Abstract
Changes in dopamine (DA) signaling have been implicated in a number of human neurologic and psychiatric disorders. Similarly, defects in DA signaling in the fruit fly, Drosophila melanogaster, have also been associated with several behavioral defects. As most genes involved in DA synthesis, transport, secretion, and signaling are conserved between species, Drosophila is a powerful genetic model organism to study the regulation of DA signaling in vivo. In this review, we will provide an overview of the genes and drugs that regulate DA biology in Drosophila. Furthermore, we will discuss the behavioral paradigms that are regulated by DA signaling in flies. By analyzing the genes and neuronal circuits that govern such behaviors using sophisticated genetic, pharmacologic, electrophysiologic, and imaging approaches in Drosophila, we will likely gain a better understanding about how this neuromodulator regulates motor tasks and cognition in humans.
Keywords: behavior, cuticle pigmentation, dopamine, Drosophila, genetics
Introduction
Dopamine (DA) is a catecholamine neurotransmitter that is highly conserved throughout evolution [126]. In mammals, DA plays key roles in motor coordination as well as motivation, reward, addiction, learning, and memory. Misregulation of DA signaling has been implicated in a variety of human disorders [29]. Most notably, loss of dopaminergic (DArgic) neurons is the principal defect in Parkinson’s disease, a neurodegenerative disease characterized by a combination of hypokinetic and hyperkinetic movements as well as more subtle sleep dysfunction and cognitive/behavioral changes. Insufficient DA signaling has also been implicated in hereditary dystonias, hypersomnia, periodic limb movement disorder/restless legs syndrome, and mood disorders. While DA agonists and reuptake inhibitors are frequently used to treat these conditions, excessive DA signaling can precipitate debilitating hyperkinetic movements such as tardive dyskinesia. The importance of precisely regulated DA signaling is further highlighted by schizophrenia, a disease in which low DA signaling is thought to mediate negative symptoms such as anhedonia and amotivation, whereas high DA signaling produces positive symptoms like hallucinations and delusions [31]. To uncover the pathophysiology of these diseases and develop more effective treatments, it is crucial to understand how DA is regulated at the molecular and cellular levels in vivo. Furthermore, uncovering the precise neuronal circuits in the brain that are modulated by DA will allow us to deepen our understanding of how imbalances of this small molecule can impact so many different neurologic functions.
In addition to mammalian experimental animals such as mice and rats, the fruit fly, Drosophila melanogaster, has been an excellent model organism to study neurologic processes in vivo through the use of sophisticated genetic techniques [10]. Furthermore, rapid advancements in genetic techniques for neuronal labeling and activity manipulation are allowing researchers to identify and characterize neuronal circuits that regulate specific behaviors [115]. Since many genes involved in DA dynamics (synthesis, transport, secretion, and metabolism) and signal transduction (receptors and downstream signaling cascades) are conserved between flies and human (Fig. 1), Drosophila studies can shed light on the molecular mechanisms underlying DA biology in higher organisms. In addition, many drugs that target the mammalian DArgic pathway have also been shown to be effective in flies [72, 74]. In this review, we will first provide an overview of DA biology in Drosophila, mainly focusing on genetic and pharmacologic approaches that have paved the way to our current understanding of DA dynamics and signaling. The similarities as well as differences between the DA systems of flies and mammals will be discussed along the way. Next, we will review the behavioral paradigms found to be regulated by DA signaling in Drosophila. Of note, genes involved in Parkinson’s disease have been extensively studied in Drosophila but will not be covered here as several recent reviews address this topic [41, 50, 82, 121]. Further isolation of novel genes that regulate DA dynamics and signaling by genetic screens and dissection of neuronal circuits that govern DA-mediated behaviors by optogenetic, electrophysiologic, and imaging techniques will likely continue to provide new insights into how DA contributes to numerous neurologic and psychiatric conditions in humans.
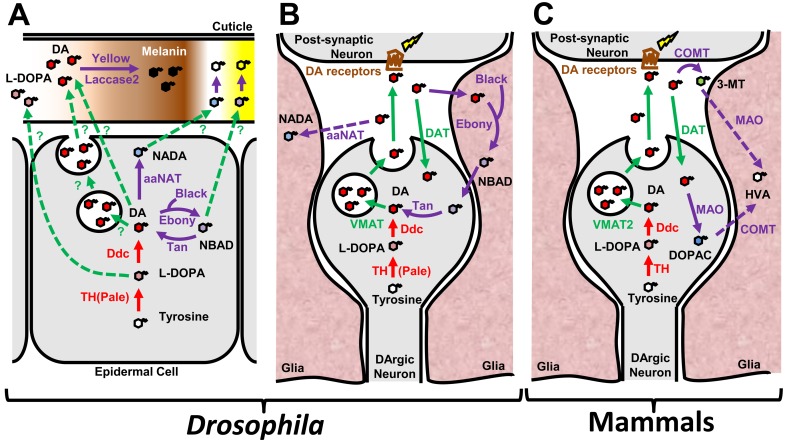
Fig. 1.
Schematic diagrams of DA dynamics and signaling in (A) Drosophila cuticle, (B) Drosophila brain, and (C) mammalian brain. (A) DA is synthesized in epidermal cells by enzymatic action of TH and Ddc upon molting or eclosion. Secreted DA becomes oxidized into melanin by phenoloxidases such as Laccase2. Yellow, a putative enzyme with unknown molecular function contributes to cuticle pigmentation. Enzymes such as Ebony, Black, Tan, and aaNAT are involved in metabolism of DA into NBAD and NADA. Melanin derived from DA (dopamine-melanin) is necessary for pigmentation, while NBAD and NADA contribute to the hardening of the cuticle. L-DOPA has also been proposed to be secreted and contributes to melanin (dopa-melanin) production. Little is known about how DA and its derivatives are secreted during this process. (B) DA is synthesized by TH and Ddc in presynaptic DArgic neurons and loaded into exocytic vesicles by VMAT. Exocytosis of DA through synaptic vesicles is considered to be the main mechanism of DA release. DA binds to DA receptors present on the postsynaptic neurons and triggers a signaling cascade. Excessive DA becomes metabolized into NADA by aaNAT. DAT mediates presynaptic DA reuptake. Ebony, Black, and Tan mediate the recycling of DA between glia cells and DArgic neurons. (C) Most genes involved in synthesis, transport, secretion, signal reception, and signal transduction are conserved between Drosophila and mammals. However, the major enzymes involved in DA metabolism are different between the two species. DA becomes metabolized into DOPAC or 3-MT and then HVA by MAO and COMT in mammalian brains. In addition, a DA recycling pathway between glia cells and neurons has not been identified in mammals.
Genes and Drugs Involved in Dopamine Dynamics and Signaling in Drosophila
DArgic neurons synthesize, secrete, reuptake, and metabolize DA using a set of enzymes and transporters, many of which are evolutionarily conserved. Secreted DA binds to receptors on postsynaptic cells and modulates intracellular signaling cascades. In addition to its role in the nervous system, DA and its metabolites also play an important role in the formation of the insects’ pigmented exoskeleton [124]. In this section, we will first describe the role of DA in cuticle pigmentation in Drosophila. Historically, mutations isolated based on altered pigmentation have lead to the identification of core genes that affect DA dynamics in the cuticle as well as the nervous system. The key molecular players in DA biology will be introduced along with the genetic and pharmacologic tools used to manipulate them in flies. Specifically, we will discuss DA synthesis, transport, and secretion in DA-expressing cells, followed by signal reception and transduction in DA-receiving cells, and finally DA metabolism and recycling.
DA in Drosophila cuticle pigmentation
The insect exoskeleton and mammalian skin both contain melanin, a dark pigment derived from DA precursors tyrosine and L-Dopa (L-3,4-dihydroxyphenylalanine) [101]. In mammals, melanin formation takes place in melanocytes, specialized pigment cells of the skin [85]. These cells contain melanosomes, lysosome-related organelles that contain enzymes responsible for melanin synthesis such as Tyrosinase. While these enzymes produce melanin from DA precursors in the skin, they are not involved in DA synthesis in the mammalian central nervous system. In contrast, insects do not have melanocytes or an identified Tyrosinase ortholog. Interestingly, genes essential for melanin synthesis in the insect cuticle also regulate DA synthesis in insect and mammalian brains (Fig. 1) [124]. Upon molting and eclosion, Drosophila epidermal cells synthesize and secrete DA, a process which will be discussed further below. The secreted DA is then incorporated into the cuticle and oxidized into melanin by phenoloxidases such as Laccase2 [86]. In addition, metabolites of DA such as NBAD (N-β-alanyl dopamine) and NADA (N-acetyl dopamine) are required for hardening of the cuticle (sclerotization). Changes in cuticle color can be directly observed in unbiased forward genetic screens, leading to the identification of a number of genes that regulate DA dynamics in both the cuticle and nervous system [124]. The names of these genes in Drosophila frequently reflect their mutant cuticle pigmentation phenotype (i.e. pale, ebony, tan).
DA synthesis
Several mutations causing impaired cuticle formation or pigmentation affect genes that function directly in DA synthesis from tyrosine. The conversion of tyrosine to L-Dopa is mediated by Tyrosine Hydroxylase (TH), which is encoded by the gene pale in Drosophila [51], and requires Tetrahydrobiopterin (BH4) as a cofactor. BH4 is synthesized from GTP via three chemical reactions, the rate limiting step of which is mediated by GTP cyclohydrolase I (GTPCH) [106]. Several GTPCH mutants were isolated based on cuticle depigmentation and were named unpigmented (now the gene is known as Punch) [73]. The second step of DA synthesis, conversion of L-Dopa to DA, is mediated by Dopa Decarboxylase (Ddc). While Drosophila Ddc mutants also exhibit depigmentation, the gene was first mapped based on biochemical assessment of enzymatic activity in flies with genomic duplications and deletions [24]. Strong loss of function alleles of TH, GTPCH, and Ddc are embryonic lethal due to the requirement of DA for cuticle synthesis. To uncouple the function of DA in the cuticle and the brain, flies specifically deficient in DA synthesis in the adult nervous system have been studied and found to exhibit numerous behavioral phenotypes [87].
Another method to circumvent the early lethality of DA synthetic genes is administration of drugs. α-methyl-p-tyrosine (AMPT, α-MT) [79] or 3-Iodo-L-tyrosine (3-I-Y, 3IY) [69] can be added to fly food to inhibit TH activity. Inhibitors of Ddc, such as α-methyldopa, can also block DA synthesis [95]; however, this manipulation is less specific since Ddc is also required for serotonin biosynthesis. Conversely, defects in DA synthesis can be bypassed by feeding flies L-Dopa [69] or DA [15]. Ingested DA can have direct effects on the nervous system in flies [15, 118], which is in direct contrast to mammals in which supplemental DA cannot cross the blood-brain barrier. These pharmacological approaches have been routinely used to identify behaviors regulated by DA signaling.
To date, several genes have been found to regulate DA synthesis, some of which have been implicated in human diseases with altered DA levels. dTorsin, the fly homolog of TOR1A (also known as DYT1) which is mutated in early-onset primary dystonia, was found to be required for GTPCH stabilization [118]. Mutations in GTPCH and TH have also been linked to dopa-responsive dystonia (DYT5) [18], suggesting that impaired DA synthesis plays a key role in the development of dystonia. Reduced DA levels are also thought to contribute to the sleep fragmentation seen with loss of dBTBD9, the fly homolog of a restless legs syndrome susceptibility factor [32, 33]. Conversely, increased DA levels are seen in the akinetic mutants in Catsup, the fly homolog of zinc transporter ZIP7 [40], which physically interacts with TH and GTPCH [99, 120]. The role of mammalian ZIP7 in DA synthesis has not been investigated, but increased zinc uptake has been associated with decreased levels of TH [97] and DA [89] in rats.
DA secretion
After DA is synthesized in the cytoplasm, it is packaged into exocytic vesicles such as synaptic vesicles and dense core vesicles. Transport of DA across the membrane is mediated by vesicular monoamine transporters (VMATs) [39]. While mammals have two VMAT-encoding genes expressed in a tissue specific manner (VMAT1 in neuroendocrine cells, VMAT2 in neurons), flies have only one VMAT gene. Reserpine, an antipsychotic drug that blocks mammalian VMATs, is also effective in Drosophila and has been used to inhibit the DA signaling in vivo [8, 17, 77]. Flies mutant for VMAT exhibit numerous behavioral abnormalities [96], but some of these phenotypes may be due to defects in other neuromodulators since this transporter also packages serotonin and octopamine [20]. Interestingly, VMAT mutant flies do not show defects in cuticle pigmentation, suggesting that DA release in epidermal cells may require an alternative transporter or be mediated by a non-vesicular mechanism. In addition, L-Dopa and the DA metabolites NADA and NBAD are also thought to be secreted from epidermal cells into the cuticle [37, 56, 104, 123]; however, the molecular mechanism remains to be elucidated.
In neurons, depolarization leads to secretion of DA mainly through exocytosis of DA-containing vesicles at presynaptic sites. In vivo modulation of DA release has been achieved by manipulation of DArgic neuron activity, regulation of vesicle trafficking, and administration of pharmacologic agents. Using the GAL4/UAS binary expression system [13], ectopic expression of Kir2.1 (an inward rectifying potassium channel) [67] or TRPA1 (a temperature activated cation channel) [55] blocks or facilitates DArgic neuron depolarization, respectively. More accurate spatial and temporal control of neuronal activation has been achieved through channelrhodopsin-dependent optogenetic techniques [131]. Alternatively, DA release has been diminished by inhibiting synaptic vesicle trafficking by Tetanus Toxin [61] or expression of dominant negative Dynamin [90]. Conversely, DA secretion can be increased pharmacologically by psychostimulants such as amphetamines [5, 83]. These drugs mediate release of a number of monoamines including DA and serotonin, likely through interactions with VMATs and DA transporter (DAT) [103], the protein that mediates presynaptic DA reuptake (see below). Thus, similar to the effect of VMAT inhibitors, some of the behavioral phenotypes observed upon stimulant administration may be attributed to other neuromodulators or to additive/synergetic effects.
DA signal reception and transduction
Upon secretion, DA binds to its receptors on the postsynaptic cell. In Drosophila, four G-protein coupled DA receptors have been identified: two D1-like receptors (DopR [38, 100] and DopR2 [30, 42]), one D2-like receptor (D2R [43]), and one non-canonical receptor (DopEcR [98]). Similar to humans, D1-like receptors act through activation of the cAMP pathway, while D2-like receptors inhibit this pathway. Therefore, the effect of DA on a specific postsynaptic neuron depends on the type of DA receptor that is expressed. A number of agonists and antagonists that target mammalian D1 and D2 receptors have been used to pharmacologically activate and inhibit Drosophila homologs in vivo. These drugs include D1 agonist SKF82958, D1 antagonists SKF83566 and SCH 23390, D2 agonist Quinpirole, and D2 antagonists Eticlopride, Haloperidol, and Racloprine [17, 64, 128]. The Drosophila non-canonical DA receptor is homologous to mammalian β-adrenergic receptors and mediates activation of cAMP and PI3K pathways [98]. DopEcR can also bind ecdysone, an insect steroid hormone. In vitro studies have shown that ecdysone binding can negatively regulate the DA-mediated activation of DopEcR, but the in vivo significance remains to be investigated.
DA reuptake and metabolism
After signaling, DA needs to be cleared from the synaptic cleft to maintain proper signaling levels in the brain. One mechanism is to take up DA back into presynaptic neurons via the transporter DAT [84]. A spontaneous mutation in DAT named fumin, meaning sleepless in Japanese, has elevated levels of DA [58]. This strain shows increased basal activity and decreased time spent asleep, consistent with increased DA signaling. DAT function can also be pharmacologically blocked by cocaine [17, 108]. Injection or volatilized administration of cocaine to flies has been reported to lead to hyperactive motor phenotypes.
In addition to direct DA reuptake by DArgic neurons, Drosophila can also recycle DA through glial cells [102]. Similar to key genes involved in biosynthesis of DA, most genes involved in DA recycling were originally identified as mutations in cuticle color. After secretion, some DA is thought to be taken up by glia cells and undergo β-alanylation by the enzymatic action of Ebony [47]. β-alanine synthesis is regulated by Black, an aspartate decarboxylase (also known as Glutamic acid decarboxylase 2) [45, 81]. The Ebony product NBAD is then passed from the glia cell to the presynaptic neuron and converted back to DA by Tan, a NBAD hydrolase [109]. Mutations in ebony, black, and tan have been shown to exhibit behavioral defects in addition to cuticle color change. While these defects may be due to defective DA recycling, the same β-alanylation pathway also recycles histamine, a neurotransmitter used by photoreceptor cells in Drosophila [12]. Orthologs of ebony and tan have not been found in the mammalian genome. However, interestingly, Ebony and DA levels are regulated in vivo by a fly homolog of Dysbindin, a human schizophrenia susceptibility gene [93]. Considering that altered DA signaling is one of the major contributors to the pathogenesis of schizophrenia [107], it is possible that DA recycling may also play a role in mammalian DA regulation.
Another mechanism to clear synaptic DA is to metabolize it into inactive compounds. In contrast to the highly conserved processes of DA synthesis, secretion, and signaling, DA breakdown in mammals and flies are significantly different. In mammals, DA is inactivated by metabolic enzymes such as MAOs (L-Monoamine Oxidases) and COMT (Catechol-O-methyltransferase) [68]. Through oxidation and methylation, DA is converted into HVA (homovanillic acid) through the intermediate products DOPAC (3,4-Dihydroxyphenylacetic acid) or 3-MT (3-methoxytyramine). In flies, however, direct orthologs of MAO and COMT genes have not been identified. Instead, fly biogenic amines are thought to be metabolized primarily through acetylation by Dopamine N-acetyltransferase, also known as arylalkylamine N-acetyltransferase (aaNAT) [44, 75]. Flies with reduced aaNAT activity show defects in sleep homeostasis, a phenotype affected by aberrant DA signaling [14, 35, 94]. Thus, while the genes involved in biosynthesis, secretion, and signaling are conserved, genes that mediate breakdown of DA seem to have diverged evolutionarily. It is important to note however that the mammalian DA oxidative products DOPAC [19, 118, 132] and HVA [32] have been detected biochemically in Drosophila, suggesting that an analogous metabolic pathway may exist in flies. In addition, N-acetylation of biogenic amines by mammalian AANAT is involved in the biosynthesis of melatonin, a hormone that influences activity and sleep according to the circadian clock [36]. Thus, studies of fly biogenic amine metabolism by N-acetylation are still important to our understanding of aminergic signaling systems in the mammalian brain.
In summary, DA is synthesized and secreted using a conserved set of genes between flies and human. Receptors and transporters involved in signal reception and DA reuptake are also conserved and can be targeted pharmacologically. However, the pathways that metabolize DA appear to have diverged evolutionarily with mammals using primarily oxidation and methylation, and flies using β-alanylation and N-acetylation.
Behavioral Paradigms Affected byDopamine in Drosophila
Out of the ∼100,000 neurons in the adult Drosophila brain, only ∼130 cells are DArgic [66]. In the larval central nervous system, this number is even smaller (70–90 cells) [91]. Despite their modest numbers, DArgic neurons project broadly and can cause widespread effects. DA has been shown to play key roles in regulating basal locomotion as well as learning and memory, courtship, and addiction in flies. More recently, the involvement of DA in more complex behaviors such as attention, decision making, and appetite have also been discussed. Intensive studies on some of these behaviors have lead to identification of the responsible DArgic neurons and neuronal circuits. In this section, we will review the literature on behavioral paradigms modulated by genetic or pharmacological manipulation of the DArgic system in Drosophila. These behavioral paradigms may serve as useful phenotypic readouts to identify new genes and drugs that affect DA dynamics and signaling in vivo.
Basal activity and locomotion
Several studies indicate that aberrant DA signaling affects basal activity and locomotion in the fly. Typically, increases in DA signaling lead to hyperactivity while decreases in DA signaling have been linked to hypoactivity. Supporting this, direct application of DA or DA agonists on an exposed nerve cord can stimulate stereotypical grooming behavior, whereas application of DA antagonists leads to akinesia [128]. In addition, subsequent studies using genetic or pharmacological manipulations to facilitate or inhibit DA secretion and signaling also show increased or decreased locomotion, respectively [28, 55, 76,77,78, 87, 110, 131]. Increases in DA reuptake by manipulation of DAT activity or treatment with cocaine are also in agreement with these studies [17, 58, 108, 110]. It is important to note, however, that increased DA levels have also been reported to lead to a reduction of larval locomotion [99] and decreased DA levels can lead to hyperactivity [2, 32] and hyperexcitability [34]. These conflicting findings are reminiscent of human Parkinson’s disease in which loss of DArgic neurons produces both hypokinetic and hyperkinetic symptoms [29]. This variability may arise from the differences in DA levels, postsynaptic neurons and receptors, or downstream signaling.
Circadian rhythm, arousal and sleep
Part of the effect of DA on basal locomotion can be attributed to the role of DA in regulating the circadian rhythm, arousal, and sleep [114]. Similar to mammals, flies maintain a circadian rhythm through an internal clock, and their basal activity changes over the course of the day. Flies tend to be more active around the dusk and dawn and sleep during the night [3, 22]. Genetic and pharmacological manipulations that increase DA signaling modulate circadian rhythm-dependent behaviors. Increases in synaptic DA by administration of methamphetamine [5] or blockage of DA reuptake [57, 58, 125] have been shown to reduce sleep and increase basal activity during the night. This arousal-promoting effect of DA is also seen with artificial activation of DArgic neurons [92]. Conversely, mutants in the DA receptor DopR show an increase in time spent sleeping [59]. In addition, mutations in ebony impair circadian rhythm-based activity [71], likely by reduced DA recycling from glia cells [102]. Furthermore, DA also mediates the wake-promoting effect of caffeine [4]. Considering that expression of some genes that regulate DA synthesis [16] and metabolism [102] have been shown to undergo diurnal variation, it is likely that arousal and sleep status in the fly is modulated by DA dynamics and signaling according to the circadian clock. Recent studies have identified specific dopaminergic neurons and neuronal connections that regulate sleep and arousal in Drosophila [63, 112]. Further genetic and molecular studies may shed light on the role of DA in human sleep disorders such as hypersomnia, REM sleep behavior disorder, and periodic limb movement disorder/restless legs syndrome.
Learning and memory
DA signaling has also been shown to play multiple key roles in learning and memory in Drosophila [117]. Flies can be trained to learn and remember both aversive and appetitive cues. While most studies agree that DA is necessary for acquisition of aversive memory, the role of DA in acquisition of appetitive memory has been debated [46, 54, 90, 91, 105]. Both increased and reduced DA levels have been shown to negatively affect memory retention, suggesting that optimal dosage or temporal-spatial regulation of DA is critical [130]. Furthermore, DA signaling is required for removal of old memories (i.e. forgetting) [11]. Interestingly, the learning and forgetting functions appear to be mediated by different DA receptors (DopR and DopR2, respectively). Despite the complexity of DA effects on memory formation, maintenance, and clearance, recent functional anatomic studies have begun to uncover specific DArgic cell populations and neuronal networks that play key roles in learning and memory [7, 11, 62]. Researchers are now able to stimulate these neurons to induce ectopic memory formation through genetic manipulations [6, 23]. In humans, advanced Parkinson’s disease is associated with significant dementia [29]; it is however unclear if this is related to the disease pathophysiology or a result of DArgic treatment.
Courtship and sexual orientation
Courtship is a classical behavioral paradigm that has been extensively characterized in Drosophila. A male fly integrates visual, olfactory, and gustatory cues to recognize a female fly and then executes stereotypical movements known as the “courtship dance” [116]. A female fly also integrates sensory cues to recognize the courting male and decides whether to accept or reject his attentions. Early studies using TH inhibitors to reduce DA levels in adults found that DA is required for female receptivity but dispensable for male courtship behavior [70]. In contrast, increased DA levels through methamphetamine administration [5] or overexpression of VMAT [17] was found to promote courtship in males. Interestingly, increased DA levels were found to specifically promote male-male courtship [64], suggesting that DA levels may play a role in sexual orientation in male flies. Paradoxically, decreased DA levels increase attractiveness of male flies towards other males [65], and a loss of function mutation in DopR also increases male-male courtship [21]. As both high and low DA levels can induce male-male courtship, further studies are necessary to pinpoint the precise role of DA in courtship and sexual orientation in male and female brains.
Reward and addiction
DA acts as a reward signal in the human brain and is strongly linked to the molecular mechanisms underlying alcohol, tobacco, and drug addiction. Excessive L-Dopa use in Parkinson’s patients is associated with hedonistic behaviors such as hypersexuality, pathologic gambling, and compulsive shopping [29]. While the major reward signal in the insect brain is considered to be octopamine rather than DA [80], some of the behavioral phenotypes induced by addictive reagents such as ethanol, nicotine, cocaine, and methamphetamine are known to be mediated by DA signaling in Drosophila. For example, reduction of DA levels by TH or VMAT inhibitors can suppress some locomotion defects caused by ethanol, nicotine, or cocaine [8]. In addition, blocking synaptic transmission or synaptic recycling in DArgic neurons is also effective in suppressing the behavioral effects caused by acute or repetitive exposure to ethanol [55, 60]. Similarly, manipulating DArgic neuron activity has been found to modulate sensitization against cocaine administration [61] and alcohol reward [52]. Thus, while DA is not considered to be the major reward signal, Drosophila still serves as an important model system to understand the molecular mechanisms underlying drug-related behavior and addiction [53].
Other complex behaviors
Recently, the role of DA in more complex Drosophila behaviors like attention, appetite, temperature preference, and aggression has begun to be investigated. Attention can be assessed in flies by measuring flight orientation towards a visual stimulus in a flight simulator [113]. Flies can also be trained to make decisions based on visual cues in this setup. Acute loss of DA signaling was associated with defects in decision making [129], and chronic loss of DA signaling impairs flight orientation, suggestive of defects in attention [127]. Another behavior regulated by DA in Drosophila is appetite or motivation to feed, a process normally enhanced by starvation or sensory stimuli such as appetitive olfactory cues. Recent studies have revealed that in both larvae [119] and adult flies [49, 67], DA plays a key role in mediating increased appetite upon changes in physiologic and sensory status. Drosophila temperature preference also appears to be affected by DA signaling. Whereas one study argues that loss of DA signaling leads to cold temperature preference [9], another reports the opposite [111], a controversy that needs to be further addressed. Finally, a small subset of DArgic neurons have recently been implicated in regulating aggressive behavior in male flies [1]. As more behavioral paradigms become investigated, it is likely that this list of behaviors impacted by DArgic signaling will expand and augment our understanding of the role of DA in high order functions and neuropsychiatric disorders.
Conclusions and Future Directions
As we have described, many similarities can be found between the DArgic systems in flies and humans. First, most genes involved in DA synthesis, secretion, and signaling are evolutionarily conserved which makes Drosophila an important model system to study the molecular function of these genes. Using unbiased forward genetic screens, many genes involved in DA dynamics and signaling have been isolated based on altered cuticle pigmentation, and future screens based on behavioral phenotypes affected by DA may identify novel regulators of signaling. Second, drugs that act on mammalian DArgic systems are also effective in flies. This suggests that high-throughput Drosophila behavioral assays may be a useful screening tool to identify new small molecules affecting DA signaling. Alternatively, screens to identify genetic modifiers of drug-induced behaviors, such as cocaine-induced hyperactivity, may provide insight into the mechanisms of pharmacologic agents and the complex behaviors they affect. Third, mutations in genes associated with human disorders with altered DArgic signaling, such as hereditary dystonia and restless legs syndrome, also affect DA-mediated behaviors in flies. Therefore, flies have the potential to undercover the molecular function of human disease genes in vivo, increasing our understanding of the pathophysiology of DA-related neuropsychiatric diseases. In addition, by identifying new conserved genes that regulate DA dynamics and signaling, fly research may be able to provide novel candidate genes for neurologic diseases without a known genetic cause as well as additional targets for drug therapy.
While there are many similarities, it is also important to consider the differences in DA dynamics and signaling in humans and flies. First, DA metabolism seems to have diverged during evolution with mammals using oxidation and methylation and flies using N-acetylation and β-alanylation. Second, flies lack the genes required to synthesize norepinephrine (noradrenaline) and epinephrine (adrenaline), two key catecholamines derived from DA that function in neuromodulation and neuroendocrine signaling in mammals. Instead, octopamine and tyramine are considered their insect counterparts [88]. Octopamine and tyramine are also derived from tyrosine and are structurally similar to norepinephrine and epinephrine. Moreover, the receptors for these molecules are homologous to mammalian adrenergic receptors. Thus, while DA does not serve as a precursor of fly adrenergic transmitters, many parallels can be drawn between mammalian and insect aminergic systems. Third, DA is necessary for epidermal melanin synthesis in flies but not mammals. Melanin synthesis in mammalian skin is mediated by a melanosomal pathway [26]; however, it is interesting to note that DA oxidation in DArgic neurons produces neuromelanin in the human brain [27]. Flies have also adapted the use of DA to other processes such as oogenesis [69], tracheal morphogenesis [48], pheromone production [122], and innate immunity [25]. Therefore, it is possible that some of the gene regulatory mechanisms present in flies to regulate DA dynamics may not be relevant in DA regulation in the mammal brain. Finally, the organization and the anatomy of the insect and human brains are considerably different. Although progress in mapping specific neurons and neurocircuits responsible for DA-regulated behaviors in flies is expected to provide insight into how behavioral networks are wired, application of such knowledge to human brains will require further investigation as well as characterization of analogous neuronal circuits in rodent model systems such as mice and rats.
In summary, use of genetic and pharmacologic tools has made Drosophila an excellent model organism to study genes, behaviors, and neuronal circuits that are regulated by DA dynamics and signaling in vivo. Despite the differences that exist between humans and flies, research using Drosophila will likely continue to bring new insight into the molecular mechanisms that regulate DA signaling and into the pathophysiology of DA-associated diseases.
Acknowledgments
We would like to apologize to those whose work has not been cited because of space constraints. We would like to thank Drs. Kartik Venkatachalam, Sheng Zhang, and Hugo Bellen for useful suggestions and critical comments. SY is a Fellow of the Jan and Dan Duncan Neurological Research Institute, Texas Children’s Hospital.
References
- 1.Alekseyenko O.V., Chan Y.B., Li R., Kravitz E.A.2013. Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. USA. 110: 6151–6156. doi: 10.1073/pnas.1303446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekseyenko O.V.,, Lee C., Kravitz E.A.2010. Kravitz, Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5: e10806. doi: 10.1371/journal.pone.0010806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allada R., Chung B.Y.2010. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 72: 605–624. doi: 10.1146/annurev-physiol-021909-135815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andretic R., Kim Y.C., Jones F.S., Han K.A., Greenspan R.J.2008. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc. Natl. Acad. Sci. USA. 105: 20392–20397. doi: 10.1073/pnas.0806776105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andretic R.,, van Swinderen B., Greenspan R.J.2005. Greenspan. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15: 1165–1175. doi: 10.1016/j.cub.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 6.Aso Y., Herb A., Ogueta M., Siwanowicz I., Templier T., Friedrich A.B., Ito K., Scholz H., Tanimoto H.2012. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 8: e1002768. doi: 10.1371/journal.pgen.1002768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aso Y., Siwanowicz I., Bräcker L., Ito K., Kitamoto T., Tanimoto H.2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20: 1445–1451. doi: 10.1016/j.cub.2010.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bainton R.J., Tsai L.T., Singh C.M., Moore M.S., Neckameyer W.S., Heberlein U.2000. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10: 187–194. doi: 10.1016/S0960-9822(00)00336-5 [DOI] [PubMed] [Google Scholar]
- 9.Bang S., Hyun S., Hong S.T., Kang J., Jeong K., Park J.J., Choe J., Chung J.2011. Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet. 7: e1001346. doi: 10.1371/journal.pgen.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellen H.J., Tong C., Tsuda H.2010. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat. Rev. Neurosci. 11: 514–522. doi: 10.1038/nrn2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry J.A., Cervantes-Sandoval I., Nicholas E.P., Davis R.L.2012. Dopamine is required for learning and forgetting in Drosophila. Neuron 74: 530–542. doi: 10.1016/j.neuron.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borycz J., Borycz J.A., Loubani M., , Meinertzhagen I.A.2002. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J. Neurosci. 22: 10549–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand A.H., Perrimon N.1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- 14.Brodbeck D., Amherd R., Callaerts P., Hintermann E., Meyer U.A., Affolter M.1998. Molecular and biochemical characterization of the aaNAT1 (Dat) locus in Drosophila melanogaster: differential expression of two gene products. DNA Cell Biol. 17: 621–633. doi: 10.1089/dna.1998.17.621 [DOI] [PubMed] [Google Scholar]
- 15.Budnik V, W C.F., White K.1989. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J. Neurosci. 9: 2866–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceriani M.F., Hogenesch J.B., Yanovsky M., Panda S., Straume M., Kay S.A.2002. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J. Neurosci. 22: 9305–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang H.Y., Grygoruk A., Brooks E.S., Ackerson L.C., Maidment N.T., Bainton R.J., Krantz D.E.2006. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol. Psychiatry 11: 99–113. doi: 10.1038/sj.mp.4001742 [DOI] [PubMed] [Google Scholar]
- 18.Charlesworth G., Bhatia K.P., Wood N.W.2013. The genetics of dystonia: new twists in an old tale. Brain 136: 2017–2037. doi: 10.1093/brain/awt138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhuri A., Bowling K., Funderburk C., Lawal H., Inamdar A., Wang Z., O’Donnell J.M.2007. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 27: 2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A., Ng F., Lebestky T., Grygoruk A., Djapri C., Lawal H.O., Zaveri H.A., Mehanzel F., Najibi R., Seidman G., Murphy N.P., Kelly R.L., Ackerson L.C., Maidment N.T., Jackson F.R., Krantz D.E.2013. Dispensable, redundant, complementary, and cooperative roles of dopamine, octopamine, and serotonin in Drosophila melanogaster. Genetics 193: 159–176. doi: 10.1534/genetics.112.142042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B., Liu H., Ren J., Guo A.2012. Mutation of Drosophila dopamine receptor DopR leads to male-male courtship behavior. Biochem. Biophys. Res. Commun. 423: 557–563. doi: 10.1016/j.bbrc.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Cirelli C., Bushey D.2008. Sleep and wakefulness in Drosophila melanogaster. Ann. NY Acad. Sci. 1129: 323–329. doi: 10.1196/annals.1417.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claridge-Chang A., Roorda R.D., Vrontou E., Sjulson L., Li H., Hirsh J., Miesenböck G.2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415. doi: 10.1016/j.cell.2009.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark W.C., Pass P.S., Venkataraman B., Hodgetts R.B.1978. Dopa decarboxylase from Drosophila melanogaster. Purification, characterization and an analysis of mutants. Mol. Gen. Genet. 162: 287–297. doi: 10.1007/BF00268854 [DOI] [Google Scholar]
- 25.Davis M.M., Primrose D.A., Hodgetts R.B.2008. A member of the p38 mitogen-activated protein kinase family is responsible for transcriptional induction of Dopa decarboxylase in the epidermis of Drosophila melanogaster during the innate immune response. Mol. Cell Biol. 28: 4883–4895. doi: 10.1128/MCB.02074-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dell’Angelica E.C., Mullins C., Caplan S., Bonifacino J.S.2000. Lysosome-related organelles. FASEB J. 14: 1265–1278. doi: 10.1096/fj.14.10.1265 [DOI] [PubMed] [Google Scholar]
- 27.Double K.L., Dedov V.N., Fedorow H., Kettle E., Halliday G.M., Garner B., Brunk U.T.2008. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol. Life Sci. 65: 1669–1682. doi: 10.1007/s00018-008-7581-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draper I., Kurshan P.T., McBride E., Jackson F.R., Kopin A.S.2007. Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev. Neurobiol. 67: 378–393. doi: 10.1002/dneu.20355 [DOI] [PubMed] [Google Scholar]
- 29.Fahr S., Jankovic J., Hallett M.2011. Principles and Practice of Movement Disorders. Elsevier, Philadelphia. [Google Scholar]
- 30.Feng G., Hannan F., Reale V., Hon Y.Y., Kousky C.T., Evans P.D., Hall L.M.1996. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J. Neurosci. 16: 3925–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer B.A., Buchanan R.W.2013. Schizophrenia: Epidemiology and pathogenesis. in UpTo Date. (D.S. Basow, eds.), UptoDate, Massachusetts. [Google Scholar]
- 32.Freeman A., Pranski E., Miller R.D., Radmard S., Bernhard D., Jinnah H.A., Betarbet R., Rye D.B., Sanyal S.2012. Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Curr. Biol. 22: 1142–1148. doi: 10.1016/j.cub.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman A.A., Mandilaras K., Missirlis F., Sanyal S.2013. An emerging role for Cullin-3 mediated ubiquitination in sleep and circadian rhythm: insights from Drosophila. Fly (Austin) 7: 39–43. doi: 10.4161/fly.23506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S.2003. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54: 618–627. doi: 10.1002/neu.10185 [DOI] [PubMed] [Google Scholar]
- 35.Ganguly-Fitzgerald I., Donlea J., Shaw P.J.2006. Waking experience affects sleep need in Drosophila. Science 313: 1775–1781. doi: 10.1126/science.1130408 [DOI] [PubMed] [Google Scholar]
- 36.Ganguly S., Coon S.L., Klein D.C.2002. Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res. 309: 127–137. doi: 10.1007/s00441-002-0579-y [DOI] [PubMed] [Google Scholar]
- 37.Gibert J.M., Peronnet F., Schlotterer C.2007. Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network. PLoS Genet. 3: e30. doi: 10.1371/journal.pgen.0030030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotzes F., Balfanz S., Baumann A.1994. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels 2: 131–141. [PubMed] [Google Scholar]
- 39.Greer C.L., Grygoruk A., Patton D.E., Ley B., Romero-Calderon R., Chang H.Y., Houshyar R., Bainton R.J., Diantonio A., Krantz D.E.2005. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J. Neurobiol. 64: 239–258. doi: 10.1002/neu.20146 [DOI] [PubMed] [Google Scholar]
- 40.Groth C., Sasamura T., Khanna M.R., Whitley M., Fortini M.E.2013. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development 140: 3018–3027. doi: 10.1242/dev.088336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo M.2012. Drosophila as a model to study mitochondrial dysfunction in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2: a009944doi:10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han K.A., Millar N.S., Grotewiel M.S., Davis R.L.1996. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 16: 1127–1135. doi: 10.1016/S0896-6273(00)80139-7 [DOI] [PubMed] [Google Scholar]
- 43.Hearn M.G., Ren Y., McBride E.W., Reveillaud I., Beinborn M., Kopin A.S.2002. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. USA. 99: 14554–14559. doi: 10.1073/pnas.202498299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hintermann E., Grieder N.C., Amherd R., Brodbeck D., Meyer U.A.1996. Cloning of an arylalkylamine N-acetyltransferase (aaNAT1) from Drosophila melanogaster expressed in the nervous system and the gut. Proc. Natl. Acad. Sci. USA. 93: 12315–12320. doi: 10.1073/pnas.93.22.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgetts R.B.1972. Biochemical characterization of mutants affecting the metabolism of -alanine in Drosophila. J. Insect. Physiol. 18: 937–947. doi: 10.1016/0022-1910(72)90031-5 [DOI] [PubMed] [Google Scholar]
- 46.Honjo K., Furukubo-Tokunaga K.2009. Distinctive neuronal networks and biochemical pathways for appetitive and aversive memory in Drosophila larvae. J. Neurosci. 29: 852–862. doi: 10.1523/JNEUROSCI.1315-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hovemann B.T., Ryseck R.P., Walldorf U., Störtkuhl K.F., Dietzel I.D., Dessen E.1998. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene 221: 1–9. doi: 10.1016/S0378-1119(98)00440-5 [DOI] [PubMed] [Google Scholar]
- 48.Hsouna A., Lawal H.O., Izevbaye I., Hsu T., O’Donnell J.M.2007. Drosophila dopamine synthesis pathway genes regulate tracheal morphogenesis. Dev. Biol. 308: 30–43. doi: 10.1016/j.ydbio.2007.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inagaki H.K., Ben-Tabou de-Leon S., Wong A.M., Jagadish S., Ishimoto H., Barnea G., Kitamoto T., Axel R., Anderson D.J.2012. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 148: 583–595. doi: 10.1016/j.cell.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaiswal M., Sandoval H., Zhang K., Bayat V., Bellen H.J.2012. Probing mechanisms that underlie human neurodegenerative diseases in Drosophila. Annu. Rev. Genet. 46: 371–396. doi: 10.1146/annurev-genet-110711-155456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jurgens G., Wieschaus E., Kluding H.1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Rouxs. Arch. Dev. Biol. 193: 283–295. doi: 10.1007/BF00848157 [DOI] [PubMed] [Google Scholar]
- 52.Kaun K.R., Azanchi R., Maung Z., Hirsh J., Heberlein U.A.2011. Drosophila model for alcohol reward. Nat. Neurosci. 14: 612–619. doi: 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaun K.R., Devineni A.V., Heberlein U.2012. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 131: 959–975. doi: 10.1007/s00439-012-1146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y.C., Lee H.G., Han K.A.2007. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 27: 7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong E.C., Woo K., Li H., Lebestky T., Mayer N., Sniffen M.R., Heberlein U., Bainton R.J., Hirsh J., Wolf F.W.2010. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5: e9954. doi: 10.1371/journal.pone.0009954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kronforst M.R., Barsh G.S., Kopp A., Mallet J., Monteiro A., Mullen S.P., Protas M., Rosenblum E.B., Schneider C.J., Hoekstra H.E.2012. Unraveling the thread of nature’s tapestry: the genetics of diversity and convergence in animal pigmentation. Pigment. Cell Melanoma Res. 25: 411–433. doi: 10.1111/j.1755-148X.2012.01014.x [DOI] [PubMed] [Google Scholar]
- 57.Kumar S., Chen D., Sehgal A.2012. Dopamine acts through Cryptochrome to promote acute arousal in Drosophila. Genes Dev. 26: 1224–1234. doi: 10.1101/gad.186338.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kume K., Park S.K., Hirsh J., Jackson F.R.2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25: 7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebestky T., Chang J.S., Dankert H., Zelnik L., Kim Y.C., Han K.A., Wolf F.W., Perona P., Anderson D.J.2009. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron 64: 522–536. doi: 10.1016/j.neuron.2009.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H.G., Kim Y.C., Dunning J.S., Han K.A.2008. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS ONE 3: e1391. doi: 10.1371/journal.pone.0001391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H., Chaney S., Roberts I.J., Forte M., Hirsh J.2000. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10: 211–214. doi: 10.1016/S0960-9822(00)00340-7 [DOI] [PubMed] [Google Scholar]
- 62.Liu C., Plaçais P.Y., Yamagata N., Pfeiffer B.D., Aso Y., Friedrich A.B., Siwanowicz I., Rubin G.M., Preat T., Tanimoto H.2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488: 512–516. doi: 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- 63.Liu Q., Liu S., Kodama L., Driscoll M.R., Wu M.N.2012. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr. Biol. 22: 2114–2123. doi: 10.1016/j.cub.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu T., Dartevelle L., Yuan C., Wei H., Wang Y., Ferveur J.F., Guo A.2008. Increased dopamine level enhances male-male courtship in Drosophila. J. Neurosci. 28: 5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T., Dartevelle L., Yuan C., Wei H., Wang Y., Ferveur J.F., Guo A.2009. Reduction of dopamine level enhances the attractiveness of male Drosophila to other males. PLoS ONE 4: e4574. doi: 10.1371/journal.pone.0004574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao Z., Davis R.L.2009. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural. Circuits 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marella S., Mann K., Scott K.2012. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 73: 941–950. doi: 10.1016/j.neuron.2011.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meiser J., Weindl D., Hiller K.2013. Complexity of dopamine metabolism. Cell Commun. Signal 11: 34. doi: 10.1186/1478-811X-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neckameyer W.S.1996. Multiple roles for dopamine in Drosophila development. Dev. Biol. 176: 209–219. doi: 10.1006/dbio.1996.0128 [DOI] [PubMed] [Google Scholar]
- 70.Neckameyer W.S.1998. Dopamine modulates female sexual receptivity in Drosophila melanogaster. J. Neurogenet. 12: 101–114. doi: 10.3109/01677069809167259 [DOI] [PubMed] [Google Scholar]
- 71.Newby L.M., Jackson F.R.1991. Drosophila ebony mutants have altered circadian activity rhythms but normal eclosion rhythms. J. Neurogenet. 7: 85–101. doi: 10.3109/01677069109066213 [DOI] [PubMed] [Google Scholar]
- 72.Nichols C.D.2006. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol. Ther. 112: 677–700. doi: 10.1016/j.pharmthera.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 73.Nusslein-Volhard C., Wieschaus E., Kluding H.1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Rouxs. Arch. Dev. Biol. 193: 267–282. doi: 10.1007/BF00848156 [DOI] [PubMed] [Google Scholar]
- 74.Pandey U.B., Nichols C.D.2011. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 63: 411–436. doi: 10.1124/pr.110.003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paxon T.L., Powell P.R., Lee H.G., Han K.A.2005. Ewing AG.Microcolumn separation of amine metabolites in the fruit fly. Anal. Chem. 77: 5349–5355. doi: 10.1021/ac050474m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pendleton R.G., Rasheed A., Hillman R.2000. Effects of adrenergic agents on locomotor behavior and reproductive development in Drosophila. Drug Dev. Res. 50: 142–146. doi: [DOI] [Google Scholar]
- 77.Pendleton R.G., Rasheed A., Paluru P., Joyner J., Jerome N., Meyers R.D., Hillman R.2005. A developmental role for catecholamines in Drosophila behavior. Pharmacol. Biochem. Behav. 81: 849–853. doi: 10.1016/j.pbb.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 78.Pendleton R.G., Rasheed A., Sardina T., Tully T., Hillman R.2002. Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behav. Genet. 32: 89–94. doi: 10.1023/A:1015279221600 [DOI] [PubMed] [Google Scholar]
- 79.Pendleton R.G., Robinson N., Roychowdhury R., Rasheed A., Hillman R.1996. Reproduction and development in Drosophila are dependent upon catecholamines. Life Sci. 59: 2083–2091. doi: 10.1016/S0024-3205(96)00562-0 [DOI] [PubMed] [Google Scholar]
- 80.Perry C.J., Barron A.B.2013. Neural mechanisms of reward in insects. Annu. Rev. Entomol. 58: 543–562. doi: 10.1146/annurev-ento-120811-153631 [DOI] [PubMed] [Google Scholar]
- 81.Phillips A.M., Salkoff L.B., Kelly L.E.1993. A neural gene from Drosophila melanogaster with homology to vertebrate and invertebrate glutamate decarboxylases. J. Neurochem. 61: 1291–1301. doi: 10.1111/j.1471-4159.1993.tb13621.x [DOI] [PubMed] [Google Scholar]
- 82.Pienaar I.S., Gotz J., Feany M.B.2010. Parkinson’s disease: insights from non-traditional model organisms. Prog. Neurobiol. 92: 558–571. doi: 10.1016/j.pneurobio.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 83.Pizzo A.B., Karam C.S., Zhang Y., Yano H., Freyberg R.J., Karam D.S., Freyberg Z., Yamamoto A., McCabe B.D., Javitch J.A.2013. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol. Psychiatry 18: 824–833. doi: 10.1038/mp.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porzgen P., Park S.K., Hirsh J., Sonders M.S., Amara S.G.2001. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol. Pharmacol. 59: 83–95. [DOI] [PubMed] [Google Scholar]
- 85.Raposo G., Marks M.S.2007. Melanosomes–dark organelles enlighten endosomal membrane transport. Nat .Rev. Mol. Cell Biol. 8: 786–797. doi: 10.1038/nrm2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riedel F., Vorkel D., Eaton S.2011. Megalin-dependent yellow endocytosis restricts melanization in the Drosophila cuticle. Development 138: 149–158. doi: 10.1242/dev.056309 [DOI] [PubMed] [Google Scholar]
- 87.Riemensperger T., Isabel G., Coulom H., Neuser K., Seugnet L., Kume K., Iché-Torres M., Cassar M., Strauss R., Preat T., Hirsh J., Birman S.2011. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA. 108: 834–839. doi: 10.1073/pnas.1010930108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roeder T.2005. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50: 447–477. doi: 10.1146/annurev.ento.50.071803.130404 [DOI] [PubMed] [Google Scholar]
- 89.Satija N.K., Seth T.D., Tandon D.S.1978. Dopamine and noradrenaline levels in the brains of lead and zinc poisoned rats. Toxicology 10: 13–16. doi: 10.1016/0300-483X(78)90050-1 [DOI] [PubMed] [Google Scholar]
- 90.Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M.2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23: 10495–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Selcho M., Pauls D., Han K.A., Stocker R.F., Thum A.S.2009. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE 4: e5897. doi: 10.1371/journal.pone.0005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shang Y., Haynes P., Pírez N., Harrington K.I., Guo F., Pollack J., Hong P., Griffith L.C., Rosbash M.2011. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat. Neurosci. 14: 889–895. doi: 10.1038/nn.2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao L., Shuai Y., Wang J., Feng S., Lu B., Li Z., Zhao Y., Wang L., Zhong Y.2011. Schizophrenia susceptibility gene dysbindin regulates glutamatergic and dopaminergic functions via distinctive mechanisms in Drosophila. Proc. Natl. Acad. Sci. USA. 108: 18831–18836. doi: 10.1073/pnas.1114569108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaw P.J., Cirelli C., Greenspan R.J., Tononi G.2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287: 1834–1837. doi: 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- 95.Sherald A.F., Wright T.R.1974. The analog inhibitor, alpha-methyl dopa, as a screening agent for mutants elevating levels of dopa decarboxylase activity in Drosophila melanogaster. Mol. Gen. Genet. 133: 25–36. doi: 10.1007/BF00268674 [DOI] [PubMed] [Google Scholar]
- 96.Simon A.F., Daniels R., Romero-Calderón R., Grygoruk A., Chang H.Y., Najibi R., Shamouelian D., Salazar E., Solomon M., Ackerson L.C., Maidment N.T., Diantonio A., Krantz D.E.2009. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics 181: 525–541. doi: 10.1534/genetics.108.094110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh B.K., Kumar A., Ahmad I., Kumar V., Patel D.K., Jain S.K., Singh C.2011. Oxidative stress in zinc-induced dopaminergic neurodegeneration: implications of superoxide dismutase and heme oxygenase-1. Free Radic. Res. 45: 1207–1222. doi: 10.3109/10715762.2011.607164 [DOI] [PubMed] [Google Scholar]
- 98.Srivastava D.P., Yu E.J., Kennedy K., Chatwin H., Reale V., Hamon M., Smith T., Evans P.D.2005. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 25: 6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stathakis D.G., Burton D.Y., McIvor W.E., Krishnakumar S., Wright T.R., O’Donnell J.M.1999. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics 153: 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sugamori K.S., Demchyshyn L.L., McConkey F., Forte M.A., Niznik H.B.1995. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 362: 131–138. doi: 10.1016/0014-5793(95)00224-W [DOI] [PubMed] [Google Scholar]
- 101.Sugumaran M.2002. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 15: 2–9. doi: 10.1034/j.1600-0749.2002.00056.x [DOI] [PubMed] [Google Scholar]
- 102.Suh J., Jackson F.R.2007. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 55: 435–447. doi: 10.1016/j.neuron.2007.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sulzer D., Sonders M.S., Poulsen N.W., Galli A.2005. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 75: 406–433. doi: 10.1016/j.pneurobio.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 104.Takahashi A.2013. Pigmentation and behavior: potential association through pleiotropic genes in Drosophila. Genes Genet. Syst. 88: 165–174. [DOI] [PubMed] [Google Scholar]
- 105.Tempel B.L., Livingstone M.S., Quinn W.G.1984. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc. Natl. Acad. Sci. USA. 81: 3577–3581. doi: 10.1073/pnas.81.11.3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thony B., Auerbach G., Blau N.2000. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347: 1–16. doi: 10.1042/0264-6021:3470001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toda M., Abi-Dargham A.2007. Dopamine hypothesis of schizophrenia: making sense of it all. Curr. Psychiatry Rep. 9: 329–336. doi: 10.1007/s11920-007-0041-7 [DOI] [PubMed] [Google Scholar]
- 108.Torres G., Horowitz J.M.1998. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse 29: 148–161. doi: [DOI] [PubMed] [Google Scholar]
- 109.True J.R., Yeh S.D., Hovemann B.T., Kemme T., Meinertzhagen I.A., Edwards T.N., Liou S.R., Han Q., Li J.2005. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 1: e63. doi: 10.1371/journal.pgen.0010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ueno T., Masuda N., Kume S., Kume K.2012. Dopamine modulates the rest period length without perturbation of its power law distribution in Drosophila melanogaster. PLoS ONE 7: e32007. doi: 10.1371/journal.pone.0032007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ueno T., Tomita J., Kume S., Kume K.2012. Dopamine modulates metabolic rate and temperature sensitivity in Drosophila melanogaster. PLoS ONE 7: e31513. doi: 10.1371/journal.pone.0031513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ueno T., Tomita J., Tanimoto H., Endo K., Ito K., Kume S., Kume K.2012. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 15: 1516–1523. doi: 10.1038/nn.3238 [DOI] [PubMed] [Google Scholar]
- 113.van Swinderen B.2011. Attention in Drosophila. Int. Rev. Neurobiol. 99: 51–85. doi: 10.1016/B978-0-12-387003-2.00003-3 [DOI] [PubMed] [Google Scholar]
- 114.van Swinderen B., Andretic R.2011. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc. Biol. Sci. 278: 906–913. doi: 10.1098/rspb.2010.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venken K.J., Simpson J.H., Bellen H.J.2011. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72: 202–230. doi: 10.1016/j.neuron.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Villella A., Hall J.C.2008. Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62: 67–184. doi: 10.1016/S0065-2660(08)00603-2 [DOI] [PubMed] [Google Scholar]
- 117.Waddell S.2010. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 33: 457–464. doi: 10.1016/j.tins.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wakabayashi-Ito N., Doherty O.M., Moriyama H., Breakefield X.O., Gusella J.F., O’Donnell J.M., Ito N.2011. Dtorsin, the Drosophila ortholog of the early-onset dystonia TOR1A (DYT1), plays a novel role in dopamine metabolism. PLoS ONE 6: e26183. doi: 10.1371/journal.pone.0026183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y., Pu Y., Shen P.2013. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 3: 820–830. doi: 10.1016/j.celrep.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 120.Wang Z., Ferdousy F., Lawal H., Huang Z., Daigle J.G., Izevbaye I., Doherty O., Thomas J., Stathakis D.G., O’Donnell J.M.2011. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J. Neurochem. 119: 1294–1305. doi: 10.1111/j.1471-4159.2011.07517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Whitworth A.J.2011. Drosophila models of Parkinson’s disease. Adv. Genet. 73: 1–50. doi: 10.1016/B978-0-12-380860-8.00001-X [DOI] [PubMed] [Google Scholar]
- 122.Wicker-Thomas C., Hamann M.2008. Interaction of dopamine, female pheromones, locomotion and sex behavior in Drosophila melanogaster. J. Insect. Physiol. 54: 1423–1431. doi: 10.1016/j.jinsphys.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 123.Wittkopp P.J., Carroll S.B., Kopp A.2003. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet. 19: 495–504. doi: 10.1016/S0168-9525(03)00194-X [DOI] [PubMed] [Google Scholar]
- 124.Wright T.R.1987. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 24: 127–222. doi: 10.1016/S0065-2660(08)60008-5 [DOI] [PubMed] [Google Scholar]
- 125.Wu M.N., Koh K., Yue Z., Joiner W.J., Sehgal A.2008. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep 31: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto K., Vernier P.2011. The evolution of dopamine systems in chordates. Front Neuroanat. 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ye Y., Xi W., Peng Y., Wang Y., Guo A.2004. Long-term but not short-term blockade of dopamine release in Drosophila impairs orientation during flight in a visual attention paradigm. Eur. J. Neurosci. 20: 1001–1007. doi: 10.1111/j.1460-9568.2004.03575.x [DOI] [PubMed] [Google Scholar]
- 128.Yellman C., Tao H., He B., Hirsh J.1997. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA. 94: 4131–4136. doi: 10.1073/pnas.94.8.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang K., Guo J.Z., Peng Y., Xi W., Guo A.2007. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science 316: 1901–1904. doi: 10.1126/science.1137357 [DOI] [PubMed] [Google Scholar]
- 130.Zhang S., Yin Y., Lu H., Guo A.2008. Increased dopaminergic signaling impairs aversive olfactory memory retention in Drosophila. Biochem. Biophys. Res. Commun. 370: 82–86. doi: 10.1016/j.bbrc.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 131.Zhang W., Ge W., Wang Z.2007. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 26: 2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y.Q., Friedman D.B., Wang Z., Woodruff E., 3rd, Pan L., O’donnell J., Broadie K.2005. Protein expression profiling of the drosophila fragile X mutant brain reveals up-regulation of monoamine synthesis. Mol. Cell Proteomics 4: 278–290. doi: 10.1074/mcp.M400174-MCP200 [DOI] [PubMed] [Google Scholar]