Abstract
Adiponectin and its receptors have been demonstrated to play important roles in regulating glucose and lipid metabolism in mice. Obesity, type II diabetes and cardiovascular disease are highly correlated with down-regulated adiponectin signaling. In this study, we generated mice overexpressing the porcine Adipor1 transgene (pAdipor1) to study its beneficial effects in metabolic syndromes as expressed in diet-induced obesity, hepatosteatosis and insulin resistance. Wild-type (WT) and pAdipor1 transgenic mice were fed ad libitum with a standard chow diet (Chow) or a high-fat/sucrose diet (HFSD) for 24 weeks, beginning at 6 to 7 weeks of age. There were 12 mice per genetic/diet/sex group. When challenged with HFSD to induce obesity, the pAdipor1 transgenic mice resisted development of weight gain, hepatosteatosis and insulin resistance. These mice had lowered plasma adiponectin, triglyceride and glycerol concentrations compared to WT mice. Moreover, we found that (indicated by mRNA levels) fatty acid oxidation was enhanced in skeletal muscle and adipose tissue, and liver lipogenesis was inhibited. The pAdipor1 transgene also restored HFSD-reduced phosphoenolpyruvate carboxykinase 1 (Pck1) and glucose transporter 4 mRNA in the adipose tissues, implying that the increased Pck1 may promote glyceroneogenesis to reduce glucose intolerance and thus activate the flux of glyceride-glycerol to resist diet-induced weight gain in the adipose tissues. Taken together, we demonstrated that pAdipor1 can prevent diet-induced weight gain and insulin resistance. Our findings may provide potential therapeutic strategies for treating metabolic syndromes and obesity, such as treatment with an ADIPOR1 agonist or activation of Adipor1 downstream targets.
Keywords: adiponectin receptor 1, diet-induced obesity, insulin resistance, pig
Introduction
Chronic intake of a high-fat/sucrose diet (HFSD) is one environmental factor responsible for the development of metabolic syndromes, including type II diabetes, insulin resistance, atherosclerosis and inflammation [1, 33, 35]. In both obese and lean mice, HFSD was induces glucose intolerance and correlates with plasma concentrations of adipokines [36]. Adipose tissues secrete a variety of factors or adipokines, such as leptin, adiponectin, resistin, interleukin-6 and tumor necrosis factor a, which have been demonstrated to play important roles in regulating insulin resistance and metabolic homeostasis. Abdominal obesity is highly correlated with plasma concentrations of these adipokines in human metabolic disorders [11, 16, 42].
Adiponectin is an anti-inflammatory adipokine that increases fatty acid oxidation, decreases gluconeogenesis, improves insulin sensitivity and regulates food intake [20]. Secretory adiponectin, especially the high-molecular-weight form, is abundant in the circulation, and is negatively associated with obesity and type II diabetes mellitus [41]. AMP-activated protein kinase (AMPK) is downstream effector mediating adiponectin action through two major receptors, adiponectin receptors 1 (ADIPOR1) and 2 (ADIPOR2) [41]. In a gene knock-out study Adipor2−/− mice are lean and resistant to a high-fat diet-induced obesity and glucose intolerance, whereas Adipor1−/− may have the opposite functions [6].
Although adenovirus infection has been used to study the functions of ADIPOR1 and ADIPOR2 [43], the molecular mechanism underlying these two receptors in diet-induced metabolic syndrome remains unclear. This laboratory has been interested in porcine adipose tissue lipid metabolism and its regulation, including studies of the cloned pAdipor1 and pAdipor2. Our previous study found that both ADIPOR1 and ADIPOR2 were highly homologous between pigs and mice, and the receptors responded to insulin via the phosphatidylinositol 3-kinase (PI3K) pathway [10, 18, 19]. We have expressed the pADIPOR1 in mice in order to ascertain its metabolic functions and to be able to compare its functions to mADIPOR1. The association of the pADIPOR1 and energy utilization in differ tissues have not been demonstrated. We proposed that pADIPOR1 may act as mADIPOR1 to mediate adiponectin’s function. Therefore, in the current study, the pAdipor1 transgenic mice were challenged with a HFSD to study underlying mechanisms in diet-induced metabolic syndromes.
Materials and Methods
Generation of pAdipor1 transgenic mice
The cDNAs of pAdipor1(Genbank no. AY578142) containing a N-terminal Kozak sequence and a C-terminal FLAG-tag were constructed into the Vitality® pIRES-hrGFP II Mammalian Expression Vector (Stratagene, La Jolla, CA, USA) by swapping the CMV promoter with that of the chicken β-actin (pCX-EGFP). The pAdipor1 is widely expressed and most abundant in the heart and skeletal muscle [10]. Hence, we utilized the chicken β-actin promoter to constantly drive the expression of pAdipor1 in mice. The humanized recombinant green fluorescent protein region was replaced with the red fluorescent protein coding region (pTRE-Tight-DsRed2 expression vector; BD Biosciences Clontech, San Jose, CA, USA) for the construction of the pAdipor1 transgene (also see Supplemental Fig. 1: refer to J-STAGE at https://www.jstage.jst.go.jp/browse/expanim). The DsRed2 was used as a reporter gene for genotyping the heterozygous and homozygous transgenic mice at birth and has no obvious negative effects in vivo [21, 32, 39].
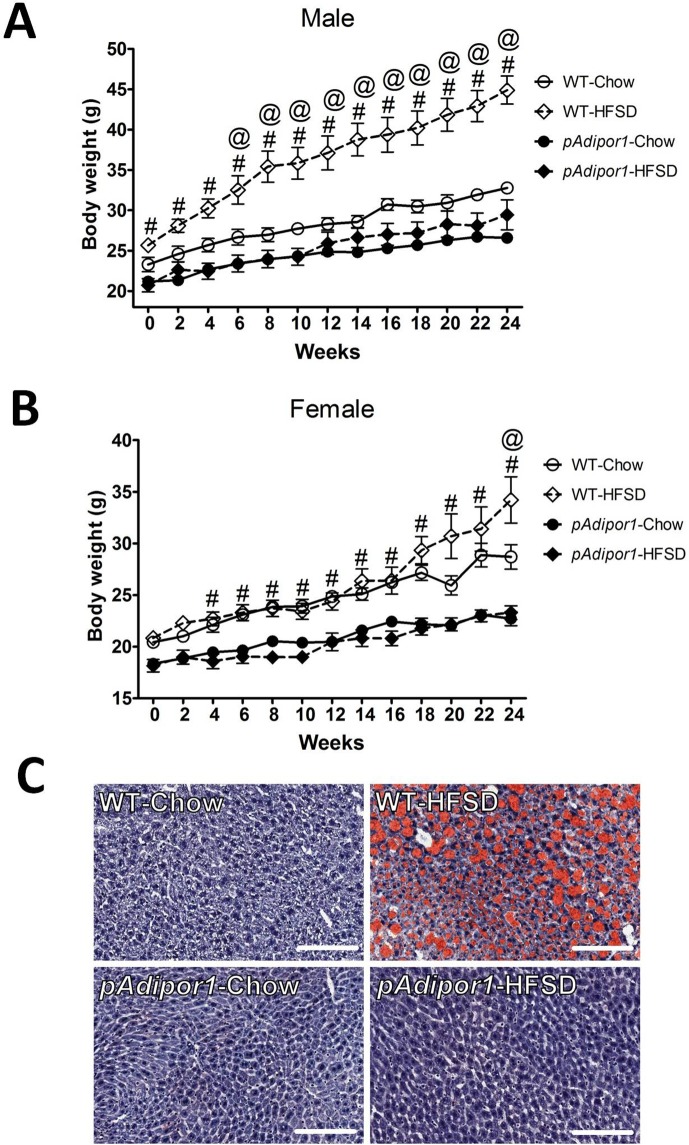
Fig. 1.
The effects of pAdipor1 transgene on HFSD-induced obesity. Wild-type (WT) and pAdipor1 transgenic mice were fed ad libitum a standard chow diet (Chow) or a high-fat/sucrose diet (HFSD) for 24 weeks, beginning at 6–7 weeks of age. There were 12 mice per genetic/diet/sex group. A: Body weights for the male mice. B: Body weights for the female mice. C: Histological sections of the livers (male) stained with Oil Red O to detect hepatic lipid droplets. Values were expressed as mean ±SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. P≤0.05: #, WT-HFSD vs. pAdipor1-HFSD; @, WT-HFSD vs. WT-Chow. The scar bars represented 100 µm.
Both C57BL/6J and FVB/N mice are commonly used inbred mouse strains and are susceptible to diet-induced obesity [25]. Here, the fertilized eggs from FVB/N donors were used because of better reproductive performance than C57BL/6J mice. The injection fragment (the β-actin-driven pAdipor1/DsRed2) was digested with HinCII and PsiI to remove the neomycin/kanamycin resistance genes, separated by agarose gel-electrophoresis, purified, and used for pronuclear microinjection. The founder mice were crossed with WT to generate the F1 heterozygous (pAdipor1+/−) offspring and then backcrossed to the WT to generate the F2. When the F2 progeny were crossed, quantitative real-time PCR (qPCR) and Southern blotting analyses were performed to identify the homologous (pAdipor1+/+) littermates of the F3 [34]. For qPCR analysis, primer pairs of DsRed2 (Table 1) were used to avoid interference by intrinsic gene expression in pAdipor1 transgenic mice.
Table 1. Primer sets for qPCR.
| Gene name | Primers 5’-3’(forward and reverse) | Length (bp) | Annealing temperature (°C) |
Reference sequence |
| DsRed2 | F: GACCCACAAGGCCCTGAAG, R: TGCTCCACGATGGTGTAGTCC |
159 | 64 | EU016077 |
| Adiponectin | F: GGCTCTGTGCTGCTCCATCT, R: AGAGTCGTTGACGTTATCTGCATAG |
101 | 55 | NM_009605 |
| Adipor1 | F: CCTGGCTCTATTACTCCTTC, R: GAACACTCCTGCTCTTGTCT |
149 | 62 | NM_028320 |
| Pparα | F: TGCTGGTATCGGCTCAATAA, R: TCCTGCCACTTGCTCACTAC |
114 | 64 | NM_011144 |
| Acox1 | F: AGTTCCAACTAGCCAGGCAT, R: GAGTGGCCTTGACCTCTGAT |
81 | 62 | NM_015729 |
| Cpt1a | F: GGTCTCAAGTAATGGGTGC, R: GAATACCAAACGGAGTTGC |
102 | 62 | BC054791 |
| Cpt1b | F: TTTGGGAACCACATCCGCCAA, R: TTATGCCTGTGAGCTGGCCAC |
262 | 60 | NM_009948 |
| CD36 | F: CAAGCTCCTTGGCATGGTAGA, R: TGGATTTGCAAGCACAATATGAA |
92 | 62 | NM_007643 |
| Ucp2 | F: CTCTTCTCTGGGAGCCAATC, R: CCCCTTCACCTCTTTAGCAG |
99 | 62 | NM_011671 |
| Srebf1 | F: GAACCAGCGGTGGGAACACAGAGC, R: GACGGCGGCAGCTCGGGTTTCTC |
224 | 57 | NM_011480 |
| Fasn | F: GGGCACTGACTGTCTGTTTTCC, R: GGATCAGGAGAGCATCAAGAGC |
200 | 60 | NM_007988 |
| Glut4 | F: TACATACCTGACAGGGCAAGG, R: TTCGGGTTTAGCACCCTTC |
131 | 58 | NM_009204 |
| Pck1 | F: GTCACCATCACTTCCTGGAAGA, R: GGTGCAGAATCGCGAGTTG |
174 | 64 | NM_011044 |
| β-actin | F: TGTTACCAACTGGGACGACA, R: CTTTTCACGGTTGGCCTTAG |
130 | 62 | NM_007393 |
Ultimately, we obtained two pAdipor1/DsRed2 lines of transgenic mice with different insertion sites, but a similar expression pattern by ANOVA analysis (expression of membrane transgenes confirmed by the ADIPOR1 polyclonal antibody targeting both mouse and pig gene products, Supplemental Fig. 1). All experiments were carried out on both male and female mice with homologous offspring from the F4 or later generations (n=6 for each line/sex).
Induction of obesity
Mice of 6 to 7 weeks old were randomly housed in cages (n=6 per cage) for each experimental group (n=12, two lines) with the light-dark cycle maintained at 12:12 h (lighting from 06:00 to 18:00 h). The wild-type (WT) mice, two lines of pAdipor1 transgenic mice were fed ad libitum with either a standard chow diet (Chow) containing 3.5 kcal/g metabolic energy (MF-18: 18% protein, 18% fat, 6% fiber, and 58% nitrogen free extract; Oriental Yeast Co., Tokyo, Japan) or a HFSD consisting (on a weight basis) of 21.3% protein, 23.6% fat, 5.8% fiber, and 41.2% carbohydrates with 4.65 kcal/g metabolic energy (45% energy from fat; St. Louis, MO, USA). Body weights of each feeding group were measured every two weeks.
Sample collection
After 25 weeks of feeding, the mice were anesthetized with 2,2,2-tribromoenthanol (intraperitoneally) and blood samples from tail vein were collected with EDTA anticoagulant for determining plasma adiponectin, insulin, triglycerides and glycerol levels. Animals were then sacrificed by CO2 and the perigonadal adipose tissues (epididymal in males and ovarian/uterine in females), skeletal muscles and livers were excised, frozen in liquid nitrogen and stored at −80°C until RNA extraction and histological examination. Plasma and tissue samples were collected from mice at 09:00~12:00 after a 12 h fast. The animal protocol was approved by the Experimental Animal Care and Use Committee at National Taiwan University.
Intraperitoneal glucose tolerance test (IPGTT)
After 24 weeks, an IPGTT was performed at 10:00 h after a 12 h fasting by injecting mice (n=6 per group) intraperitoneally with 2 mg/g body weight of glucose. Blood samples were taken from the tail vein at 0, 15, 30, 60 and 120 min after glucose injection for the determination of plasma glucose levels (Accu-Chek® Active; Roche Diagnostics, Mannheim, Germany).
Plasma adiponectin, insulin, triglyceride and glycerol levels
Plasma adiponectin level was determined using a Mouse/Rat Adiponectin ELISA Kit (UM-100201, Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). Plasma insulin was measured by a mouse insulin ELISA kit (10-1149-01, Mercodia, Uppsala, Sweden). Plasma triglyceride was measured by a Triglyceride Colorimetric Assay Kit (10010303, Cayman Chemical Co., Ann Arbor, MI, USA). Plasma glycerol was measured by a Glycerol Colorimetric Assay Kit (10010755, Cayman Chemical Co. Ann Arbor, MI, USA). All plasma samples were assayed in duplicate and determinations were according to the manufacturer’s instructions.
Liver histology
Liver frozen for histology was imbedded in Optimum Cutting Temperature Compound (4583, Sakura Finetek USA, Inc., Torrance, CA, USA) and sectioned at 6-µm thickness. The liver tissue slices were then fixed in 10% (v/v) buffered formalin and stained with hematoxylin and Oil Red O for the detection of lipid droplets [31]. The lipid contents were then quantified using ImageJ 1.46r software.
Quantitative PCR (qPCR) analysis
Total RNAs were extracted from tissue samples in TRI REAGENT® (Molecular Research Center, Inc., Cincinnati, OH, USA) by homogenization using a ZrSiO beads-based homogenizer (Next Advance Inc., Averill Park, NY, USA). For the qPCR analysis, first-strand cDNA was synthesized from TURBO™ DNase-treated (Applied Biosystems, Foster, CA, USA) total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The cDNA for individual genes was amplified using the RealQ-PCR Master Mix Kit (250507, Ampliqon, Copenhagen, Denmark) with paired forward and reverse primers (Table 1) designed from UniSTS database in National Center for Biotechnology Information. Amplification of specific transcripts was further confirmed by melting curve profile analysis. The pAdipor1 primers could detect both mouse and pig target genes. The relative expression levels were calculated according to the formula 2–ΔCT and normalized using the expression of the β-actin housekeeping gene in the same sample.
Statistical analysis
Numerical values were expressed as the mean ± SEM. Results involving more than two groups (genotype, diet, time and sex effects) were assessed by two-way ANOVA procedure. The Dunnett’s post-hoc test was followed to evaluate differences among means (SAS Inst., Inc., Cary, NC, USA) for multiple comparisons. A significant difference was indicated at P≤0.05.
Results
pAdipor1 transgenic mice were small and resistant to HFSD-induced hepatosteatosis
The pAdipor1 transgenic mice were smaller than WT mice (Fig. 1A and B) and deposition of white adipose tissue around with gonads was limited (data not indicated). In Chow-fed mice, total adipose tissue Adipor1 and Adipor2 mRNA levels were much greater than in other tissues (Supplemental Fig. 1B). Total Adipor2 mRNA in liver and muscle were depressed. Consistent with its role in modulating energy homeostasis, we found that the pAdipor1 transgenic mice fed the Chow diet were significantly smaller than the WT mice (Fig. 1A and B). HFSD induced obesity in the WT mice (with a stronger effect in the males); the pAdipor1 transgenic mice were leaner than the WT mice (Fig. 1A and B). More importantly, while HFSD yielded extensive hepatic fat deposition in the WT mice (55% increase in lipid content − data not indicated), this symptom was not observed in pAdipor1 transgenic mice (Fig. 1C; male), confirming the beneficial effects of pAdipor1 transgene on hepatosteatosis.
Glucose intolerance is not seem in HFSD-fed pAdipor1 transgenic mice
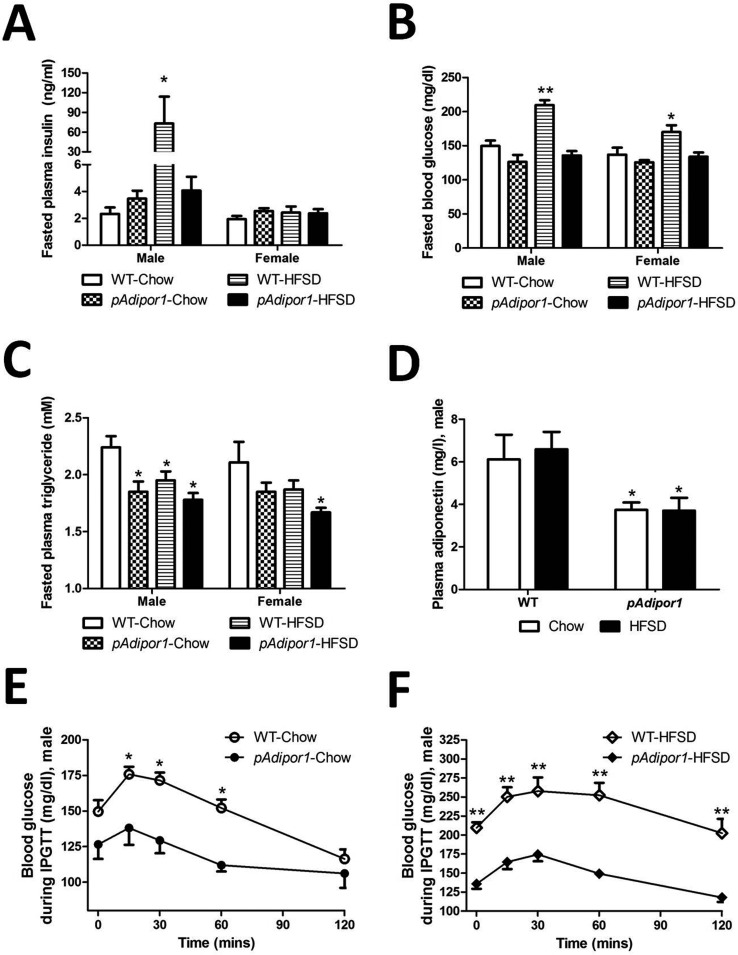
As shown in Fig. 2B, 24-week-HFSD-feeding induced hyperglycemia in WT mice. However, only the male mice developed higher fasting insulin levels, indicating that insulin resistance was developing in this treatment group (Fig. 2A). By sharp contrast, HFSD-fed pAdipor1 transgenic mice had lower fasting plasma glucose levels than HFSD-fed WT mice (Fig. 2B). Furthermore, the highly elevated plasma insulin levels seem in male HFSD-fed WT mice were not present in pAdipor1 transgenic mice, confirming an anti-diabetic role for pAdipor1 transgene (Fig. 2A and 2B).
Fig. 2.
Plasma values and intraperitoneal glucose tolerance test. The genetic and diet groups are as indicated in Fig. 1. There were 12 mice per genetic/diet/sex group. A: Fasted plasma insulin concentration. B: Fasted blood glucose concentration. C: Fasted plasma triglyceride concentration. D: Fasted plasma adiponectin concentration. Fasted insulin, triglyceride and adiponectin concentrations were measured after 25 weeks of feeding the diets to obviate the stress from the intraperitoneal glucose tolerance test. E: Intraperitoneal glucose tolerance test in male mice fed the Chow diets for 24 weeks. F: Intraperitoneal glucose tolerance test in male mice fed the HFSD diets for 24 weeks. All values were expressed as mean ±SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. Groups A−D were compared statistically to the control mice (WT-Chow). Groups E and F were compared statistically at each time point. P≤0.05: *, and P≤0.01: **.
Plasma triglyceride levels (Fig. 2C) were lower in pAdipor1 transgenic male mice than in WT male mice when fed Chow. HFSD lowered plasma triglyceride in male WT mice and in female pAdipor1 transgenic mice. These results suggest that the male mice were more sensitive to the HFSD challenge and had poorer glucose tolerance and thus a greater tendency to develop obesity and diabetes than the females. We also found that for the males, the plasma levels of total adiponectin were down-regulated in pAdipor1 transgenic mice, regardless of the dietary treatment (Fig. 2D).
We further quantified the degree of glucose intolerance using IPGTT and found that HFSD fed male WT mice showed impairment in glucose tolerance (Fig. 2E and 2F), as evidenced by a substantial increase in the incremental glucose area under curve (AUC; WT-Chow: 17957 ± 49.2 mg/dl/2h, WT-HFSD: 28553 ± 57.4 mg/dl/2h. WT-Chow vs. WT-HFSD: P≤0.001). By contrast, male pAdipor1 transgenic mice had better glucose tolerance when fed with either Chow or HFSD (pAdipor1-Chow: 14153 ± 24.5 mg/dl/2h, pAdipor1-HFSD: 17670 ± 27.9 mg/dl/2h in glucose AUC index, WT-HFSD vs. pAdipor1-HFSD: P≤0.001, WT-HFSD vs. pAdipor1-Chow: P≤0.001, WT-Chow vs. pAdipor1-Chow: P≤0.05 and pAdipor1-Chow vs. pAdipor1-HFSD: P≤0.05). It should also be noted that pAdipor1 transgenic mice had lower plasma glucose levels after a 14-h fast compared to male WT mice when fed with HFSD (Supplemental Fig. 2: refer to J-STAGE at https://www.jstage.jst.go.jp/browse/expanim), indicating an insulin-sensitizing effect for the pAdipor1 transgene.
Effects of pAdipor1 transgene on the expression profile of metabolic genes in the adipose tissues of HFSD-fed mice
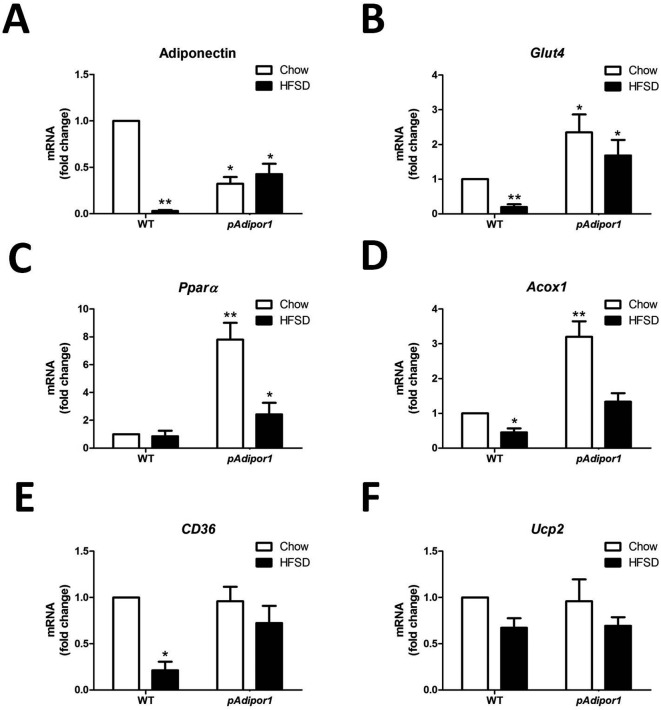
To explore the mechanisms underlying pADIPOR1 action on HFSD treatment, we analyzed the expression profiles of metabolism-associated genes in metabolic tissues. Due to a more profound effect of pAdipor1 transgene on HFSD-induced weight gain and plasma insulin level in the males, we chose the male mice to determine its effects on the expression profiles of genes in adipose tissue of HFSD-fed animals. Consistent with previous findings, HFSD sharply reduced adiponectin mRNA levels in WT mice (Fig. 3A). Adiponectin mRNA levels were lower in pAdipor1 mice (compared to WT mice) and there was no effect of HFSD.
Fig. 3.
The effects of the pAdipor1 transgene on the expression profile of metabolic genes in the adipose tissue. The mice and diets were as indicated in Fig. 1. There are 12 mice per genetic/diet/sex group. Diets were fed for 25 weeks. A: Expression of adiponectin mRNA. B: Expression of Glut4 mRNA. C: Expression of Pparα mRNA. D: Expression of Acox1 mRNA. E: Expression of CD36 mRNA. F: Expression of Ucp2 mRNA. All values were expressed as mean ± SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. All groups were compared to the control mice (WT-Chow) for statistically significant differences. P≤0.05: *, and P≤0.01: **.
The insulin-sensitive glucose transporter 4 (Glut4) mRNA was decreased by HFSD in WT mice, whereas the pAdipor1 transgene raised Glut4 mRNAs in both the Chow and HFSD groups (Fig. 3B), suggesting increased glucose uptake that may lead to improve glucose tolerance in adipose tissue of HFSD-fed pAdipor1 transgenic mice (Fig. 2E and F).
Peroxisome proliferator activated receptor alpha (Pparα) mRNA was increased in pAdipor1 compared to WT mice with the effect being greater in the Chow-fed compared to the HFSD-fed mice (Fig. 3C). The acyl-CoA oxidase 1 (Acox1) mRNA was decreased in the HFSD-fed mice compared to Chow-fed mice regardless of genotype; the mRNA was increased in Chow-fed pAdipor1 compared to WT mice (Fig. 3D). These results suggest an enhanced fatty acid oxidation in adipose tissue by the pAdipor1 transgene. The pAdipor1 transgene also prevented the down-regulation of fatty acids translocase (CD36) mRNA by HFSD (Fig. 3E). However, the expression of a Pparα target gene, mitochondrial uncoupling protein 2 (Ucp2), was not changed by either diet or genotype (Fig. 3F), suggesting that thermogenesis of the white adipose tissue is not the target of pAdipor1 for the prevention of obesity and insulin resistance.
Effects of pAdipor1 transgene on the expression profile of metabolic genes in the skeletal muscles of HFSD-fed mice
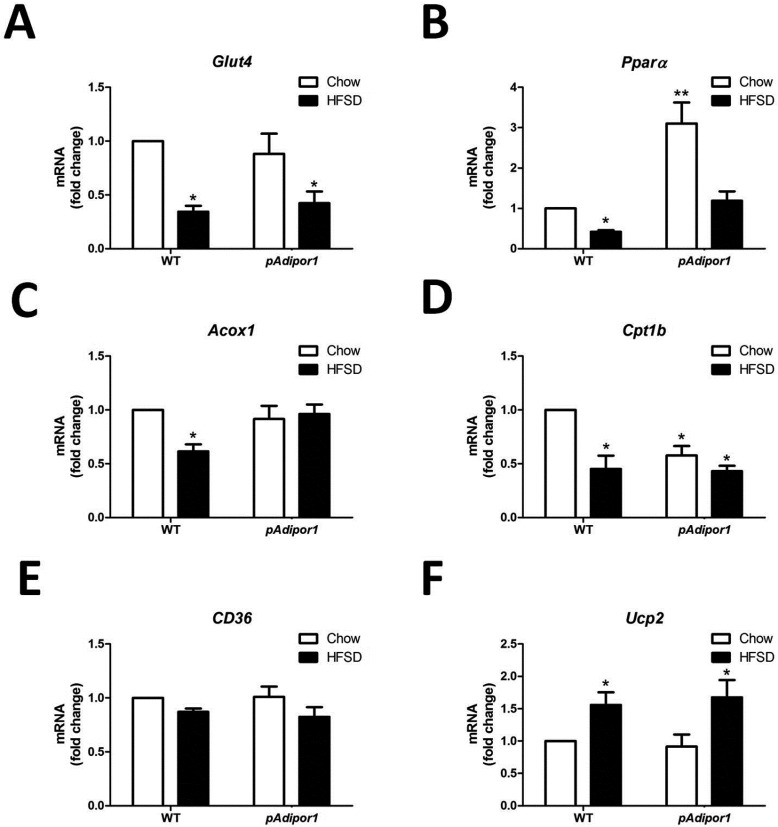
In the skeletal muscles, we found that the expressions of genes associated with glucose uptake (Fig. 4A) and fatty acid oxidation (Fig. 4B, 4C and 4D) were decreased by HFSD in both WT and pAdipor1 transgenic mice, except Acox1 and carnitine palmitoyltransferase 1b (Cpt1b), suggesting that HFSD impaired the functions of glucose and lipid metabolism in the skeletal muscles. Moreover, pAdipor1 increased Pparα mRNA, but decreased Cpt1b mRNA in Chow-fed mice (Fig. 4B and 4D). These findings suggest that pAdipor1 transgene has the opposite effects on fatty acid oxidation and β-oxidation in the skeletal muscles of HFSD-fed mice. Moreover, HFSD had no effect in the gene expression of CD36 (Fig. 4E) but increased the gene expression of Ucp2 (Fig. 4F) in the skeletal muscles of both WT and pAdipor1 transgenic mice, suggesting that thermogenesis was induced by HFSD.
Fig. 4.
The effects of the pAdipor1 transgene on the expression profile of metabolic genes in the skeletal muscle. The mice and diets were as indicated in Fig. 1. There are 12 mice per genetic/diet/sex group. Diets were fed for 25 weeks. A: Expression of Glut4 mRNA. B: Expression of Pparα mRNA. C: Expression of Acox1 mRNA. D: Expression of Cpt1b mRNA. E: Expression of CD36 mRNA. F: Expression of Ucp2 mRNA. All values were expressed as mean ± SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. All groups were compared to the control mice (WT-Chow) for statistically significant differences. P≤0.05: *, and P≤0.01: **.
Effects of pAdipor1 transgene on the expression profile of metabolic genes in the livers of HFSD-fed mice
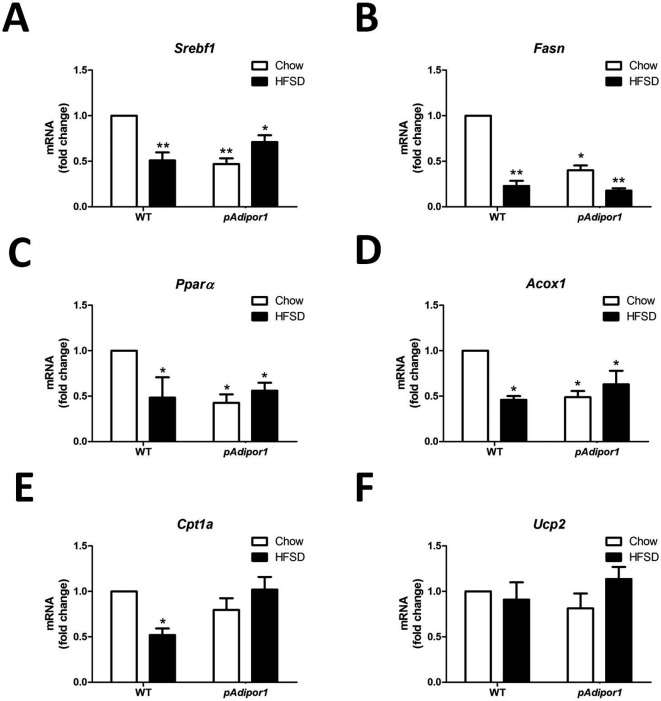
In the liver, HFSD decreased gene expression of sterol regulatory element-binding transcription factor 1 (Srebf1) in WT, but not in pAdipor1 mice (Fig. 5A). The pAdipor1 transgene suppressed the Srebf1 mRNA regardless of diet. For fatty acid synthase (Fasn), a Srebf1 target gene, the pAdipor1 transgene suppressed mRNA expression and HFSD further suppressed the mRNA, suggesting that liver lipogenesis was decreased by Adipor1 (Fig. 5B). The pAdipor1 transgene down regulated Pparα and Acox1, but not Cpt1a or Ucp2 mRNA (Fig. 5C–F). The HFSD decreased Pparα, Acox1 and Cpt1a, but not Ucp2 mRNA in WT, but not in pAdipor1 mice (Fig. 5C–F). These results suggest that the over-expressed pAdipor1 has the opposite effect between skeletal muscle and liver under an HFSD challenge. As in adipose tissue, the expression of Ucp2 gene in the liver was not affected by pAdipor1 or HFSD (Fig. 5F).
Fig. 5.
The effects of the pAdipor1 transgene on the expression profile of metabolic genes in the liver. The mice and diets were as indicated in Fig. 1. There are 12 mice per genetic/diet/sex group. Diets were fed for 25 weeks. A: Expression of Srebf1 mRNA. B: Expression of Fasn mRNA. C: Expression of Pparα mRNA. D: Expression of Acox1 mRNA. E: Expression of Cpt1a mRNA. F: Expression of Ucp2 mRNA. All values were expressed as mean ± SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. All groups were compared to the control mice (WT-Chow) for statistically significant differences. P≤0.05: *, and P≤0.01: **.
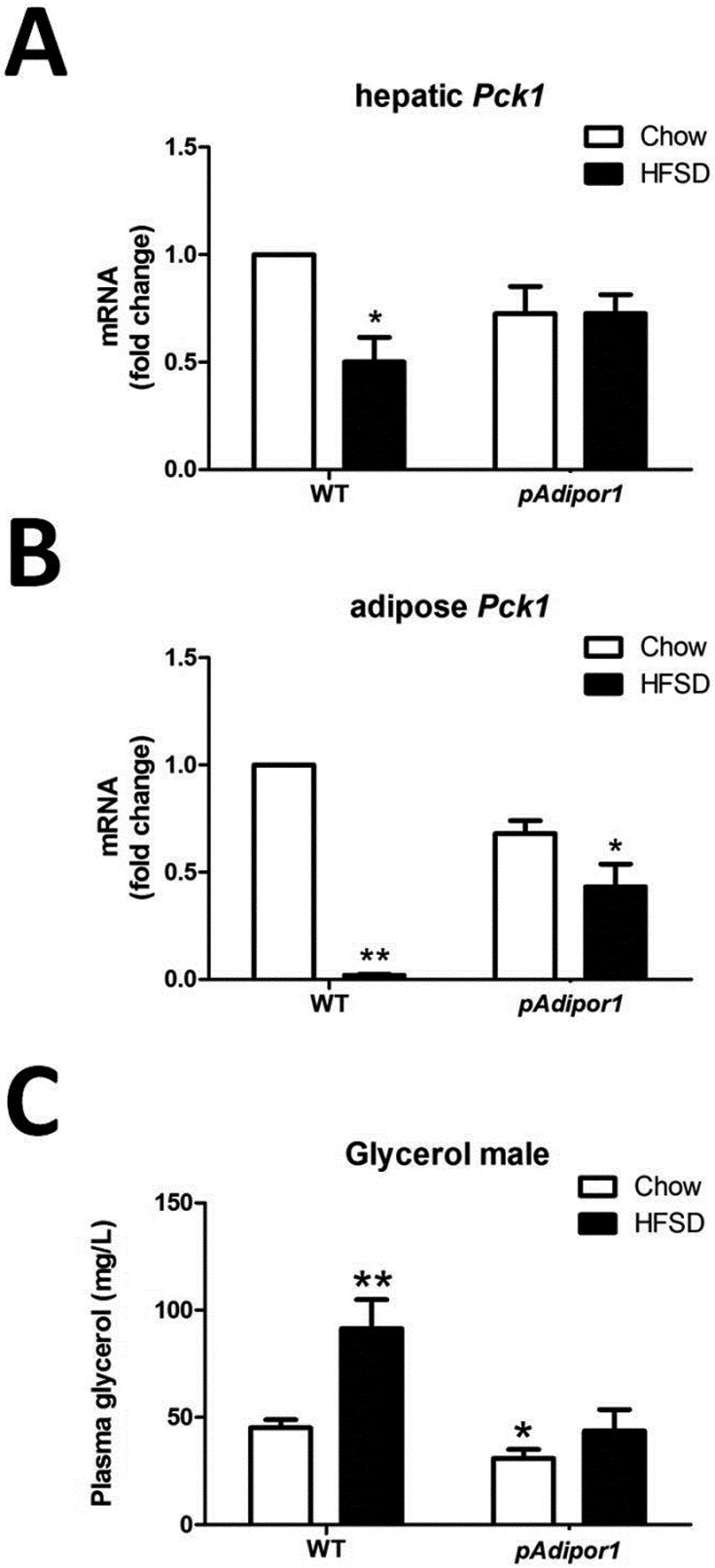
pAdipor1 transgene prevented the HFSD-downregulated Pck1 mRNA
Expression of phosphoenolpyruvate carboxykinase 1 (Pck1) mRNA (PCK1 being a gluconeogenic and glyceroneogenic enzyme) in the liver was decreased by HFSD in WT, but not pAdipor1 transgenic mice (Fig. 6A). It was decreased to an even greater extent in adipose tissue of HFSD-fed WT, but not in pAdipor1 transgenic mice (Fig. 6B). Plasma glycerol levels were increased by HFSD in WT, but not in pAdipor1 transgenic mice (Fig. 6C). The increased plasma glycerol levels and reduced Pck1 mRNA in the liver and adipose tissue of WT suggests that the reesterification of triacylglycerol was dysregulated by HFSD, which can be ameliorated by pAdipor1 transgene.
Fig. 6.
The glyceroneogenesis of the pAdipor1 transgene in the liver and adipose tissue. The genetic and diet groups are indicated in Fig. 1. There were 12 male mice per genetic/diet group. A: Expression of Pck1 mRNA in the liver. B: Expression of Pck1 mRNA in the adipose tissue. C: Plasma glycerol concentrations. All values were expressed as mean ± SEM. Differences among means for multiple comparisons were evaluated by two-way ANOVA and post-hoc test. All groups were compared to the control mice (WT-Chow) for statistically significant differences. P≤0.05: *, and P≤0.01: **.
Discussion
Adiponectin has been recognized as an insulin-sensitizing adipokine that may have a role in preventing obesity and type II diabetes. Both adiponectin and its receptors are negatively associated with obesity and diabetes in human and rodent studies [2, 30, 40]. Our previous study indicated that pAdipor1 is widely and consistently expressed in many tissues and to a greater extent than pAdipor2. Only one human study demonstrated that overexpression of mAdipor1 in macrophages enhanced the actions of adiponectin to reduce body weight and fat accumulation [22]. However, the underlying mechanisms and variations between adiponectin and its receptors are still unclear in diet-induced obesity. In the current study, we hypothesized that Adipor1 may have important regulatory functions when mice were fed HFSD. We generated pAdipor1 transgenic mice and challenged them with HFSD to demonstrate that Adipor1 can prevent diet-induced weight gain and hepatosteatosis.
The expression level of adiponectin is recognized to negatively associate with obesity and diabetes [2]. Long-term (16 weeks) consumption of HFSD has been shown to increase circulating adiponectin, but decreases its mRNA levels in the adipose tissues of C57BL/6J mice and rats [7, 26, 44]. Similar effects were found in this study; when WT mice were fed HFSD (24 weeks), the adiponectin mRNA level in the adipose tissue was markedly reduced (Fig. 3A), but plasma adiponectin was maintained (Fig. 2D). Recent studies demonstrate that the actions of circulating adiponectin depend on which of the multiple active forms mediate energy homoeostasis [20]. Hence, the level of total plasma adiponectin is not a good marker to monitor the diet-induced obesity. Adipor1 mRNA and the AMPK pathway in the muscle are decreased by HFSD, suggesting the inactivation of the signaling pathway for adiponectin [7]. Some studies indicated that there was the opposite, or no, effect on the expression of adiponectin and its receptors after HFSD feeding [8, 15]. These discrepancies may be due to the different treatment periods and extent of obesity or type II diabetes. We found that pAdipor1 transgenic mice had decreased circulating adiponectin and mRNA in the adipose tissue. The pAdipor1 transgene also reduced total Adipor2 mRNA in the liver and muscle. These results suggest that there exists a negative feedback mechanism between adiponectin and its receptor. The pAdipor1 transgene increased the membrane-bound ADIPOR1, but had no effects on the expression of pAdipor1 mRNA in the liver and muscle (Supplemental Fig. 1B and 1C). This discrepancy suggested that the transgene product of ADIPOR1 may translocate quickly when synthesized in these tissues. Our findings that long-term feeding of HFSD resulted in weight gain, hepatosteatosis, glucose intolerance, hyperglycemia and hyperinsulinemia in FVB/N mice, but not in pAdipor1 transgenic mice, suggest that the pAdipor1 transgene improves glucose tolerance and prevents these symptoms. The Adipor1−/− mice have increased adiposity and decreased glucose tolerance [6]. In the current study, the pAdipor1 transgenic mice were smaller than the WT mice even after long-term feeding of HFSD, suggesting that pAdipor1 is involved in preventing weight gain and the metabolic syndrome.
Obesity and type II diabetes have been recognized to induce insulin resistance and disturb metabolic homeostasis. We found that the pAdipor1 transgenic mice were smaller and had improved fatty acid oxidation associated genes expression in adipose tissue and skeletal muscle, but not in liver. Although there were opposite effects of fatty acid oxidation and fatty acid β-oxidation related genes expression in the liver and skeletal muscle of pAdipor1 mice, the pAdipor1 transgenic mice had reduced plasma triglyceride and maintained plasma glycerol concentration when fed with HFSD. The expression of genes associated with lipogenesis in the liver was also down-regulated by pAdipor1 transgene and probably resulted in the lower level of plasma triglyceride. However, in the skeletal muscle, both glucose uptake and fatty acid β-oxidation associated genes expression were not up-regulated by the pAdipor1 transgene. These results, suggest that the expression profile of metabolic genes in the adipose tissue of pAdipor1 mice may play important roles in resisting diet-induced obesity.
ADIPOR1 activates the AMPK pathway and ADIPOR2 promotes the PPARα pathway in the liver of mice [43]. The same team further demonstrated that adiponectin and ADIPOR1 coordinated to activate peroxisome proliferator-activated receptor γ coactivator-1α (PGC1) in myocytes [17]. Hence, pAdipor1 transgene increased the gene expression of Pparα in adipose tissue and skeletal muscle and may activate via the PGC1 pathway [23]. The induction of PGC1 and PPARα directly promoted the expression and activity of glycerol kinase, which may control the homeostasis of triacylglycerol hydrolysis and fatty acid re-esterification in human adipocytes [23]. The HFSD increased plasma glycerol perhaps as a result of decreased triacylglycerol synthesis in liver and adipose tissue.
One of adiponectin functions is to decrease the expression of PCK1 and reduce hepatic gluconeogenesis in diabetic and WT mice [5, 9]. However, other evidences also indicate that glyceroneogenesis (the synthesis of glyceride-glycerol from sources other than glycerol and glucose) but not gluconeogenesis is the major action of PCK1 in the adipose tissue and is linked to diabetes and obesity [3, 4, 12, 24, 27]. Triacylglycerol turnover in the liver and adipose tissue affects the concentrations of plasma fatty acids and leads to glucose intolerance, insulin resistance and type II diabetes in mammals [28]. We found that the pAdipor1 transgene only increased total Adipor1 and Adipor2 mRNA in the adipose tissue (Supplemental Fig. 1B). In the mice, the expression of Adipor2 mRNA was highly expressed in the liver, not in the adipose tissue. Hence, the major action of PCK1 in our pAdipor1 mice might act in the adipose tissue. Dysregulated glyceroneogenesis induces obesity, lipodystrophy, hepatosteatosis and type II diabetes in both Pck1 gene-knockout and -overexpressing mice [12, 14, 29, 38]. Anti-diabetic PPARγ2 ligands increase the expression of Pck1 mRNA and concomitantly increase the rate of glyceroneogenesis in adipose tissues [13, 37]. In the current study, we found that the expression of Pck1 mRNA in the liver and adipose tissue were improved in pAdipor1 mice fed with HFSD. Both adenovirus-mediated expression and targeted disruption of Adipor1 indicate that Adipor1 decreases the expression of Pck1 and Srebf1 mRNAs and leads to the inhibition of glucose production in the liver [43]. We found similar results in the liver and that the expression of Glut4 mRNA was up-regulated in adipose tissue by the pAdipor1 transgene, suggesting that pAdipor1 was involved in moving glucose to the peripheral tissues. In brief, pAdipor1 transgenic mice may increase Pck1 and Glut4 to promote glyceroneogenesis in adipose tissue to improve glucose tolerance.
In conclusion, our pAdipor1 transgene prevented mice from developing diet-induced weight gain, hepatosteatosis and insulin resistance. The function of the over-expressed pAdipor1 may be enhanced by maintaining high levels of Pparα mRNA in skeletal muscle and adipose tissue, and inhibiting the lipogenesis genes expression in liver. The pAdipor1 may increase glyceroneogenesis as the result of up-regulation of Pck1 and increase Glut4 in the adipose tissue to increase glucose tolerance. These findings may lead to the development of novel therapeutic strategies for treating metabolic syndromes and obesity.
Acknowledgments
The authors would like to express our gratitude to the lab members for their help and input during the study. The study was supported in part by grants from the National Science Council in Taiwan. C.-C. CHEN was supported by the postdoctoral fellowship grant (NSC099-2811-B-029-003) from the National Science Counsel in Taiwan.
References
- 1.Bartels E.D., Bang C.A., Nielsen L.B.2009. Early atherosclerosis and vascular inflammation in mice with diet-induced type 2 diabetes. Eur. J. Clin. Invest. 39: 190–199. doi: 10.1111/j.1365-2362.2009.02086.x [DOI] [PubMed] [Google Scholar]
- 2.Bauer S., Weigert J., Neumeier M., Wanninger J., Schaffler A., Luchner A., Schnitzbauer A.A., Aslanidis C., Buechler C.2010. Low-abundant adiponectin receptors in visceral adipose tissue of humans and rats are further reduced in diabetic animals. Arch. Med. Res. 41: 75–82. doi: 10.1016/j.arcmed.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 3.Beale E.G., Harvey B.J., Forest C.2007. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem. Biophys. 48: 89–95. doi: 10.1007/s12013-007-0025-6 [DOI] [PubMed] [Google Scholar]
- 4.Beale E.G., Hammer R.E., Antoine B., Forest C.2004. Disregulated glyceroneogenesis: PCK1 as a candidate diabetes and obesity gene. Trends Endocrinol. Metab. 15: 129–135. doi: 10.1016/j.tem.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Berg A.H., Combs T.P., Du X., Brownlee M., Scherer P.E.2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7: 947–953. doi: 10.1038/90992 [DOI] [PubMed] [Google Scholar]
- 6.Bjursell M., Ahnmark A., Bohlooly Y.M., William-Olsson L., Rhedin M., Peng X.R., Ploj K., Gerdin A.K., Arnerup G., Elmgren A., Berg A.L., Oscarsson J., Linden D.2007. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56: 583–593. doi: 10.2337/db06-1432 [DOI] [PubMed] [Google Scholar]
- 7.Bonnard C., Durand A., Vidal H., Rieusset J.2008. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 34: 52–61. doi: 10.1016/j.diabet.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 8.Bullen J.W., Jr, Bluher S., Kelesidis T., Mantzoros C.S.2007. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 292: E1079–E1086. doi: 10.1152/ajpendo.00245.2006 [DOI] [PubMed] [Google Scholar]
- 9.Combs T.P., Berg A.H., Obici S., Scherer P.E., Rossetti L.2001. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108: 1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding S.T., Liu B.H., Ko Y.H.2004. Cloning and expression of porcine adiponectin and adiponectin receptor 1 and 2 genes in pigs. J. Anim. Sci. 82: 3162–3174. [DOI] [PubMed] [Google Scholar]
- 11.Fantuzzi G.2005. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115: 911–919, quiz 920. doi: 10.1016/j.jaci.2005.02.023 [DOI] [PubMed] [Google Scholar]
- 12.Franckhauser S., Munoz S., Pujol A., Casellas A., Riu E., Otaegui P., Su B., Bosch F.2002. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 51: 624–630. doi: 10.2337/diabetes.51.3.624 [DOI] [PubMed] [Google Scholar]
- 13.Glorian M., Duplus E., Beale E.G., Scott D.K., Granner D.K., Forest C.2001. A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie 83: 933–943. doi: 10.1016/S0300-9084(01)01343-8 [DOI] [PubMed] [Google Scholar]
- 14.Hakimi P., Johnson M.T., Yang J., Lepage D.F., Conlon R.A., Kalhan S.C., Reshef L., Tilghman S.M., Hanson R.W.2005. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. (Lond) 2: 33. doi: 10.1186/1743-7075-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada K., Shen W.J., Patel S., Natu V., Wang J., Osuga J., Ishibashi S., Kraemer F.B.2003. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am. J. Physiol. Endocrinol. Metab. 285: E1182–E1195. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil G.S., Shargill N.S., Spiegelman B.M.1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91. doi: 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 17.Iwabu M., Yamauchi T., Okada-Iwabu M., Sato K., Nakagawa T., Funata M., Yamaguchi M., Namiki S., Nakayama R., Tabata M., Ogata H., Kubota N., Takamoto I., Hayashi Y.K., Yamauchi N., Waki H., Fukayama M., Nishino I., Tokuyama K., Ueki K., Oike Y., Ishii S., Hirose K., Shimizu T., Touhara K., Kadowaki T.2010. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464: 1313–1319. doi: 10.1038/nature08991 [DOI] [PubMed] [Google Scholar]
- 18.Liu B.H., Wang P.H., Wang Y.C., Cheng W.M., Mersmann H.J., Ding S.T.2008. Fasting regulates the expression of adiponectin receptors in young growing pigs. J. Anim. Sci. 86: 3377–3384. doi: 10.2527/jas.2008-0971 [DOI] [PubMed] [Google Scholar]
- 19.Liu B.H., Wang Y.C., Wu S.C., Mersmann H.J., Cheng W.T., Ding S.T.2008. Insulin regulates the expression of adiponectin and adiponectin receptors in porcine adipocytes. Domest. Anim. Endocrinol. 34: 352–359. doi: 10.1016/j.domaniend.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Liu M., Liu F.2010. Transcriptional and post-translational regulation of adiponectin. Biochem. J. 425: 41–52. doi: 10.1042/BJ20091045 [DOI] [PubMed] [Google Scholar]
- 21.Livet J., Weissman T.A., Kang H., Draft R.W., Lu J., Bennis R.A., Sanes J.R., Lichtman J.W.2007. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450: 56–62. doi: 10.1038/nature06293 [DOI] [PubMed] [Google Scholar]
- 22.Luo N., Chung B.H., Wang X., Klein R.L., Tang C.K., Garvey W.T., Fu Y.2013. Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis 228: 124–135. doi: 10.1016/j.atherosclerosis.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzucotelli A., Viguerie N., Tiraby C., Annicotte J.S., Mairal A., Klimcakova E., Lepin E., Delmar P., Dejean S., Tavernier G., Lefort C., Hidalgo J., Pineau T., Fajas L., Clement K., Langin D.2007. The transcriptional coactivator peroxisome proliferator activated receptor (PPAR) gamma coactivator-1 alpha and the nuclear receptor PPAR alpha control the expression of glycerol kinase and metabolism genes independently of PPAR gamma activation in human white adipocytes. Diabetes 56: 2467–2475. doi: 10.2337/db06-1465 [DOI] [PubMed] [Google Scholar]
- 24.Millward C.A., Desantis D., Hsieh C.W., Heaney J.D., Pisano S., Olswang Y., Reshef L., Beidelschies M., Puchowicz M., Croniger C.M.2010. Phosphoenolpyruvate carboxykinase (Pck1) helps regulate the triglyceride/fatty acid cycle and development of insulin resistance in mice. J. Lipid. Res. 51: 1452–1463. doi: 10.1194/jlr.M005363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery M.K., Hallahan N.L., Brown S.H., Liu M., Mitchell T.W., Cooney G.J., Turner N.2013. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56: 1129–1139. doi: 10.1007/s00125-013-2846-8 [DOI] [PubMed] [Google Scholar]
- 26.Naderali E.K., Estadella D., Rocha M., Pickavance L.C., Fatani S., Denis R.G., Williams G.2003. A fat-enriched, glucose-enriched diet markedly attenuates adiponectin mRNA levels in rat epididymal adipose tissue. Clin. Sci. (Lond) 105: 403–408. doi: 10.1042/CS20030094 [DOI] [PubMed] [Google Scholar]
- 27.Nye C.K., Hanson R.W., Kalhan S.C.2008. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 283: 27565–27574. doi: 10.1074/jbc.M804393200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nye C., Kim J., Kalhan S.C., Hanson R.W.2008. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19: 356–361. doi: 10.1016/j.tem.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 29.Olswang Y., Cohen H., Papo O., Cassuto H., Croniger C.M., Hakimi P., Tilghman S.M., Hanson R.W., Reshef L.2002. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc. Natl. Acad. Sci. U.S.A. 99: 625–630. doi: 10.1073/pnas.022616299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Y., Rideout D., Rakita S., Sajan M., Farese R., You M., Murr M.M.2009. Downregulation of adiponectin/AdipoR2 is associated with steatohepatitis in obese mice. J. Gastrointest. Surg. 13: 2043–2049. doi: 10.1007/s11605-009-1032-2 [DOI] [PubMed] [Google Scholar]
- 31.Ramirez-Zacarias J.L., Castro-Munozledo F., Kuri-Harcuch W.1992. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 97: 493–497. doi: 10.1007/BF00316069 [DOI] [PubMed] [Google Scholar]
- 32.Schmid R.S., Yokota Y., Anton E.S.2006. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia. 53: 345–351. doi: 10.1002/glia.20274 [DOI] [PubMed] [Google Scholar]
- 33.Schreyer S.A., Wilson D.L., LeBoeuf R.C.1998. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 136: 17–24. doi: 10.1016/S0021-9150(97)00165-2 [DOI] [PubMed] [Google Scholar]
- 34.Shitara H., Sato A., Hayashi J., Mizushima N., Yonekawa H., Taya C.2004. Simple method of zygosity identification in transgenic mice by real-time quantitative PCR. Transgenic Res. 13: 191–194. doi: 10.1023/B:TRAG.0000026084.32492.eb [DOI] [PubMed] [Google Scholar]
- 35.Sone H., Kagawa Y.2005. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48: 58–67. doi: 10.1007/s00125-004-1605-2 [DOI] [PubMed] [Google Scholar]
- 36.Sumiyoshi M., Sakanaka M., Kimura Y.2006. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J. Nutr. 136: 582–587. [DOI] [PubMed] [Google Scholar]
- 37.Tordjman J., Chauvet G., Quette J., Beale E.G., Forest C., Antoine B.2003. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J. Biol. Chem. 278: 18785–18790. doi: 10.1074/jbc.M206999200 [DOI] [PubMed] [Google Scholar]
- 38.Valera A., Pujol A., Pelegrin M., Bosch F.1994. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. U.S.A. 91: 9151–9154. doi: 10.1073/pnas.91.19.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vintersten K., Monetti C., Gertsenstein M., Zhang P., Laszlo L., Biechele S., Nagy A.2004. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40: 241–246. doi: 10.1002/gene.20095 [DOI] [PubMed] [Google Scholar]
- 40.Wade T.E., Mathur A., Lu D., Swartz-Basile D.A., Pitt H.A., Zyromski N.J.2009. Adiponectin receptor-1 expression is decreased in the pancreas of obese mice. J. Surg. Res. 154: 78–84. doi: 10.1016/j.jss.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Lam K.S., Chan L., Chan K.W., Lam J.B., Lam M.C., Hoo R.C., Mak W.W., Cooper G.J., Xu A.2006. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J. Biol. Chem. 281: 16391–16400. doi: 10.1074/jbc.M513907200 [DOI] [PubMed] [Google Scholar]
- 42.Wellen K.E., Hotamisligil G.S.2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., Ito Y., Kamon J., Tsuchida A., Kumagai K., Kozono H., Hada Y., Ogata H., Tokuyama K., Tsunoda M., Ide T., Murakami K., Awazawa M., Takamoto I., Froguel P., Hara K., Tobe K., Nagai R., Ueki K., Kadowaki T.2007. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13: 332–339. doi: 10.1038/nm1557 [DOI] [PubMed] [Google Scholar]
- 44.Yang B., Chen L., Qian Y., Triantafillou J.A., McNulty J.A., Carrick K., Clifton L.G., Han B., Geske R., Strum J., Brown K.K., Stimpson S.A., Pahel G.2006. Changes of skeletal muscle adiponectin content in diet-induced insulin resistant rats. Biochem. Biophys. Res. Commun. 341: 209–217. doi: 10.1016/j.bbrc.2005.12.172 [DOI] [PubMed] [Google Scholar]