Abstract
Here, to determine the effects of transport stress on blood parameters in dogs, we investigated the changes in hematologic and serum chemical parameters in healthy beagle dogs transported from Beijing, China, to Osaka, Japan, to obtain the background data. Only the activity of serum alkaline phosphatase increased clearly upon arrival, a change attributed to transport stress, but the activity gradually reduced afterward. No marked changes in levels of other blood parameters were noted. Our findings here suggest that alkaline phosphatase is a useful tool for studying transport stress.
Keywords: alkaline phosphatase, blood parameter, dog, transport stress
Introduction
Astellas Pharma Inc. was accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International in 2008. For any animal, veterinary care is important for keeping them healthy [14]. To maintain top quality of data in our experiments using healthy animals, we perform blood tests for hematology and blood chemistry twice a year as part of our health-monitoring program.
Many studies require transportation of animals from relatively far-away locations, and the Kashima Facilities of Astellas Pharma Inc. are located in Osaka, Japan, approximately 2,000 km from the breeding colony of dogs in Beijing, China.
Here, in order to prepare background data on blood parameters for future pharmacological studies, we evaluated the response of blood parameters in beagle dogs before and after transportation.
Materials and Methods
Animals
All animal experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc. Furthermore, the Kashima Facilities at Astellas Pharma Inc. have been awarded Accreditation Status by the AAALAC International.
Healthy male beagle dogs at the age of 6 months (6–8 kg) that were born and raised in China were purchased from Marshall BioResources Japan (Tsukuba, Japan). The dogs were driven to Beijing Capital International Airport, flown to Kansai International Airport, and placed in a single cage. Animals were transported from Kansai International Airport to our animal facility in Osaka on a highway with traffic under controlled temperature conditions (15–25°C) [13], which took approximately 3 h. In our laboratory, the animals were maintained on a 12:12 h light-dark cycle (light on from 07:00 to 19:00) in a controlled temperature (23 ± 1°C) and humidity (55 ± 5%) environment. Animals were given standard laboratory food (250–300 g/animal/day, TC-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum.
Blood parameters measurements
Blood was collected from the cephalic vein of the forelimb using a disposable syringe between 09:00 and 11:00. Whole blood collected into K2EDTA-coated tubes was analyzed using an ADVIA 120 system (Siemens Japan K.K., Tokyo, Japan) for hematologic parameters. For blood chemistry measurements, whole blood was collected in plastic tubes, allowed to clot, and then centrifuged at 3,000 rpm for 10 min at 4°C to separate serum. The resulting supernatant, serum, was assayed using the Automatic Analyzer 7170S (Hitachi Ltd., Tokyo, Japan). Blood samples before transport were collected from 6-month-old healthy male beagle dogs at the breeding facility in Beijing in 2011 and assayed using a Bayer ADVIA 2120 Analyzer for hematologic parameters or an Olympus AU400 Analyzer for blood chemistry measurements.
Results are expressed as the mean ± standard error of the mean (SEM).
Results
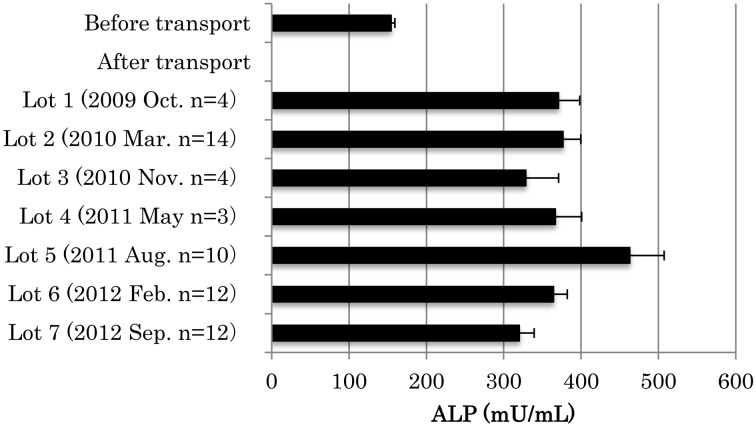
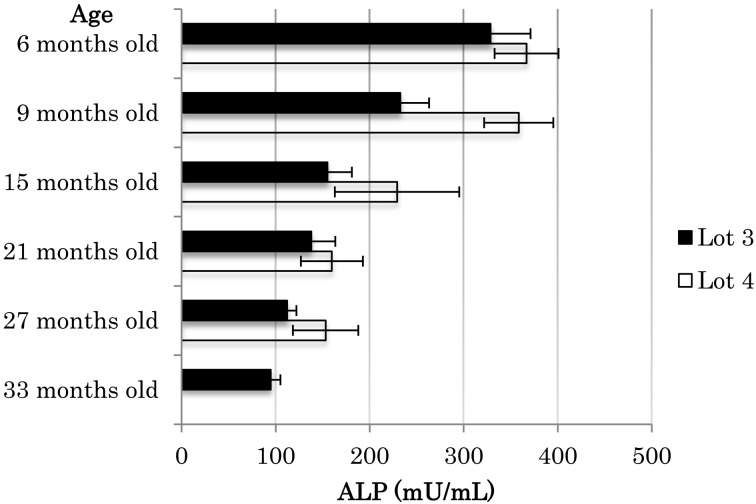
Serum alkaline phosphatase (ALP; EC 3.1.3.1) activity increased from a mean ± SEM (minimum-maximum) of 151 ± 3.9 (93–240) mU/ml before transportation to 340 ± 10.4 (198–601) mU/ml 7 days after arrival. Figure 1 indicates the effects of transport stress on serum ALP activity in 6-month-old beagle dogs purchased in October 2009 (Lot 1; n=4), March 2010 (Lot 2; n=14), November 2010 (Lot 3; n=4), May 2011 (Lot 4; n=3), August 2011 (Lot 5; n=10), February 2012 (Lot 6; n=12), and September 2012 (Lot 7; n=12). The activity of serum ALP in the beagle dogs increased upon arrival relative to the levels before transportation. Figure 2 shows the change in serum ALP activity in beagle dogs with age after arrival at our facility. The activity of serum ALP gradually decreased between 6 and 27 months of age after arrival.
Fig. 1.
Activity of serum ALP in peripheral blood before and after transportation in male beagle dogs at the age of 6 months. Values are means ± SEM.
Fig. 2.
Change in serum ALP activity in peripheral blood after transportation in male beagle dogs purchased at the age of 6 months. Values are means ± SEM.
In contrast, no marked changes were noted in the level of other blood parameters, namely, red blood cells, hemoglobin, hematocrit, mean cell volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets, white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, basophils, leukocytes, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, glucose, triglyceride, total bilirubin, or creatinine (data not shown).
Discussion
Transport can be a stressful experience, both physically and mentally, for many animals [6, 8, 11]. In particular, the loading experience and noise levels are environmental changes that strongly influence transport stress [1]. Here, we investigated the effects of transport stress on the levels of blood parameters in dogs. Serum ALP activity in healthy dogs increased clearly after transport by air. Although different analyzers were used for the analysis, the same method of measuring p-nitrophenol, the product of p-nitrophenol phosphate, was employed to determine serum ALP activity both in the Beijing facility and our laboratory in Japan. Our preliminary study revealed that serum ALP activities upon arrival and 7 days after arrival were 389 ± 21 and 364 ± 18 mU/ml, respectively. These data exclude the possibility that high ALP activity was caused by the environmental differences in the two facilities. Instead, our observations indicate that the change in serum ALP activity is associated with transportation.
Transport stress induces the production of adrenocorticotropin (ACTH) from immune cells such as lymphocytes, eliciting an extremely strong reaction by the adrenocortical system [3, 4]. ACTH subsequently stimulates the release of ALP in adrenocortical cells [9]. Surprisingly, relatively little attention has been given to the study of transport stress in dogs. Our finding using healthy dogs shows an increase in ALP activity after transportation, suggesting that serum ALP may be a useful marker for transport stress in dogs.
ALP is distributed in various tissues, including the liver, bone, intestine, kidneys, mammary glands, and placenta [5]. Serum ALP is used primarily as a biochemical marker of hepatic disease [7], and dogs with osteosarcoma have increased serum ALP activity [10]. Further, ALP activity in dogs is a well-studied indicator of disease. However, the beagle dogs analyzed in this study were normal and healthy throughout the study based on clinical signs and blood parameters expect for ALP.
Increased ALP activity is seen more in young dogs than adults due to bone growth [12, 15]. In addition, ALP activity is highest at birth, and begins to decrease within 10 days [2]. The present study monitored age-related changes in serum ALP activity after arrival at our facility as background data. During the period between the ages of 6 and 27 months, the serum activity of ALP in healthy dogs, which was about 350 mU/ml at 6 months, steadily decreased to about 100–150 mU/ml at 27 months. This gradual decrease in serum ALP activity may reflect not only recovery from the transport stress but also physiological age-dependent changes.
In conclusion, measuring serum ALP activity may be useful as a biochemical marker for transport stress in dogs. To confirm this conclusion, further studies on the relationship between transport stress and serum ALP are needed.
Acknowledgments
The authors would like to thank Mr. H. Abe, Marshall BioResources Japan, for his assistance in collecting the data.
References
- 1.Agnes F., Sartorelli P., Abdi B.H., Locatelli A.1990. Effect of transport loading or noise on blood biochemical variables in calves. Am. J. Vet. Res. 51: 1679–1681. [PubMed] [Google Scholar]
- 2.Center S.A., Randolph J.F., ManWarren T., Slater M.1991. Effect of colostrum ingestion on gamma-glutamyltransferase and alkaline phosphatase activities in neonatal pups. Am. J. Vet. Res. 52: 499–504. [PubMed] [Google Scholar]
- 3.Dixit V.D., Marahrens M., Parvizi N.2001. Transport stress modulates adrenocorticotropin secretion from peripheral bovine lymphocytes. J. Anim. Sci. 79: 729–734. [DOI] [PubMed] [Google Scholar]
- 4.Fazio E., Medica P., Aronica V., Grasso L., Ferlazzo A.2008. Circulating beta-endorphin, adrenocorticotrophic hormone and cortisol levels of stallions before and after short road transport: stress effect of different distances. Acta. Vet. Scand. 50: 6. doi: 10.1186/1751-0147-50-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez N.J., Kidney B.A.2007. Alkaline phosphatase: beyond the liver. Vet. Clin. Pathol. 36: 223–233. doi: 10.1111/j.1939-165X.2007.tb00216.x [DOI] [PubMed] [Google Scholar]
- 6.Fernström A.L., Sutian W., Royo F., Westlund K., Nilsson T., Carlsson H.E., Paramastri Y., Pamungkas J., Sajuthi D., Schapiro S.J., Hau J.2008. Stress in cynomolgus monkeys (Macaca fascicularis) subjected to long-distance transport and simulated transport housing conditions. Stress 11: 467–476. doi: 10.1080/10253890801903359 [DOI] [PubMed] [Google Scholar]
- 7.Fukui Y., Sato J., Sato R., Yasuda J., Naito Y.2006. Canine serum thermostable alkaline phosphatase isoenzyme from a dog with hepatocellular carcinoma. J. Vet. Med. Sci. 68: 1129–1132. doi: 10.1292/jvms.68.1129 [DOI] [PubMed] [Google Scholar]
- 8.González L.A., Schwartzkopf-Genswein K.S., Bryan M., Silasi R., Brown F.2012. Factors affecting body weight loss during commercial long haul transport of cattle in North America. J. Anim. Sci. 90: 3630–3639. doi: 10.2527/jas.2011-4786 [DOI] [PubMed] [Google Scholar]
- 9.Martini C.N., Vaena de Avalos S.G., Vila M.2004. ACTH stimulates the release of alkaline phosphatase through Gi-mediated activation of a phospholipase C and the release of inositolphosphoglycan. Mol. Cell Biochem. 258: 191–199. doi: 10.1023/B:MCBI.0000012855.94291.dd [DOI] [PubMed] [Google Scholar]
- 10.Ryseff J.K., Bohn A.A.2012. Detection of alkaline phosphatase in canine cells previously stained with Wright-Giemsa and its utility in differentiating osteosarcoma from other mesenchynal tumors. Vet. Clin. Pathol. 41: 391–395. doi: 10.1111/j.1939-165X.2012.00445.x [DOI] [PubMed] [Google Scholar]
- 11.Sackett G.P.1981. Pregnancy outcome following jet transport stress in nonhuman primates. J. Med. Primatol. 10: 149–154. [DOI] [PubMed] [Google Scholar]
- 12.Sanecki R.K., Hoffmann W.E., Hansen R., Schaeffer D.J.1993. Quantification of bone alkaline phosphatase in canine serum. Vet. Clin. Pathol. 22: 17–23. doi: 10.1111/j.1939-165X.1993.tb00871.x [DOI] [PubMed] [Google Scholar]
- 13.Schrama J.W., van der Hel W., Gorssen J., Henken A.M., Verstegen M.W.A.1996. Required thermal thresholds during transport of animals. Vet. Q. 18: 90–95. doi: 10.1080/01652176.1996.9694624 [DOI] [PubMed] [Google Scholar]
- 14.Suckow M.A., Doerning B.J.2000. Assessment of veterinary care. pp.447–464. In: The IACUC Handbook (Silverman, J., Suckow, MA., and Muethy, S., eds.), CRC Press, Washngton, D.C. [Google Scholar]
- 15.Syakalima M., Takiguchi M., Yasuda J., Hashimoto A.1997. The age dependent levels of serum ALP isoenzymes and the diagnostic significance of corticosteroid-induced ALP during long-term glucocorticoid treatment. J. Vet. Med. Sci. 59: 905–909. doi: 10.1292/jvms.59.905 [DOI] [PubMed] [Google Scholar]