Abstract
There are over 1 million hospitalizations for heart failure (HF) annually in the United States alone, and a similar number has been reported in Europe. Recent clinical trials investigating novel therapies in patients with hospitalized HF (HHF) have been negative, and the post-discharge event rate remains unacceptably high. The lack of success with HHF trials stem from problems with understanding the study drug, matching the drug to the appropriate HF subgroup, and study execution. Related to the concept of study execution is the importance of including appropriate study sites in HHF trials. Often overlooked issues include consideration of the geographic region and the number of patients enrolled at each study center. Marked differences in baseline patient co-morbidities, serum biomarkers, treatment utilization and outcomes have been demonstrated across geographic regions. Furthermore, patients from sites with low recruitment may have worse outcomes compared to sites with higher enrollment patterns. Consequently, sites with poor trial enrollment may influence key patient end points and likely do not justify the costs of site training and maintenance. Accordingly, there is an unmet need to develop strategies to identify the right study sites that have acceptable patient quantity and quality. Potential approaches include, but are not limited to, establishing a pre-trial registry, developing site performance metrics, identifying a local regionally involved leader and bolstering recruitment incentives. This manuscript summarizes the roundtable discussion hosted by the Food and Drug Administration between members of academia, the National Institutes of Health, industry partners, contract research organizations and academic research organizations on the importance of selecting optimal sites for successful trials in HHF.
Keywords: Heart failure, Site selection, Clinical trials, FDA
Introduction: an open forum
Recent clinical trials investigating therapies for patients hospitalized for heart failure (HF) have failed to show benefit with respect to key end points [1–3]. Possible explanations include an inadequate understanding of the study drug properties, failure to match the study drug with the correct patient population and poor study execution. However, the main reason for this continued lack of success may be related to the majority of studies focusing on short-term therapies to improve signs and symptoms during hospitalization that are already known to improve with standard therapy [4]. Few studies have concentrated on the unacceptably high post-discharge event rate, which has been shown to occur despite the use of current evidence based therapies [1, 5]. Accordingly, there is an unmet need to develop new therapies to improve post-discharge outcomes in this patient population. This is even more important in patients with hospitalized heart failure (HHF) with preserved ejection fraction for whom no evidence-based therapies exist [6].
Multiple reasons for the repeated failure of clinical trials have been addressed in prior meetings [7–10]. However, one topic of increasing importance with global clinical trials that has yet to be addressed in an open forum is the importance and method of quality site selection in trials of HHF. This manuscript summarizes the roundtable discussion between members of academia, the National Institutes of Health (NIH), various industry partners, contract research organizations (CRO) and academic research organizations (ARO) on the importance of selecting optimal sites for a successful trial. This meeting was hosted by the Food and Drug Administration (FDA) on January 12, 2012 in Silver Spring, Maryland (see Appendix 1 and 2). For clarity, a summative impression is provided for each individual participant.
Trials in patients hospitalized for heart failure to date: an overview
Mihai Gheorghiade
There are over one million hospitalizations for HF in the United States alone [11], and a similar number is seen in Europe. Despite available therapies, mortality and rehospitalization rates can be as high as 15 and 30 %, respectively, within 60–90 days post-discharge [12]. The majority of these patients are elderly (median age 75 years) and have a history of cardiovascular (coronary artery disease [CAD] ~60 %, hypertension ~70 %, atrial fibrillation ~40 %) and non-cardiovascular comorbidities (diabetes ~40 %, severe renal impairment ~30 % and chronic obstructive pulmonary disease ~25 %) [13–15].
To date, the majority of trials conducted in HHF have focused on improving early signs and symptoms with short-term treatments. These trials have targeted therapies for patients at time of initial presentation and during the hospitalization and are thus classified as Stage A and Stage B trials, respectively [8, 10, 16]. Unfortunately, with the exception of the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) and the ongoing ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trials [1, 17], there have been few Stage C trials where therapies are initiated before or soon after discharge and effects on post-discharge event rates are evaluated. while standard in-hospital therapies may improve symptoms in the majority of patients, there is an apparent dissociation between these improvements and post-discharge outcomes [10]. We now realize that the major goal in HHF is to improve the unacceptably high post-discharge event rate rather than clinical signs and symptoms [18].
Additional problems with HHF trials stem from the aforementioned problems with understanding the study drug [19], matching the drug to the correct patient [4, 10], and study execution. Overall, the properties of pharmacologic therapies in HHF patients are poorly characterized. Even widely available guideline recommended agents for chronic HF have not been specifically studied in the setting of HHF. Furthermore, many new HHF trials are limited by investigating novel molecules with poorly described properties and mechanisms of action.
The heterogeneity of clinical profiles, etiologies, substrates and precipitants in HHF patients necessitates new trials to only include the subset of patients most appropriate for a given therapy. By analogy, progress in the treatment of acute coronary syndromes (ACS) has been aided by the recognition of discrete subpopulations (ST-segment elevation myocardial infarction vs. non-ST-segment elevation myocardial infarction vs. unstable angina) and the realization that effective therapies for one subgroup (such as thrombolytics for ST-segment elevation myocardial infarction) are not helpful for another (such as unstable angina) [8].
However, even when a study drug is well characterized and matched with the appropriate HHF subpopulation, proper study execution remains a significant obstacle. Recently, even the TIMI (Thrombolysis in Myocardial Infarction) Study Group, among the most experienced AROs in cardiology with an impressive track record, has encountered problems in trial execution. The Rivaroxaban in ACS ATLAS-TIMI 51 program found that rivaroxaban safely reduced the primary efficacy end point, but the drug was subsequently not approved by the FDA due to missing study data [20]. These difficulties encountered by an experienced and well-established ARO highlight how challenging sound study execution can be.
Related to study execution is the importance of including appropriate study sites in HHF trials. The EVEREST trial illustrated that with site selection, it is necessary to consider both geographic region and the number of patients enrolled at a center [1]. Preliminary unpublished data from EVEREST demonstrated marked differences in baseline patient co-morbidities (i.e. CAD, hyperlipidemia, diabetes and chronic kidney disease), serum B-type natriuretic peptide (BNP) and QRS duration across geographic regions. Regional differences also existed in regard to HF treatment utilization and rates of death and HF hospitalization. Furthermore, patients from sites with low recruitment (10 or fewer during the trial duration) had poorer outcomes compared to sites with higher enrollment patterns. Overall, EVEREST randomized 4133 patients from 359 sites, corresponding to an enrollment rate of only 0.41 patients/site/month. This enrollment rate is consistent with other large HF trials such as ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Subjects with Decompensated Heart Failure) and EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure) [2, 21].
As depicted in EVEREST, sites with poor trial enrollment may influence key patient end points making evaluation of the study drug implicitly difficult. From the administrative standpoint, each individual site and investigator may have proportionally less training, infrastructure and investment. Low volume recruitment likely does not justify the costs of site training and maintenance. Accordingly, there is an unmet need to develop strategies to identify quality study centers that have acceptable patient quantity and quality. One proposed solution involves the use of a pre-trial registry and the development of site performance metrics. Such a system has several advantages including characterization of the target study population (i.e. number of patients, demographic variables, HF etiologies), the clinical course of disease (i.e. length of hospital stay, post-discharge patient disposition) and the post-discharge event rate at each potential study site. If a center is determined to be under-performing or an outlier in one or more of these areas, corrective efforts may be employed or the center may be excluded from the subsequent trial. Furthermore, a pre-trial registry would help ensure adequate trial enrollment to achieve the necessary study power and also provides centers the opportunity for a “dry-run” as they become familiar with data collection, protocol and terminology.
Other fields in medicine such as oncology have had more success with trial recruitment and patient participation. The fundamental difference between oncology and cardiology/ HF in terms of clinical trial participation revolves around perception and messaging (Table 1). Despite similar rates of 1-year mortality in many cases, cancer patients are aware of their limited therapeutic options, and oncologists reinforce that clinical trials may offer them the only possible solution; hence, trial participation is high. Similarly, patients experiencing acute myocardial infarctions (AMI) are recognized to have poor outcomes and thus are actively and promptly enrolled in clinical trials. In contrast, HHF patients are rarely made aware of their poor prognosis, and since symptomatic relief is achieved fairly readily with use of diuretic strategies, patients and physicians do not perceive clinical worsening and necessity for clinical trial enrollment. In addition, clear subgroups have been established in cancer patient cohorts (genetic-based) and AMI patients (ST-segment elevation vs. non-ST-segment elevation). Unfortunately, in HHF, the lack of established subgroups make specific trial enrollment difficult. Cancer investigators have utilized a series of disease-oriented networks to help identify and select appropriate patients for individual trials.
Table 1.
Differences between oncology and cardiology clinical trials
| HF research | AMI research | Oncology research | |
|---|---|---|---|
| Perception of the poor prognosis |
Low | High | High |
| Treatment efficacy on symptoms |
High | Low | Low |
| Concomitant background therapy |
Well-established, complex and Effective |
Well-established and effective | Less developed, less beneficial |
| Subgroup selection | Absent | Fair (ST elevation, troponins) | High (Genetic) |
| Surrogate endpoints | Still uncertain | Present (i.e. troponins) | Present |
| Pre-trial clinical research models |
Uncertain | Established | Present [33] |
| Trial endpoints | Independent from treatment (mortality) |
Independent from treatment (mortality, reinfarction) |
Flexible: Treatment/disease- specific |
AMI acute myocardial infarction; HF heart failure
Geographical disparities in acute heart failure trials
Marco Metra
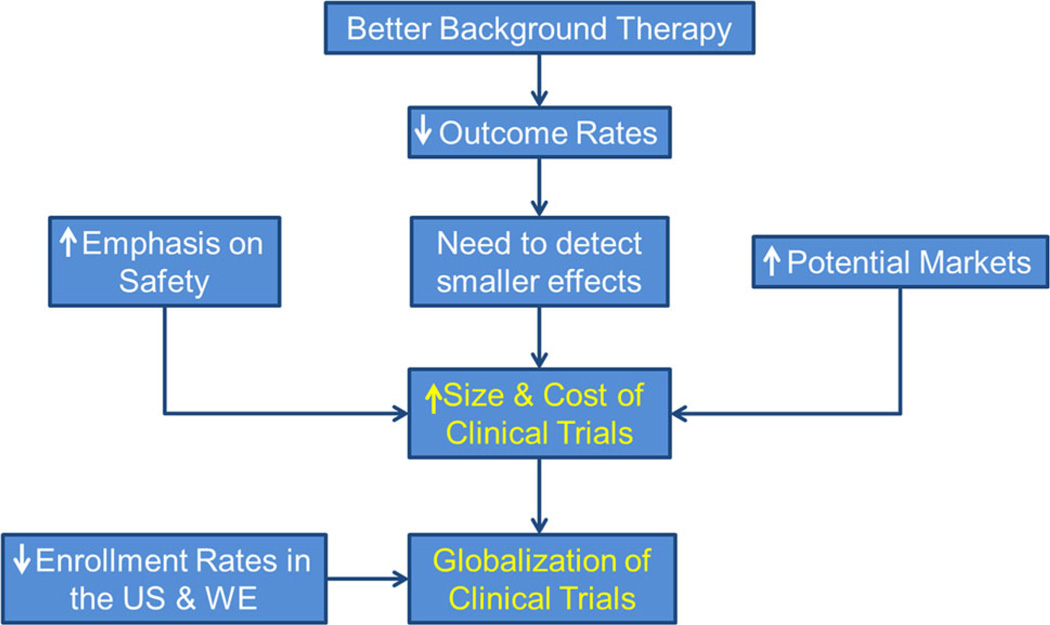
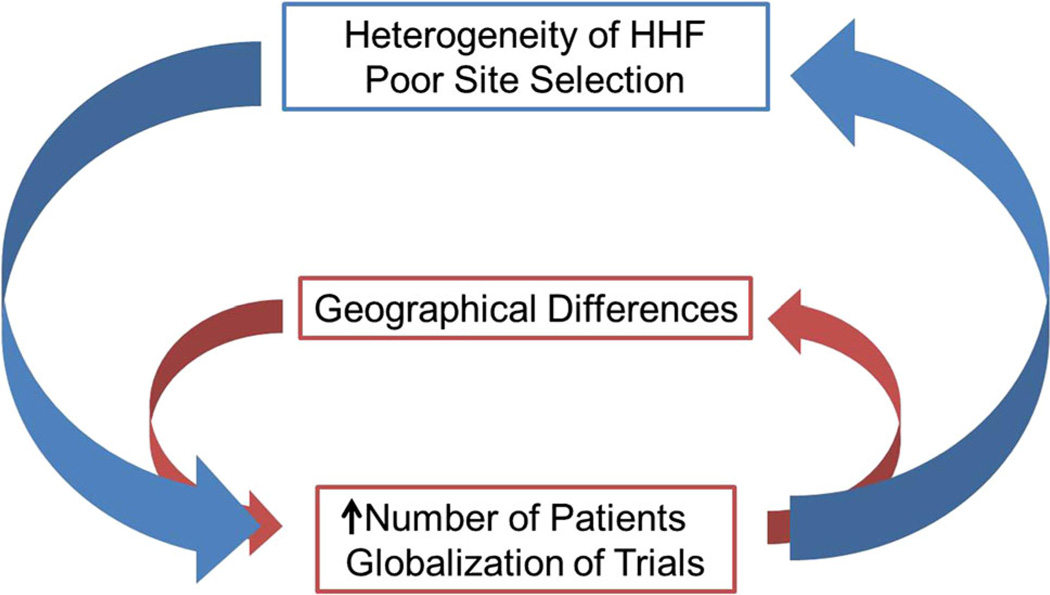
Historically, trials in cardiology prior to 1990 had significantly fewer sites and more success with patient recruitment per site. There have been a number of important changes in this area including rapid globalization of HHF trials in recent years. Improved background therapy for patients with chronic HF has reduced overall event rates and forced detection of smaller effect sizes in trial settings. This change, in combination with the need for unquestionable safety data and the goal to broaden the potential market, has contributed to dramatic increases in the size and cost of clinical trials (Fig. 1). Concurrently, we have seen a reduction in overall enrollment rates in the US and Western Europe [22] and rapid rise in globalization of HHF trials. Indeed, this pattern of trial enrollment with marked geographical variation has been observed in a number of disease conditions. However, increased geographic differences in patient baseline characteristics and outcomes are now recognized, and the need to further increase the number of patients and the duration of randomized controlled trials creates a positive feedback loop toward further heterogeneity of a study population (Fig. 2). Globalization of HHF trials and increased disparities between different geographical areas, associated with the intrinsic heterogeneity of HHF and the lack of well-characterized knowledge of HHF sites, have increased the intrinsic variability of the study population, thus making the detection of a meaningful drug effect less likely.
Fig. 1.
Mechanisms for the globalization of clinical trials in heart failure [32]. (US United States, WE Western Europe)
Fig. 2.
Interaction between heart failure site selection, geographical differences and trial enrollment. HHF hospitalized heart failure
Characteristics of an “Ideal Site”
Gadi Cotter
In North America and Western Europe, enrollment in clinical HHF studies has decreased in the last decade. Typical sites now enroll 1–2 patients per year out of hundreds of HF admissions. Low enrollment suggests increased selectivity such that the study patients may not represent the real-world HF population, especially if enrollment is biased by socioeconomic status and adherence. Increasing variability in the study population may translate into inaccurate estimates of the treatment effect in the target population, and greater dilution of the drug effect. Lower enrolling centers may have less infrastructure and fail to properly select patients and favor the inclusion of patients less likely to benefit from the studied therapy. These centers may be less familiar or adherent to strict protocols, have less structured recourses to conduct the study, and may have too many competing studies or higher dropout rates and poor follow-up. Hence, accurate interpretation of treatment effect estimates across all enrolling sites is complex. Does a trial with 100 centers enrolling 4,000 patients over 2 years produce different findings from a trial with 1,000 centers enrolling the same number of patients over the same time? There is a tenuous balance between enrolling a sufficient number of patients and selecting the “right” patient (quality vs. quantity). Furthermore, there is increasing recognition of the impact of regional differences on outcomes in HF trials [23]. As low enrollment and regional differences interact with unpredictable ways, there is concern for further bias of trial results.
Low enrolling sites may result in high cost, reduced innovation and poor quality. The “fixed cost” of opening a site ($30,000–$50,000) and keeping a site open ($3000– $5000/month) make studies enrolling thousands of sites prohibitively expensive. Many sites with few or no patients will not justify the cost of training and site maintenance. Sites may not have sufficient resources (study physicians, nurse/site coordinators), expertise in the disease area, prior trial experience or a patient population meeting study selection criteria to allow for adequate recruitment. Since reimbursement and recognition for increased recruiting is poor, sites lack motivation and become overworked without adequate compensation. Furthermore, too many sites create costly logistical issues. These high costs limit the number of new interventions that will be examined in phase III clinical studies, causing early abandonment of potentially effective interventions.
Clearly, there is no one ideal site for all studies. For instance, studies enrolling patients with acute pulmonary edema may do less well in trials testing an agent targeting refractory, inotrope-dependent HF. Do there need to be fundamentally different approaches to identifying centers and investigators for participating in HHF trials as compared to chronic HF trials? As a general rule, site enrollment needs to be tailored depending on the specific intervention under investigation.
In order to successfully conduct studies, site personnel should be interested in study involvement at multiple levels, including the program director (cardiology, heart failure and emergency department), sub-investigators (junior faculty and fellows), and perhaps most importantly, the study coordinator. To maximize interest, studies should exhibit scientific merit, be well designed and, most importantly, be simple to execute.
Experienced, motivated and well-compensated study personnel are key to successful enrollment. First, site personnel should be experienced in conducting such studies, especially those in HHF. Second, personal relationships between local staff and operational leadership are crucial to maintain motivation. Third, local leadership through regional leaders is critical. Selecting the wrong country PI can lead to poor motivation of his/her colleagues. Finally, compensation of investigators, coordinators and co-investigators is essential as there needs to be strong motivation for sites to enroll patients. However, the specific methods of such techniques are unclear in a climate with low reimbursement, a lack of site recognition of the merit of conducting trials (e.g. increasing workload without compensation or authorship on trial manuscripts) and concerns that monetary incentives for investigators may encourage protocol violations.
Food and drug administration
Norman L. Stockbridge
Heart failure trialists often compare the public health burden of their disease to the one treated by oncologists, but the former fail to learn the latter’s lesson. A high proportion of practicing oncologists participate in clinical research on a continuing basis [24], and they foster that culture among their patients.
Contract research organization
Chris Giordano
Current practices of site enrollment are strongly driven by timelines and target overall enrollment rates, missing key steps along the site enrollment process. New models should occur in three distinct stages. The first stage is the site and patient selection stage. Large populations of patients must be analyzed to find patients’ meeting enrollment criteria and to determine the sites with access to these patients. For efficiency and quality, these should not be created in a vacuum, but rather compiled by a cohort of organizations including ARO networks, the National Lead network (part of the ARO contribution) and CROs. Well-performing sites that have previously investigated the study agent or recruited the study population of interest should also be specially invited to participate. The second stage is evaluating feasibility—the scientific/academic leadership should provide a draft protocol and detailed questionnaire to help determine site and patient criteria. Logistical elements such as the anticipated number of sites, length of recruitment and potential challenges should be identified. Site participation in this developmental stage can help gauge site interest/engagement. This participation further allows us to inspire a sense of “ownership” of the trial. Sites that demonstrate that they can build the cross-specialization/department network and create “ownership” of the trial within their institution will properly recruit into the trial and may yield the required patients since these same two factors drive subject volume and appropriateness. The final stage and perhaps the most important is the verification phase. Although site selection visits can be costly and time-consuming, they may prove invaluable to avoid downstream unanticipated problems/issues. During this final stage, these site selection visits can be targeted at fewer, more qualified sites that have been identified in previous stages, thus potentially reducing the overall costs of site monitoring.
Although it has been proposed that utilization of a registry instrument may aid in the recruitment of quality sites and patients, this approach presents some concerns. Some quality sites interested in a Phase II/III study may not be interested in investing time and burden in a registry. Furthermore, sites willing to participate in a pre-trial-specific registry may not be predisposed toward a nationwide HHF registry in which their performance (exams and treatments) may be measured in a non-anonymous manner. The registry only provides a snapshot of a site profile/ capacity/performance which is likely to be dependent on site personnel. Unfortunately, emergency department (ED) site personnel experience high turnover.
Academic research organizations
W. Frank Peacock
While an ARO may exemplify process and infrastructure unique from that of their CRO counterpart, ultimately, they share nearly identical needs. Specifically, both types of research organizations require reasonable clinical and economic efficiency from the institutions they partner with. Without this efficiency, poor protocol compliance and high rates of adverse events from non-standard clinical care increase the study population and costs needed to determine the experimental intervention’s effect. Ideally, a smaller number of efficient centers would have important impacts on study costs, enrollment goals and potentially clinical outcomes.
The historical strategy of increasing the number of sites to reach enrollment targets is doomed to failure without considering financial and clinical efficiency. Very low enrollment is usually the consequence of a lack of infrastructure, not lack of patients. Knowledge of the ED census can approximate a site’s potential acute HF population. In 2012, there will be an estimated 130 million US emergency department visits. Of these, 1 % (1.3 million) will suffer acute HF, and 1.04 million will be hospitalized [25]. Since acute HF is for the most part uniformly distributed across the North American continent, we can predict a site’s potential acute HF population. To recruit these potential participants, infrastructure needs to be where the patients are, when the patients are there. Most patients present to the ED at non-business hours. A 9a–5p research nurse in the department of heart failure is in the wrong department and available at the wrong time to enroll many acute heart failure patients.
Further, although most HF research is performed by HF cardiologists, most HF patients are not immediately hospitalized under the care of a HF cardiologist. After an ED evaluation, most HF patients are admitted to internal medicine, family practice or a hospitalist service. HF specialists may not care for the patient until after trial enrollment eligibility has expired.
The ED is the one common location of the dispersed population of the one million US acute HF patients. Importantly, although the presence and location of acute HF patients are predictable, it too is challenging. In a 75,000 annual visit ED, there will be approximately 750 annual acute HF presentations. This is about 2 patients per day, both of whom may arrive after 11 pm. Ultimately, larger centers (i.e. more potential candidates for study entry) with more hours of ED research coverage per day (i.e. greater probability of capture) will have greater enrollment success than centers that do not have these characteristics. Finally, potential site selection in future research projects requires objective prospective evaluation of their probability for success. Although not validated, metrics that may be useful appear in Table 2.
Table 2.
Heart failure study metrics
| HF Study Metrics | Emergency department metrics |
|---|---|
| Prior performance | Dedicated ED research staff |
| Monthly screening | Annual ED census |
| ED door to screening time | Prior ED HF study participation |
| ED door to enrollment time | Point of care lab |
| Daily hours of research team availability |
ED physician site co-principal investigator |
| Days per week of research team availability |
|
| ED HF admissions by month for prior year |
|
| Research call system for off hours | |
| 24/7 enrollment |
ED emergency department; HF heart failure
Faiez Zannad: Fight-HF Alliance
Certain inherent challenges exist with HHF trial execution. The definition of HHF is commonly based on gradual or rapid onset of HF signs and symptoms requiring urgent therapy. The patient population is highly heterogeneous, and the definition is dependent on clinical judgment, open to selection bias and does not categorize the multiple phenotypes. The severity of symptoms that mandates patient admission is very subjective and may vary widely across various healthcare systems. There are no robust diagnostic ascertainment criteria such as electrocardiogram and enzymes for myocardial infarction. The use of BNP as an inclusion criterion is not standard practice and has limitations. Patients proceed through a continuum of stages from the ED to post-discharge, and the place to screen for and optimally enroll patients with HHF may vary from EDs, intensive care units (ICU), general ward, internal medicine, cardiology departments or HF units, according to the stage of the disease that is targeted in specific trials. The TIMI group experience has capitalized on the structuring of healthcare systems worldwide for the specific coordinated and integrated management of ACS worldwide that led to ICUs in the seventies. A similar structuring effort for the management of HHF, a condition that has grown to become twice as frequent and more severe than ACS, is an unmet need. Only such structuring may ultimately lead to the emergence of HHF—minded and trained dedicated physicians with experience in clinical trials and acute management of HF. Large regional variation exists and the individual investigators magnify this heterogeneity. The treatment and management of patients with HHF is largely multi-disciplinary, and thus no particular treating physician takes ownership. Follow-up after hospitalization remains particularly challenging and is also influenced by regional differences in healthcare systems.
Fight-HF Alliance represents a global ARO aimed at optimizing execution of HHF trials. The principles underlying this organization include establishing a better understanding of the disease and its heterogeneity across clinical practices, specialties, regions and healthcare systems. It tries to provide a durable and extensive global registry database available for queries to allow for trial design optimization, identification of target populations, pre-trial market research and health economics/analysis. This provides a huge opportunity for training and investigator consortium building. Furthermore, Fight-HF provides academic expertise in designing clinical trials and maximizing knowledge production. It aims at identifying, creating, training and certifying networks of multidisciplinary investigators/teams profiled to the specific needs of HHF trials, led by active lead country investigators.
Dirk J. van Veldhuisen
The reasons underlying low patient recruitment may be low financial incentives and low motivation levels from participating sites. This may partially be due to anonymous relations with sponsor and CRO. Furthermore, if inclusion/ exclusion criteria are very strict, and the screen failure rate is high, a trial may become unattractive financially for a site. Payment for screen logs may be considered and will sometimes be used to check commitment to the study. However, it is also costly and does not add to inclusion.
The potential advantages of an ARO include, but are not limited to, more local/regional commitment. Often, an academic group is not able to keep all patients in their own hospital because their capacity (both in-hospital and in their out-patient clinic) is limited, but it can work together with local/regional non-academic sites and make agreements about referring patients for specific trials. Agreements can be made about authorships and participation in writing articles. In most AROs, the primary incentive is scientific and not financial. Such cooperation may work very well with investigator initiated studies, which have a lower budget, but it can also work in industry trials. In general, lines are shorter and less expensive in AROs, and small-to-medium size studies can be managed (up to 1,000 patients more or less). Clearly, the ARO needs to have the same quality standards as commercial, non-academic CROs. Commercial sponsors (industry) may sometimes use an ARO (or a group of them) instead of a commercial CRO.
National Heart Lung and Blood Institute
Monica Shah
The primary goals of the National Heart Lung and Blood Institute (NHLBI) for site selection are to conduct high-quality, streamlined and cost-efficient trials by selecting fewer, but better performing sites. However, there is no official policy for site selection. There is a peculiar paradox that exists in trial execution—we perform clinical trials to generate evidence to improve patient outcomes; however, we conduct clinical trials like anecdotal medicine: (1) we do what we think works; (2) we rely on experience and judgment; and (3) limited data to support best practices.
The NHLBI site selection process involves two phases: peer-review and after peer-review. Peer reviewers evaluate site performance and selection based on experience and judgment in regard to feasibility. Specific requests for applications or requests for proposals may have more detailed criteria for site selection. For example, the Heart Failure Clinical Research Network Regional Clinical Centers details the following performance requirements: number of patients recruited, timeliness of enrollment, end point completion, patient retention, data quality and timeliness. Peer reviewers are also required to evaluate information about potential study populations in terms of women and minorities (National Institutes of Health Revitalization Act 1993). Recruitment targets for all clinical trials are 50 % women and 30 % total minority component. If the proportion of women or minorities is lower, then the reason(s) for the discrepancy must be identified.
After the peer-review process is complete, NHLBI staff assists in reviewing and monitoring site selection. With investigators, NHLBI staff develops trial-specific criteria to optimize site selection and ensure women and minority targets are addressed. Site selection criteria include: scientific integrity of sites; experience with large, randomized trials; prior performance, recruitment, data quality; case mix and clinical practice; availability of essential resources including study coordinators, research pharmacy; availability of specialized resources including imaging; experience with unique trial procedures such as waiver of informed consent, surgical technique; diversity of patient population and; geographic representation. International sites are selected based on heterogeneity of patient population, prevalence of disease, background therapies utilized and healthcare systems in place. The National Institutes of Health has specific policies as well for international recruitment: (1) International recruitment can be counted toward target enrollment; (2) Information about women and minority recruitment must be reported separately for US and international sites; and (3) International reports should include the same categories as US sites.
Thus, the NHLBI uses general guidelines, but possesses no official policy for site selection. It largely relies on experience and judgment of peer reviewers, investigators and staff. However, it includes an official policy for women and minority recruitment. Selection and performance metrics would certainly enhance conduct of trials. A future possibility to help bolster trial participation and enrollment efficiency is the creation of HF-specific NHBLI-sponsored standing networks. This system may assist in building upon prior success in this area to guide future HHF clinical trials.
Industry
Frank Misselwitz: Bayer
In the past, trials were prohibitively expensive without enrolling enough quality patients. In order to avoid a high number of sites with only 1–2 patients, we must reduce the geographic footprint, not overextend our established resources, and focus on including sites with a proven track record (high enrolling, high-quality sites). We must anticipate downstream problems in the trial by performing dry runs, pre-screening, pre-trial registries, etc.
There needs to be a new paradigm of how industry, academia and CROs work together. Conventional performance (and payment) metrics of CROs typically favor opening multiple sites with frequent visits even in the absence of patients. It is much better to invest into operational capabilities of sites (study nurses for example)— which perhaps is the most important single lever to improve our trials. AROs typically have the full scientific background and access to high volume sites; however, clinical operations, quality assurance and rigid payment schedules based on key performance indicators are not (yet) engrained in (most) AROs.
Industry fully supports the initiatives of academia to establish AROs or Academic Research Teams. Together, we must seek to determine the best agents for the best sub-populations of patients (patient segmentation). We should search for and validate surrogate markers and different imaging modalities (cardiac magnetic resonance imaging, echo, etc.,) that can be utilized in clinical trials. Metrics that can be used to help select high-quality sites include past performance, benchmark trials, scientific interest, operational capabilities, general interest in measuring quality of patient care and improving healthcare delivery (hospitalist, epidemiology, quality systems in place), high number of eligible patients (referral site), functioning systems to follow-up patients after discharge/intervention long-term, and known and operationalized patient flows with clear responsibilities and adequate staffing.
A pre-trial registry may be an effective strategy to help overcome these stated problems. It could assist in determining patient volume and patient flow. The instrument could stratify/subsegment patients into subgroups. It could determine availability, training and willingness of personnel to operate clinical trials. Finally, it could determine the ability to perform required diagnostic procedures in due time and the ability to produce high-quality, reproducible procedures/intervention results.
Eva Muhlhofer: Bayer
In most studies, 50 % of the countries involved contribute 90 % of the patients, as many sites enroll zero to two patients. Furthermore, with less experience, low recruiting sites have many protocol violations which have a major impact on costs, study duration and affect study outcome.
New tools to improve site selection and study management are needed. There are initiatives to use electronic health record systems and to link them to clinical trial systems, but all are at an early stage, and there is no harmonization of these systems. Another option would be to use a clinical registry, either an ongoing prospective registry or a trial-specific one. Many topics regarding this must be addressed. Should it be trial-specific? What is the ideal duration of the registry? Should it include HHF and/or chronic HF patients? What population size could be representative for global studies? Should it include hospital and/or outpatient data? Other challenges including time delay, funding and logistics need discussion. Depending on the overall frame of the registry, it could support drug development plans, study protocol development, site selection (feasibility checks), patient selection (capture of patient profiles and patients’ flow data) and safety assessments (current pre-intervention data), and, in the end, deliver real-life data for reimbursement discussions. A registry has the opportunity be a tool to support global medical research, improve healthcare and enhance patient safety.
The HHF target population is very difficult to define especially if many countries are participating (different patient care/therapy, length of ICU/hospital stay, early or late mobilization, etc.). If you harmonize too much, you limit both your population and your recruitment and fail to reflect real life. If you are too broad, you may miss the population who benefits. It is not an easy to balance.
Andrew Zalewski: Novartis
Disappointing results of almost all trials in HHF stem from many factors. The operational issues are compounded by fragmented patient care that does not allow for a simple identification of investigators within hospital practices. The pathways of in-hospital care may differ not just in the very acute stage (e.g. presentation to the ED, acute care facilities) but also during the subsequent hospital stay (e.g. care provided by internists, cardiologists or commonly other specialties). Other challenges arise from the often forgotten fact that symptoms of HHF are precipitated by non-cardiac disease. Analogous to trial planning for acute MI, trial planning in HHF needs to involve both ED physicians and physicians who will follow the patients during the hospital stay. For treatments applied beyond the first few hours of presentation and designed to be continued for weeks or months, it is important to broaden patient pool in clinical trials beyond that available in cardiology practices (which may skew patient population to lower risk patients).
Other issues that plagued some trials conducted in HHF relate to excessive protocol complexity. Multiple assessments (biomarkers, imaging) may phenotypically better define patient population but often reduce enrollment into the trials (“hassle factor”) or shift patient randomization toward later time point when the benefit of therapy will be more difficult to ascertain. The sponsors and the academic leadership of the trial should discipline themselves to simplify inclusion and exclusion criteria, avoid complex assessments, particularly if the intervention for HHF is to be delivered very early after presentation. Overloading the trials with sub-studies, although intellectually always attractive, minimizes the chance of success around the primary hypothesis. In addition, careful pre-trial feasibility is required to assure that biomarker criteria are readily available in global trial settings.
Fabio Baschiera: Novartis
The validity of a clinical research lays in its application to the general population. In order to enroll a homogeneous population in HHF clinical trials, it is necessary to select sites responding to defined characteristics. What are the key ones? We can recognize 3 main features: (1) the site should be in a hospital operating as an integrated healthcare provider, with tight interconnections among departments (e.g. ED); (2) electronic records accessible from all departments with historical data allowing real time admissions scrutiny as well as reliable estimates of recruitment capabilities; and (3) HF specialists available for an early diagnosis. Regulatory agencies and sponsors need to recognize the growing challenges of clinical trials from patient to complex protocol procedures. Such recognition may contribute to the demand of cost-containment with effects that translate to reduced drug development costs and a reduced final on-the-shelf price of new drugs.
North American registries
Gregg C. Fonarow
Heart failure registries have been highly successful in enrolling HHF patients at academic and community based hospitals in the US and elsewhere, even when requiring informed consent and post-discharge data collection [26]. In the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) registry, 48,612 patients were able to be enrolled in less than 24 months from 259 centers [27]. Among 91 sites selected to obtain patient informed consent and capture 60–90 day post-discharge follow-up, 5791 unique HHF patients were enrolled. The majority of patients screened for enrollment in the registry are able to be successfully enrolled. Heart failure registries have also been organized to be inclusive of heart failure specialists, general cardiologists, ED physicians, hospital medicine physicians, primary care physicians, nurses, allied healthcare personnel and hospital administration [26]. In contrast to the broad enrollment criteria utilized by registries, most acute HF clinical trials have applied extensive inclusion and exclusion criteria, resulting in a highly select patient population. Beyond posing challenges to the identification and recruitment of trial subjects, the external validity and generalizability of resulting findings generated from highly select clinical trial patient populations may be significantly limited. There are many lessons from HHF registries that can be utilized to improve trial design, site selection, evaluation of site performance and study conduct. Large scale randomized trials for ACS have been successful in part due to applying lessons learned from ACS registries such as the National Registry of Myocardial Infarction (NRMI) and aggregating dataset from multiple randomized trials.
North American trialists
Kirkwood F. Adams, Jr. MD
Study process management is a critical element to excellent study execution. Many sites are plagued by inadequate attention to process management of the study. Variation in recruitment process is considered acceptable, ignoring the fact that successful systems share many fundamental methods of work. Registries can promote common practice of key recruitment strategies, lead to refinement of strategies and directly assess site performance. If properly designed, conducted and funded, a Patient- and Site-Based Registry can be used to improve enrollment and quality of HHF trials. This registry can help assess site process for a trial, ensure that enrollment shows integrated function of research team at the site and provides funding mechanism to support trials and offers academic opportunity. Registry enrollment may help guide site selection. Design features of this registry rely on the premise that both patient and site data are collected, patients are formally consented for involvement and a detailed log of screening process is maintained.
John R. Teerlink
Trial execution has appeared to be more successful in the setting of ACS for several reasons. A clearly defined pathophysiological target is present (“the clot”). There is a widely held perception among healthcare providers, patients and the community at-large of a life-threatening disease, and, accordingly, early and continuous involvement of treating cardiologists is beneficial and is supported by national performance measures. In contrast, HHF presents unique problems to study conduct and design. A complex pathophysiological target exists. There is an overwhelming lack of perception that HHF is a life-threatening condition and multiple care-providers are involved with the management of these patients. There have been no substantial data supporting early intervention and no performance measures reaffirming this concept. Due to these underlying differences, an entirely different approach should be mandated in HHF. We must broaden site selection by including increased enrollment from non-academic US community sites with support for continued site development. Different funding mechanisms should be utilized to allow for overhead to create and maintain infrastructure for early screening and capture of patients. Broader inclusion of non-cardiologists (hospitalists, ED physicians) as site PIs and investigational teams would assist with recruiting. Simplifying Clinical Report Forms (CRFs) and increased funding (with careful definition) for screening “failures” may also increase enrollment at the ground-level. Realistic expectations by sponsor (cost and recruitment/timelines) should be established. Clear academic changes must occur to assist with the trial process. We must work with academic centers to provide protected time/credit for enrolling patients in trials. Finally, a re-evaluation of the social contract between the medical research centers, healthcare payers, pharmaceutical and device sponsors, and governmental agencies is overdue. All of us benefit from the developments of medical research, especially in diseases with such high morbidity and mortality like HF.
Malcolm Arnold
Many efforts have been expended but, for many reasons, clinical sites often enroll only a few patients at a high setup and monitoring cost. The efficiency of HHF trials may benefit from attention to the detailed process of patient flow. Does the ED team directly contact the HHF team? How soon does the HHF research team get to the patient? What is the knowledge and training of housestaff and trainees? Have residents been trained to present and obtain informed consent for a specific trial? Has the chart been reviewed to measure gaps in the timeline when patients are “just waiting”? Selecting sites for clinical trials in HHF will require extensive evidence of the enrollment patterns among sites that reflect organization/patterns of practice and feasibility. If we focus on high enrollment strategies in previously successful sites, we may skew the management profile of the patients that is less generalizable. The selection of new sites may rely on prior experience with other cardiovascular clinical trials, documented number of HHF patients per year (along with baseline characteristic snapshot), reviewing existing PIs and staff, available protected time, letters of support, level of interest and timely response to inquiries. Due to the narrow window of enrollment, similar to ACS trials, basic and simple metrics should help guide patient recruitment (e.g. time from arrival in ED to first IV treatment, door to consent time, door to first study treatment time and completeness of records) (Table 3). An administrator must assist in removing apparent barriers, change ED protocols as needed, promote involvement and ownership, make clinical pathways and logistical flow sheet available for use.
Table 3.
Impact of patient flow on heart failure trial efficiency: metrics for improvement
| Phase of hospital care | Metric |
|---|---|
| ED evaluation and treatment for suspected worsening HF (CXR, O2, diuretics, nitrates, etc.) |
What treatments were provided to the patient? |
| Evaluation for other potential conditions (pneumonia, COPD, PE, etc.) | What conditions from the differential diagnosis were excluded? |
| Consult with, or transfer care to, other physicians | How long did this take? |
| Admission to hospital ward | Utilization of hospital resources |
| Complications during hospital stay | Renal dysfunction? Hypotension? Death? |
| Discharge and plan for outpatient care | Post-discharge readmission rate survival |
COPD chronic obstructive pulmonary disease, CXR chest X-ray, ED emergency department, PE pulmonary embolism, HF heart failure
European trialists
John G. Cleland
Investigators need support, encouragement and nurture from all types of regulatory bodies. Regulations currently focus too much on industry and do not recognize the huge extra burden this places on investigators. Research is becoming too complex and too bureaucratic. This discourages potential investigators from participating in trials (Fig. 3). Protocols and recruitment strategies should be designed so that centers can recruit >30–50 patients per year. Sites have little interest in recruiting 5–10 patients into a study since this will seldom cover the research costs. The current strategies are not economically viable as, without subsidized labor (e.g. by “stealing” staff from services or other projects), studies will result in a net loss to the department. The current reimbursement pattern for CROs favors increasing the complexity and workload of clinical trials. Of particular concern are the poorly remunerated costs of screening. This can be by far the largest cost to the center in a clinical trial but is often poorly reimbursed, if at all.
Fig. 3.
Complexity of the clinical trial enterprise
There are several possible solutions to this problem. One solution would be to conduct all clinical trials in Denmark (population ~5 million). Trialists in Denmark are able to recruit 7,000 patients with MI in 2–3 years with 35 hospitals in a 150-mile radius and obtain a clear mortality result [28]. If we are not able to move all trials to Denmark, we can at least start with the Danish system. This system of clinical trial execution relies on relatively few, well-resourced centers with dedicated full-time staff that can enroll consecutive patients. These centers could also act as ‘National Health Laboratories’ recording the nature and volume of disease in particular common disease areas. This would ensure their value and continued existence if a hiatus developed in the trials program and prevent the “stop-start” culture inherent in existing strategies.
Stefan D. Anker
Site selection is too often based on historic data that is not relevant since data are outdated, often applied for a different indication and acquired under a different staff system. Site selection is also based on no data at all and only based on private relationships and important names. Also, the selection process is underfunded, and forms are either too complicated (and hence not filled in properly) or filled in by junior staff and/or simply guesses rather than data driven. Company staff managing trials are sometimes underappreciating the importance of site selection (e.g. it often happens that when a country PI is selected, already all sites have been chosen). The free sharing of information regarding high-quality sites is typically not done. Studies often spread themselves too thin hoping they will include a few good sites.
Furthermore, the economics of the current clinical trial paradigm does not promote high enrollment. Recruitment requires at least one full-time person, which costs approximately 60,000 dollars per year. If the study pays 3,000 dollars per patient, then the site must enroll >15 to make the process sustainable. However, site contracts often only aim for 6 or 10 patients; hence, trial work cannot be economically viable. Ultimately, one good research fellow given time and support makes all the difference. There may also be problematic protocols that could use revision. Often, current trial protocols receive input from too many experts by committee. There is a need for smaller executive committees to design the trial, which is subsequently approved by a larger body. Exclusion criteria should be stringently reviewed; patients should be in the trial unless there is a good reason not to be included. The companies need to develop long-term relationships with investigators, instead of shorter term need-based interactions. Larger academic centers should be able to deliver >30 or >50 patients, not 5 or 10.
Japanese trialists
Naoki Sato
Regarding the quality of the study, the lack of infrastructure of the participating centers is one of critical issues, and it might be related to characteristics and the number of enrolled patients. Based on data from the ATTEND (Acute Decompensated Heart Failure Syndromes) registry, an ongoing HHF registry in Japan [29], the patient characteristics were quite different between daytime- and nighttime-admitted patients, that is, higher blood pressure and heart rate were observed in patients admitted at nighttime who were also more likely to have orthopnea, lower pulse oximetry saturation and less peripheral edema [30]. In Japan, the number of daytime-admitted patients was larger than that of nighttime-admitted patients, but it would be different in each region or site. Thus, the characteristics and number of enrolled patients would be influenced by the infrastructure of the participating center and cause bias in the study.
There is another important issue related to site selection: the regional transportation system. The transportation time is clearly related to outcome of HHF patients, which was demonstrated with data from the Tokyo CCU network [31]. In HHF patients, the longer transportation time, the poorer outcome. Therefore, it might be necessary to evaluate also the transporting system in the area where the participating sites exist. If regional differences in transporting system exist, it would be one of major biases in the results of the study.
Incentives
Javed Butler
As a moderator, I was impressed with the robust discussion around the issue of incentives to participate in clinical trials in academic medical centers. Currently, it is deemed unethical by most institutional review boards to provide any incentives to boost enrollment in clinical trials. These include both incentives to patients and to the investigators and coordinators. The fear about patient incentives is that it may cloud patients’ judgment and they may agree to take risks that they may otherwise not consent to. The concern for investigators and coordinators are that incentives may alter unfavorably the process of complete disclosure of risks to patients and strict adherence to the inclusion and exclusion criteria for the clinical trial. Industry and academic legal counsels are concerned regarding incentives of any sort for both ethical reasons but also the potential for negative consequences from government, regulators and funding agencies. These concerns have resulted in a situation where almost all incentives are prohibited for anyone involved in clinical trials. Even small incentives, for example, providing a medical textbook to trainees to encourage screening patients, are deemed unacceptable. Though the concerns related to incentives are theoretically true, such deliberate or random unwanted consequences are not inevitable. The current processes already in place for evaluation, initiation and conduct of trials include contract negotiations, billing compliance, institutional review board approval, and in many institutions, a clinical trial oversight and monitoring committee. These processes have significant labor and financial startup costs, and the process can last several months. However, built within these steps are mechanisms that can mitigate the chances for abuse of incentives.
It was generally felt that investigators did not get adequate protected time or salary support needed for the successful conduct of clinical trials. The investigators then depend on a variety of staff and colleagues, who in turn uniformly have almost no incentive to make the trial a success. Currently, the coordinator’s salary cannot be tied to the rate of enrollment. There are a series of activities that the coordinators perform that can be enhanced, provided these were linked to incentives build into their performance metric, for example, extra effort to screen patients from a wider outpatient and inpatient settings than only the cardiology floors, be enthusiastically available to other physicians and patients’ families for all questions related to trials, develop mechanisms with information technology, hospital medicine, emergency department to maximize enrollment possibilities, develop good communication with various clinics and hospital floor staff, utilize time efficiently, enroll patients despite knowing the fact that per protocol some of the procedure may have to be performed off usual duty hours based on when the patient presented to the hospital, etc. These and multiple other steps are dependent on motivation of an individual. If the coordinator’s salary is unaffected regardless of the performance on these steps and they cannot be incentivized in any form, then the only real incentive, besides the motivation to do the “right” thing, is that non-productivity may lead to cessation of the research program. However, this negative, as opposed to positive, incentive likely drives lowest possible bar as performance target.
Finally, most of the site investigators will not be authors on manuscripts, do not drive monetary benefits from participation, do not have protected time, and continue to be stretched in multiple directions in the rapidly changing healthcare environment. However, the investigator assumes regulatory and legal risks. During the discussion, it was generally felt that in the current economic and healthcare reform environment, the institutional academic leadership does not value participation in multi-center clinical trials by the faculty, unless it is an investigator initiated trial that brings substantial funding to the institution.
Although the group recognized the potential problems with incentives, the general consensus was that completely prohibiting all incentives to all individuals, especially the coordinators, is a significant impediment to success. It was recommended that beyond the investigators, the dialog around incentives in clinical trials should also include other critical stakeholders including the legal counsels from government, academia, and industry, ethicists, and business leaders. This dialog will provide a comprehensive view on the promises and pitfalls of incentives. This will inform the potential considerations for changing the current paradigm from no incentives to acceptable incentives while strictly maintaining ethically sound boundaries in conjunction with patient safety and autonomy.
Impediments to patient recruitment into clinical trials of HHF
Hani N. Sabbah
In the United States as well as in Western Europe, enrollment of patients into clinical trials of HHF and, for that matter, all other HF trials has markedly dropped in the last decade. While most clinical trial sponsors, clinical trialists, AROs and CROs are aware of this drop, none have undertaken a systematic review of the underlying impediments to patient recruitment into clinical trials for HHF. The following is a list of real or perceived impediments to the recruitment of patients into such clinical trials.
The adverse impact of changing healthcare policies on physician compensation
In recent years, healthcare organizations and hospitals have linked “compensation” to clinical care performance. In the United States, this has taken the form of “Relative Value Units” or RVUs. Physicians are required to generate a minimum number of RVUs per year to maintain their annual compensation level. RVUs accumulated above this minimum can often trigger additional compensation in the form of “bonuses”. These RVUs, however, are earned for work performed in the course of patient care and not for work performed as an investigator in a clinical trial. For this reason, many academic physicians who are named investigators on clinical trials spend most, if not all their time, generating so-called clinical RVUs and devote less of their time on clinical trials. This healthcare policy shift has created a culture of “disincentiveness” on the part of physicians to devote precious time to research endeavors.
The adverse impact of changing healthcare policies on research coordinators
Changes in physician compensation as a result of the changing healthcare policies have also had a negative impact of the effectiveness of the traditional clinical trial team of “physician leader and support coordinators/nurses”. The lack of availability of the physician leader on a regular basis to help recruit patients into clinical trials has left this entire responsibility to site coordinators. While these individuals are often hard working and efficient, they cannot replace the role of the physician in “consenting patients” for a clinical trial. Patients, regardless of their level of illness, want assurances from their physicians that their participation in a clinical trial is in their best interest. Lack of physician interaction in recruitment process often leads to refusal of informed consent by the patient. The resulting “low enrollment” into a clinical trial at a trial site can lead to a “blame game” between principal investigator and coordinators that promotes further ineffectiveness and further deterioration of patient recruitment.
The fading support of fellow faculty physicians
An issue in many academic institutions is the participation of other faculty physicians within the institution that are not officially part of a given clinical trial, in helping in the patient recruitment process. There is an ever increasing discontent among physicians and nurses who are not active parts of a clinical trials to feel that their contributions to patient recruitment brings them nothing in return for their efforts. This situation has given rise to the frequent rhetorical response of “what is in it for me” when asked to refer patients to the trial. Principal investigators on clinical trials are perceived as having the “privilege” of travel, authorship of academic publications and presentation at scientific conferences when the referring physician is often denied such participation. The absence of reward of any kind aside from the goodwill of fellow physician contributes to a climate of “disincentiveness”.
The conflict of interest barriers
In recent years, universities, major medical centers and hospitals have introduced policies to limit so-called “conflicts of interest” between members of their faculty and the medical industry in general. The barriers erected by both medical institutions to limit “perceived conflicts” have taken their toll on the conduct of clinical trials and on the recruitment of patients into such trials. In many instances, physicians serving as site principal investigators are prohibited from participating in any other activities organized by the sponsor of the trial on which they serve as a principal investigator. Other faculty members from a clinical trial site who are not principal investigators on the trial are often also prevented from participating in activities of the trial such as Data and Safety Monitoring Boards and Steering Committees for fears of perceived conflicts. These issues have created an environment of distrust and “disincentiveness” on the part of key opinion leaders from encouraging participation in clinical trials for fear of being found in conflict with institutional policies. These barriers, while well intended and perhaps necessary, have removed the desire on the part of young clinicians and future medical opinion leaders from seeking participating in the “clinical trial” forum and from contributing effectively to this essential activity.
The institution view of the clinical trial enterprise
There were times when academic medical centers looked forward to participating in clinical trials. Their reward was articulated in the form of contribution to the advancement of science and patient care and the value of notoriety among peers. In fact, many institutions worked at creating the necessary environment and infrastructure to ensure the success of clinical trial activities. In recent years, this positive attitude has been tempered considerably. Many institutions view the participation in clinical trials only in terms of its “revenue potential”. Institutional indirect costs and start-up costs have increased dramatically to maximize such revenues. No test conducted as part of a clinical trial is left unaccounted for in the final budget. This transformation of “no support for unfunded clinical research” has also helped nourish a state of “disincentiveness” among the medical and scientific faculty.
The paradoxical influence of high standards of care
Last but by no means least is the problem of identifying the proper patients that can be recruited into a clinical trial of HHF. In the United States, regulatory agencies mandate that patients entered into clinical trials must be optimally medicated as per practice guidelines. Patients are often excluded because of inadequate therapy at time of screening and often require additional months of therapy before becoming eligible. Over the years, the exclusion criteria for entry into clinical trials have also expanded to ensure that only those who are likely to benefit from the test article are included. This focus on sub-populations, while appropriate to better select effective therapies for each subset of patients with HHF, makes it more difficult to recruit patients that meet all inclusion and exclusion criteria. Patients with HF also live longer as a result of marked advancement in therapy. As a result, the population is aging, and the frequency of comorbid conditions is on the rise, making it yet more difficult to recruit patients that fulfill the needs of the trial.
Concluding remarks
Mihai Gheorghiade
In summary, the key components necessary to deliver successful HHF trials include knowing the drug, matching the drug with the right patient population, and utilizing the appropriate investigators and sites. Current standards for trial conduct have established low benchmarks for patient recruitment. The experiences in recent international trials of HHF have routinely shown the vast majority of site centers either do not enroll any patients or deliver very low total enrollment counts.
This meeting will serve as a guide to help identify the “right” sites for HHF trials in the setting of increasing globalization. This document reflects the range of viewpoints present at a roundtable discussion hosted by the FDA. Members from academia, the NIH, industry, CROs, AROs and trialists have offered their unique perspectives and potential solutions to this critical issue. Most agree that there is an unmet need to develop strategies to identify the right study sites that have acceptable patient quantity and quality. Common themes that were identified include, but are not limited to, establishing a pre-trial registry, developing site performance metrics, identifying a local regionally involved leader and bolstering recruitment incentives.
Although Albert Einstein wisely stated that “we cannot solve problems by using the same kind of thinking we used when we created them” and that “if I had an hour to save the world, I would spend 59 min defining the problem and 1 min finding solutions”, we have now come to the minute in which we need to find solutions for HHF trials and for HHF patients.
Acknowledgments
Consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, Cor-Thera, Cytokinetics, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical and Trevena Therapeutics. Stefan D Anker: Consultant for Vifor International, Amgen, Bayer, Bosch GmbH, PsiOxus Therapeutics, Professional Dietetics, Novartis. Research support from Vifor International, PsiOxus Therapeutics. John G F Cleland: Received an honorarium from Bayer for an advisory board. Gregg C Fonarow: Consultant to Medtronic, Novartis, Gambro and Ortho-McNeill. Marco Metra: Received honoraria from Bayer, Corthera, Novartis, and Servier for speeches or participation in advisory boards/steering committees. Frank Misselwitz and Eva Mühlhofer: Employed by Bayer Healthcare. Hani N Sabbah: Research Grants and Consultant to Bayer Healthcare. Naoki Sato: Consultant to Chugai Pharmaceutical Company and honoraria from Daiichi-Sankyo, Otsuka, Philips Respironics, Astellas, Ono, Eisai, Chugai, Mitsubishi-Tanabe, Sanofi-Aventis, and Novartis pharmaceuticals. John R Teerlink: Research Grants from Corthera, Novartis, Cytokinetics, Amgen, NIH. Consultant to Amgen, Anexon, Cardio3 Bioscience, CardioMEMS, Cytokinetics, Gambro, Johnson and Johnson, Merck, NovaCardia, Novartis, Scios, Sorbent Therapeutics, St. Jude Medical, Teva, Theravance, Trevena. Faiez Zannad: Reports receiving consultant honoraria from Servier, Pfizer, Novartis, ResMed, Takeda, and Boston Scientific, and that his institution receives research grants from Roche Diagnostics and BG Medicine. Dirk J van Veldhuisen: Board Membership fees from Amgen, BG Medicine, Pfizer, Sorbent and Vifor. Javed Butler: Consultant to Cardiomems, Trevena, Bayer Healthcare, Takeda, Gambro, Ono Pharma, and Amgen; Events Committee for WorldHeart, Corthera, Abbott Vascular, Celladon, Research Support, NIH, GE Healthcare and Medtronic.
Appendix 1
See Table 4.
Table 4.
Selecting Investigators and Sites for global clinical trials in patients hospitalized for heart failure (HHF): current state and future direction—Program Agenda
| Location: | FDA White Oak Campus, Building 22, Room 2205, Silver Spring, Maryland | |
|---|---|---|
| Date: | Thursday, January 12, 2012 8:00 AM–2:30 PM | |
| Time | Topic | Presenter |
| 8:00–8:10 | Welcome | Norman Stockbridge |
| 8:10–8:30 | Trials in HHF to date: an overview | Mihai Gheorghiade |
| 8:30–8:50 | Geographical Disparities in HHF Trials | Marco Metra |
| Perspectives on the current process of site selection | ||
| Moderator: Marco Metra | ||
| 8:50–9:00 | Characteristics of an “ideal site” | Gadi Cotter |
| 9:00–9:10 | Contract research organization | Chris Giordano |
| 9:10–9:20 | Academic Research Organization | Frank Peacock |
| 9:20–9:30 | National Heart Lung and Blood Institute | Monica Shah/Alice Mascette |
| 9:30–10:00 | Coffee Break | |
| 10:00–10:10 | Industry | Andrew Zalewski |
| 10:10–10:20 | North American trialist | Kirkwood Adams |
| 10:30–10:40 | European trialist | Stefan Anker |
| 10:50–11:30 | Group discussion | |
| 11:30–12:15 | Lunch | |
| Opportunities to improve site selection | ||
| Moderator: Javed Butler | ||
| 12:15–12:30 | A Proposal: A Pre-Trial Registry/Instrument | Mihai Gheorghiade |
| 12:30–12:40 | A Contract Research Organization view | Chris Giordano |
| 12:40–12:50 | An Academic Research Organization view | Dirk van Veldhuisen |
| 12:50–13:00 | A National Heart Lung and Blood Institute view | Monica Shah/Alice Mascette |
| 13:00–13:10 | Industry view | Frank Misselwitz/Eva Muehlhofer |
| 13:10–13:20 | North American trialist view | John Teerlink/Malcolm Arnold |
| 13:20–13:30 | European trialist view | Stefan Anker/Marco Metra |
| 13:30–13:40 | FDA view | Norman Stockbridge |
| 13:40–13:55 | TIMI study group experience | Eugene Braunwald |
| 13:55–14:25 | Group discussion | |
| 14:25–14:30 | Concluding remarks | M. Gheorghiade and N. Stockbridge |
Appendix 2
| Group | Members | |
|---|---|---|
| Non-industry | ||
| Physicians | Adams Jr., Kirkwood | Mentz, Robert |
| Anker, Stefan | Metra, Marco | |
| Arnold, Malcom | Nodari, Savina | |
| Butler, Javed | O’Connor, Chris | |
| Cotter, Gadi | Peacock, Frank | |
| Cotter-Davison, Beth A. |
Pitt, Bertram | |
| Daum, Douglas | Ruschitka, Frank | |
| Domanski, Michael | Sabbah, Hani | |
| Gheorghiade, Mihai | Sato, Naoki | |
| Greenberg, Barry | Teerlink, John | |
| Holzmeister, Johannes | Van Veldhuisen, Dirk | |
| Liu, Peter | Yancy, Clyde | |
| Massie, Barry | Zannad, Faiez | |
| NIH | Gordon, David | Mascette, Alice |
| Desvigne-Nickens, Patrice |
Shah, Monica | |
| Industry | ||
| Abbot POC | Morgan, Jayne | Sethuraman, Barathi |
| Amgen | Kim, Jae | |
| Bayer | Cook Bruns, Nancy | Misselwitz, Frank |
| Delvers, Thomas | Muehlhofer, Eva | |
| Ewald, Silke | Oechsner, Sven | |
| Nowak, Christina | Roessig, Lothar | |
| Cardiorentis | Schnee, Elmar | |
| Cytokinetics | Woolf, Andrew | |
| J&J | Barret, Terrance | Gengo, Peter |
| Porter, Brandon | Peters, Gary | |
| Burton, Paul | Radziszewski, Waldemar |
|
| Medtronic | Hill, Michael R. S. | |
| Group | Members | |
| Merck | Cody, Robert | Koglin, Joerg |
| Novartis | Zalewski, Andrew | |
| Ono Pharma | Kang, Sok | Ross, Douglas |
| Kiyoshi, Hidekazu | Soumaoro, Ibrahima | |
| Otsuka | Krasa, Holly | Zimmer, Chris |
| Quintiles | Mahaux, Veronique | |
| SigmaTau | Giuseppi, Bianchi | |
| Sticares | Yun, Chris | |
| Takeda | Knapp, Beth Anne | Kupfer, Stuart |
| Trevena | Swiggard, Pamela | |
| Meeting coordinators |
Kozeli, Devi | Mallery, Michelle |
Footnotes
Conflict of interest Mihai Gheorghiade: All other authors have no conflicts of interest to declare.
Contributor Information
Mihai Gheorghiade, Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, 645 N. Michigan Avenue, Suite 1006, Chicago 60611, IL, USA, m-gheorghiade@northwestern.edu.
Muthiah Vaduganathan, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Stephen J. Greene, Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, 645 N. Michigan Avenue, Suite 1006, Chicago 60611, IL, USA
Robert J. Mentz, Division of Cardiology, Department of Medicine, Duke University Medical Center, Durham, NC, USA
Kirkwood F. Adams, Jr, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Stefan D. Anker, Centre for Clinical and Basic Research, IRCCS San Raffaele, Rome, Italy
Malcolm Arnold, Division of Cardiology, University of Western Ontario, London, ON, Canada.
Fabio Baschiera, Novartis Pharma AG, Basel, Switzerland.
John G. F. Cleland, Castle Hill Hospital, Hull York Medical School, Kingston-Upon-Hull, UK
Gadi Cotter, Momentum-Research, Inc., Durham, NC, USA.
Gregg C. Fonarow, Division of Cardiology, University of California, Los Angeles, CA, USA
Christopher Giordano, Quintiles, Cardiovascular and Metabolic Therapeutic Delivery Unit, Durham, NC, USA.
Marco Metra, Division of Cardiology, University of Brescia, Brescia, Italy.
Frank Misselwitz, Bayer Healthcare Pharmaceuticals, Berlin, Germany.
Eva Mühlhofer, Bayer Vital GmbH, Leverkusen, Germany.
Savina Nodari, Division of Cardiology, University of Brescia, Brescia, Italy.
W. Frank Peacock, Department of Emergency Medicine, Baylor College of Medicine, Houston, TX, USA.
Burkert M. Pieske, Division of Cardiology, Medical University of Graz, Graz, Austria
Hani N. Sabbah, Department of Medicine, Henry Ford Hospital, Detroit, MI, USA
Naoki Sato, Department of Internal Medicine and Cardiology, Nippon Medical School Musashi-Kosugi Hospital, Kanagawa, Japan.
Monica R. Shah, National Heart Lung and Blood Institute, Bethesda, MD, USA
Norman L. Stockbridge, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD, USA
John R. Teerlink, Section of Cardiology, San Francisco Veterans Affairs Medical Center, University of California, San Francisco, CA, USA
Dirk J. van Veldhuisen, Department of Cardiology, University of Groningen, Groningen, The Netherlands
Andrew Zalewski, Novartis Pharmaceutical Corporation, East Hanover, NJ, USA.
Faiez Zannad, Inserm CIC 9501 and U961, Universite´ de Lorraine, CHU Cardiology, Nancy, France.
Javed Butler, Division of Cardiology, Emory University School of Medicine, Atlanta, GA, USA.
References
- 1.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orland C Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. New England J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. New England J Med. 2010;363(15):1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 4.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, Masip J, Frank Peacock W, Sliwa K, Gayat E, Filippatos G, Cleland JG, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31(7):832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 5.Allen LA, Hernandez AF, O’Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol. 2009;53(24):2248–2258. doi: 10.1016/j.jacc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Pang PS, O’Connor CM, Prasad K, McMurray J, Teerlink JR, Fiuzat M, Sabbah H, Komajda M. Clinical development of pharmacologic agents for acute heart failure syndromes: a proposal for a mechanistic translational phase. Am Heart J. 2011;161(2):224–232. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Pang PS, Adams KF, Cleland JG, Cotter G, Dickstein K, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O’Connell J, O’Connor CM, Pina IL, Ponikowski P, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Gheorghiade M. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circ Heart Fail. 2010;3(2):314–325. doi: 10.1161/CIRCHEARTFAILURE.109.893222. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Adams KF, Cleland JG, Cotter G, Felker GM, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O’Connell J, O’Connor CM, Pang PS, Pina IL, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Stockbridge N. Phase III clinical trial end points in acute heart failure syndromes: a virtual roundtable with the Acute Heart Failure Syndromes International Working Group. Am Heart J. 2009;157(6):957–970. doi: 10.1016/j.ahj.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112(25):3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA, J Am Med Assoc. 2006;296(18):2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, Committee ASA, Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of C (2003) The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 24(5):442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Adams KF, Cleland JG, Cotter G, Felker GM, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O’Connell J, O’Connor CM, Pang PS, Pina IL, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Stockbridge N Acute Heart Failure Syndromes International Working G. Phase III clinical trial end points in acute heart failure syndromes: a virtual roundtable with the Acute Heart Failure Syndromes International Working Group. Am Heart J. 2009;157(6):957–970. doi: 10.1016/j.ahj.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A, Investigators A, study c. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) Eur J Heart Fail. 2011;13(1):100–106. doi: 10.1093/eurjhf/hfq209. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Braunwald E. Hospitalizations for heart failure in the United States-a sign of hope. JAMA. 2011;306(15):1705–1706. doi: 10.1001/jama.2011.1510. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Pang PS, O’Connor CM, Prasad K, McMurray J, Teerlink JR, Fiuzat M, Sabbah H, Komajda M. Clinical development of pharmacologic agents for acute heart failure syndromes: a proposal for a mechanistic translational phase. Am Heart J. 2011;161(2):224–232. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. New England J Med. 2012;366(1):9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 21.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 22.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA. Ethical and scientific implications of the globalization of clinical research. New England J Med. 2009;360(8):816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 23.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52(20):1640–1648. doi: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Finn R. Oncologist’s role critical to clinical trial enrollment. J Natl Cancer Inst. 2000;92(20):1632–1634. doi: 10.1093/jnci/92.20.1632. [DOI] [PubMed] [Google Scholar]
- 25.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonarow GC. Improving quality of care and outcomes for heart failure: role of registries. Circ J. 2011;75(8):1783–1790. doi: 10.1253/circj.cj-11-0582. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167(14):1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 28.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. New England J Med. 1995;333(25):1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 29.Sato N, Kajimoto K, Asai K, Mizuno M, Minami Y, Nagashima M, Murai K, Muanakata R, Yumino D, Meguro T, Kawana M, Nejima J, Satoh T, Mizuno K, Tanaka K, Kasanuki H, Takano T. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 2010;159(6):949–955. doi: 10.1016/j.ahj.2010.03.019. e941. [DOI] [PubMed] [Google Scholar]
- 30.Minami Y, Kajimoto K, Sato N, Yumino D, Mizuno M, Aokage T, Murai K, Munakata R, Asai K, Sakata Y, Keida T, Hagiwara N, Mizuno K, Kasanuki H, Takano T. Admission time, variability in clinical characteristics, and in-hospital outcomes in acute heart failure syndromes: findings from the ATTEND registry. Int J Cardiol. 2011;153(1):102–105. doi: 10.1016/j.ijcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi M, Kohsaka S, Miyata H, Yoshikawa T, Takagi A, Harada K, Miyamoto T, Sakai T, Nagao K, Sato N, Takayama M. Association between prehospital time interval and short-term outcome in acute heart failure patients. J Cardiac Fail. 2011;17(9):742–747. doi: 10.1016/j.cardfail.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Massie BM. Globalization of clinical trials how should we interpret differences in outcomes? J Am Coll Cardiol. 2011;58(9):923–924. doi: 10.1016/j.jacc.2011.04.027. [DOI] [PubMed] [Google Scholar]