Abstract
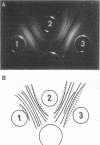
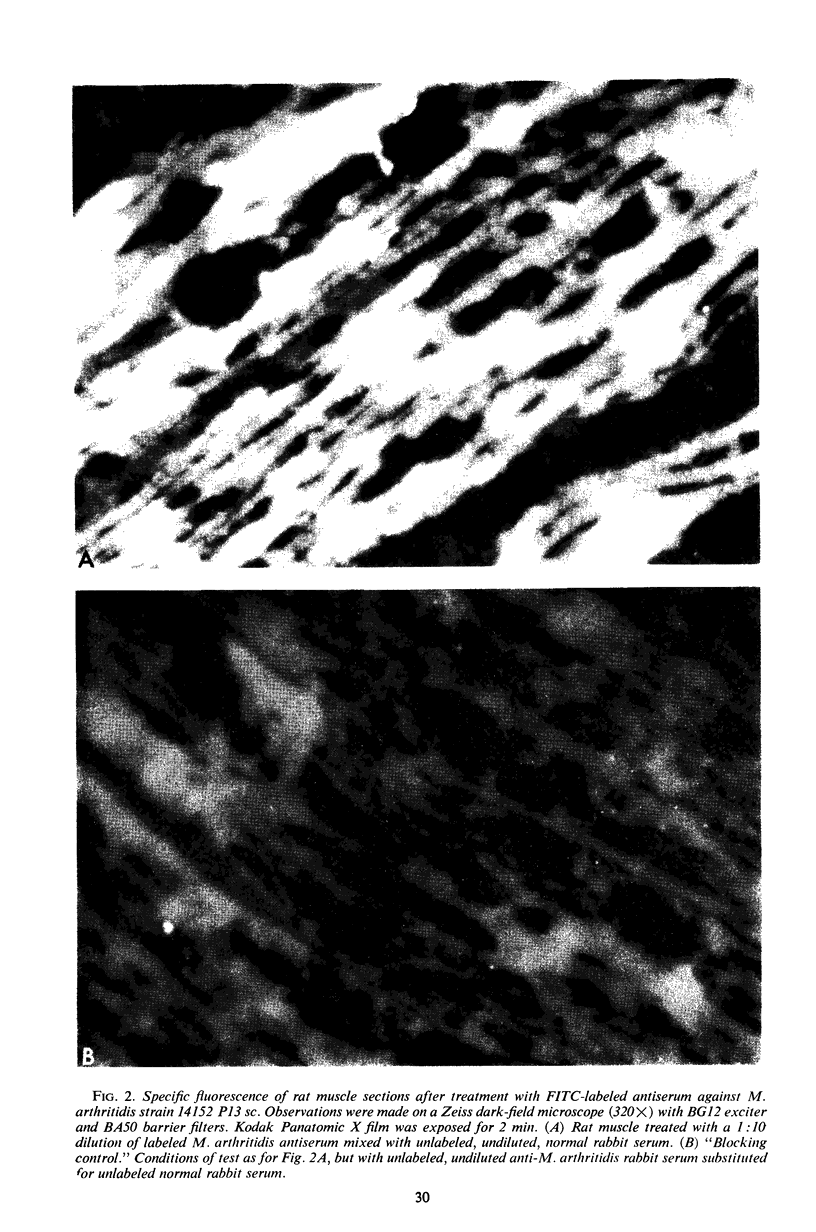
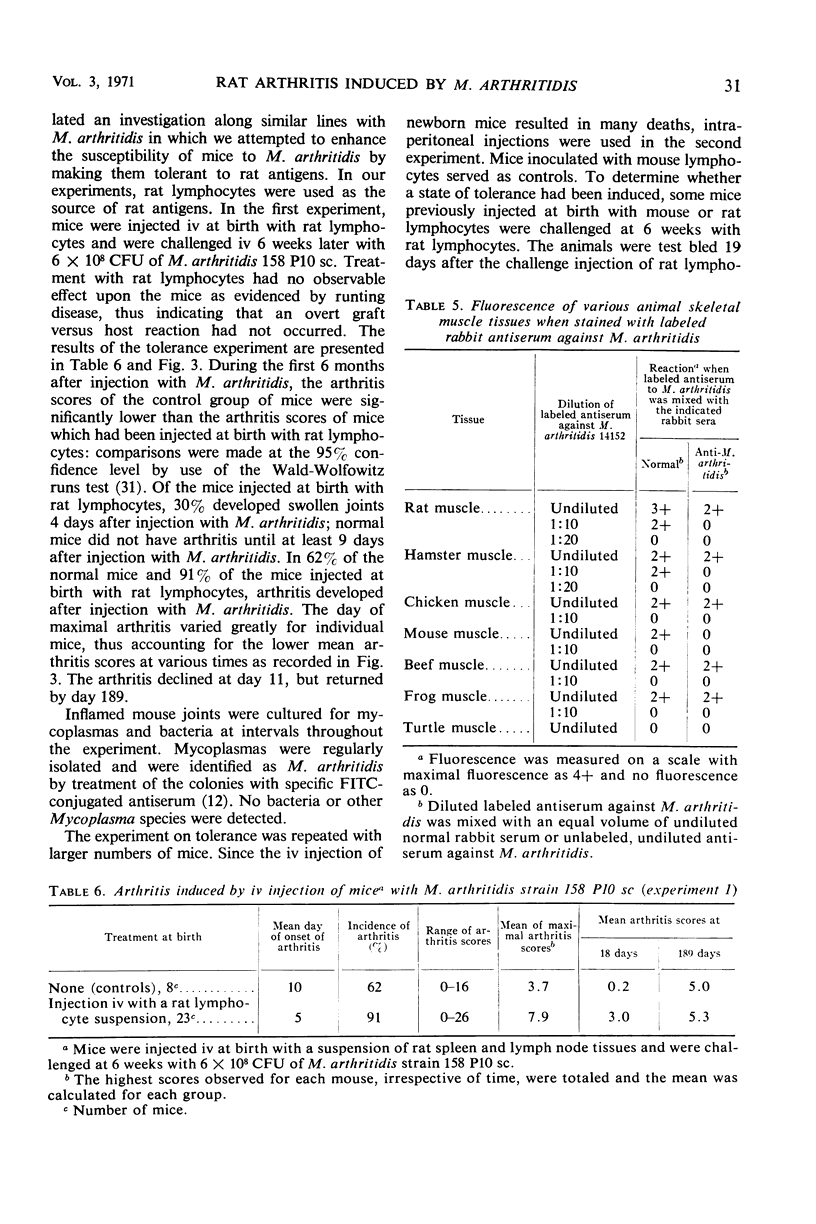
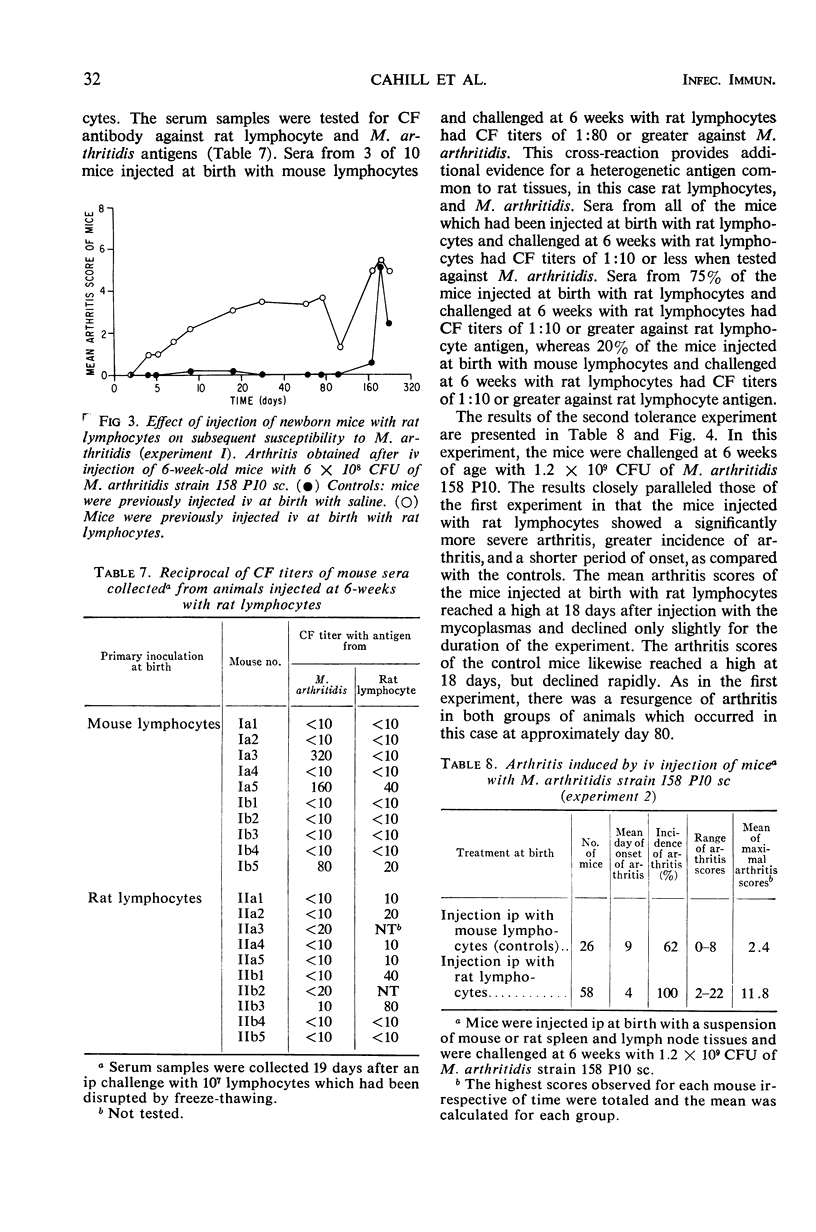
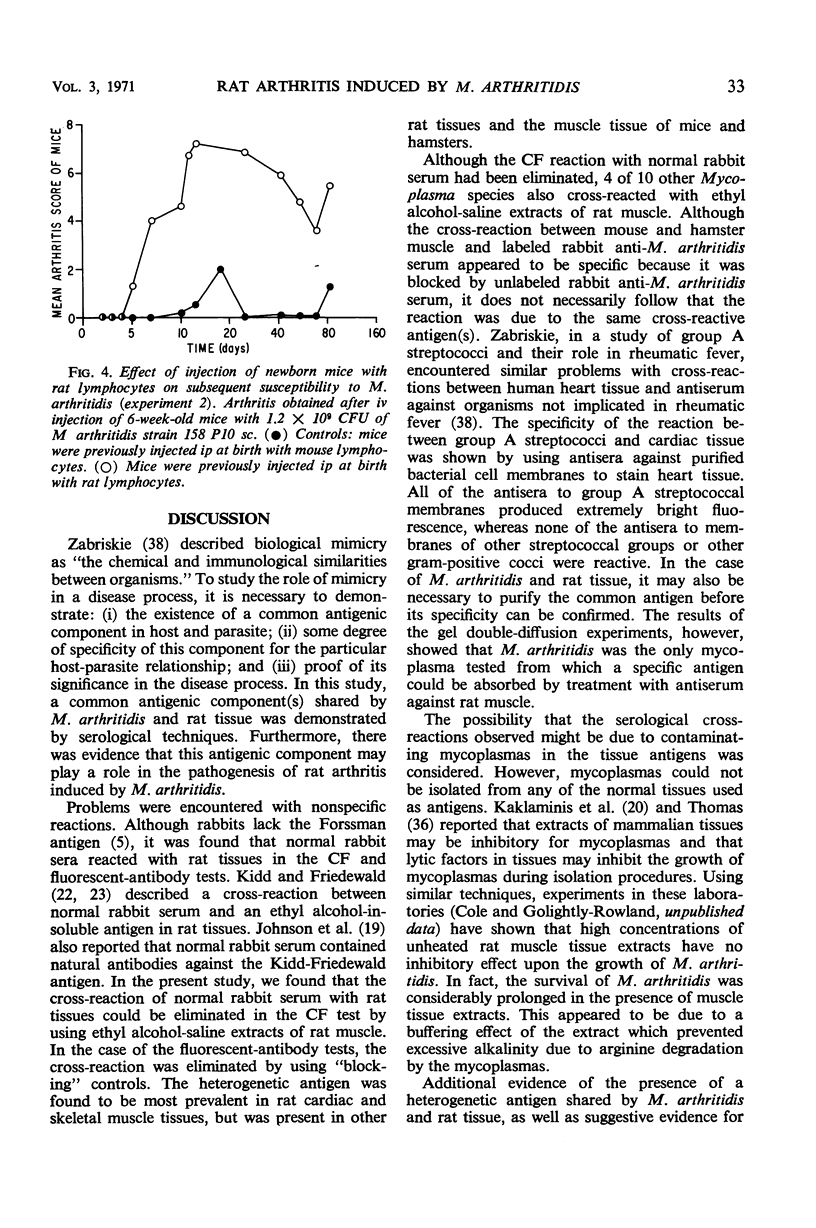
Complement fixation (CF), immunofluorescence, and agar gel double-diffusion tests were used to demonstrate an antigenic relationship between rat tissues and Mycoplasma arthritidis. Rabbit antisera against six strains of M. arthritidis exhibited positive reactions in the CF test with an ethyl alcohol-saline extract of rat muscle, whereas only 6 of 18 antisera against other Mycoplasma species were positive. With the use of gel diffusion techniques, absorption of various M. arthritidis antigens with antiserum against rat muscle removed at least one precipitin band when the absorbed mycoplasma antigens were reacted against homologous antisera. Rabbit antiserum against M. arthritidis was conjugated with fluorescein isothiocyanate and reacted against frozen sections of muscle tissues of various animals. As controls, unlabeled normal rabbit serum and rabbit anti-M. arthritidis serum were included to determine the specificity of the reaction. Rat, hamster, and mouse skeletal muscle exhibited specific fluorescence, whereas chicken, beef, frog, and turtle muscles exhibited no specific fluorescence. Mice injected at birth with rat lymphocytes were found to be more susceptible to subsequent infection by M. arthritidis than were normal mice or mice injected at birth with mouse lymphocytes. These results indicate the occurrence of a heterogenetic antigen(s) common to M. arthritidis and rat tissues. Preliminary evidence suggests that this heterogenetic antigen(s) may enable the mycoplasmas to become established in their host.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld G. Mycoplasma pneumoniae infections and antibodies to brain. Lancet. 1969 Nov 29;2(7631):1202–1202. doi: 10.1016/s0140-6736(69)92530-6. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Cahill J. F., Wiley B. B., Ward J. R. Immunological responses of the rat to Mycoplasma arthritidis. J Bacteriol. 1969 Jun;98(3):930–937. doi: 10.1128/jb.98.3.930-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly L., Ward J. R. Characterization of mycoplasma strains from cats. J Bacteriol. 1967 Nov;94(5):1451–1458. doi: 10.1128/jb.94.5.1451-1458.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Miller M. L., Ward J. R. A comparative study on the virulence of Mycoplasma arthritidis and "Mycoplasma hominis, type 2" strains in rats. Proc Soc Exp Biol Med. 1967 Jan;124(1):103–107. doi: 10.3181/00379727-124-31676. [DOI] [PubMed] [Google Scholar]
- Del Giudice R. A., Robillard N. F., Carski T. R. Immunofluorescence identification of Mycoplasma on agar by use of incident illumination. J Bacteriol. 1967 Apr;93(4):1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N., Shifrine M. Antigenic cross-reactions between the galactan from Mycoplasma mycoides and polysaccharides from other sources. J Comp Pathol. 1966 Oct;76(4):417–425. doi: 10.1016/0021-9975(66)90063-6. [DOI] [PubMed] [Google Scholar]
- HARRIS R. J., SIMONSEN M. Induced susceptibility of turkeys to the Rous virus after treatment in the embryonic stage with normal chicken blood. Acta Pathol Microbiol Scand Suppl. 1956;39(Suppl 111):53–54. doi: 10.1111/j.1600-0463.1956.tb06728.x. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- JENKIN C. R. An antigenic basis for virulence in strains of Salmonella typhimurium. J Exp Med. 1962 Apr 1;115:731–743. doi: 10.1084/jem.115.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON G. D., ASHERSON G. L., KAKLAMANIS E., DUMONDE D. C. DEMONSTRATION BY IMMUNOFLUORESCENCE OF AUTO-ANTIBODY IN THE SERUM OF RABBITS GIVEN INJECTIONS OF RAT TISSUE. J Pathol Bacteriol. 1963 Oct;86:521–525. doi: 10.1002/path.1700860226. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H., SVEC K. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. III. PRESENCE IN HUMAN SERA OF STREPTOCOCCAL ANTIBODY CROSS-REACTIVE WITH HEART TISSUE. ASSOCIATION WITH STREPTOCOCCAL INFECTION, RHEUMATIC FEVER, AND GLOMERULONEPHRITIS. J Exp Med. 1964 Apr 1;119:651–666. doi: 10.1084/jem.119.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaklamanis E., Thomas L., Stavropoulos K., Borman I., Boshwitz C. Mycoplasmacidal action of normal tissue extracts. Nature. 1969 Mar 1;221(5183):860–862. doi: 10.1038/221860b0. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ A. S., ARMSTRONG S. H., Jr, KUSHNER D. S. Immunological relationships between the rat glomerulus and nephritogenic streptococci. Nature. 1960 Sep 24;187:1095–1097. doi: 10.1038/1871095a0. [DOI] [PubMed] [Google Scholar]
- Morton H. E., Roberts R. J. Production of anti-Mycoplasma (PPLO) antibodies in rabbits. Proc Soc Exp Biol Med. 1967 Jun;125(2):538–543. doi: 10.3181/00379727-125-32140. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Hammarström S., Lagercrantz R., Gustafsson B. E. Antigen from colon of germfree rats and antibodies in human ulcerative colitis. Ann N Y Acad Sci. 1965 Jun 30;124(1):377–394. doi: 10.1111/j.1749-6632.1965.tb18972.x. [DOI] [PubMed] [Google Scholar]
- Peterson O. L., Ham T. H., Finland M. COLD AGGLUTININS (AUTOHEMAGGLUTININS) IN PRIMARY ATYPICAL PNEUMONIAS. Science. 1943 Feb 12;97(2511):167–167. doi: 10.1126/science.97.2511.167. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., JENKIN C. R. Antigenic cross-reaction between host and parasite as a possible cause of pathogenicity. Nature. 1962 Jan 13;193:151–154. doi: 10.1038/193151a0. [DOI] [PubMed] [Google Scholar]
- SIMONSEN M. Artificial production of immunological tolerance; induced tolerance to heterologous cells and induced susceptibility to virus. Nature. 1955 Apr 30;175(4461):763–764. doi: 10.1038/175763a0. [DOI] [PubMed] [Google Scholar]
- Shifrine M., Gourlay R. N. Serological relationship between galactans from normal bovine lung and from Mycoplasma mycoides. Nature. 1965 Oct 30;208(5009):498–499. doi: 10.1038/208498a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., SOMERSON N. L., TURNER H. C., CHANOCK R. M. SEROLOGICAL RELATIONSHIPS AMONG HUMAN MYCOPLASMAS AS SHOWN BY COMPLEMENT-FIXATION AND GEL DIFFUSION. J Bacteriol. 1963 Jun;85:1261–1273. doi: 10.1128/jb.85.6.1261-1273.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Berry D. M. The evaluation of the metabolic-inhibition technique for the study of Mycoplasma gallisepticum. J Gen Microbiol. 1969 Jan;55(1):127–137. doi: 10.1099/00221287-55-1-127. [DOI] [PubMed] [Google Scholar]
- Thomas L. Mechanisms of pathogenesis in Mycoplasma infection. Harvey Lect. 1969;63:73–98. [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. Mimetic relationships between group A streptococci and mammalian tissues. Adv Immunol. 1967;7:147–188. doi: 10.1016/s0065-2776(08)60128-5. [DOI] [PubMed] [Google Scholar]