markers and intracellular IFN-γ of NKT cells
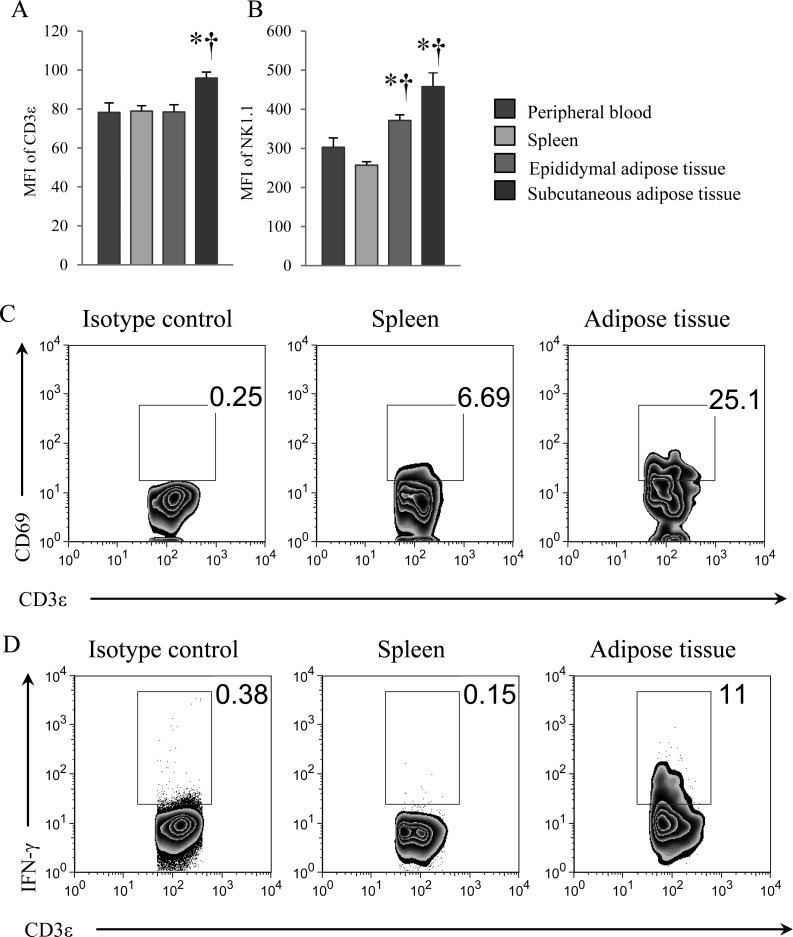
CD3ε and NK1.1 are cell-surface molecules on NKT cells [3, 20, 31]. To characterize NKT cells in adipose tissues, the expression levels of CD3ε and NK1.1 were determined. CD3ε expression on NKT cells in subcutaneous adipose tissue was significantly higher than in the spleen and peripheral blood (Fig. 3A), whereas expression of NK1.1 in epididymal and subcutaneous adipose tissues was significantly higher than in the spleen and peripheral blood (Fig. 3B). We also examined the expression level of CD69, an activation marker of lymphocytes. High expression of CD69 was observed in NKT cells in adipose tissue in comparison with NKT cells in the spleen (Fig. 3C). Furthermore, we investigated whether NKT cells in adipose tissue of nonobese young mice retained the potential to produce cytokines. Surprisingly, some NKT cells in adipose tissue produced a large amount of IFN-γ in comparison with those in the spleen (Fig. 3D). These results suggest that NKT cells in adipose tissue are functionally activated compared with NKT cells in the spleen.
Fig. 3.
Flow cytometric analysis of cell-surface markers and intracellular cytokine of NKT cells. (A) Mean fluorescence intensity (MFI) of CD3ε in NKT cells, and (B) MFI of NK1.1 in NKT cells from peripheral blood, the spleen, and epididymal and subcutaneous adipose tissues. MFI determinations were performed by flow cytometry using 20-week-old mice. (C) Flow cytometric analysis of CD69 expression in NKT cells of spleen and epididymal adipose tissue. (D) Flow cytometric analysis of intracellular IFN-γ in NKT cells of spleen and epididymal adipose tissue. The fraction of NKT cells was gated by CD3ε+ and NK1.1+ cells. Mice were 8 weeks-old. Data represent means ± SEM (n=5).*P<0.05 vs. peripheral blood; †P<0.05 vs. spleen.
TCR Vβ repertoire of NKT cells
Almost all iNKT cells have an invariant TCR α-chain, Vα14Jα18 in mice, and limited β-chains, which consist primarily of either Vβ8, Vβ7, or Vβ2. However, the high levels of expression of NKT cell-surface markers in adipose tissues and the potency with regard to producing massive cytokines of NKT cells in adipose tissues suggest that some endogenous antigens of NKT cells are present in nonobese adipose tissue and that NKT cells in adipose tissues express a distinct TCR Vβ repertoire compared with that of NKT cells in peripheral tissues. We therefore assessed the TCR Vβ repertoire of NKT cells in adipose tissues, the spleen and peripheral blood by flow cytometry. NKT cells expressing TCR Vβ8.1 and Vβ8.2 formed the largest group, followed by those expressing Vβ2 and Vβ7, which also accounted for a considerable proportion of cells, particularly in the spleen, peripheral blood and epididymal adipose tissue, whereas NKT cells in subcutaneous adipose tissue showed a different pattern of Vβ repertoire compared with NKT cells in other adipose tissue (Fig. 4). These results indicate that NKT cells in abdominal adipose tissue are composed of similar subsets of NKT cells in the spleen and peripheral circulation, whereas NKT cells in subcutaneous adipose tissue are a distinct subset.
Fig. 4.
TCR Vβ repertoire of NKT cells. NKT cells were isolated from the spleen, peripheral blood and epididymal, perirenal and subcutaneous adipose tissues. Evaluation of TCR Vβ repertoire was performed by flow cytometry. The antibodies used were anti-CD3ε (145-2C11), anti-NK1.1 (PK136) and anti-Vβ2 (B20.6), Vβ3 (KJ25), Vβ4 (KT4), Vβ5 (MR9-4), Vβ6 (44-22-1), Vβ7 (TR310), Vβ8.1, 8.2 (KJ-16), Vβ9 (MR10-2), Vβ10b (KT10b), and Vβ11 (RR3-15) (n=5).
Effects of obesity on NKT cell subsets in adipose tissue
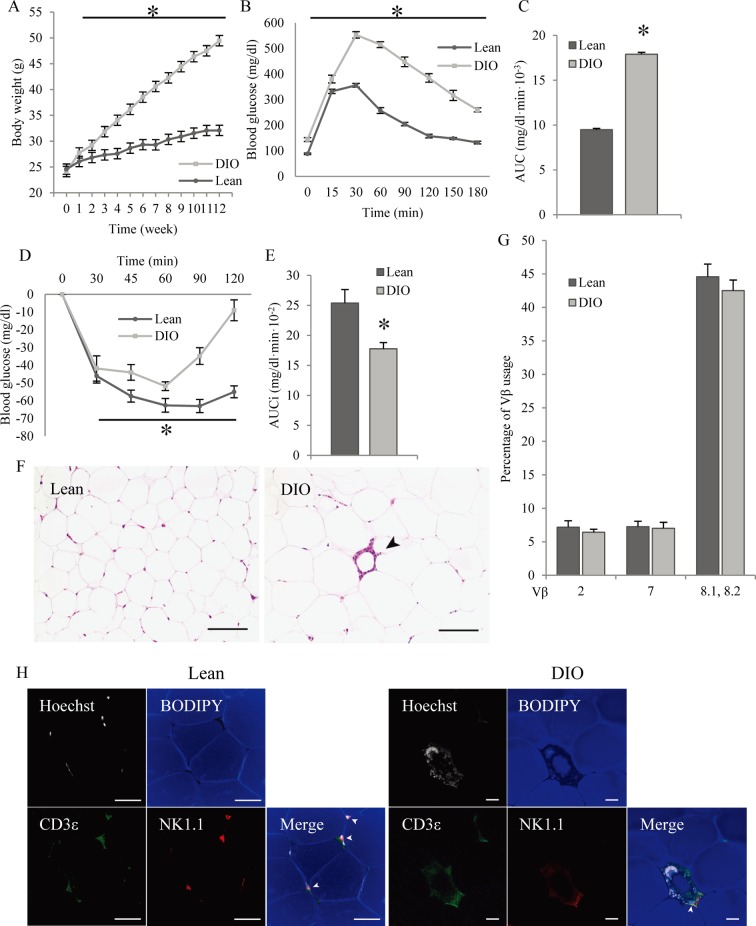
Adipose tissue inflammation, in particular that of epididymal adipose tissue, is an important subject area in immunology [28]. We determined whether adipose tissue inflammation influenced NKT cell subsets in epididymal adipose tissue. We prepared DIO mice as an obesity and type 2 diabetes model by feeding 8-week-old mice a high-fat diet (HFD) for 12 weeks. After 12 weeks, the body weights of the DIO mice reached 49.45 ± 1.33 g, while those of the lean mice reached 32.08 ± 0.67 g. DIO mice gained significant weight compared with lean mice from the first week following the start of the HFD (Fig. 5A).
Fig. 5.
Effects of obesity on NKT subpopulations. Mice were fed a normal diet or a high-fat diet from 8 to 20 weeks-old. (A) Body weight increases over time in lean and DIO mice from 8 to 20 weeks-old (n=6–7). (B) Blood glucose concentration; a glucose-tolerance test was performed after a 16-h fast and intraperitoneal administration of 2 g/kg glucose. Lean and DIO mice were 20 weeks-old, and fed either a normal or high-fat diet for 12 weeks (n=6–7). (C) Calculation of the areas under the curve (AUC) of blood glucose concentrations (Fig. 5B) (n=6–7). (D) Blood glucose concentration; insulin-tolerance test performed after a 16-h fast and intraperitoneal administration of 0.5 IU / kg insulin. Lean and DIO mice were 20 weeks-old and fed either a normal or high-fat diet for 12 weeks (n=6–7). (E) Calculation of the areas under the curve inverse (AUCi) of blood glucose concentrations (Fig. 5D) (n=6–7). (F) Histology of epididymal adipose tissues from 20-week-old lean mice (left) and DIO mice (right). The arrowhead shows a CLS. Bar=100 µm. (G) Proportions of Vβ2-, Vβ7-, Vβ8.1- and Vβ8.2-positive NKT cells in total NKT cells from lean and DIO mice (n=5–6). (H) Three-dimensional adipose tissue imaging of epididymal adipose tissue from 20-week-old lean and DIO mice, performed by confocal microscopy. Nuclei (white), lipid droplets (blue), CD3ε (green), and NK1.1 (red) were counterstained. Arrowheads show NKT cells. White bar=50 µm. All data represent means ± SEM. *P<0.05 vs. lean mice.
To confirm resistance to glucose and insulin, we performed glucose- and insulin-tolerance tests in lean and DIO mice. Blood glucose concentrations in DIO mice were significantly higher than those in lean mice in terms of both the glucose- and insulin-tolerance tests (Fig. 5B and 5D). The area under the curve for the glucose-tolerance test and the incremental area under the curve for the insulin-tolerance test were significantly larger than those in lean mice (Fig. 5C and 5E). These results indicate that DIO mice were sufficiently resistant to glucose and insulin.
Additionally, histopathological examination indicated induction of adipose tissue inflammation in DIO mice. Adipose tissue macrophages aggregated around dead adipocytes to form a distinct crown-like structure (CLS) lesion. In addition, the diameter of adipocytes in DIO mice was significantly larger than that in lean mice (Fig. 5F). These results indicate that feeding an HFD for 12 weeks induced adipose tissue inflammation.
NKT cells expressing TCR Vβ8.1 and Vβ8.2, Vβ7, and Vβ2 formed the major groups in adipose tissues in lean mice (Fig. 4). We thus determined whether NKT cells in inflamed adipose tissues were also composed of NKT cells expressing TCR Vβ8.1 and Vβ8.2, Vβ7, and Vβ2. Although DIO mice clearly exhibited adipose tissue inflammation and type 2 diabetes with glucose and insulin resistance, the proportion of NKT cells expressing TCR Vβ8.1 and Vβ8.2, Vβ7, and Vβ2 in inflamed adipose tissues did not change (Fig. 5G). To investigate whether NKT cells take part in adipose tissue inflammation, we performed adipose tissue imaging. NKT cells (CD3ε+ NK1.1+) were present in adipose tissue of lean and DIO mice, being observed between mature adipocytes in nonobese adipose tissue and composed partially of a CLS lesion in obese adipose tissue (Fig. 5H). These results suggest that NKT cells surely take part in adipose tissue inflammation but that the fraction of NKT cells does not undergo a drastic change as a result of inflammation.
Discussion
The relationship between NKT cells and adipose tissue inflammation is controversial. Some researchers have reported that NKT cells aggravate adipose tissue inflammation [27], while others insist that NKT cells have no effect on adipose tissue inflammation [21]. In this paper, we characterized the TCR Vβ repertoire of NKT cells in both lean and DIO mice and found that NKT cells in adipose tissues of lean mice expressed a higher amount of not only cell-surface CD69 but also intracellular IFN-γ.
NKT cells were thought to exist at lower levels compared with T and B cells in lymphoid tissues, but to form a large proportion of the T cells in the small and large intestines (4–10%), liver (>30%), and the lung (~7%) [1, 11, 16, 23, 25]. However, NKT cells also accounted for ~10% of cells in adipose tissues. This suggests that adipose tissues should be recognized as another site in which NKT cells are resident.
iNKT cells are the most common subset of NKT cells. iNKT cells have a limited semifixed TCR, Vα14Jα18 and either Vβ8, Vβ7, or Vβ2 [12, 25]. Most NKT cells express TCR Vβ8.1 and Vβ8.2, Vβ7, or Vβ2 in adipose tissue, which suggests that iNKT cells represent the major subset in adipose tissues as well as in the spleen and peripheral blood. We assumed that obesity would alter the main subset of NKT cells, and that a specific subset of NKT cells in adipose tissues of DIO mice would affect inflammation via the production of cytokines and growth factors. However, contrary to our expectations, the main subset of NKT cells in adipose tissues of DIO mice seemed to be similar to that of lean mice. These results suggest two possibilities: 1) NKT cells play a limited role in adipose tissue inflammation, or 2) the effects of NKT cells are masked by those of other immune cells.
We were surprised that activation markers on NKT cells in adipose tissues were expressed at higher levels than those in the spleen and peripheral blood. It is well-known that the activation via TCR signaling, for example, by treatment with anti-CD3ε antibody, downregulates CD3ε expression in T cells [14], and that stimulation by an exogenous antigen also induced downregulation of cell-surface receptors on iNKT cells [3, 31]. Therefore, we thought that this result may suggest the functions of NKT cells in adipose tissue were suppressed in comparison with peripheral NKT cells. However, NKT cells in adipose tissue were unpredictably potent with regard to secretion of large amounts of Th1 cytokines compared with NKT cells in the spleen, and in fact, the expression of CD69, an activation marker, was high in NKT cells in adipose tissue in comparison with NKT cells in the spleen. These data suggests that NKT cells were physiologically activated in adipose tissues even in the absence of inflammation. In lean mice, adipose and other tissues are thought to be maintained in an anti-inflammatory and insulin-sensitive state [28]. NKT cells are stimulated by the proinflammatory cytokines IL-12 and IL-18 and by TCR signaling. It is likely that in lean mice, these proinflammatory cytokines are produced at very low concentrations in adipose tissues [30]. Hence, antigen recognition via CD1d may be associated with the activation of NKT cells in normal adipose tissue. Recently, it has been reported that gram-positive-bacteria-derived antigens can activate NKT cells, but that adipose tissue presents a sanctuary from infectious diseases, since to date, few infectious organisms have been reported in adipose tissues [18]. Therefore, the probability of exogenous antigens being presented to NKT cells without inflammation of the adipose tissue is extremely slim. Our findings suggest the presentation of novel endogenous antigens to NKT cells situated in normal adipose tissue. Endogenous antigens of NKT cells are also present in the thymus, and one is known to promote production of IL-4 [9]. Endogenous antigens in adipose tissues, as well as in the thymus, also regulate NKT cells in the anti-inflammatory state. In this study, the activated NKT cells we described were stimulated by an unknown endogenous antigen and contributed to maintenance of adipose tissue homeostasis.
Acknowledgments
We thank Dr. Takashi Sekiya for technical assistance. This work was supported by JSPS KAKENHI Grant Number 23650230.
References
- 1.Bannai M., Kawamura T., Naito T., Kameyama H., Abe T., Kawamura H., Tsukada C., Watanabe H., Hatakeyama K., Hamada H., Nishiyama Y., Ishikawa H., Takeda K., Okumura K., Taniguchi M., Abo T.2001. Abundance of unconventional CD8(+) natural killer T cells in the large intestine. Eur. J. Immunol. 31: 3361–3369. doi: [DOI] [PubMed] [Google Scholar]
- 2.Brake D.K., Smith C.W.2008. Flow cytometry on the stromal-vascular fraction of white adipose tissue. Methods Mol. Biol. 456: 221–229. doi: 10.1007/978-1-59745-245-8_16 [DOI] [PubMed] [Google Scholar]
- 3.Braun N.A., Mendez-Fernandez Y.V., Covarrubias R., Porcelli S.A., Savage P.B., Yagita H., Van Kaer L., Major A.S.2010. Development of spontaneous anergy in invariant natural killer T cells in a mouse model of dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 30: 1758–1765. doi: 10.1161/ATVBAHA.110.206045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brutkiewicz R.R., Sriram V.2002. Natural killer T (NKT) cells and their role in antitumor immunity. Crit. Rev. Oncol. Hematol. 41: 287–298. doi: 10.1016/S1040-8428(01)00198-6 [DOI] [PubMed] [Google Scholar]
- 5.Caspar-Bauguil S., Cousin B., Galinier A., Segafredo C., Nibbelink M., André M., Casteilla L., Pénicaud L.2005. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 579: 3487–3492. doi: 10.1016/j.febslet.2005.05.031 [DOI] [PubMed] [Google Scholar]
- 6.Crowe N.Y., Coquet J.M., Berzins S.P., Kyparissoudis K., Keating R., Pellicci D.G., Hayakawa Y., Godfrey D.I., Smyth M.J.2005. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202: 1279–1288. doi: 10.1084/jem.20050953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donath M.Y., Shoelson S.E.2011. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11: 98–107. doi: 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 8.Duwaerts C.C., Gregory S.H.2011. Targeting the diverse immunological functions expressed by hepatic NKT cells. Expert. Opin. Ther. Targets 15: 973–988. doi: 10.1517/14728222.2011.584874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facciotti F., Ramanjaneyulu G.S., Lepore M., Sansano S., Cavallari M., Kistowska M., Forss-Petter S., Ni G., Colone A., Singhal A., Berger J., Xia C., Mori L., De Libero G.2012. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 13: 474–480. doi: 10.1038/ni.2245 [DOI] [PubMed] [Google Scholar]
- 10.Faulds M.H., Dahlman-Wright K.2012. Metabolic diseases and cancer risk. Curr. Opin. Oncol. 24: 58–61. doi: 10.1097/CCO.0b013e32834e0582 [DOI] [PubMed] [Google Scholar]
- 11.Godfrey D.I., Hammond K.J.L., Poulton L.D., Smyth M.J., Baxter A.G.2000. NKT cells: facts, functions and fallacies. Immunol. Today 21: 573–583. doi: 10.1016/S0167-5699(00)01735-7 [DOI] [PubMed] [Google Scholar]
- 12.Godfrey D.I., Stankovic S., Baxter A.G.2010. Raising the NKT cell family. Nat. Immunol. 11: 197–206. doi: 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- 13.Heikkinen S., Argmann C.A., Champy M.F., Auwerx J.2007. Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. Chapter 29: Unit 29B.3. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch R., Eckhaus M., Auchincloss H., Sachs D.H., Bluestone J.A.1988. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J. Immunol. 140: 3766–3772. [PubMed] [Google Scholar]
- 15.Ibrahim M.M.2010. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 11: 11–18. doi: 10.1111/j.1467-789X.2009.00623.x [DOI] [PubMed] [Google Scholar]
- 16.Ishimoto Y., Tomiyama-Miyaji C., Watanabe H., Yokoyama H., Ebe K., Tsubata S., Aoyagi Y., Abo T.2004. Age-dependent variation in the proportion and number of intestinal lymphocyte subsets, especially natural killer T cells, double-positive CD4(+) CD8(+) cells and B220(+) T cells, in mice. Immunology 113: 371–377. doi: 10.1111/j.1365-2567.2004.01961.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Y., Sun S., Xu A., Bhargava P., Yang L., Lam K.S., Gao B., Lee C.H., Kersten S., Qi L.2012. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via the IL-4/STAT6 signaling axis in obesity. J. Biol. Chem. 287: 13561–13571. doi: 10.1074/jbc.M112.350066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinjo Y., Illarionov P., Vela J.L., Pei B., Girardi E., Li X., Li Y., Imamura M., Kaneko Y., Okawara A., Yesilkaya H., Andrew P.W., Wong C.H., Kawakami K., Nizet V., Besra G.S., Tsuji M., Zajonc D.M., Kronenberg M.2011. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat. Immunol. 12: 966–974. doi: 10.1038/ni.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Divoux A., Sun J., Zhang J., Clement K., Glickman J.N., Sukhova G.K., Wolters P.J., Du J., Gorgun C.Z., Doria A., Libby P., Blumberg R.S., Kahn B.B., Hotamisligil G.S., Shi G.P.2009. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15: 940–945. doi: 10.1038/nm.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljutic B., Carlyle J.R., Filipp D., Nakagawa R., Julius M., Zuniga-Pflucker J.C.2005. Functional requirements for signaling through the stimulatory and inhibitory mouse NKR-P1 (CD161) NK cell receptors. J. Immunol. 174: 4789–4796. [DOI] [PubMed] [Google Scholar]
- 21.Mantell B.S., Stefanovic-Racic M., Yang X., Dedousis N., Sipula I.J., O’Doherty R.M.2011. Mice Lacking NKT Cells but with a Complete Complement of CD8(+) T-Cells Are Not Protected against the Metabolic Abnormalities of Diet-Induced Obesity. PLoS ONE 6: e19831. doi: 10.1371/journal.pone.0019831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer E.H., DeKruyff R.H., Umetsu D.T.2008. T cells and NKT cells in the pathogenesis of asthma. Annu. Rev. Med. 59: 281–292. doi: 10.1146/annurev.med.59.061506.154139 [DOI] [PubMed] [Google Scholar]
- 23.Middendorp S., Nieuwenhuis E.E.S.2009. NKT cells in mucosal immunity. Mucosal Immunol. 2: 393–402. doi: 10.1038/mi.2009.99 [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R.2009. CD8(+) effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15: 914–920. doi: 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 25.Novak J., Griseri T., Beaudoin L., Lehuen A.2007. Regulation of type 1 diabetes by NKT cells. Int. Rev. Immunol. 26: 49–72. doi: 10.1080/08830180601070229 [DOI] [PubMed] [Google Scholar]
- 26.Oh S.J., Chung D.H.2011. Invariant NKT Cells Producing IL-4 or IL-10, But Not IFN-gamma, Inhibit the Th1 Response in Experimental Autoimmune Encephalomyelitis, Whereas None of These Cells Inhibits the Th17 Response. J. Immunol. 186: 6815–6821. doi: 10.4049/jimmunol.1003916 [DOI] [PubMed] [Google Scholar]
- 27.Ohmura K., Ishimori N., Ohmura Y., Tokuhara S., Nozawa A., Horii S., Andoh Y., Fujii S., Iwabuchi K., Onoe K., Tsutsui H.2010. Natural Killer T Cells Are Involved in Adipose Tissues Inflammation and Glucose Intolerance in Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 30: 193–199. doi: 10.1161/ATVBAHA.109.198614 [DOI] [PubMed] [Google Scholar]
- 28.Osborn O., Olefsky J.M.2012. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18: 363–374. doi: 10.1038/nm.2627 [DOI] [PubMed] [Google Scholar]
- 29.Rhost S., Sedimbi S., Kadri N., Cardell S.L.2012. Immunomodulatory type II natural killer T (NKT) lymphocytes in health and disease. Scand. J. Immunol. 76: 246–255. doi: 10.1111/j.1365-3083.2012.02750.x [DOI] [PubMed] [Google Scholar]
- 30.Strissel K.J., DeFuria J., Shaul M.E., Bennett G., Greenberg A.S., Obin M.S.2010. T-Cell Recruitment and Th1 Polarization in Adipose Tissue During Diet-Induced Obesity in C57BL/6 Mice. Obesity (Silver Spring) 18: 1918–1925. doi: 10.1038/oby.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson M.T., Johansson C., Olivares-Villagómez D., Singh A.K., Stanic A.K., Wang C.R., Joyce S., Wick M.J., Van Kaer L.2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. U.S.A. 100: 10913–10918. doi: 10.1073/pnas.1833166100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer D.A., Winer S., Shen L., Wadia P.P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M.G., Alonso M.N., Leong H.X., Glassford A., Caimol M., Kenkel J.A., Tedder T.F., McLaughlin T., Miklos D.B., Dosch H.M., Engleman E.G.2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617. doi: 10.1038/nm.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., Maezawa Y., Drucker D.J., Engleman E., Winer D., Dosch H.M.2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15: 921–929. doi: 10.1038/nm.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organisation Fact sheet: obesity and overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/print.html Accessed 15 October 2012.