Abstract
Neisseria meningitidis causes a severe, frequently fatal sepsis when it enters the human blood stream. Infection leads to extensive damage of the blood vessels resulting in vascular leak, the development of purpuric rashes and eventual tissue necrosis. Studying the pathogenesis of this infection was previously limited by the human specificity of the bacteria, which makes in vivo models difficult. In this protocol, we describe a humanized model for this infection in which human skin, containing dermal microvessels, is grafted onto immunocompromised mice. These vessels anastomose with the mouse circulation while maintaining their human characteristics. Once introduced into this model, N. meningitidis adhere exclusively to the human vessels, resulting in extensive vascular damage, inflammation and in some cases the development of purpuric rash. This protocol describes the grafting, infection and evaluation steps of this model in the context of N. meningitidis infection. The technique may be applied to numerous human specific pathogens that infect the blood stream.
Keywords: Infection, Issue 86, Disease Models, Bacteria, Bacterial Infections and Mycoses, Neisseria meningitidis, purpura, vascular infection, humanized model
Introduction
Meningococcal sepsis is a frequently fatal blood born infection caused by the bacterial pathogen Neisseria meningitidis. Meningococcal sepsis patients often present with a petechial or purpuric rash on their skin that has previously been associated with vascular destruction caused by circulating bacteria and bacterial products1. Skin biopsies from clinical patients show bacteria associated with microvessels, often filling the vessels2. Apart from the bacteria, extensive thrombosis, coagulation, congestion and vascular leak is seen in the purpuric regions3-5. This vascular damage can lead to extensive necrosis of the skin and surrounding tissues, resulting in debridement and even amputation in meningococcal survivors. Understanding how infection causes this vascular damage is important to optimize prevention and treatment strategies. The majority of research on meningococcal sepsis has been performed in vitro on human cell lines due to the human specificity of N. meningitidis. Many aspects of infection have been studied in vitro including bacterial adhesion, host cell response as well as cytokine response6-9. Type IV pili (Tfp) have been implicated as the major adhesion organelle for N. meningitidis on both epithelial and endothelial cells10. It has also been shown that adhesion of N. meningitidis to host cells is shear stress dependent and is therefore thought to be related to blood flow rates in the microvasculature11. This suggests the dynamic stresses the bacteria face in vivo are crucial to pathogenesis. It is however very difficult to model the microenvironment of small vessels in vitro.
The adhesion receptor for Neisseria Tfp is still unknown and therefore knock-in strategies to achieve bacterial adhesion in an animal model cannot be envisaged at this time. CD46 was suggested to be the Tfp receptor and transgenic animals were produced to act as mouse models. However, infection in these animals does not lead to extensive infection or to rash development12,13. Other animal models that have been described for the bacteremia aspect of Neisseria infection take into account the bacterial preference for human transferrin as an iron source14,15. Either supplementing human transferrin or expressing it from a transgene results in an increased bacterial load in the blood stream over an extended time period, but this model shows no bacterial adhesion or rash development16,17.
In this protocol, we describe a humanized mouse model in which human skin, including the dermal microvasculature, is transplanted onto immunocompromised mice18,19. This results in functional human vessels, perfused with the mouse circulation. Combined with human transferrin supplementation, this model accounts for at least two of the human specific aspects of N. meningitidis, i.e. human endothelium and human transferrin, in an in vivo environment. N. meningitidis introduced intravenously into this model adhere specifically to the human endothelium, producing a pathology that is similar to what is reported in clinical patients, including vascular damage and purpuric rash development18.
Protocol
1. Risks and Permissions
Local ethics committees must approve all animal protocols. This protocol was conducted in accordance with guidelines established by the French and European regulations for the care and use of laboratory animals (Décrets 87-848, 2001-464, 2001-486 and 2001-131 and European Directive 2010/63/UE) and approved by the local ethical committee Comité d'Ethique en matière d'Expérimentation Animale, Universite Paris Descartes, Paris, France. No: CEEA34.GD.002.11.
For human skin, written informed patient consent was obtained and all procedures were performed according to French national guidelines and approved by the local ethical committee, Comité d'Evaluation Ethique de l'INSERM IRB 00003888 FWA 00005881, Paris, France Opinion: 11-048.
N. meningitidis is a potentially harmful human pathogen and all necessary precautions must be taken when handling the organism. These precautions include vaccination and working under Biosafety level 2 conditions.
2. Skin Graft
Collect human skin as close to grafting time as possible. The majority of skin in this work comes from plastic surgery operations involving the abdominal area. Other sources such as breast, leg and even face have been used with success.
Prepare the human skin sample by cleaning skin surface with 70% EtOH and lying it flat on a well-cleaned surface, preferably under a flow hood.
Slice 200-400 µm slices from the top of the skin using a dermatome with preset thickness.
Check the skin slices for integrity and cut them into 2 cm x 2 cm squares, then place in PBS (without divalent cations) if grafting immediately or at 4 °C in DMEM with 10% serum for short-term storage (3-12 hr).
Anaesthetize 6-8 week old SCID.bg mice (approximately 20 g) with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) injected i.p.
Once the mice are anaesthetized, check for pain response by performing a toe pinch, a standard method for establishing pain reflex. Apply gel to eyes to prevent corneal drying and shave the left side of the animal behind the shoulder, where the graft will be placed. Keep the mouse warm on a heat pad throughout the procedure.
After prepping mouse for surgery, clean the shaved area with 70% EtOH.
Apply local anesthetic topically to the graft site (Tronothane) and prepare the graft site by carefully removing the superficial layer of mouse skin with a pair of curved scissors. Be careful not to disrupt the underlying vasculature.
Remove skin in an area a bit smaller than the area of the human skin slice.
Remove skin slice from PBS (or DMEM) and place over graft bed, ensuring the epidermal side is facing up. Steps 2.8-2.10 should be performed as quickly as possible, ensuring the graft bed does not dry out.
Align one corner or edge and fix with tissue glue. Carefully fit the rest of the skin slice to the graft bed, placing small amounts of tissue glue around the edges. Always trim the human skin to size rather than make the graft bed larger.
Do not pull the skin too tight over the graft bed as the mouse skin is very flexible.
Make sure the graft is fixed with tissue glue in each corner.
Clean the area with an iodine solution.
Apply a Band-Aid with the cushion area over the graft. Make sure the Band-Aid is firm but not tight and that the animal's breathing is not affected.
Fix the Band-Aid with dressing tape.
Allow the animal to recover on a warm surface.
Once awake, return the animal to its cage. Animals are housed 2-3 per cage.
- Check mice regularly during recovery for signs of pain or distress. 10-14 days after grafting, remove the Band-Aid, being careful not to disrupt the graft. In our experience postoperative pain is minimal. Nevertheless animals should be monitored every day in particular in the few days following operation. Criteria for pain or distress are the following.
- Loss of weight over 10%
- Physical appearance, fur, hunchback position, eyes
- Increased respiration or heart rates
- Decreased or unusual movements.
- Rise in body temperature
- In case signs of pain are detected paracetamole is administered orally (300 mg/kg, every 4 hr). Humane endpoints are strictly adhered to if the pain persists.
If the graft looks dry apply baby oil every 2-3 days to keep the skin supple.
The graft is ready for experimentation 21 days post graft.
3. Infection
The day before infection, prepare bacterial strain by streaking onto GCB agar plates with appropriate antibiotics. Grow overnight at 37 °C in 5% CO2.
- Bacteria preparation
- Reinoculate bacteria into 5 ml of Human endothelium SFM media with 10% serum to an OD600 0.05.
- Incubate while shaking for 2 hr in incubator (37 °C in 5% CO2) with loose lid, until a bacterial density of approximately OD600 0.1.
- Centrifuge the bacteria for 3 min 15,000 rpm.
- Remove supernatant and resuspend in PBS, then centrifuge again.
- Repeat the wash step (2.2.4-2.2.5) twice.
- Resuspend bacterial pellet in PBS.
- Measure OD600.
- Make a culture of either OD600 0.1 (108 CFU/ml), or OD600 1.0 (109 CFU/ml). From this known concentration, dilute to 107 CFU/ml. Try to prepare bacteria as close to injection time as possible.
- Mice
- Weigh mice.
- Anaesthetize mice with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) injected i.p.
- Once asleep, check that the pain response is absent by a toe-pinch, keep the mice warm with a heat pad and put eye ointment on to prevent drying.
- Give each mouse 8 mg of human transferrin, resuspended in saline or PBS, injected i.p.
- Take a small drop of blood from the tail and spread onto an agar plate to check blood is sterile prior to infection.
- Inject 100 µl 107 CFU/ml bacterial culture (106 CFU total) intravenously via the retro-orbital or tail vein. Measurements of CFU taken 5 min post injection are consistent regardless of injection site.
- After a few minutes, snip the tail end and spread blood sample onto agar plates with appropriate antibiotics with one 10-fold dilution to establish blood CFU immediately following infection.
- Seal the tail with tissue glue.
- After injection, dilute the bacterial inoculum and spread onto agar plates with appropriate antibiotics to establish CFU.
- Allow the mice to recover on a heat pad until they are up and moving
- At 6 hr post infection draw a small amount of blood from the tail, dilute and plate to establish CFU at 6 hr.
- Incubate all agar plates at 37 °C in 5% CO2 overnight.
4. Sacrifice
At the chosen time of infection, anaesthetize mice with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) injected i.p.
Once asleep, keep the mouse warm with a heat pad.
When the pain reflex has gone (toe-pinch), snip end of tail and draw blood.
Spread blood onto agar plates with appropriate antibiotics; 5 μl direct and 10 µl 1:10 dilution to establish CFU.
Sacrifice the mouse by cervical dislocation.
After sacrificing the mouse according to all relevant institutional and ethical guidelines, confirm death by lack of heart beat. Make a midline incision, cut through rib cage, and make an incision in the heart.
Collect as much blood as possible from the heart/chest cavity and place in a sterile tube.
Remove organs (e.g. liver, spleen, brain) into a sterile plate.
Remove the skin graft and a section of mouse skin into the sterile plate.
Dispose of the mouse body.
After the collected blood has been allowed to clot for 15 min, centrifuge it 15 min at 3,000 rpm.
Remove plasma from blood and store at -80 °C for later analysis, for example for cytokine detection by ELISA or FACS based methods.
5. Organ CFU Counts
Weigh homogenizing tubes containing 500 μl of PBS each prior to taking biopsy samples.
Take 4 mm biopsy samples from each tissue using a sterile biopsy punch.
Place biopsies into preweighed homogenizing tubes containing 500 µl PBS. Homogenize skin samples with 2 mm beads, use smaller beads for other organs.
Place the remaining tissues in 4% Paraformaldehyde (PFA) and store at 4 °C for microscopy (see below).
Weigh homogenizing tubes containing the biopsies to establish biopsy weight (for later calculation of CFU/mg tissue).
- Homogenize samples.
- Homogenize liver, spleen and brain, at 6,000 rpm for 30 sec.
- Homogenize skin samples at 6,000 rpm for 30 sec and repeat if necessary.
Spread dilutions from each homogenizing tube onto agar plates and grow overnight at 37 °C in 5% CO2 to establish organ CFU counts.
6. Histology/Immunohistochemistry
- Fixation
- Fix the tissues in 4% PFA overnight at 4 °C. The fixation step may be longer; if storing for longer periods place the tissues in 0.25% PFA.
- Rinse the tissues in PBS and then place in 20% sucrose solution and return to 4 °C overnight or until the tissues have sunk in the solution.
- Wash the tissues in PBS, cut to size and place in OCT solution (either in a tissue mold or adhered to a base board).
- Quickly freeze the tissues on a Petri dish floating in liquid nitrogen.
- After freezing, put the tissues onto dry ice before transfer to -80 °C for storage.
- Slicing
- Remove tissues from -80 °C and allow to equilibrate to -20 °C in a cryostat.
- Slice tissues with a thickness of approximately 10-15 µm and place onto coated microscopy slides.
- Store slides at -20 °C until needed.
- Staining
- Allow slides to come to room temperature.
- Draw around the slice on the slide with a hydrophobic pen. Wait 5-10 min.
- Rehydrate/wash slice in PBS for 5 min, repeat twice.
- Permeabilize slice in 0.1% Triton in PBS for 10 min.
- Wash in PBS 2-3x for 5 min.
- Block in PBS 0.1% Triton 1% BSA for between 10-60 min depending on the antibody or stain to be used.
- Remove blocking buffer and add primary antibody diluted as required in block buffer. Incubate for determined time. (Often 1 hr at room temperature or overnight at 4 °C).
- Wash in PBS 2-3x for 5 min.
- Apply secondary antibody diluted as appropriate in bock buffer; DAPI is typically included in this step. Incubate for determined time, often 1-2 hr room temperature, in the dark.
- Wash in PBS 2-3x for 5 min.
- Mount in fade protecting mounting media with a cover glass and seal with nail varnish.
- Image stained slides immediately or store at 4 °C.
- Perform imaging with a confocal or fluorescent microscopy set up with filters/lasers appropriate to fluorophores used.
- Histology
- Allow slides to be used for histology to come to room temperature.
- Stain slides using a standard hematoxylin /eosin staining protocol.
- Place the slides in slide rack.
- Transfer rack with slides between solutions in the following order:
- 2 sec into running tap water.
- 3 min in filtered hematoxylin solution.
- 10 sec into running water.
- 10 sec into distilled water.
- 10 sec into ethanol 100%.
- 2 sec into filtered eosin.
- 10 sec into running water.
- 10 sec into ethanol 100%
- 2 min into xylene x 3 (bath I, II, III).
- Transfer immediately and add few drops of fixation glue onto each slides, place cover slip on top, remove air bubbles, let dry for a few hours.
- Image slides under a standard white light microscope.
Representative Results
CFU counts
The N. meningitidis strain used in these representative results is N. meningitidis 8013 clone 12, a serogroup C clinical isolate, expressing a class I SB pilin, Opa-, Opc-, PilC1+/PilC2+ 20. The strain had been engineered to express green fluorescent protein (GFP) from a chromosomal insertion18. Bacterial colony forming unit counts are established by counting the number of colonies on the agar plates and calculating either CFU/ml of blood or CFU/mg of tissue from the known volumes plated. Blood counts showed that 5 min after i.v. injection of 106 CFU bacteria there was an average of 1.5 x 105 CFU/ml circulating in the blood (Figure 1A). After 6 hr the counts averaged 4.8 x 104 CFU/ml. By 24 hr the average counts were 2.4 x 104 CFU/ml but in this group of 10 mice, 5 had no detectable circulating bacteria while the other 5 had relatively high counts (Figure 1A). CFU counts taken from the skin samples show the majority of mice having considerable counts in the human skin, averaging 2.1 x 104 CFU/mg of tissue at 6 hr and 4.4 x 102 CFU/mg of tissue at 24 hr (Figure 1B). The contralateral mouse skin samples showed no bacterial counts at 24 hr and only a very low number at 6 hr, demonstrating the strong preference for N. meningitidis to the human vessels in the grafted skin (Figure 1B). In general bacterial counts were very low to nondetectable in the other organs sampled although 2 animals did show counts which may be attributed to contamination from the blood, as they correlated with high circulating bacterial numbers (Figure 1C). The model can also be used to determine the role of virulence factors in vivo, for example the type IV pili. Bacterial strains with defined mutations in the pilD gene21, resulting in a strain lacking Tfp , as well as the pilC1 gene8, lacking the proposed Tfp 'adhesin' were introduced into the model. These mutations resulted in no bacterial adhesion in the human skin graft, confirming the crucial role Tfp is playing in adhesion in vivo (Figure 1D).
Immunohistochemistry/Histology
In these experiments we used N. meningitidis strains that express green fluorescence protein (GFP) to enable fluorescent detection without the need for secondary staining. Human vessels were stained using a marker for human endothelium, either CD31 (PECAM-1) or the lectin Ulex europaeus agglutinin (UEA)18,22.The UEA was conjugated to rhodamine allowing one-step staining (Figures 2A-C). Cell nuclei can be stained with DAPI to help identify tissue structures (Figures 2A-B).Histology was performed using a standard hematoxylin/eosin staining. The epidermal/dermal border of the skin was clearly identifiable. Inflammation, thrombosis and vascular leak were concentrated to vessels close to this border 24 hr after infection (Figure 2D). Thrombosis was visible in several small dermal vessels, often accompanied by congestion and inflammation. Leakage of red blood cells out into the tissues could be seen, indicating an extensive level of vessel damage. The histopathology of grafted skin in noninfected mice appeared normal with no distinguishable inflammation18. In about 30% of the infections bacterial adhesion in the skin leads to the development of macroscopically detectable purpura (Figures 2E and 2F).
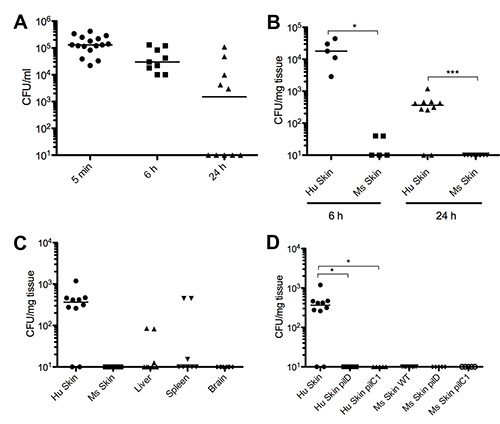 Figure 1. CFU counts. (A) Bacterial CFU counts per ml of blood at 5 min, 6 hr, and 24 hr post infection. (B) Bacterial CFU counts from skin samples comparing human skin (HS) and mouse skin (MS) at both 6 hr and 24 hr post infection. (C) Bacterial CFU counts from other organs taken 24 hr post infection. (D) Comparing bacterial CFU counts from human and mouse skin samples taken at 24 hr post infection from mice infected with the wild type N. meningitidis 2C43 stain (WT), a strain with a mutation in the pilD gene (pilD) or a strain with a mutation in the pilC1 gene (pilC1). All graphs are shown as raw data with median. Figure is modified from Melicanet al.18 Click here to view larger image.
Figure 1. CFU counts. (A) Bacterial CFU counts per ml of blood at 5 min, 6 hr, and 24 hr post infection. (B) Bacterial CFU counts from skin samples comparing human skin (HS) and mouse skin (MS) at both 6 hr and 24 hr post infection. (C) Bacterial CFU counts from other organs taken 24 hr post infection. (D) Comparing bacterial CFU counts from human and mouse skin samples taken at 24 hr post infection from mice infected with the wild type N. meningitidis 2C43 stain (WT), a strain with a mutation in the pilD gene (pilD) or a strain with a mutation in the pilC1 gene (pilC1). All graphs are shown as raw data with median. Figure is modified from Melicanet al.18 Click here to view larger image.
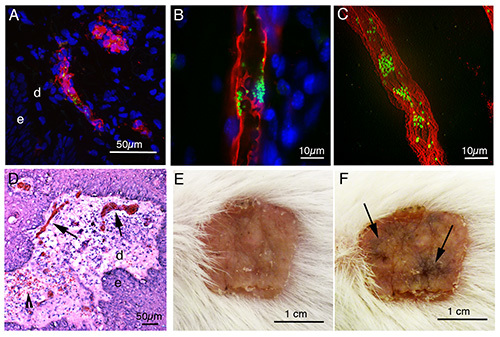 Figure 2. Microscopy. (A) Projection of a confocal stack showing a human microvessel stained with UEA lectin (red) close to the dermal (d) epidermal (e) border. The vessel is infected with N. meningitidis (green) 2 hr post infection. (B) An optical slice and a slice projection (C) showing N. meningitidis microcolonies (green) infection a human vessel (UEA – red) within a skin graft 2 hr post infection. (D) Haematoxylin/Eosin staining of human skin graft infected for 24 hr with N. meningitidis. The epidermal (e), dermal (d) border is clearly visible and extensive thrombosis and congestion of microvessels (arrows) is seen in the dermis. Inflammation and some vascular leak (arrowhead) can also be identified. (E) Human skin graft prior to infection. (F) The same skin graft as (E) 24 hr post infection with N. meningitidis showing purpuric regions (arrows). Figures 2B, 2D, 2E, and 2F are modified from Melican et al.18 Click here to view larger image.
Figure 2. Microscopy. (A) Projection of a confocal stack showing a human microvessel stained with UEA lectin (red) close to the dermal (d) epidermal (e) border. The vessel is infected with N. meningitidis (green) 2 hr post infection. (B) An optical slice and a slice projection (C) showing N. meningitidis microcolonies (green) infection a human vessel (UEA – red) within a skin graft 2 hr post infection. (D) Haematoxylin/Eosin staining of human skin graft infected for 24 hr with N. meningitidis. The epidermal (e), dermal (d) border is clearly visible and extensive thrombosis and congestion of microvessels (arrows) is seen in the dermis. Inflammation and some vascular leak (arrowhead) can also be identified. (E) Human skin graft prior to infection. (F) The same skin graft as (E) 24 hr post infection with N. meningitidis showing purpuric regions (arrows). Figures 2B, 2D, 2E, and 2F are modified from Melican et al.18 Click here to view larger image.
Discussion
Animal models are critically important to bacterial pathogenesis research. It is impossible to fully mimic the in vivo environment in cell culture and it is becoming apparent that host-pathogen interaction is influenced by many dynamic factors. The human specificity of some clinically important pathogens, such as N. meningitidis, HIV, HCV, Plasmodium falciparum, Listeria monocytogenes, and Salmonella typhi has limited the use of in vivo models for these infections. However, as we begin to understand which infectious steps are involved in the specificity, humanized models are being developed. The protocol described here is a demonstration of this with the introduction of human microvessels into mice, allowing for extensive in vivo infection with N. meningitidis, resulting in vascular damage and occasionally the development of purpuric rashes.
Using this model, we have been able to define that the adhesive properties of Tfp are involved in vascular colonization in vivo by using bacterial mutants and that the vascular damage is reduced in the absence of adhesion18. Previously, circulating bacterial products have been implicated in this damage but our results suggest a decisive role for local adhesion and vascular colonization. This opens up new possibilities for the development of novel treatment targets. If adhesion of pathogenic bacteria could be blocked pharmaceutically it could possibly prevent the development of dermal lesions and lead to better outcomes for meningococcal survivors in terms of tissue necrosis, debridement and amputations. The work has also demonstrated the complexity of the infection and the involvement of the immune response and coagulation cascade. We identified human cytokine signaling in the serum of infected mice despite the relatively small amount of human endothelium present18. This indicated a significant cytokine response, along with the infiltration of mouse immune cell populations into the area.
Animal models can of course never fully replicate human disease and all results garnered from them must be considered with this in mind. For instance, in this model the blood and circulating cells are of mouse origin and we cannot discount that they may behave differently to human cells. An advantage of this however, as demonstrated in our recent publication18, is the ability to differentiate signaling originating from the human endothelium from that of the circulating mouse cells. The immunocompromised background of the mice used in this model would also allow for the allogenic transfer of human immune cell populations, adding a further 'humanization' aspect. The immunocompromised background of the mice may however mask a role for NK, T or B cells, which are all lacking or defective in this model. The relatively short timeframe (24 hr) used in this model mainly concerns the innate response, but for longer-term infections and the development of immunity other options may need to be explored.
The skin is an important infection site for N. meningitidis but having a relatively small amount of human vessels also means that extrapolating the data to a systemic infection involving numerous organs is difficult. While this model allows for the study of dermal lesion development, important steps of meningococcal infection such as epithelial and blood-brain crossing are not included. Further development of these humanized models is needed to address these other aspects of infection. Nevertheless this model offers great potential for numerous human specific pathogens, particularly those targeting the blood vessels.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
The authors would like to thank all members of the Dumenil lab, particularly Silke Silva for critical reading of the manuscript. The surgery department at Hôpital Européen Georges-Pompidou (HEGP), Dr. David Maladry. Michael Hivelin and Dr. Patrick Bruneval, Pathology Department at HEGP. The animal facility at PARCC, headed by Elizabeth Huc. This work was supported by the following grant agencies: Marie Curie IEF fellowship no. 273223 (KM), ATIP-Avenir Grant from INSERM, CODDIM equipment grant (Ile de France Region), FRM (fondation pour la recherche médicale) equipment grant, the IBEID Laboratory of excellence consortium, ANR (Agence Nationale pour la Recherche) grant “Bugs-in-flow”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Davis CE, Arnold K. Role of meningococcal endotoxin in Meningococcal purpura. J. Exp. Med. 1974;140:159–171. doi: 10.1084/jem.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairey E, et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood-brain barrier. J. Exp. Med. 2006;203:1939–1950. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, van Deuren M. Classification and pathogenesis of meningococcal infections. Methods Mol. Biol. 2012;799:21–35. doi: 10.1007/978-1-61779-346-2_2. [DOI] [PubMed] [Google Scholar]
- Guarner J, et al. Pathogenesis and diagnosis of human meningococcal disease using immunohistochemical and PCR assays. Am. J. Clin. Pathol. 2004;122:754–764. doi: 10.1309/A7M2-FN2T-YE6A-8UFX. [DOI] [PubMed] [Google Scholar]
- Hill WR, Kinney TD. The cutaneous lesions in acute meningococcemia; a clinical and pathologic study. J. Am. Med. Assoc. 1947;134:513–518. doi: 10.1001/jama.1947.02880230023006. [DOI] [PubMed] [Google Scholar]
- Capecchi B, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 2005;55:687–698. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- Merz AJ, Enns CA, So M. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- Morand PC, Tattevin P, Eugene E, Beretti JL, Nassif X. The adhesive property of the type IV pilus-associated component PilC1 of pathogenic Neisseria is supported by the conformational structure of the N-terminal part of the molecule. Mol. Microbiol. 2001;40:846–856. doi: 10.1046/j.1365-2958.2001.02452.x. [DOI] [PubMed] [Google Scholar]
- Taha MK. Neisseria meningitidis induces the expression of the TNF-alpha gene in endothelial cells. Cytokine. 2000;12:21–25. doi: 10.1006/cyto.1999.0506. [DOI] [PubMed] [Google Scholar]
- Kirchner M, Meyer TF. The PilC adhesin of the Neisseria type IV pilus-binding specificities and new insights into the nature of the host cell receptor. Mol. Microbiol. 2005;56:945–957. doi: 10.1111/j.1365-2958.2005.04600.x. [DOI] [PubMed] [Google Scholar]
- Mikaty G, et al. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, et al. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- Kirchner M, Heuer D, Meyer TF. CD46-independent binding of Neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect. Immun. 2005;73:3072–3082. doi: 10.1128/IAI.73.5.3072-3082.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, et al. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012;483:53–58. doi: 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers AB, Gonzalez GC. Receptors for transferrin in pathogenic bacteria are specific for the host's protein. Can. J. Microbiol. 1990;36:145–147. doi: 10.1139/m90-026. [DOI] [PubMed] [Google Scholar]
- Oftung F, Lovik M, Andersen SR, Froholm LO, Bjune G. A mouse model utilising human transferrin to study protection against Neisseria meningitidis serogroup B induced by outer membrane vesicle vaccination. FEMS Immunol. Med. Microbiol. 1999;26:75–82. doi: 10.1111/j.1574-695X.1999.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Zarantonelli ML, et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect. Immun. 2007;75:5609–5614. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K, Michea Veloso P, Martin T, Bruneval P, Dumenil G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AG, et al. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9146–9150. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X, et al. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 1993;8:719–725. doi: 10.1111/j.1365-2958.1993.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Floquet S, Metais A, Nassif X, Pelicic V. Large-scale analysis of the meningococcus genome by gene disruption: resistance to complement-mediated lysis. Genome Res. 2003;13:391–398. doi: 10.1101/gr.664303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Hickey MJ, Murray AG, Andonegui G, Kubes P. Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J. Exp. Med. 2000;192:1205–1211. doi: 10.1084/jem.192.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]


