Abstract
We overviewed the pathophysiological features of diabetes and its complications in obese type 2 diabetic rat models: Otsuka Long-Evans Tokushima fatty (OLETF) rat, Wistar fatty rat, Zucker diabetic fatty (ZDF) rat and Spontaneously diabetic Torii (SDT) fatty rat. Pancreatic changes with progression of diabetes were classified into early changes, such as islet hypertrophy and degranulation of β cells, and degenerative changes, such as islet atrophy and fibrosis of islet with infiltration of inflammatory cells. Renal lesions in tubuli and glomeruli were observed, and nodular lesions in glomeruli were notable changes in OLETF and SDT fatty rats. Among retinal changes, folding and thickening were interesting findings in SDT fatty rats. A decrease of motor nerve conduction velocity with progression of diabetes was presented in obese diabetic rats. Other diabetic complications, osteoporosis and sexual dysfunction, were also observed. Observation of bone metabolic abnormalities, including decrease of osteogenesis and bone mineral density, and sexual dysfunction, including hypotestosteronemia and erectile dysfunction, in obese type 2 diabetic rats have been reported.
Keywords: animal model, diabetes, diabetic complication, obese, SDT fatty rat
Introduction
The growing population of patients with type 2 diabetes has resulted in a rapid increase in the number of patients who have diabetic complications [22, 73]. In addition to the adverse effects on the quality of life of such patients, the growing number of patients with complications such as nephropathy, retinopathy, and neuropathy contributes importantly to rising medical costs [5, 12]. Despite much effort to develop means to prevent or arrest the incidence and the progression of the disease, the effects and results remain poor.
Various animal models of type 2 diabetes have been established to improve understanding of the pathophysiology in diabetes and its complications [5]. Most of these models have abnormalities of single or multiple genes related to obesity, glucose intolerance, and/or insulin resistance leading to high blood glucose levels [36]. The development and progression of diabetic complications are affected by various factors, including obesity, insulin resistance, hyperglycemia, and hyperlipidemia [5].
In this review, we examined the pathophysiology of diabetic complications in obese type 2 diabetic rats, including Otsuka Long-Evans Tokushima fatty (OLETF) rats, Wistar fatty rats, and Zucker diabetic fatty (ZDF) rats, and attempted to elucidate the characteristics of the complications in Spontaneously Diabetic Torii (SDT) fatty rats, a novel obese type 2 diabetic rat, by comparing with the other diabetic models. The SDT fatty rat was established by introducing the fa allele of the Zucker fatty rat into the SDT normal rat genome [49]. The SDT fatty rat shows overt obesity, and hyperglycemia, hypertriglyceridemia and hypercholesterolemia are observed at a young age as compared with the lean rat. Furthermore, with early incidence of diabetes mellitus, diabetes-associated complications in the SDT fatty rat are seen at younger age than those in lean rat [31, 52, 57].
Pancreatic Lesions
Pancreatic lesions do not belong to the category of diabetic complications, but an understanding of the pancreatic changes is very important for elucidating the development of diabetes mellitus. Hyperglycemia is attributed to two major defects, namely, insulin resistance and depletion of insulin secretion from pancreas [68, 80]. The pancreatic β cells are key regulators of glucose homeostasis, and type 2 diabetes evolves due to an inability of the islets to adapt the β cell mass to the increased insulin demand [3, 34, 66]. Pancreatic lesions in diabetic animal models are largely related to the incidence or the development of diabetes mellitus. It is reported that pathological changes in islets, such as hypertrophy, atrophy, and fibrosis, are observed in obese type 2 diabetic rats. In human, the relative β-cell volume with both impaired fasting glucose and type 2 diabetes is decreased and the islet amyloid is increased [4]. It is important to note the changes in β-cell mass with progress of diabetes in animal models and human.
In male OLETF rats, an impaired glucose tolerance was observed from 8 weeks of age, and the plasma glucose level became higher from 18 weeks of age [17, 36]. The histological changes of pancreatic islets can be classified into three stages: 1) an early stage (from 6 to 20 weeks of age), 2) a hyperplastic stage (20 to 40 weeks of age), and 3) a final stage (after 40 weeks of age) [32, 36]. In the early stage, mild to moderate lymphocyte infiltration in or around pancreatic islets was observed, and degenerative changes and necrosis of islets also became apparent after 12 weeks of age. After 20 weeks of age, fibrosis of the islets was observed, and the islets were divided or completely replaced by fibrotic fibers. After 40 weeks of age, the pancreatic islets were replaced by connective tissues.
In male Wistar fatty rats, glucose intolerance accompanied by exaggerated insulin secretion and an increase of basal plasma glucose level were observed at 8 weeks of age. Histological observations were performed at 14 weeks of age [26]. Wistar fatty rats at 14 weeks of age showed high levels of plasma glucose and insulin, and insulin, and hypertrophied islets appeared to increase in the pancreas. Also, aldehyde fuchsin staining revealed degranulation of β cells. The pancreas of Wistar fatty rats was considered to be in the active state of insulin secretion and synthesis.
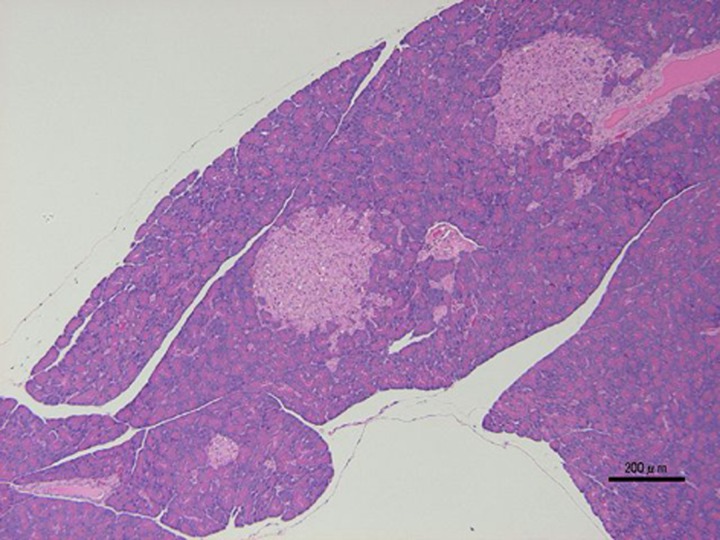
ZDF rats developed progressive insulin resistance and glucose intolerance between 3 and 8 weeks of age and become overtly diabetic between 8 and 10 weeks of age. Islet morphology differed between the ZDF rats and the lean control rats at 7 weeks of age (prediabetic), and these differences were more pronounced at 12 weeks of age (diabetic). The islets of ZDF rats were larger and had irregular boundaries (Fig. 1) [24, 77, 84]. There was a good correspondence between the increase in islet DNA content and serum insulin levels, suggesting that islet hyperplasia plays a role in the development of hyperinsulinemia in ZDF rats [84].
Fig. 1.
Histological analysis. HE stain. Pathological findings such as hypertrophy and irregular boundaries in pancreatic islets of male ZDF rat at 9 weeks of age. Bar=200 µm.
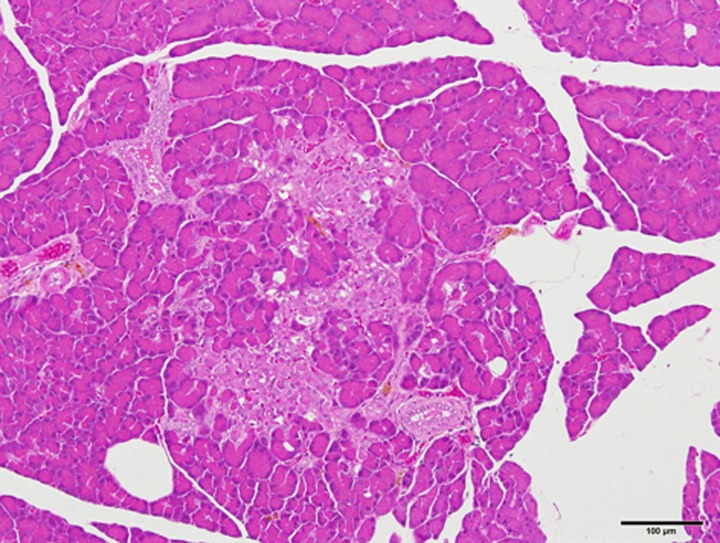
SDT fatty rats of both sexes showed a significant hyperphagia and obesity after weaning. Serum glucose levels in SDT fatty rats of both sexes were elevated from 6 weeks, and lipid parameters such as serum triglyceride and total cholesterol levels in the rats were elevated from 4 weeks of age. The hyperglycemia, hypertriglyceridemia and hypercholesterolemia were sustained for a long time afterwards [31, 52]. The male SDT fatty rats showed hyperinsulinemia from 4 to 8 weeks of age, but after 16 weeks their insulin levels decreased to levels similar to those in SDT rats. In the female rats, hyperinsulinemia was shown from 4 to 12 weeks of age, and the insulin levels decreased gradually. In a glucose tolerance test conducted at 9 weeks of age, SDT fatty rats showed higher serum glucose levels after glucose loading, without any response of plasma insulin [30]. Those impaired glucose tolerance and insulin secretion were deteriorated with aging. In pancreatic islets of female SDT fatty rats, pathological findings such as vacuolation, hypertrophy, and hemorrhage were observed from 8 weeks of age, and findings such as atrophy and fibrosis in the islets were observed from 24 weeks of age (Fig. 2) [31]. The hemorrhage in islets was specific in SDT fatty rats, and the change was also observed in SDT rats, a non-obese type 2 diabetic model [50].
Fig. 2.
Histological analysis. HE stain. Pathological findings such as atrophy and fibrosis in pancreatic islets of female SDT fatty rat at 24 weeks of age. Bar=100 µm.
In pancreas of obese type 2 diabetic models, early changes such as islet hypertrophy and degranulation of β cells are caused by development of hyperinsulinemia. Sustained hyperglycemia or development of diabetes induced degenerative changes, such as islet atrophy and fibrosis of islet with infiltration of inflammatory cells, and, finally, the islets were divided and replaced by connective tissues.
Renal Lesions
The increase in number of patients with obesity-associated type 2 diabetes has resulted in a rapid increase in patients who have End Stage Renal Disease (ESRD) and require dialytic life support [22, 73]. Despite efforts to develop means to prevent or arrest the progression of the disease, the long-term prognosis of patients with established nephropathy remains poor [67]. In this context, better understanding of the pathophysiology of renal lesions in diabetic models will be beneficial in the design and development of therapies. It is reported that pathological changes in renal tubule, glomerulus, and tubulointerstitium are observed in obese type 2 diabetic models.
Urinary protein and albumin levels in male OLETF rats were higher than those in lean rats at 10 weeks of age, and the proteinuria and the albuminuria rose progressively with aging, from 20 to 30 weeks of age [37]. Moreover, the glomerular filtration rate (GFR) was increased compared to that in lean rats at 30 weeks of age.
In histological analysis, pathological changes such as diffuse glomerulosclerosis and nodular lesion were observed in male OLETF rats [35, 37, 64, 73]. In OLETF rats at 15 weeks of age, there were no obvious alternations in glomeruli, and mesangial cell proliferation was observed at 25 weeks of age. After 40 weeks of age, mesangial expansion accompanied by accumulation of extracellular matrix and thickening of glomerular capillary walls was observed. These changes were suggestive of those of diffuse glomerulosclerosis. After 65 weeks of age, fully developed diabetic glomerulopathy accompanied by nodular sclerosis was observed. The nodular lesions expanded to the proliferated mesangial matrix. Some tubules were dilated with flat epithelium, and mononuclear cell filtration and fibrosis were observed around atrophic tubuli. Moreover, renal hypertrophy was seen at 55 weeks of age [36].
Urinary albumin excretion (UAE) in male ZDF rats was slightly higher than that in lean rats at 6 weeks of age. Thereafter, albuminuria in ZDF rats rose progressively with aging [88]. GFR was elevated until 12 weeks of age, but fell to the level seen in lean rats by 28 weeks of age. Proteinuria started to rise during the period of increased GFR, and increased further after GFR had fallen to the lean level [25]. Renal hypertrophy was slightly observed at 12 weeks of age, and the renal hypertrophy was more prominent by 16 weeks of age [88]. In histological analysis, pathological changes such as glomerulosclerosis and tubulointerstitial scarring/inflammation were observed in male ZDF rats. ZDF rats at 8 weeks of age showed neither glomerulosclerosis nor evidence of tubulointerstitial lesions. Compared with lean rats at 8 weeks of age, glomeruli in ZDF rats seemed slightly hypertrophied, with further increment at 22 weeks of age along with mesangial expansion [7]. Furthermore, tubulointerstitial scarring and inflammation were observed in ZDF rats at 22 weeks of age.
In Wistar fatty rats, renal disease was marked by onset of albuminuria and decreased GFR at 20 weeks of age. Progressive increases in albuminuria occurred through 7 months of age. Kidney enlargement and glomerular hypertrophy were observed at 20 and 42 weeks of age [87]. Histological changes, such as a deposition of PAS-positive granules in the epithelial cells, a diffuse thickening of the mesangial area and renal tubular lesions, were observed. ICAM-1 expression on the glomeruli is associated with the development of nephropathy: it is weak at 5 weeks, becomes markedly strong at 15 weeks, and progresses further at 29 weeks of age [51].
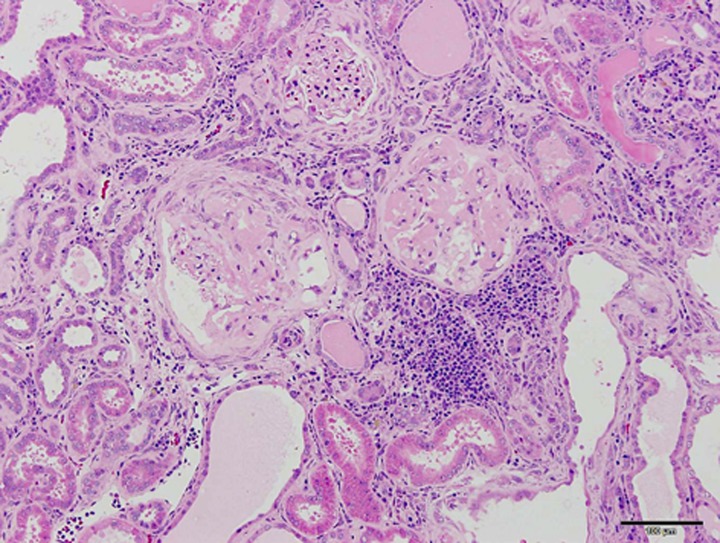
In SDT fatty rats of both sexes, a remarkable rise in renal parameters such as urine volume and urine protein was shown after 4 weeks of age [52]. With early incidence of diabetes mellitus, diabetes-associated complications in SDT fatty rats were seen at younger ages than those in the SDT rats. In male SDT fatty rats, histopathological examination of the kidneys revealed changes in the glomeruli from 16 weeks, and in the renal tubules from 8 weeks of age [52]. In the glomeruli, glomerulosclerosis was observed from 16 weeks of age, and the sclerosis progressed with aging. Nodular lesions were observed at 40 weeks of age. In the renal tubules, glycogen deposition in the tubular epithelium (Armanni-Ebstein lesions) and tubular dilation were noted from 8 weeks of age, and the change progressed from 8 to 16 weeks of age. In female SDT fatty rats, a qualitatively equal change was observed in histopathological findings of kidneys [31]. The female rats revealed changes in the glomeruli from 32 weeks of age, and in the renal tubules from 16 weeks, and the changes progressed with aging. Furthermore, we investigated histopathological characteristics of the kidneys in male and female SDT fatty rats at 60 weeks of age. Diffuse glomerulosclerosis, including increased mesangial matrix and glomerular hypertrophy, was severely progressed in the SDT fatty rats. Moreover, tubular and interstitial lesions, including fibrosis and inflammatory cell filtration, were progressed in the SDT fatty rats (Fig. 3). There were no clear sex differences in the morphological characteristics of the renal lesions.
Fig. 3.
Histological analysis. HE stain. Severely progressed glomerular and tubulointerstitial lesions in male SDT fatty rat at 60 weeks of age. There are completely sclerotic glomeruli and atrophic tubules with many inflammatory cells in the interstitium. Bar=100 µm.
In the obese diabetic rats, albuminuria and proteinuria were increased progressively with the sustained hyperglycemia and aging. The increase of GFR and the renal hypertrophy were observed in early stage of diabetic nephropathy. Glycogen deposition in the tubular epithelium (Armanni-Ebstein lesions) was also observed with the hyperglycemia. Glomerular pathological changes such as diffuse glomerulosclerosis and nodular lesion were observed in the obese diabetic rats. The expansion of nodular lesions to mesangial matrix is characteristic in OLETF rats and SDT fatty rats. The reason SDT fatty rats showed a significant nodular lesion might be related with an elevation of blood pressure, one of the characteristics in SDT fatty rats [28, 29]. The tubulointerstitial scaring and inflammation were observed in ZDF rats and SDT fatty rats. Glomerular lesions such as diffuse glomerulosclerosis and nodular lesion, and tubulointerstitial lesions were also observed in human nephropathy [5]. However, there is no animal model that leads to ESRD, a final stage in patients with nephropathy.
Occular Lesions
There are approximately 93 million people with diabetic retinopathy, 17 million with proliferative diabetic retinopathy, 21 million with diabetic macular edema, and 28 million with vision-threatening diabetic retinopathy worldwide [94]. Diabetic retinopathy is the leading cause of blindness among working-aged adults around the world [42, 71]. Diabetic retinopathy is subdivided into nonproliferative retinopathy, which is characterized by intraretinal microaneurysms, hemorrhage, nerve-fiber-layer infarcts, hard exudates, and microvascular abnormalities; and proliferative retinopathy, which is characterized by neovascularization arising either from the disk or from retinal vessels [14, 15, 72]. Although the major risk factors for diabetic retinopathy, such as hyperglycemia, hyperlipidemia, and hypertension, have been examined in many epidemiologic studies and clinical trials [42], there is considerable variation in the consistency, pattern, and strength of these risk factors [94]. Moreover, cataract, which is characterized by cloudiness or opacification of the eye lens, is also a leading cause of blindness [39]. Although the pathogenesis of diabetic cataract is not known, various biochemical pathways, such as the polyol pathway, the generation of advanced glycation end-product, and oxidative stress, have been implicated [45]. It is considered that experimental animal models will play a pivotal role in establishment of novel therapeutic methods to prevent progression to blindness.
Retinal capillary changes in OLETF rats were examined after 56 weeks of age [48, 55]. In the retinal capillaries, basement membranes were thicker, and the ratio of pericyte area to the capillary cross-section area was lower than that of lean rats. The endothelial cell cytoplasm was degenerated. The caliber irregularity, narrowing, tortuosity, and loop formations of capillaries were also observed. Moreover, the peak latency of oscillatory potential, the earliest electroretinographic manifestation of diabetic retina, was prolonged in OLETF rats at 35 weeks of age [53, 75]. On the other hand, acellular capillaries and pericyte ghosts, characteristic morphological changes in early diabetic retinopathy in humans, were not observed in OLETF rats [53]. Regarding cataract, slight lens fiber swelling was observed in the anterior and/or posterior subcapsular regions in OLETF rats at 40 weeks of age [44].
In ZDF rats, the retinal capillaries demonstrated hypercellularity, and the retinal capillary basement membrane thickness revealed thicker membrane as compared with lean rats [11, 93]. However, other lesions typical of diabetic retinopathy in humans, such as pericyte degeneration, microaneurysms, and acellular capillaries, were not observed in ZDF rats. In ZDF rats at 20 weeks of age, the lens displayed cataract formation, and the apoptotic cell death in lens was increased as compared with lean rats [39].
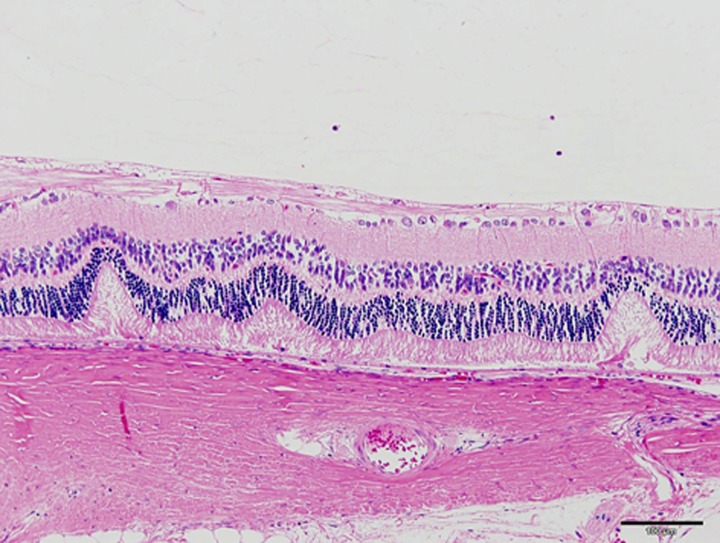
SDT fatty rats after 16 weeks of age showed a prolongation of the peak latencies of oscillatory potentials as compared with the lean rats. Histopathological findings in lens, including hyperplasia of epithelium, vacuolation of fiber, and occurrence of Morgagnian globules, were observed from 8 weeks of age in male SDT fatty rats, and these changes progressed with aging. The female rats showed similar changes from 16 weeks of age. Also, retinal lesions, such as folding and thickening, were observed with aging in male and female SDT fatty rats (Fig. 4) [31, 52].
Fig. 4.
Histological analysis. HE stain. Retinal lesions such as folding and thickening in male SDT fatty rats at 50 weeks of age. Bar=100 µm.
In the retinal capillaries of the obese diabetic rats, the basement membrane thickness and the hypercellularity were observed. Although typical lesions of diabetic retinopathy in humans, such as acellular capillaries and pericyte ghosts, were not observed in nearly diabetic rats, retinal changes such as folding and thickening in SDT fatty rats were interesting findings (Table 1). However, the development of ischemia is not observed in the diabetic rats, and there are some differences in the pathological changes of diabetic retinopathy between animal models and human [5]. The obese diabetic rats showed cataract and delay of peak latency of oscillatory potential with the sustained hyperglycemia.
Table 1. Pathophysiological characteristics of obese type 2 diabetic models (summary).
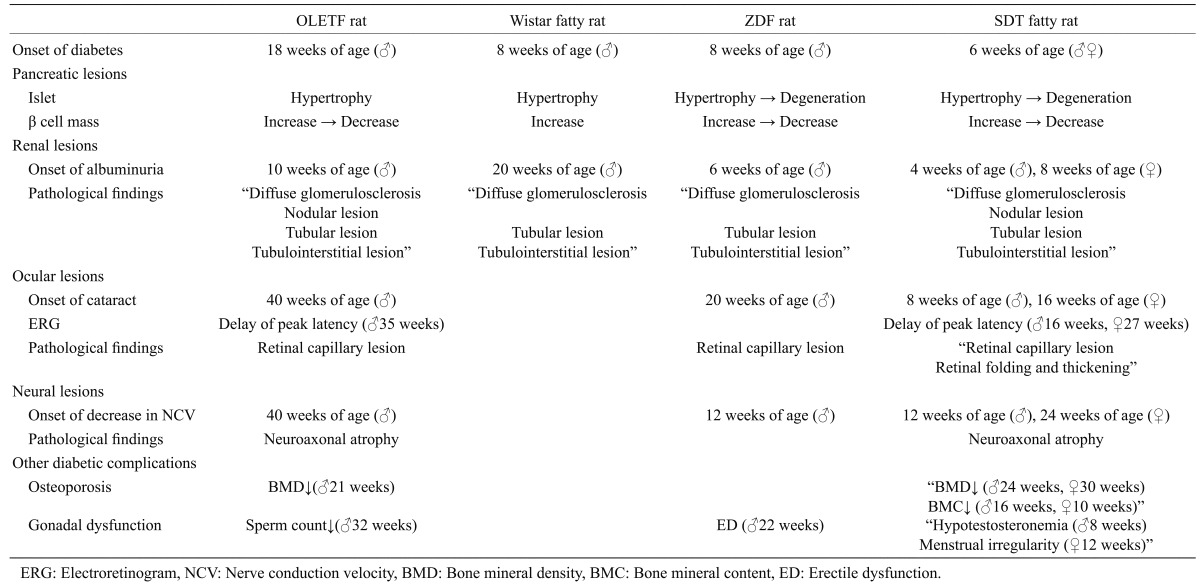
Neural Lesions
Diabetic neuropathy is a common and serious complication in diabetic patients. However, the exact mechanisms by which the neuropathy develops have not yet been elucidated [59]. The current therapeutic possibilities in neuropathy are divided into two groups, pathogenetically oriented therapy and symptomatic therapy [86]. Pathogenetically oriented therapy may delay, stop, or reverse the progression of the neuropathy, and thereby alleviate symptoms. Various hypotheses according the pathogenesis of diabetic neuropathy have been raised [19], and they are classified into metabolic and vascular categories [59]. A hypothesis in which increased polyol pathway activity and the related myo-inositol depletion induces a decrease in Na+/K+-ATP-ase activity has received considerable attention as the leading metabolic category [58, 83, 85, 95]. Moreover, recent studies have shown a relationship between polyol pathway hyperactivity and vascular factors in the etiology of diabetic neuropathy [6, 79, 81]. Most of the studies on the pathogenesis of diabetic neuropathy have been conducted mainly with type 1 diabetic animal models, including streptozotocin (STZ)-induced diabetic rats. However, the majority of human diabetes is type 2 diabetes mellitus, and especially diabetes with obesity, incidence of which has rapidly increased [78]. Therefore, it is very important to establish useful animal models of obese type 2 diabetes mellitus to investigate the pathophysiology of diabetic neuropathy.
In examination of peripheral nerve functions in male OLETF rats, cadual motor nerve conduction velocity (MNCV) tended to decrease after about 40 weeks of age as compared with lean rats [60]. Sucrose-fed OLETF rats prominently showed a decrease of MNCV at about 40 weeks of age [59, 60]. Furthermore, a decrease of sciatic nerve blood flow and an increase of ADP-induced platelet aggregation were observed in sucrose-fed OLETF rats at about 40 weeks of age. The morphologic abnormalities in sucrose-fed OLETF rats were not obvious, but the morphometric analyses showed significant differences. The sucrose-fed OLETF rats showed smaller mean myelinated fiber size and a greater fiber density as compared with lean rats [60].
In examination of sciatic nerve functions in male ZDF rats, MNCV was decreased after 12 weeks of age, and Ach-mediated vascular relaxation of epineurial arterioles of the sciatic nerve was impaired after 8 weeks of age [65, 76]. In histopathological analyses at 24–28 weeks after the onset of diabetes, ZDF rats did not show sympathetic neuroaxonal dystrophy [74].
Diabetic peripheral neuropathy was evaluated at 8, 24, and 40 weeks of age in male SDT fatty rats. Caudal MNCV in the SDT fatty rat was delayed at 24 weeks of age and was further decreased at 40 weeks of age as compared with lean rats [92]. Histopathologically, at 40 weeks of age, the fiber number was significantly decreased, and SDT fatty rats revealed significant atrophy in myelinated nerve.
Measurement of MNCV is a common method as a functional assay in neuropathy. The measurement is generally performed on the caudal or sciatic nerve. Since the sensitivity of measurement on sciatic nerve is higher than that on caudal nerve, nerve functional abnormalities in ZDF rats can be observed at an early age, about 12 weeks. In diabetic patients, pathological changes such as distal and sensory predominant nerve fiber degeneration, axonal loss, and endoneural microangiopathy, are observed in peripheral nervous system [90]. There are few reports in which histopathological analyses in obese diabetic rats were sufficiently performed. Examinations on the pathogenesis of neuropathy have been conducted mainly with STZ diabetic rats, and in further study, it is necessary to promote histological analyses using the obese type 2 diabetic rats.
Other Diabetic Complications
Osteoporosis
Diabetes mellitus is a chronic metabolic disorder with substantial morbidity and mortality, and osteoporosis is a silent disease, also with harmful impact on morbidity and mortality [1, 20]. Osteoporosis leads to increase in skeletal fragility and microarchitecture deterioration of bone tissue with a decrease in bone mineral density (BMD), bone quality, and strength [9, 62]. Along with an increased risk of diabetic complications, strong evidence for reduced BMD in type 1 diabetes mellitus, which might increase the risk of osteoporosis and its related diseases in later life, is reported [54, 61]. However, the relationship between type 2 diabetes mellitus and osteoporosis remains controversial. Obesity is strongly associated with higher BMD, probably through mechanical loading and hormonal factors, including insulin, estrogen, and leptin [16, 82, 89]. On the other hand, it is reported that AGEs in collagen may come into intact with bone to reduce bone strength, resulting in osteoporosis in patients with diabetes [91].
In male ZDF rats at 21 weeks of age, BMD was lower at the distal femur and the lumbar spine as compared with lean rats, and evaluation of histomorphometric indexes showed lower mineralized bone volume/tissue volume, trabecular thickness, and trabecular number [23]. The osteoblast differentiation was also impaired. In male SDT fatty rats, decreases in both serum osteocalcin and urine deoxypyridinoline levels, indicators of low bone turnover, were observed from 8 to 40 weeks of age [40]. Moreover, the SDT fatty rats showed lower BMD and bone mineral content (BMC) of the whole tibia, and shortening of the tibia and femur compared to age-matched control rats [41]. Deterioration in bone geometrical properties of the femur midshaft, such as cortical thickness and minimum moment of inertia, was observed in the SDT fatty rats. Furthermore, trabecular bone volume of the distal femur was lower in the SDT fatty rats. These negative effects on bone in the SDT fatty rats caused severe decreases in maximum load, stiffness, and energy absorption of the femur. Female SDT fatty rats also showed lower BMC and BMD of the whole tibia from 8 to 25 weeks of age. Bone metabolic abnormalities, including osteoblast dysfunction and a decrease of BMD or BMC, were observed in both obese diabetic rat models, ZDF rat and SDT fatty rat.
Sexual dysfunction
It was reported that subnormal free testosterone concentrations were related with inappropriately low luteinizing hormone (LH) and follicle-stimulating hormone (FSH) concentrations and a normal response to gonadotropin-releasing hormone (GnRH) of LH and FSH in type 2 diabetes [10, 13]. These abnormalities were independent of the duration and severity of hyperglycemia. Type 2 diabetic men with low testosterone levels showed a high prevalence of symptoms suggestive of hypogonadism, such as fatigability and erectile dysfunction (ED) [33]. Moreover, total testosterone and free testosterone concentrations were inversely related to body mass index (BMI) and age. Hypogonadism is considered to be associated with upper abdominal adiposity, insulin resistance, and metabolic syndrome [21, 46].
In OLETF rats, biochemical parameter levels, such as free testosterone, 17β-estradiol, LH, and sex hormone-binding globulin (SHBG), were not changed from 4 to 64 weeks of age, as compared with lean rats [43]. Testis weight was decreased in OLETF rats at 32 and 64 weeks of age, and the sperm counts at 64 weeks of age were also decreased as compared with lean rats. Histologically, seminiferous tubule atrophy was observed at 64 weeks of age. A negative correlation between testis weight and fasting blood glucose level, as well as homeostasis model assessment (HOMA) index, was recognized in OLETF rats. In the other obese diabetic rats, ED was observed at 22 weeks of age in ZDF rats [18], and hypotestostronemia was confirmed at 24 weeks of age in SDT fatty rats [38]. Moreover, it is reported that rats or mice defective in leptin or its receptor show profound infertility, in addition to obesity [8, 69].
Polycystic ovary syndrome (PCOS) is a common condition in reproductive-aged women, associated with glucose intolerance, type 2 diabetes, and metabolic syndrome [47, 56]. Insulin resistance is proposed as a key pathophysiological feature of PCOS, contributing to both reproductive and metabolic abnormalities. Reproductively, insulin resistance increases hyperandrogenism through an increase of ovarian androgen production and a reduction of hepatic SHBG [2, 70]. Women with PCOS are also proposed to have a more rapid conversion from impaired glucose tolerance (IGT) to type 2 diabetes mellitus [63]. SDT fatty rats at 12 weeks of age showed menstrual irregularity and histopathological changes, such as atrophy in uterus and inflammation in vagina, but the pathological changes of PCOS were not observed [27]. Also, in the other obese diabetic rats, there are few reports about PCOS.
Future Prospects
With increased prevalence of diabetes mellitus, the diabetic rat models have played key roles to elucidate the pathogenesis of human diabetes and its complication, such as nephropathy, retinopathy, and neuropathy. It has recently been noted that various diabetic complications, including cancer, dementia, osteoporosis, and gonadal dysfunction, are increased. Although osteoporosis and gonadal dysfunction were focused on in this review, it is necessary to investigate the relationships between diabetes mellitus and the other complications such as cancer and dementia in further study. Furthermore, the diabetic rat models are essential for developing novel drugs for diabetes and its complications. The importance of the diabetic rat models will be a constant in the future, and it is indispensable to use these models with better understanding of differences in pathophysiology between animal models and human.
References
- 1.Abdulameer S.A., Sulaiman S.A., Hassali M.A., Subramaniam K., Sahib M.N.2012. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence. 6: 435–448. doi: 10.2147/PPA.S32745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri R.L., Makris A., Randall R.W., Daniels G., Kistner R.W., Ryan K.J.1986. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J. Clin. Endocrinol. Metab. 62: 904–910. doi: 10.1210/jcem-62-5-904 [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S., Li W.C., Ouziel-Yahalom L., Guo L., Weir G.C., Sharma A.2010. Beta-cell growth and regeneration: replication is only part of the story. Diabetes 59: 2340–2348. doi: 10.2337/db10-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler A.E., Janson J., Soeller W.C., Butler P.C.2003. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52: 2304–2314. doi: 10.2337/diabetes.52.9.2304 [DOI] [PubMed] [Google Scholar]
- 5.Calcutt N.A., Cooper M.E., Kern T.S., Schmidt A.M.2009. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat. Rev. Drug Discov. 8: 417–429. doi: 10.1038/nrd2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron N.E., Cotter M.A.1992. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia 35: 1011–1019. doi: 10.1007/BF02221675 [DOI] [PubMed] [Google Scholar]
- 7.Chander P.N., Gealekman O., Brodsky S.V., Elitok S., Tojo A., Crabtree M., Gross S.S., Goligorsky M.S.2004. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J. Am. Soc. Nephrol. 15: 2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C [DOI] [PubMed] [Google Scholar]
- 8.Charlton H.M.1984. Mouse mutants as models in endocrine research. Q J Exp Physiol. 69: 655–676. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C., Melton L.J., 3rd1992. Epidemiology of osteoporosis. Trends Endocrinol. Metab. 3: 224–229. doi: 10.1016/1043-2760(92)90032-V [DOI] [PubMed] [Google Scholar]
- 10.Dandona P., Dhindsa S.2011. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 96: 2643–2651. doi: 10.1210/jc.2010-2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danis R.P., Yang Y.1993. Microvascular retinopathy in the Zucker diabetic fatty rat. Invest. Ophthalmol. Vis. Sci. 34: 2367–2371. [PubMed] [Google Scholar]
- 12.DeFronzo R.A., Abdul-Ghani M.2011. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care. 34:(Suppl 2): S202–S209. doi: 10.2337/dc11-s221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhindsa S., Prabhakar S., Sethi M., Bandyopadhyay A., Chaudhuri A., Dandona P.2004. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J. Clin. Endocrinol. Metab. 89: 5462–5468. doi: 10.1210/jc.2004-0804 [DOI] [PubMed] [Google Scholar]
- 14.Diabetic Retinopathy Study Research Group.1979. Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. The Diabetic Retinopathy Study Research Group. Arch. Ophthalmol. 97: 654–655. doi: 10.1001/archopht.1979.01020010310003 [DOI] [PubMed] [Google Scholar]
- 15.Early Treatment Diabetic Retinopathy Study Research Group.1991. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98: 786–806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 16.Felson D.T., Zhang Y., Hannan M.T., Anderson J.J.1993. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J. Bone Miner. Res. 8: 567–573. doi: 10.1002/jbmr.5650080507 [DOI] [PubMed] [Google Scholar]
- 17.Fujita Y., Kojima H., Hidaka H., Fujimiya M., Kashiwagi A., Kikkawa R.1998. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima Fatty rats. Diabetologia 41: 1459–1466. doi: 10.1007/s001250051092 [DOI] [PubMed] [Google Scholar]
- 18.Garcia M.M., Fandel T.M., Lin G., Shindel A.W., Banie L., Lin C.S., Lue T.F.2010. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J. Sex Med. 7: 89–98. doi: 10.1111/j.1743-6109.2009.01541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene D.A., Sima A.A., Stevens M.J., Feldman E.L., Lattimer S.A.1992. Complications: neuropathy, pathogenetic considerations. Diabetes Care 15: 1902–1925. doi: 10.2337/diacare.15.12.1902 [DOI] [PubMed] [Google Scholar]
- 20.Hadjidakis D.J., Mylonakis A.M., Sfakianakis M.E., Raptis A.E., Raptis S.A.2005. Diabetes and premature menopause: is their co-existence detrimental to the skeleton? Eur. J. Endocrinol. 152: 437–442. doi: 10.1530/eje.1.01853 [DOI] [PubMed] [Google Scholar]
- 21.Haffner S.M.2000. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int. J. Obes. Relat. Metab. Disord. 24:(Suppl 2): S56–S58. doi: 10.1038/sj.ijo.0801279 [DOI] [PubMed] [Google Scholar]
- 22.Hall J.E., Kuo J.J., da Silva A.A., de Paula R.B., Liu J., Tallam L.2003. Obesity-associated hypertension and kidney disease. Curr. Opin. Nephrol. Hypertens. 12: 195–200. doi: 10.1097/00041552-200303000-00011 [DOI] [PubMed] [Google Scholar]
- 23.Hamann C., Goettsch C., Mettelsiefen J., Henkenjohann V., Rauner M., Hempel U., Bernhardt R., Fratzl-Zelman N., Roschger P., Rammelt S., Gunther K.P., Hofbauer L.C.2011. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am. J. Physiol. Endocrinol. Metab. 301: E1220–E1228. doi: 10.1152/ajpendo.00378.2011 [DOI] [PubMed] [Google Scholar]
- 24.Hayek A., Woodside W.1979. Correlation between morphology and function in isolated islets of the Zucker rat. Diabetes 28: 565–569. doi: 10.2337/diab.28.6.565 [DOI] [PubMed] [Google Scholar]
- 25.Hoshi S., Shu Y., Yoshida F., Inagaki T., Sonoda J., Watanabe T., Nomoto K., Nagata M.2002. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab. Invest. 82: 25–35. doi: 10.1038/labinvest.3780392 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda H., Shino A., Matsuo T., Iwatsuka H., Suzuoki Z.1981. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes 30: 1045–1050. doi: 10.2337/diab.30.12.1045 [DOI] [PubMed] [Google Scholar]
- 27.Inaba N., Nakamura S., Hirao T., Ryumon N., Chiba K., Miyajima K., Ohta T.2012. Basic study on reproductive science in Spontaneously Diabetic Torii-Leprfa (SDT-fa/fa) female rat. J. Exp. Anim. Technol. 47: 3–10 [Google Scholar]
- 28.Ishii Y., Maki M., Yamamoto H., Sasase T., Kakutani M., Ohta T.2010. Evaluation of blood pressure in Spontaneously Diabetic Torii-Lepr(fa) rats. Exp. Anim. 59: 525–529. doi: 10.1538/expanim.59.525 [DOI] [PubMed] [Google Scholar]
- 29.Ishii Y., Maki M., Yamamoto H., Sasase T., Kakutani M., Ohta T.2011. Blood pressure characteristics of female spontaneously diabetic Torii-Lepr(fa) rats. J. Vet. Med. Sci. 73: 501–505. doi: 10.1292/jvms.10-0453 [DOI] [PubMed] [Google Scholar]
- 30.Ishii Y., Ohta T., Sasase T., Morinag H., Miyajima K., Kakutani M.2011. Effects of food restriction on pancreatic islets in Spontaneously Diabetic Torii fatty rats. J. Vet. Med. Sci. 73: 169–175. doi: 10.1292/jvms.10-0283 [DOI] [PubMed] [Google Scholar]
- 31.Ishii Y., Ohta T., Sasase T., Morinaga H., Ueda N., Hata T., Kakutani M., Miyajima K., Katsuda Y., Masuyama T., Shinohara M., Matsushita M.2010. Pathophysiological analysis of female Spontaneously Diabetic Torii fatty rats. Exp. Anim. 59: 73–84. doi: 10.1538/expanim.59.73 [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa M., Tanaka A., Nomoto S., Shirabe S., Hukuda G., Arai K., Notoya Y., Hayashi T., Komeda K., Kanazawa Y.1997. Alterations of insulin and glucagon secretion from the perfused pancreas before, at the onset and after the development of diabetes in male Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Diabetes Res. Clin. Pract. 38: 161–167. doi: 10.1016/S0168-8227(97)00102-2 [DOI] [PubMed] [Google Scholar]
- 33.Kapoor D., Aldred H., Clark S., Channer K.S., Jones T.H.2007. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30: 911–917. doi: 10.2337/dc06-1426 [DOI] [PubMed] [Google Scholar]
- 34.Karaca M., Castel J., Tourrel-Cuzin C., Brun M., Geant A., Dubois M., Catesson S., Rodriguez M., Luquet S., Cattan P., Lockhart B., Lang J., Ktorza A., Magnan C., Kargar C.2009. Exploring functional beta-cell heterogeneity in vivo using PSA-NCAM as a specific marker. PLoS ONE. 4: e5555. doi: 10.1371/journal.pone.0005555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawano K., Hirashima T., Mori S., Natori T.1994. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 24:(Suppl): S317–S320. doi: 10.1016/0168-8227(94)90269-0 [DOI] [PubMed] [Google Scholar]
- 36.Kawano K., Hirashima T., Mori S., Saitoh Y., Kurosumi M., Natori T.1992. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422–1428. doi: 10.2337/diab.41.11.1422 [DOI] [PubMed] [Google Scholar]
- 37.Kawano K., Mori S., Hirashima T., Man Z.W., Natori T.1999. Examination of the pathogenesis of diabetic nephropathy in OLETF rats. J. Vet. Med. Sci. 61: 1219–1228. doi: 10.1292/jvms.61.1219 [DOI] [PubMed] [Google Scholar]
- 38.Kemmochi Y., Fukui K., Maki M., Kimura S., Ishii Y., Sasase T., Miyajima K., Ohta T.2013. Metabolic disorders and diabetic complications in Spontaneously Diabetic Torii Lepr (fa) rat: a new obese type 2 diabetic model. J. Diabetes. Res. 2013: 948257. doi: 10.1155/2013/948257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., Kim C.S., Sohn E., Kim H., Jeong I.H., Kim J.S.2011. KIOM-79 prevents lens epithelial cell apoptosis and lens opacification in Zucker diabetic fatty rats. Evid. Based Complement. Alternat. Med. 2011: 717921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura S., Sasase T., Ohta T., Matsushita M.2011. Effects of ovariectomy on bone metabolism and bone mineral density in spontaneously diabetic Torii-Lepr(fa) rats. J. Vet. Med. Sci. 73: 1025–1029. doi: 10.1292/jvms.11-0064 [DOI] [PubMed] [Google Scholar]
- 41.Kimura S., Sasase T., Ohta T., Sato E., Matsushita M.2012. Characteristics of bone turnover, bone mass and bone strength in Spontaneously Diabetic Torii-Lepr fa rats. J. Bone. Miner. Metab. 30: 312–320. doi: 10.1007/s00774-011-0324-2 [DOI] [PubMed] [Google Scholar]
- 42.Klein B.E.2007. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 14: 179–183. doi: 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 43.Komaki K., Ohno Y., Aoki N.2005. Gonadal hormones and gonadal function in type 2 diabetes model OLETF (Otsuka Long Evans Tokushima Fatty) rats. Endocr. J. 52: 345–351. doi: 10.1507/endocrj.52.345 [DOI] [PubMed] [Google Scholar]
- 44.Kubo E., Maekawa K., Tanimoto T., Fujisawa S., Akagi Y.2001. Biochemical and morphological changes during development of sugar cataract in Otsuka Long-Evans Tokushima fatty (OLETF) rat. Exp. Eye. Res. 73: 375–381. doi: 10.1006/exer.2001.1046 [DOI] [PubMed] [Google Scholar]
- 45.Kyselova Z., Stefek M., Bauer V.2004. Pharmacological prevention of diabetic cataract. J. Diabetes Complications 18: 129–140. doi: 10.1016/S1056-8727(03)00009-6 [DOI] [PubMed] [Google Scholar]
- 46.Laaksonen D.E., Niskanen L., Punnonen K., Nyyssonen K., Tuomainen T.P., Salonen R., Rauramaa R., Salonen J.T.2003. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur. J. Endocrinol. 149: 601–608. doi: 10.1530/eje.0.1490601 [DOI] [PubMed] [Google Scholar]
- 47.Liang F., Koya D.2010. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes. Metab. 12: 555–569. doi: 10.1111/j.1463-1326.2009.01192.x [DOI] [PubMed] [Google Scholar]
- 48.Lu Z.Y., Bhutto I.A., Amemiya T.2003. Retinal changes in Otsuka long-evans Tokushima Fatty rats (spontaneously diabetic rat)–possibility of a new experimental model for diabetic retinopathy. Jpn. J. Ophthalmol. 47: 28–35. doi: 10.1016/S0021-5155(02)00631-7 [DOI] [PubMed] [Google Scholar]
- 49.Masuyama T., Katsuda Y., Shinohara M.2005. A novel model of obesity-related diabetes: introgression of the Lepr(fa) allele of the Zucker fatty rat into nonobese Spontaneously Diabetic Torii (SDT) rats. Exp. Anim. 54: 13–20. doi: 10.1538/expanim.54.13 [DOI] [PubMed] [Google Scholar]
- 50.Masuyama T., Komeda K., Hara A., Noda M., Shinohara M., Oikawa T., Kanazawa Y., Taniguchi K.2004. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem. Biophys. Res Commun. 314: 870–877. doi: 10.1016/j.bbrc.2003.12.180 [DOI] [PubMed] [Google Scholar]
- 51.Matsui H., Suzuki M., Tsukuda R., Iida K., Miyasaka M., Ikeda H.1996. Expression of ICAM-1 on glomeruli is associated with progression of diabetic nephropathy in a genetically obese diabetic rat, Wistar fatty. Diabetes Res. Clin. Pract. 32: 1–9. doi: 10.1016/0168-8227(96)01209-0 [DOI] [PubMed] [Google Scholar]
- 52.Matsui K., Ohta T., Oda T., Sasase T., Ueda N., Miyajima K., Masuyama T., Shinohara M., Matsushita M.2008. Diabetes-associated complications in Spontaneously Diabetic Torii fatty rats. Exp. Anim. 57: 111–121. doi: 10.1538/expanim.57.111 [DOI] [PubMed] [Google Scholar]
- 53.Matsuura T., Yamagishi S., Kodama Y., Shibata R., Ueda S., Narama I.2005. Otsuka Long-Evans Tokushima fatty (OLETF) rat is not a suitable animal model for the study of angiopathic diabetic retinopathy. Int. J. Tissue React. 27: 59–62. [PubMed] [Google Scholar]
- 54.Melton L.J., 3rd, Atkinson E.J., O’Fallon W.M., Wahner H.W., Riggs B.L.1993. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J. Bone Miner. Res. 8: 1227–1233. doi: 10.1002/jbmr.5650081010 [DOI] [PubMed] [Google Scholar]
- 55.Miyamura N., Bhutto I.A., Amemiya T.1999. Retinal capillary changes in Otsuka Long-Evans Tokushima fatty rats (spontaneously diabetic strain). Electron-microscopic study. Ophthalmic. Res. 31: 358–366. doi: 10.1159/000055559 [DOI] [PubMed] [Google Scholar]
- 56.Moran L.J., Misso M.L., Wild R.A., Norman R.J.2010. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 16: 347–363. doi: 10.1093/humupd/dmq001 [DOI] [PubMed] [Google Scholar]
- 57.Morinaga H., Ohta T., Matsui K., Sasase T., Fukuda S., Ito M., Ueda M., Ishii Y., Miyajima K., Matsushita M.2009. Effect of food restriction on adipose tissue in spontaneously diabetic Torii fatty rats. Exp. Diabetes Res. 2009: 715057. doi: 10.1155/2009/715057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura J., Del Monte M.A., Shewach D., Lattimer S.A., Greene D.A.1992. Inhibition of phosphatidylinositol synthase by glucose in human retinal pigment epithelial cells. Am. J. Physiol. 262: E417–E426. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura J., Hamada Y., Sakakibara F., Hara T., Wakao T., Mori K., Nakashima E., Naruse K., Kamijo M., Koh N., Hotta N.2001. Physiological and morphometric analyses of neuropathy in sucrose-fed OLETF rats. Diabetes Res. Clin. Pract. 51: 9–20. doi: 10.1016/S0168-8227(00)00205-9 [DOI] [PubMed] [Google Scholar]
- 60.Nakamura J., Koh N., Sakakibara F., Hamada Y., Wakao T., Sasaki H., Mori K., Nakashima E., Naruse K., Hotta N.1997. Diabetic neuropathy in sucrose-fed Otsuka Long-Evans Tokushima fatty rats: effect of an aldose reductase inhibitor, TAT. Life Sci. 60: 1847–1857. doi: 10.1016/S0024-3205(97)00145-8 [DOI] [PubMed] [Google Scholar]
- 61.Nevitt M.C., Johnell O., Black D.M., Ensrud K., Genant H.K., Cummings S.R.1994. Bone mineral density predicts non-spine fractures in very elderly women. Study of Osteoporotic Fractures Research Group. Osteoporos. Int. 4: 325–331. doi: 10.1007/BF01622192 [DOI] [PubMed] [Google Scholar]
- 62.New S.A.1999. Bone health: the role of micronutrients. Br Med Bull. 55: 619–633. doi: 10.1258/0007142991902501 [DOI] [PubMed] [Google Scholar]
- 63.Norman R.J., Masters L., Milner C.R., Wang J.X., Davies M.J.2001. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum. Reprod. 16: 1995–1998. doi: 10.1093/humrep/16.9.1995 [DOI] [PubMed] [Google Scholar]
- 64.Okada M., Takemura T., Yanagida H., Yoshioka K.2002. Response of mesangial cells to low-density lipoprotein and angiotensin II in diabetic (OLETF) rats. Kidney Int. 61: 113–124. doi: 10.1046/j.1523-1755.2002.00107.x [DOI] [PubMed] [Google Scholar]
- 65.Oltman C.L., Coppey L.J., Gellett J.S., Davidson E.P., Lund D.D., Yorek M.A.2005. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am. J. Physiol. Endocrinol. Metab. 289: E113–E122. doi: 10.1152/ajpendo.00594.2004 [DOI] [PubMed] [Google Scholar]
- 66.Perl S., Kushner J.A., Buchholz B.A., Meeker A.K., Stein G.M., Hsieh M., Kirby M., Pechhold S., Liu E.H., Harlan D.M., Tisdale J.F.2010. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95: E234–E239. doi: 10.1210/jc.2010-0932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersen J., Ross J., Rabkin R.1988. Effect of insulin therapy on established diabetic nephropathy in rats. Diabetes 37: 1346–1350. doi: 10.2337/diab.37.10.1346 [DOI] [PubMed] [Google Scholar]
- 68.Pfeifer M.A., Halter J.B., Porte D., Jr1981. Insulin secretion in diabetes mellitus. Am. J. Med. 70: 579–588. doi: 10.1016/0002-9343(81)90579-9 [DOI] [PubMed] [Google Scholar]
- 69.Piper M.L., Unger E.K., Myers M.G., Jr, Xu A.W.2008. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol. 22: 751–759. doi: 10.1210/me.2007-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plymate S.R., Matej L.A., Jones R.E., Friedl K.E.1988. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J. Clin. Endocrinol. Metab. 67: 460–464. doi: 10.1210/jcem-67-3-460 [DOI] [PubMed] [Google Scholar]
- 71.Porta M., Maldari P., Mazzaglia F.2011. New approaches to the treatment of diabetic retinopathy. Diabetes Obes. Metab. 13: 784–790. doi: 10.1111/j.1463-1326.2011.01415.x [DOI] [PubMed] [Google Scholar]
- 72.Rand L.I., Prud’homme G.J., Ederer F., Canner P.L.1985. Factors influencing the development of visual loss in advanced diabetic retinopathy. Diabetic Retinopathy Study (DRS) Report No. 10. Invest Ophthalmol. Vis. Sci. 26: 983–991. [PubMed] [Google Scholar]
- 73.Ritz E., Stefanski A.1996. Diabetic nephropathy in type II diabetes. Am. J. Kidney Dis. 27: 167–194. doi: 10.1016/S0272-6386(96)90538-7 [DOI] [PubMed] [Google Scholar]
- 74.Schmidt R.E., Dorsey D.A., Beaudet L.N., Peterson R.G.2003. Analysis of the Zucker Diabetic Fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am. J. Pathol. 163: 21–28. doi: 10.1016/S0002-9440(10)63626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segawa Y., Shirao Y., Yamagishi S., Higashide T., Kobayashi M., Katsuno K., Iyobe A., Harada H., Sato F., Miyata H., Asai H., Nishimura A., Takahira M., Souno T., Segawa Y., Maeda K., Shima K., Mizuno A., Yamamoto H., Kawasaki K.1998. Upregulation of retinal vascular endothelial growth factor mRNAs in spontaneously diabetic rats without ophthalmoscopic retinopathy. A possible participation of advanced glycation end products in the development of the early phase of diabetic retinopathy. Ophthalmic. Res. 30: 333–339. doi: 10.1159/000055493 [DOI] [PubMed] [Google Scholar]
- 76.Shimoshige Y., Ikuma K., Yamamoto T., Takakura S., Kawamura I., Seki J., Mutoh S., Goto T.2000. The effects of zenarestat, an aldose reductase inhibitor, on peripheral neuropathy in Zucker diabetic fatty rats. Metabolism 49: 1395–1399. doi: 10.1053/meta.2000.17723 [DOI] [PubMed] [Google Scholar]
- 77.Shino A., Matsuo T., Iwatsuka H., Suzuoki Z.1973. Structural changes of pancreatic islets in genetically obese rats. Diabetologia 9: 413–421. doi: 10.1007/BF01239438 [DOI] [PubMed] [Google Scholar]
- 78.Sima A.A., Nathaniel V., Bril V., McEwen T.A., Greene D.A.1988. Histopathological heterogeneity of neuropathy in insulin-dependent and non-insulin-dependent diabetes, and demonstration of axo-glial dysjunction in human diabetic neuropathy. J. Clin. Invest 81: 349–364. doi: 10.1172/JCI113327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevens M.J., Dananberg J., Feldman E.L., Lattimer S.A., Kamijo M., Thomas T.P., Shindo H., Sima A.A., Greene D.A.1994. The linked roles of nitric oxide, aldose reductase and, (Na+,K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J. Clin. Invest 94: 853–859. doi: 10.1172/JCI117406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor S.I., Accili D., Imai Y.1994. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 43: 735–740. doi: 10.2337/diab.43.6.735 [DOI] [PubMed] [Google Scholar]
- 81.Tesfamariam B., Palacino J.J., Weisbrod R.M., Cohen R.A.1993. Aldose reductase inhibition restores endothelial cell function in diabetic rabbit aorta. J. Cardiovasc. Pharmacol 21: 205–211. doi: 10.1097/00005344-199302000-00004 [DOI] [PubMed] [Google Scholar]
- 82.Thomas T., Burguera B., Melton L.J., 3rd, Atkinson E.J., O’Fallon W.M., Riggs B.L., Khosla S.2001. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29: 114–120. doi: 10.1016/S8756-3282(01)00487-2 [DOI] [PubMed] [Google Scholar]
- 83.Thomas T.P., Feldman E.L., Nakamura J., Kato K., Lien M., Stevens M.J., Greene D.A.1993. Ambient glucose and aldose reductase-induced myo-inositol depletion modulate basal and carbachol-stimulated inositol phospholipid metabolism and diacylglycerol accumulation in human retinal pigment epithelial cells in culture. Proc. Natl. Acad. Sci. USA. 90: 9712–9716. doi: 10.1073/pnas.90.20.9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tokuyama Y., Sturis J., DePaoli A.M., Takeda J., Stoffel M., Tang J., Sun X., Polonsky K.S., Bell G.I.1995. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 44: 1447–1457. doi: 10.2337/diab.44.12.1447 [DOI] [PubMed] [Google Scholar]
- 85.Tomlinson D.R., Willars G.B., Carrington A.L.1992. Aldose reductase inhibitors and diabetic complications. Pharmacol. Ther. 54: 151–194. doi: 10.1016/0163-7258(92)90031-T [DOI] [PubMed] [Google Scholar]
- 86.Varkonyi T., Kempler P.2008. Diabetic neuropathy: new strategies for treatment. Diabetes Obes. Metab. 10: 99–108. [DOI] [PubMed] [Google Scholar]
- 87.Velasquez M.T., Kimmel P.L., Michaelis O.E.t.1990. Animal models of spontaneous diabetic kidney disease. FASEB J. 4: 2850–2859. [DOI] [PubMed] [Google Scholar]
- 88.Vora J.P., Zimsen S.M., Houghton D.C., Anderson S.1996. Evolution of metabolic and renal changes in the ZDF/Drt-fa rat model of type II diabetes. J. Am. Soc. Nephrol. 7: 113–117. [DOI] [PubMed] [Google Scholar]
- 89.Wakasugi M., Wakao R., Tawata M., Gan N., Koizumi K., Onaya T.1993. Bone mineral density measured by dual energy x-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone 14: 29–33. doi: 10.1016/8756-3282(93)90252-6 [DOI] [PubMed] [Google Scholar]
- 90.Yagihashi S., Matsunaga M.1979. Ultrastructural pathology of peripheral nerves in patients with diabetic neuropathy. Tohoku J. Exp. Med. 129: 357–366. doi: 10.1620/tjem.129.357 [DOI] [PubMed] [Google Scholar]
- 91.Yamagishi S., Nakamura K., Inoue H.2005. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med Hypotheses 65: 1013–1015. doi: 10.1016/j.mehy.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi T., Sasase T., Mera Y., Tomimoto D., Tadaki H., Kemmochi Y., Ohta T., Sato E., Matsushita M.2012. Diabetic Peripheral Neuropathy in Spontaneously Diabetic Torii-Lepr(fa) (SDT Fatty) Rats. J. Vet. Med. Sci. 74: 1669–1673. doi: 10.1292/jvms.12-0149 [DOI] [PubMed] [Google Scholar]
- 93.Yang Y.S., Danis R.P., Peterson R.G., Dolan P.L., Wu Y.Q.2000. Acarbose partially inhibits microvascular retinopathy in the Zucker Diabetic Fatty rat (ZDF/Gmi-fa). J. Ocul. Pharmacol. Ther. 16: 471–479. doi: 10.1089/jop.2000.16.471 [DOI] [PubMed] [Google Scholar]
- 94.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E., Klein R., Krishnaiah S., Mayurasakorn K., O’Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y.2012. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu X., Eichberg J.1990. 1,2-diacylglycerol content and its arachidonyl-containing molecular species are reduced in sciatic nerve from streptozotocin-induced diabetic rats. J. Neurochem. 55: 1087–1090. doi: 10.1111/j.1471-4159.1990.tb04604.x [DOI] [PubMed] [Google Scholar]