Abstract
A model animal showing spontaneous onset is a useful tool for investigating the mechanism of disease. Here, I would like to introduce two aging model animals expected to be useful for neuroscience research: the senescence-accelerated mouse (SAM) and the klotho mouse. The SAM was developed as a mouse showing a senescence-related phenotype such as a short lifespan or rapid advancement of senescence. In particular, SAMP8 and SAMP10 show age-related impairment of learning and memory. SAMP8 has spontaneous spongy degeneration in the brain stem and spinal cord with aging, and immunohistochemical studies reveal excess protein expression of amyloid precursor protein and amyloid β in the brain, indicating that SAMP8 is a model for Alzheimer’s disease. SAMP10 also shows age-related impairment of learning and memory, but it does not seem to correspond to Alzheimer’s disease because senile plaques primarily composed of amyloid β or neurofibrillary tangles primarily composed of phosphorylated tau were not observed. However, severe atrophy in the frontal cortex, entorhinal cortex, amygdala, and nucleus accumbens can be seen in this strain in an age-dependent manner, indicating that SAMP10 is a model for normal aging. The klotho mouse shows a phenotype, regulated by only one gene named α-klotho, similar to human progeria. The α-klotho gene is mainly expressed in the kidney and brain, and oxidative stress is involved in the deterioration of cognitive function of the klotho mouse. These animal models are potentially useful for neuroscience research now and in the near future.
Keywords: aging, klotho mouse, model animal, neuroscience research, senescence-accelerated mouse
Introduction
In the field of experimental biology, a research hypothesis must be proven by scientific experiments. For this reason, it is as important to investigate how the phenomena going on in vitro are reflected to the vital responses as to resolve the individual behavior into the molecular mechanisms. Using a suitable animal model for a preferred study is a powerful tool in this process. In recent years, studies using animals in which a target gene is artificially altered have been prevalent and have made outstanding progress; they are excellent ways of determining the function of the target gene. However, such a deductive methodology does not work well in some pathological cases because diseases are generally attributed to a combination of a lot of factors. A model animal showing spontaneous onset similar to that of human disease is useful in this regard, and there are a variety of model animals from mice to monkeys addressing the increase in demands. Of course, it should be noted that experimenters always need to check whether the pathophysiological features of the model animals correspond to those of humans. Hypertension, obesity, or diabetes model animals, for example, are relatively simple to apply to disease cases in humans using index parameters. On the other hand, model animals for neuronal diseases, such as dementia, amnesia, or depression model animals, are rather difficult to correlate to cases in humans because it is not easy to evaluate these neurological disorders in animals; we sometimes decide the definition of neural diseases in animals on our own by observing them. Therefore, we need to keep accumulating collateral evidence with these animals from various aspects for continued development of new model animals.
Recently, aging research is attracting attention. Here, I would like to introduce two aging animal models expected to be useful for neuroscience research: the senescence-accelerated mouse (SAM), of which two series are available for neuronal aging, and the klotho mouse. The purpose of this review is to introduce the characteristic of these model animals and to discuss advantages and disadvantages of using these model animals with our findings or data from earlier studies.
SAMP8
The senescence-accelerated mouse (SAM) was developed at Kyoto University through the selective inbreeding of the AKR/J strain [34]. To date, 9 senescence-prone inbred strains (SAMP) and 3 senescence-resistant inbred strains (SAMR) have been established. SAMPs seem to show a senescence-related phenotype such as short lifespan or rapid advancement of senescence [34]. SAMP8 is an especially interesting model animal in terms of mechanism of learning and memory, because it has age-related impairment of learning and memory. It shows short avoidance latency in the retention test for passive avoidance response and long escape latency in the Morris water maze task compared with SAMR1, indicating that SAMP8 shows age-related deterioration of ability in learning and memory [5, 21, 24, 37, 40]. Age-related impairment of this strain is expressed in difficult tasks compared with simple ones [8]. Furthermore, histological techniques revealed that SAMP8 has spontaneous spongy degeneration in the brain stem and spinal cord, in which hypertrophic astrocytes increase with aging [39]. In addition, decreases in muscarinic acetylcholine receptors, alpha 2-adrenoceptors, N-methyl-D-aspartate receptors, and L-type Ca2+ channels in the cerebral cortex and hippocampus were reported [16]. These results indicate that the number of neurons and activity of the neural network, which are relevant to cognitive function, were attenuated in SAMP8.
Another feature of SAMP8 is its Alzheimer’s disease-like pathogenesis such as excess protein expression of amyloid precursor protein (APP) and amyloid β (Aβ), increased oxidative stress, and tau phosphorylation [7, 25, 28]. Antisense oligonucleotides against the Aβ region of APP reduced the levels of APP, which induced oxidative stress, and restored learning and memory [18, 25, 29]. Moreover, altered levels of ApoE (epsilon 4 allele is known to be related to synaptic plasticity or Alzheimer’s disease [14]) and presenilin-2 (polymorphism of presenilin-2 seems to be a risk factor of sporadic Alzheimer’s disease [4]) were reported [18, 38]. Piecing all these findings together, SAMP8 seems to be a good model animal not only for basic research on learning and memory or aging study but also for elucidation of the pathophysiological mechanism of Alzheimer’s disease.
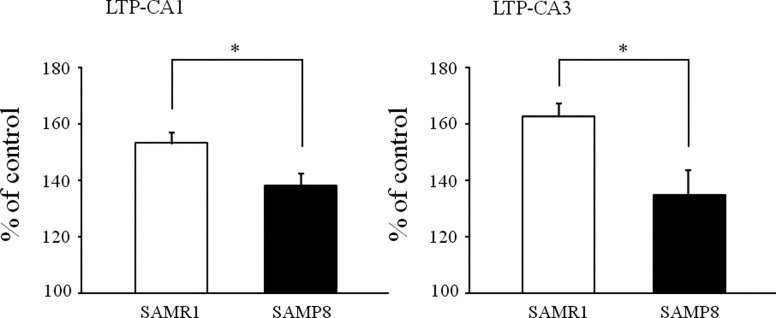
We also use SAMP8 for neuroscience study in our laboratory now. Not many studies about the learning ability of SAMP8 have been performed at the electrophysiological level compared with the studies at the behavioral level as mentioned above. We focus on long-term potentiation (LTP), which is thought to be one of the synaptic plasticities related to learning and memory, since the excitatory postsynaptic potential lasts for hours after theta burst stimulation [1]. An earlier study reported that SAMP8 shows a smaller degree of LTP compared with age-matched SAMR1 after 6 months of age [41]. On the other hand, we record LTP at the hippocampal Schaffer collateral-CA1 synapses and mossy fiber-CA3 synapses in young (2–3 weeks old) animals. There are two reasons why we use young animals; one is to know when senescence starts in SAM strains, and the other is to make it easy to compare the features with ‘‘normal’’ mice, since we already have a lot of data for young normal mice [12]. In our studies, SAMP8 shows attenuated LTP compared with SAMR1 at both CA1 and mossy fiber synapses in 2- to 3-week-old brain slices (Fig. 1, unpublished data). In studies by Katsuki et al., SAMP8 showed attenuated LTP in 10-month-old mice compared with 5-month-old mice as in SAMR1 and no significant changes in LTP between SAMP8 and SAMR1 both at CA1 and CA3 synapses [13]. Although these discrepancies seem to depend on the age of the mice used in the experiments, no phenotypic differences have been reported in 2- to 3-week-old SAMP8 until now. Our results suggest that SAM series may have some genetic backgrounds besides the accelerated senescence-related ones. Because it is an interesting model for gerontological study in addition to the view of the neuroscience of aging, further studies to explore the features of the SAM are expected.
Fig. 1.
Comparison of degree of LTP between 2- to 3-week-old SAMP8 and age-matched SAMR1 mice. LTP levels increase in SAMP8 compared with SAMR1 at both CA1 and CA3 excitatory synapses. (A) LTP at Schaffer collateral-CA1 synapses. (B) LTP at mossy fiber-CA3 synapses.
SAMP10
The other SAM that shows brain dysfunction is SAMP10. This strain of SAM also shows impairment of ability with regard to learning and memory in the passive avoidance test, active avoidance test, and Morris water maze test [23, 27, 32]. However, senile plaques primarily composed of amyloid β or neurofibrillary tangles primarily composed of phosphorylated tau were not observed in SAMP10 [31]. Instead, severe atrophy in the frontal cortex, entorhinal cortex, amygdala, and nucleus accumbens can be seen in this strain in an age-dependent manner [31], indicating that SAMP10 is a model for normal aging rather than Alzheimer’s disease. This atrophy seems to be attributed to the age-related retraction of neuronal dendrites and decrease in the number of spines from a morphological standpoint [33]. Recently, it has been reported that the concentration of catecholamine is decreased in the SAMP10 cortex despite catecholamine metabolites levels that are comparable with SAMR1 [22]. This study suggests that diminished catecholamine synthesis cause impairment of learning and memory in SAMP10. Interestingly, the hippocampus does not show severe atrophy, although performance associated with learning and memory declines. Although the reason for the impairment of ability with regard to learning and memory is partially attributed to reduced motivation and depression [23], there is no electrophysiological data such as LTP pertaining directly to it.
In the brain of SAMP10, pro-inflammatory cytokines such as IL-1β, IL-6, IFN-γ, and TNF-α were more highly expressed than in that of SAMR1 in terms of the mRNA level [17]. However, neuroinflammation induced by intraperitoneal injection of kainate did not induce upregulation of IFN-γ and its receptors in SAMP10 [9]. These studies indicate that neuroinflammation is involved in neurodegeneration in SAMP10 and that cytokine interactions relating to neuroprotection are defective in the brain of this mouse. Recently, it was reported that the superoxide level in the brain of SAMP10 is higher than that of SAMR1 in an age-dependent manner [30]. In addition, antioxidative enzyme glutathione peroxidase activity decreases in an age-dependent manner [15]. Taken together with the results showing that antioxidant catechin intake improves brain functions [35, 36], it is suggested that oxidative damage results in neurodegeneration or atrophy. Further neuroscientific studies using SAMP10 is expected to continue in the future.
Klotho Mouse
The klotho mouse was found by Nabeshima et al. in the process of making transgenic mice in 1997 [19]. This mouse shows a phenotype similar to human progeria such as short lifespan, gait abnormality, hypokinesia, infertility, arteriosclerosis, and osteoporosis. Thus, the klotho mouse is expected to be a human aging model animal. It is interesting that these phenotypes are regulated by only one gene named α-klotho.
The α-klotho gene encodes a single-pass transmembrane protein [19]. Cleaved by α- and β-secretase, which also cleave amyloid precursor protein (APP), Klotho protein function is divided into two types: the membrane type and secreted type. Secreted Klotho is a member of family 1 glycosidases and acts on the transient receptor potential vanilloid 5 (TRPV5) or renal outer medullary potassium channel 1 (ROMK1) on the kidney cell membrane to remove sialic acids [2, 3]. Galactose appears after this removal and binds galectin-1, and this complex suppresses the internalization of TRPV5 and ROMK1, resulting in an increase in Ca2+ influx and K+ efflux, respectively. Secreted Klotho not only regulates ion channel activity but also type 2a sodium-dependent phosphate cotransporter (Npt2a), and suppresses renal transepithelial phosphate reabsorption [10]. These results indicate that secreted Klotho protein is involved in the regulation of glycoproteins in the kidney. Membrane Klotho is known to make a receptor of fibroblast growth factor (FGF) 23 with the FGF receptor [20]. FGF23, a bone-derived phosphaturic hormone, binds to the FGF receptor-Klotho complex to activate transcription of the klotho gene through the mitogen-activated protein kinase (MAPK)-Egr1 signaling pathway [6]. This membrane Klotho converts into secreted Klotho to regulate phosphate homeostasis. In addition to this paracrine system, phosphate homeostasis is also under endocrine control. Phosphate metabolism was thought to be regulated by calcium-regulating hormones such as calcitriol (1,25-dihydroxyvitamin D3) and parathyroid hormones (PTH). But Klotho is expressed in both the kidney and parathyroid to make the FGF receptor-Klotho complex, and FGF23 is secreted by bone, suggesting that the bone-kidney-parathyroid axis maintains phosphate homeostasis instead of the calcium-regulating hormones. Actually, the klotho mouse shows hypervitaminosis D, hyperphosphatemia, and hypercalcemia [19], and the klotho mouse and FGF23-deficient mouse show similar phenotypes exhibiting premature aging [20]. As the Klotho mutation in humans is involved in severe tumoral calcinosis [11], it is likely that Klotho is an important factor for tissue calcification associated with aging.
The α-klotho gene is mainly expressed at the kidney and the brain [19], hence it is likely that the klotho mouse shows diminished performance associated with brain aging. Nagai et al. investigated the cognitive function of the klotho mouse [26]. In their study, object recognition ability and fear conditioning were impaired at 7 weeks of age. At the same time, apoptotic cells were observed in the hippocampus, and oral administration of an antioxidant rescued this apoptosis. Furthermore, an antioxidant restored the cognitive function impaired in the klotho mouse. These data indicate that oxidative stress is involved in the deterioration of the cognitive function of the klotho mouse. However, 6-week-old mice did not show any impairment of learning performance, although increases in oxidative lipid and DNA damage were already observed in 5-week-old mice. Although the cause of this discrepancy still remains unknown, the term between 5th and 6th weeks after birth seems to be a critical period for learning and memory in the klotho mouse.
In this review, I have discussed two model animals that are potentially useful for neuroscience research now and in the near future. As mentioned above, when we apply animal models to neuroscience investigation, we need to verify the pros and cons and carefully pick the models fit for the studies. Although this process is relatively difficult, there is no doubt that the model animal is a powerful tool, so it is desirable for researchers to continue checking for animal characteristics corresponding to human diseases to produce more available models.
References
- 1.Bliss T.V., Lomo T.1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232: 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha S.K., Hu M.C., Kurosu H., Kuro-o M., Moe O., Huang C.L.2009. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol. Pharmacol. 76: 38–46. doi: 10.1124/mol.109.055780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha S.K., Ortega B., Kurosu H., Rosenblatt K.P., Kuro-O. M., Huang C.L.2008. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. U.S.A. 105: 9805–9810. doi: 10.1073/pnas.0803223105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C., Zhou Z., Li M., Qu M., Ma Q., Zhong M., Zhang Y., Yu Z.2012. Presenilin-2 polymorphisms and risk of sporadic AD: evidence from a meta-analysis. Gene 503: 194–199. doi: 10.1016/j.gene.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Chen G.H., Wang Y.J., Wang X.M., Zhou J.N.2004. Accelerated senescence prone mouse-8 shows early onset of deficits in spatial learning and memory in the radial six-arm water maze. Physiol. Behav. 82: 883–890. [DOI] [PubMed] [Google Scholar]
- 6.Choi B.H., Kim C.G., Lim Y., Lee Y.H., Shin S.Y.2010. Transcriptional activation of the human Klotho gene by epidermal growth factor in HEK293 cells; role of Egr-1. Gene 450: 121–127. doi: 10.1016/j.gene.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Del Valle J., Duran-Vilaregut J., Manich G., Casadesús G., Smith M.A., Camins A., Pallàs M., Pelegrí C., Vilaplana J.2010. Early amyloid accumulation in the hippocampus of SAMP8 mice. J. Alzheimers. Dis. 19: 1303–1315. [DOI] [PubMed] [Google Scholar]
- 8.Flood J.F., Morley J.E.1992. Early onset of age-related impairment of aversive and appetitive learning in the SAM-P/8 mouse. J. Gerontol. 47: B52–B59. doi: 10.1093/geronj/47.2.B52 [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa-Ishii S., Takei S., Inaba M., Umegaki H., Chiba Y., Furukawa A., Kawamura N., Hosokawa M., Shimada A.2011. Defects in cytokine-mediated neuroprotective glial responses to excitotoxic hippocampal injury in senescence-accelerated mouse. Brain Behav. Immun. 25: 83–100. doi: 10.1016/j.bbi.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 10.Hu M.C., Shi M., Zhang J., Pastor J., Nakatani T., Lanske B., Razzaque M.S., Rosenblatt K.P., Baum M.G., Kuro-o M., Moe O.W.2010. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 24: 3438–3450. doi: 10.1096/fj.10-154765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichikawa S., Imel E.A., Kreiter M.L., Yu X., Mackenzie D.S., Sorenson A.H., Goetz R., Mohammadi M., White K.E., Econs M.J.2007. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117: 2684–2691. doi: 10.1172/JCI31330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K., Contractor A., Swanson G.T.2004. Attenuated plasticity of postsynaptic kainate receptors in hippocampal CA3 pyramidal neurons. J. Neurosci. 24: 6228–6236. doi: 10.1523/JNEUROSCI.1302-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuki H., Ishihara K., Shimada A., Takeda T., Satoh M.1990. Age-related deterioration of long-term potentiation in the CA3 and CA1 regions of hippocampal slices from the senescence-accelerated mouse. Arch. Gerontol. Geriatr. 11: 77–83. doi: 10.1016/0167-4943(90)90058-E [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Basak J.M., Holtzman D.M.2009. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63: 287–303. doi: 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishido T., Unno K., Yoshida H., Choba D., Fukutomi R., Asahina S., Iguchi K., Oku N., Hoshino M.2007. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology 8: 423–430. doi: 10.1007/s10522-007-9085-7 [DOI] [PubMed] [Google Scholar]
- 16.Kitamura Y., Zhao X.H., Ohnuki T., Nomura Y.1989. Ligand-binding characteristics of [3H]QNB, [3H]prazosin, [3H]rauwolscine, [3H]TCP and [3H]nitrendipine to cerebral cortical and hippocampal membranes of senescence accelerated mouse. Neurosci. Lett. 106: 334–338. doi: 10.1016/0304-3940(89)90186-9 [DOI] [PubMed] [Google Scholar]
- 17.Kumagai N., Chiba Y., Hosono M., Fujii M., Kawamura N., Keino H., Yoshikawa K., Ishii S., Saitoh Y., Satoh M., Shimada A., Hosokawa M.2007. Involvement of pro-inflammatory cytokines and microglia in an age-associated neurodegeneration model, the SAMP10 mouse. Brain Res. 1185: 75–85. doi: 10.1016/j.brainres.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 18.Kumar V.B., Farr S.A., Flood J.F., Kamlesh V., Franko M., Banks W.A., Morley J.E.2000. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides 21: 1769–1775. doi: 10.1016/S0196-9781(00)00339-9 [DOI] [PubMed] [Google Scholar]
- 19.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y.I.1997. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 20.Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.C., Moe O.W., Kuro-o M.2006. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281: 6120–6123. doi: 10.1074/jbc.C500457200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., Cheng H., Zhang X., Shang X., Xie H., Zhang X., Yu J., Han J.2013. Hippocampal neuron loss is correlated with cognitive deficits in SAMP8 mice. Neurol. Sci. 34: 963–969. doi: 10.1007/s10072-012-1173-z [DOI] [PubMed] [Google Scholar]
- 22.Miyajima M., Numata T., Minoshima M., Tanaka M., Nishimura R., Hosokawa T., Kurasaki M., Saito T.2013. Deficiency of catecholamine syntheses caused by downregulation of phosphorylation of tyrosine hydroxylase in the cerebral cortex of the senescence-accelerated mouse prone 10 strain with aging. Arch. Gerontol. Geriatr. 56: 68–74. doi: 10.1016/j.archger.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto M.1997. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 32: 139–148. doi: 10.1016/S0531-5565(96)00061-7 [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto M., Kiyota Y., Yamazaki N., Nagaoka A., Matsuo T., Nagawa Y., Takeda T.1986. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 38: 399–406. doi: 10.1016/0031-9384(86)90112-5 [DOI] [PubMed] [Google Scholar]
- 25.Morley J.E., Kumar V.B., Bernardo A.E., Farr S.A., Uezu K., Tumosa N., Flood J.F.2000. β-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides 21: 1761–1767. doi: 10.1016/S0196-9781(00)00342-9 [DOI] [PubMed] [Google Scholar]
- 26.Nagai T., Yamada K., Kim H.C., Kim Y.S., Noda Y., Imura A., Nabeshima Y., Nabeshima T.2003. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 17: 50–52. [DOI] [PubMed] [Google Scholar]
- 27.Okuma Y., Murayama T., Tha K.K., Yamada C., Hosokawa M., Ishikawa A., Watanabe R., Maekawa M., Nomura Y.2000. Learning deficiency and alterations in acetylcholine receptors and protein kinase C in the brain of senescence-accelerated mouse (SAM)-P10. Mech. Ageing Dev. 114: 191–199. doi: 10.1016/S0047-6374(00)00103-2 [DOI] [PubMed] [Google Scholar]
- 28.Pallas M., Camins A., Smith M.A., Perry G., Lee H.G., Casadesus G.2008. From aging to Alzheimer’s disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). J. Alzheimers Dis. 15: 615–624. [DOI] [PubMed] [Google Scholar]
- 29.Poon H.F., Joshi G., Sultana R., Farr S.A., Banks W.A., Morley J.E., Calabrese V., Butterfield D.A.2004. Antisense directed at the Aβ region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 1018: 86–96. doi: 10.1016/j.brainres.2004.05.048 [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T., Unno K., Tahara S., Shimada A., Chiba Y., Hoshino M., Kaneko T.2008. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell 7: 459–469. doi: 10.1111/j.1474-9726.2008.00394.x [DOI] [PubMed] [Google Scholar]
- 31.Shimada A.1999. Age-dependent cerebral atrophy and cognitive dysfunction in SAMP10 mice. Neurobiol. Aging 20: 125–136. doi: 10.1016/S0197-4580(99)00044-5 [DOI] [PubMed] [Google Scholar]
- 32.Shimada A., Ohta A., Akiguchi I., Takeda T.1993. Age-related deterioration in conditional avoidance task in the SAM-P/10 mouse, an animal model of spontaneous brain atrophy. Brain Res. 608: 266–272. doi: 10.1016/0006-8993(93)91467-7 [DOI] [PubMed] [Google Scholar]
- 33.Shimada A., Tsuzuki M., Keino H., Satoh M., Chiba Y., Saitoh Y., Hosokawa M.2006. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 32: 1–14. doi: 10.1111/j.1365-2990.2006.00632.x [DOI] [PubMed] [Google Scholar]
- 34.Takeda T., Hosokawa M., Higuchi K.1991. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J. Am. Geriatr. Soc. 39: 911–919. [DOI] [PubMed] [Google Scholar]
- 35.Unno K., Fujitani K., Takamori N., Takabayashi F., Maeda K., Miyazaki H., Tanida N., Iguchi K., Shimoi K., Hoshino M.2011. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 45: 966–974. doi: 10.3109/10715762.2011.566869 [DOI] [PubMed] [Google Scholar]
- 36.Unno K., Sugiura M., Ogawa K., Takabayashi F., Toda M., Sakuma M., Maeda K., Fujitani K., Miyazaki H., Yamamoto H., Hoshino M.2011. Beta-cryptoxanthin, plentiful in Japanese mandarin orange, prevents age-related cognitive dysfunction and oxidative damage in senescence-accelerated mouse brain. Biol. Pharm. Bull. 34: 311–317. doi: 10.1248/bpb.34.311 [DOI] [PubMed] [Google Scholar]
- 37.Wang F., Chen H., Sun X.2009. Age-related spatial cognitive impairment is correlated with a decrease in ChAT in the cerebral cortex, hippocampus and forebrain of SAMP8 mice. Neurosci. Lett. 454: 212–217. doi: 10.1016/j.neulet.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 38.Wei X., Zhang Y., Zhou J.1999. Alzheimer’s disease-related gene expression in the brain of senescence accelerated mouse. Neurosci. Lett. 268: 139–142. doi: 10.1016/S0304-3940(99)00396-1 [DOI] [PubMed] [Google Scholar]
- 39.Yagi H., Irino M., Matsushita T., Katoh S., Umezawa M., Tsuboyama T., Hosokawa M., Akiguchi I., Tokunaga R., Takeda T.1989. Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. J. Neuropathol. Exp. Neurol. 48: 577–590. doi: 10.1097/00005072-198909000-00008 [DOI] [PubMed] [Google Scholar]
- 40.Yagi H., Katoh S., Akiguchi I., Takeda T.1988. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res. 474: 86–93. doi: 10.1016/0006-8993(88)90671-3 [DOI] [PubMed] [Google Scholar]
- 41.Yang S., Qiao H., Wen L., Zhou W., Zhang Y.2005. D-serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci. Lett. 379: 7–12. doi: 10.1016/j.neulet.2004.12.033 [DOI] [PubMed] [Google Scholar]