Abstract
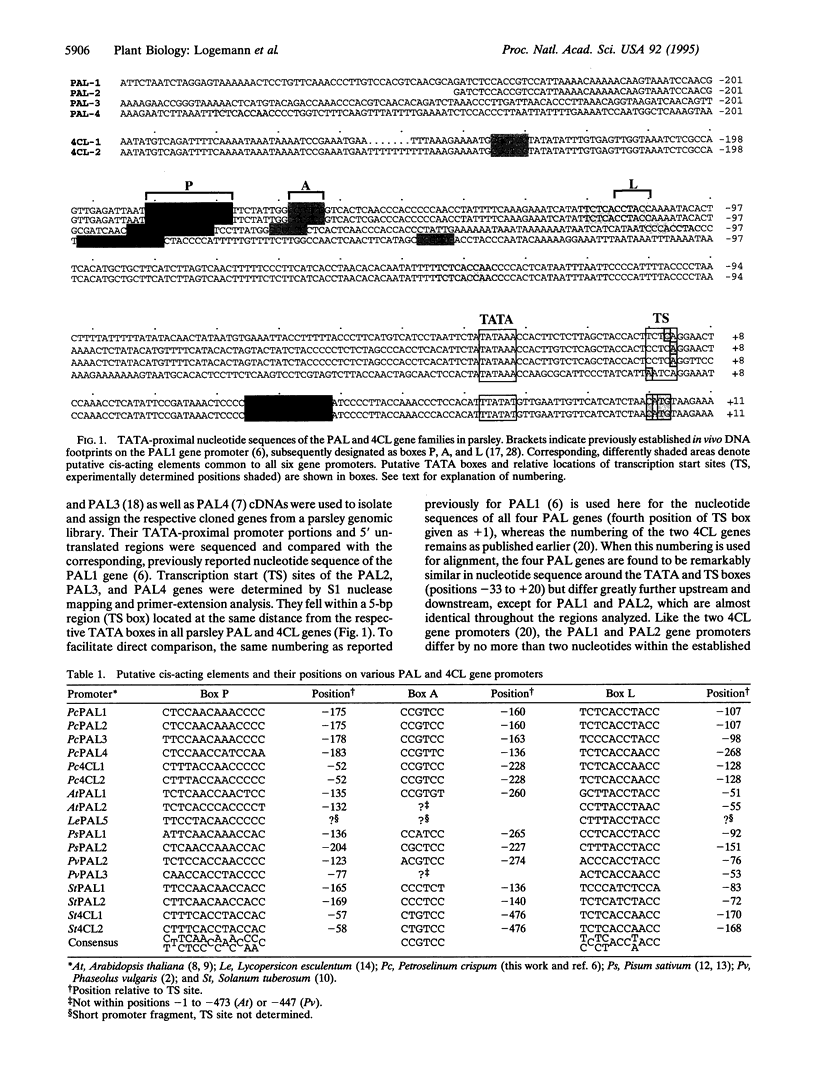
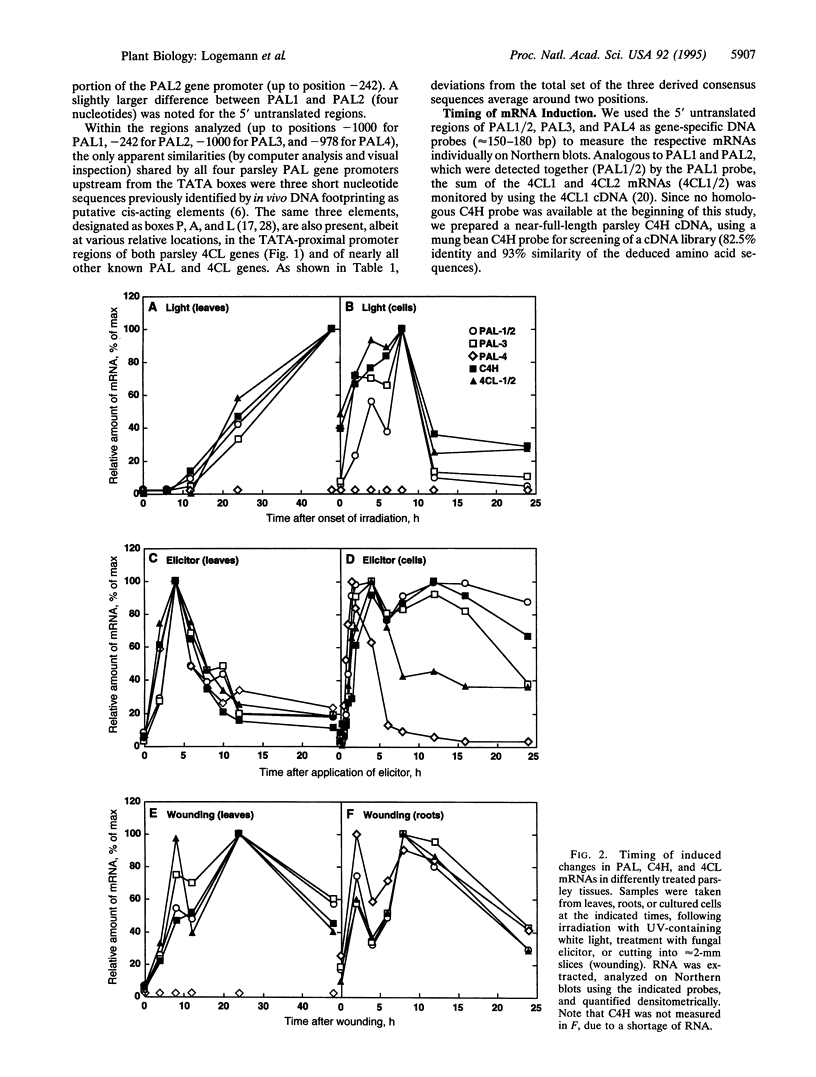
We describe a complete gene family encoding phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) in one particular plant species. In parsley (Petroselinum crispum), the PAL gene family comprises two closely related members, PAL1 and PAL2, whose TATA-proximal promoter and coding regions are almost identical, and two additional members, PAL3 and PAL4, with less similarity to one another and to the PAL1 and PAL2 genes. Using gene-specific probes derived from the 5' untranslated regions of PAL1/2, PAL3, and PAL4, we determined the respective mRNA levels in parsley leaves and cell cultures treated with UV light or fungal elicitor and in wounded leaves and roots. For comparison, the functionally closely related cinnamate 4-hydroxylase (C4H) and 4-coumarate:CoA ligase (4CL) mRNAs were measured in parallel. The results indicate various degrees of differential responsiveness of PAL4 relative to the other PAL gene family members, in contrast to a high degree of coordination in the overall expression of the PAL, C4H, and 4CL genes. The only significant sequence similarities shared by all four PAL gene promoters are a TATA-proximal set of three putative cis-acting elements (boxes P, A, and L). None of these elements alone, or the promoter region containing all of them together, conferred elicitor or light responsiveness on a reporter gene in transient expression assays. The elements appear to be necessary but not sufficient for elicitor- or light-mediated PAL gene activation, similar to the situation previously reported for 4CL.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert C., Logemann E., Hahlbrock K., Schmid J., Amrhein N. Structural and catalytic properties of the four phenylalanine ammonia-lyase isoenzymes from parsley (Petroselinum crispum Nym.). Eur J Biochem. 1994 Oct 1;225(1):491–499. doi: 10.1111/j.1432-1033.1994.00491.x. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Schulze-Lefert P., Hahlbrock K. Structural comparison, modes of expression, and putative cis-acting elements of the two 4-coumarate: CoA ligase genes in potato. J Biol Chem. 1991 May 5;266(13):8551–8559. [PubMed] [Google Scholar]
- Douglas C. J., Hauffe K. D., Ites-Morales M. E., Ellard M., Paszkowski U., Hahlbrock K., Dangl J. L. Exonic sequences are required for elicitor and light activation of a plant defense gene, but promoter sequences are sufficient for tissue specific expression. EMBO J. 1991 Jul;10(7):1767–1775. doi: 10.1002/j.1460-2075.1991.tb07701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C., Hoffmann H., Schulz W., Hahlbrock K. Structure and elicitor or u.v.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J. 1987 May;6(5):1189–1195. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Scheel D., Logemann E., Nürnberger T., Parniske M., Reinold S., Sacks W. R., Schmelzer E. Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauffe K. D., Lee S. P., Subramaniam R., Douglas C. J. Combinatorial interactions between positive and negative cis-acting elements control spatial patterns of 4CL-1 expression in transgenic tobacco. Plant J. 1993 Aug;4(2):235–253. doi: 10.1046/j.1365-313x.1993.04020235.x. [DOI] [PubMed] [Google Scholar]
- Hauffe K. D., Paszkowski U., Schulze-Lefert P., Hahlbrock K., Dangl J. L., Douglas C. J. A parsley 4CL-1 promoter fragment specifies complex expression patterns in transgenic tobacco. Plant Cell. 1991 May;3(5):435–443. doi: 10.1105/tpc.3.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H. J., Hahlbrock K. Phenylalanine ammonia-lyase in potato (Solanum tuberosum L.). Genomic complexity, structural comparison of two selected genes and modes of expression. Eur J Biochem. 1992 Mar 1;204(2):621–629. doi: 10.1111/j.1432-1033.1992.tb16675.x. [DOI] [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- Lee S. W., Robb J., Nazar R. N. Truncated phenylalanine ammonia-lyase expression in tomato (Lycopersicon esculentum). J Biol Chem. 1992 Jun 15;267(17):11824–11830. [PubMed] [Google Scholar]
- Leyva A., Liang X., Pintor-Toro J. A., Dixon R. A., Lamb C. J. cis-element combinations determine phenylalanine ammonia-lyase gene tissue-specific expression patterns. Plant Cell. 1992 Mar;4(3):263–271. doi: 10.1105/tpc.4.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Hahlbrock K. Differential wound activation of members of the phenylalanine ammonia-lyase and 4-coumarate:CoA ligase gene families in various organs of parsley plants. Z Naturforsch C. 1992 Jan-Feb;47(1-2):90–94. doi: 10.1515/znc-1992-1-216. [DOI] [PubMed] [Google Scholar]
- Minami E., Ozeki Y., Matsuoka M., Koizuka N., Tanaka Y. Structure and some characterization of the gene for phenylalanine ammonia-lyase from rice plants. Eur J Biochem. 1989 Oct 20;185(1):19–25. doi: 10.1111/j.1432-1033.1989.tb15075.x. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Ward E., DiMaio J., Ohta D., Ryals J., Sato R. Molecular cloning and sequencing of a cDNA encoding mung bean cytochrome P450 (P450C4H) possessing cinnamate 4-hydroxylase activity. Biochem Biophys Res Commun. 1993 Feb 15;190(3):875–880. doi: 10.1006/bbrc.1993.1130. [DOI] [PubMed] [Google Scholar]
- Ohl S., Hedrick S. A., Chory J., Lamb C. J. Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell. 1990 Sep;2(9):837–848. doi: 10.1105/tpc.2.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz W., Eiben H. G., Hahlbrock K. Expression in Escherichia coli of catalytically active phenylalanine ammonia-lyase from parsley. FEBS Lett. 1989 Dec 4;258(2):335–338. doi: 10.1016/0014-5793(89)81687-4. [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P., Becker-André M., Schulz W., Hahlbrock K., Dangl J. L. Functional architecture of the light-responsive chalcone synthase promoter from parsley. Plant Cell. 1989 Jul;1(7):707–714. doi: 10.1105/tpc.1.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shufflebottom D., Edwards K., Schuch W., Bevan M. Transcription of two members of a gene family encoding phenylalanine ammonia-lyase leads to remarkably different cell specificities and induction patterns. Plant J. 1993 Jun;3(6):835–845. doi: 10.1111/j.1365-313x.1993.00835.x. [DOI] [PubMed] [Google Scholar]
- Subramaniam R., Reinold S., Molitor E. K., Douglas C. J. Structure, inheritance, and expression of hybrid poplar (Populus trichocarpa x Populus deltoides) phenylalanine ammonia-lyase genes. Plant Physiol. 1993 May;102(1):71–83. doi: 10.1104/pp.102.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner L. A., Li G., Ware D., Somssich I. E., Davis K. R. The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana. Plant Mol Biol. 1995 Jan;27(2):327–338. doi: 10.1007/BF00020187. [DOI] [PubMed] [Google Scholar]
- Weisshaar B., Armstrong G. A., Block A., da Costa e Silva O., Hahlbrock K. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 1991 Jul;10(7):1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten R. W., Sederoff R. R. Phenylalanine ammonia-lyase from loblolly pine : purification of the enzyme and isolation of complementary DNA clones. Plant Physiol. 1992 Jan;98(1):380–386. doi: 10.1104/pp.98.1.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Sriprasertsak P., Kato H., Hashimoto T., Shimizu H., Shiraishi T. Functional analysis of the promoters of phenylalanine ammonia-lyase genes in pea. Plant Cell Physiol. 1994 Sep;35(6):917–926. [PubMed] [Google Scholar]
- da Costa e Silva O., Klein L., Schmelzer E., Trezzini G. F., Hahlbrock K. BPF-1, a pathogen-induced DNA-binding protein involved in the plant defense response. Plant J. 1993 Jul;4(1):125–135. doi: 10.1046/j.1365-313x.1993.04010125.x. [DOI] [PubMed] [Google Scholar]
- van de Löcht U., Meier I., Hahlbrock K., Somssich I. E. A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO J. 1990 Sep;9(9):2945–2950. doi: 10.1002/j.1460-2075.1990.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



