Abstract
Influenza viruses initiate infection by attaching to sialic acid receptors on the surface of host cells. It has been recognized for some time that avian influenza viruses usually bind to terminal sialic acid that is linked in the α2-3 configuration to the next sugar while human viruses show preference for α2-6 linked sialic acid. With developments in synthetic chemistry and chemo-enzymatic methods of synthesizing quite complex glycans, it has become clear that the binding specificity extends beyond the sialic acid, and this has led to considerable interest in developing glycan reagents that could be used either as a diagnostic tool for particular influenza viruses, or to identify cells that are susceptible to infection by certain influenza viruses. Here we describe the use of the Consortium for Functional Glycomics Glycan Array to investigate binding specificity of influenza hemagglutinin and cleavage by neuraminidase, using seasonal and pandemic H1N1 influenza viruses as examples, and compare the results with published data using other array methods.
Keywords: Influenza virus, hemagglutinin, neuraminidase, Glycan Array, Consortium for Functional Glycomics
1. Introduction
Influenza viruses initiate infection by attaching the viral hemagglutinin to sialic acid receptors on the surface of host cells. It has been recognized for some time that avian influenza viruses usually bind to terminal sialic acid that is linked in the α2-3 configuration to the next sugar while human viruses show preference for α2-6 linked sialic acid [9], but further studies on contribution of downstream sugars to binding have been restricted to available reagents; until recently these were small oligosaccharides such as sialyllactose, sialyl-Lewis antigens, gangliosides [28], and sialylated milk oligosaccharides such as LSTa and LSTc [10]. The types of experiments that could be done were restricted by the low affinity (mM) of an HA subunit for a single sialylated species because the interaction did not survive washing steps. This was overcome by coupling sialylated glycans to multivalent supports such as polyacrylamide [11] but the reagent pool remained quite small. The establishment of the Consortium for Functional Glycomics provided impetus and resources to develop new chemo-enzymatic methods to synthesize quite complex glycans. The ensuing rapid expansion of available reagents was exploited to make a Glycan Array [3] that was capable of rapid screening of binding specificity of viruses as well as expressed hemagglutinin. It has become clear that the binding specificity extends beyond the sialic acid, and is variable from virus to virus. This has led to considerable interest in developing glycan reagents that could be used either as a diagnostic tool for particular influenza viruses, or to identify cells that are susceptible to infection by certain influenza viruses. Here we describe the use of the Consortium for Functional Glycomics Glycan Array to investigate binding specificity of influenza hemagglutinin and cleavage by neuraminidase, using seasonal and pandemic H1N1 influenza viruses as examples.
2. Materials and Methods
2.1 Viruses
Seasonal H1N1 strains A/Oklahoma/447/2008 and A/Oklahoma/1138/2009, and pandemic H1N1 (pdmH1N1) A/Oklahoma/3052/2009, were patient isolates from The Children’s Hospital of Oklahoma. The viruses were isolated in primary rhesus monkey cells and then grown in Madin-Darby canine kidney (MDCK) cells. A/OK/3052/09 was grown in embryonated chicken eggs for some experiments. Vaccine strains A/Brisbane/59/07 (H1N1) and A/Uruguay/716/2007 X-175 (H3N2) were obtained from CDC and grown in chicken eggs. Cell debris was centrifuged down at low speed then viruses in the clarified supernatant were sedimented, resuspended, and purified by centrifugation on a 10–40% sucrose gradient [12; 20]. Virus was assayed by hemagglutination titer using turkey red blood cells and expressed as hemagglutinating units (HAU) or log2 HAU.
2.2 Glycan Array
To determine binding specificity we used the Consortium for Functional Glycomics Glycan Array, versions 3.1 to 4.2 as indicated in the Figure legends. These contained from 377 to 511 synthetic glycans, made by combinations of chemical and enzymatic methods [3]. The glycans were attached to an amine-active linker and covalently printed on to N-hydroxysuccinimide-activated glass slides. A variety of linkers have been used to distance the glycan from the slide matrix. The lists of glycans and their linkers on the different versions of the array can be found at: http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh.shtml. Alexa488-labeled viruses were incubated on the array slide in TSM binding buffer (20 mM tris-HCl pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2,1% BSA and 0.05% Tween 20) at 4°C for 1 hour and the slide washed and read [13].
2.3 Labeling of viruses and binding to array
Purified viruses were labeled with Alexa448 succinimidyl ester (Alexa488-SE) as described previously [12; 13].
2.4 Neuraminidase activity
Neuraminidase enzyme assays used the fluorescent substrate 4-methylumbelliferyl-N-acetylneuraminic acid (MUN) by the method of Potier [23] scaled down to a 96-well plate format. For assays with other substrates, we used a 96-well plate version of the Warren thiobarb assay that measures released sialic acid [29].
To evaluate the substrate range of neuraminidase, we used digestion of the Glycan Array. Alexa488 labeled virus was incubated on the Array for 1–4 hours at 37° in the TSM binding buffer to allow the neuraminidase to cleave sialic acids, then cooled to 4° for 1 hour to allow re-binding before washing and reading. In other experiments, Alexa488 virus was diluted in 100 mM acetate buffer pH 5.5 with addition of 2 mM CaCl2, 2 mM MgCl2, 1% BSA and 0.05% Tween 20 then incubated with the glycan array for 1 to 4 h at 37°C to allow neuraminidase to cleave under optimal conditions, the slide was washed, dried and scanned, then fresh Alexa-labeled virus added for 1 hour at 4°C in TSM binding buffer pH 7, washed and the binding read.
2.5 Mass Spectrometry
The proteins of Alexa-488-labeled viruses were separated by gel electrophoresis and the HA1 band excised, treated with iodoacetic acid, digested with trypsin or chymotrypsin, and the peptides analyzed by mass spectrometry. Products were searched for masses that corresponded to lysines modified by Alexa488 or not modified.
3. Results and Discussion
The CFG Glycan Array [3] is based on an NHS-activated glass slide to which glycans are covalently attached via a spacer moiety to separate the glycan from the glass surface. The various versions of this array have been used in many studies of binding specificity of influenza viruses, for example [4; 6; 12; 16; 22; 26]. The experimental approaches range from purified recombinant HA (cross-linked with an anti-His tag monoclonal antibody and then by anti-Fc antibody to give a multimer of 4 HA trimers) to applying whole virus to the array either directly labeled or detected by fluorescently-labeled antibody methods. All of our experiments have used purified, whole virus particles directly labeled with Alexa488 attached to lysine residues. We first describe some important control experiments, then show results of array studies on binding of seasonal and pandemic H1N1 viruses and discuss how our results compare to published experiments using other methods. We also describe the use of the array in studies of specificity of viral neuraminidase (NA).
3.1. Does direct labeling of virus affect the binding (HA) or sialidase (NA) activities?
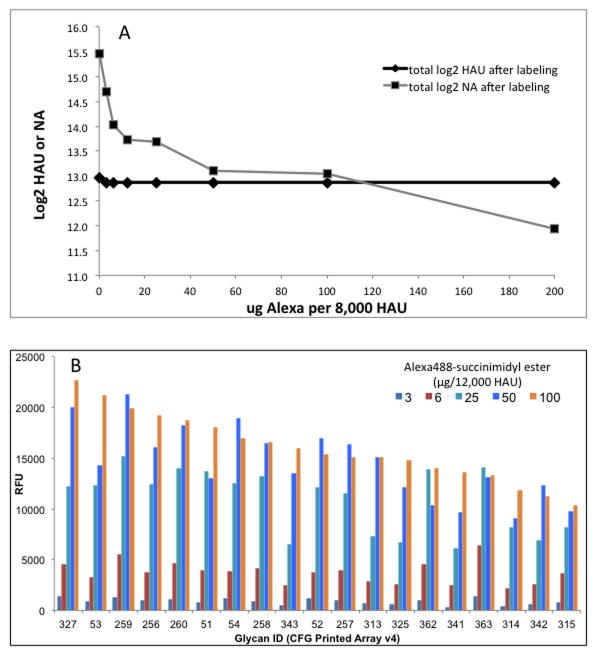
A question often asked is whether direct labeling of virus with the fluorescent tag will affect its binding activity. Figure 1A shows the effect of increasing concentration of Alexa488 succinimidyl ester on the hemagglutination (HA) titer and on NA activity of A/Oklahoma/3052/2009 (pdmH1N1). Up to 200 μg of reagent per 8,000 HAU of virus has no effect on HA activity, but the NA activity decreases with increasing Alexa concentration. Other viruses we have tested showed little decrease in NA activity, but the pdmH1N1 NA activity has been reported to be low compared to other subypes [4; 32], perhaps indicating that this NA is less stable and therefore more affected by Alexa488 modification. Binding to the array increases with increasing Alexa488 up to 25–50 μg/12,000 HAU but does not drop off up to 100 μg (Figure 1B). At 200 μg Alexa488 per 12,000 HAU the results became highly erratic, possibly due to non-covalent binding of the high concentration of dye to the virus particle. The stability of HA binding seems surprising, since we might expect that all lysines on the surface of the HA can be modified and a high level of modification would be expected to interfere with biological activity. However, Laver showed that however high the concentration of modifying reagents, no biological activity was lost, and this was because only a few of the Lys, His or Tyr residues of Memphis/1/71 HA or Tokyo/67 NA were modified [17; 18]. We therefore used mass spectrometry to investigate which lysines in the HA of viruses we are currently studying are reactive with Alexa488. We were not able to quantify the results, but only about 50% of the lysines of three different virus HAs studied were modified to a significant extent by Alexa488, even though high concentrations were used (Table 1). Examination of the crystal structures of HA and NA suggests that lysine ε amino groups that are involved in hydrogen bonding to other sidechains or mainchain carbonyl oxygen atoms are resistant to modification while only those that are free can react with Alexa488. Lysines near the receptor binding site are involved in interactions and are not modified.
Figure 1. Effect of Alexa488 modification on biological activity and binding activity of pandemic H1N1 influenza virus.
A. Varying amounts of Alexa488 succinimidyl ester were reacted with purified virions (8,000 HAU) of A/Oklahoma/3052/2009 (pdmH1N1). Excess reagent was dialyzed away and residual HA and NA activities were measured using turkey red blood cells and MUN substrate respectively.
B. Binding of viruses (5,000 HAU/ml) to the Glycan Array v4.0 after reaction with varying amounts of Alexa488 as indicated. The y axis is fluorescent signal (RFU) of virus bound to the individual glycans on the array. The glycans that bind (ID numbers on the x axis) are sorted from high to low at 100 μg Alexa.
Table 1.
Summary of lysines in Alexa488 succinimidyl ester modified HA1 identified as Alexa-labeled or unlabeled by mass spectrometry of tryptic or chymotryptic peptides1. The numbering is from the N-terminus of HA1.
| A/OK/3052/09 (pdm H1N1) | A/Brisbane/59/07 (H1N1) | A/Uruguay/716/07 X-175 (H3N2) | DNP-labeled A/Memphis/1/71 (H3N2)2 | ||||
|---|---|---|---|---|---|---|---|
| Labeled | Unlabeled | Labeled | Unlabeled | Labeled | Unlabeled | Labeled | Unlabeled |
| 22 | 22?3 | 2 | |||||
| 36 | 40 | 27 | 27 | ||||
| 40 | 45 | 82 | 50 | ||||
| 43 | 73 | 83 | 53 | ||||
| 54 | 82 | 92 | 92 | ||||
| 119 | 119 | 119 | 158 | 158 | 140 | ||
| 130 | 153 | 160 | 156 | ||||
| 142 | 142 | 168 | 173 | 173 | 176 | ||
| 146 | 170 | 176 | 238 | ||||
| 153 | 188 | 207 | 259 | ||||
| 160 | 208 | 238 | 264 | ||||
| 163 | 218 | 259 | 259 | 292 | |||
| 169 | 273 | 264 | 264 | 299 | |||
| 171 | 277 | 276 | 307 | ||||
| 208 | 304 | 292 | 310 | ||||
| 209 | 209 | 310 | 310 | 310 | 315 | ||
| 211 | 315 | 315 | 326 | ||||
| 219 | 219 | 326 | |||||
| 239 | |||||||
| 283 | |||||||
| 302 | |||||||
| 305 | |||||||
| 308 | |||||||
| 311 | 311 | ||||||
C.L. Feasley, J.M. Johnson, C.M. West and G.M. Air, manuscript in preparation.
Data from Laver et al. using FDNB (Sanger’s reagent) [17]
? = peptide not found
Therefore, although it seemed likely that the direct Alexa488 labeling of virus would interfere in its binding activity, we found we can use high levels of reagent with no reduction of binding activity, because even at high Alexa/HAU ratios (170 μg/10,000 HAU), only some lysines are reactive. Nevertheless, for each virus we are studying we always titrate both HA and NA activity before and after labeling and only use samples that show no reduction in these activities. Usually up to 5 μg Alexa per 1000 HAU is satisfactory for both activity and signal strength.
3.2 What is the effect of virus concentration?
With increasing interest in using Glycan Arrays to look at binding specificity, one important aspect is often overlooked. Unlike a gene expression array, where all interactions are equal, protein glycan interactions have a wide range of affinities. So a probe for “specificity” is actually a probe for “affinity”, or indeed avidity since only multivalent interactions are detectable. Binding to the glycan array is not plus or minus, but a range of signals, different for each glycan species. This means that the interpretation of a glycan array experiment is dependent on the amount of virus or HA applied to the array. Figure 2 shows that glycans 362, 363 and 260 have the top binding signals at 20,000 HAU/ml but have relatively low affinity and their signals drop below glycans 52, 54 and 259 at 10,000 HAU/ml. So if laboratory A runs the experiment at 20,000 HAU/ml they will report the highest binding is to relatively short sialylated glycans (362, Neu5Acα2-6GlcNAcβ1-4GlcNAc-Sp21; 363, Neu5Acα2-6GlcNAcβ1-4GlcNAcβ1-4GlcNAcSp21; 260, Neu5Acα2-6Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcb-Sp0), but laboratory B running an experiment at 10,000 HAU/ml will report that the highest binding is to long or sulfated glycans: (52, 54, Neu5Acα2-6Galβ1-4GlcNAcβ1-2Manα1-3(Neu5Acα2-6Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAc with different linkers; and 259, Neu5Acα2-6Galβ1-4GlcNAcβ1-3Galβ1-4(Fuca1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0).
Figure 2.
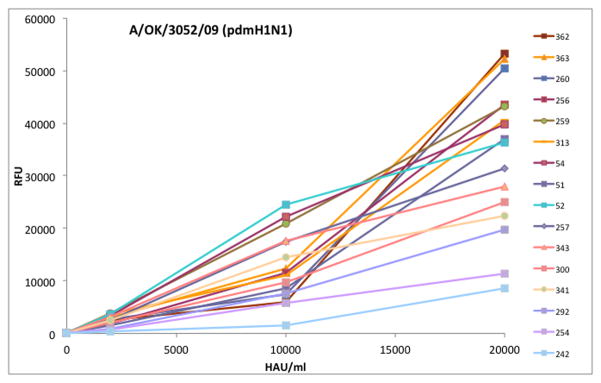
Dose response of influenza virus A/Oklahoma/3052/2009 (pdmH1N1) discriminates high and low affinity binding to the CFG Glycan Array v4.0. The x axis is the concentration of virus applied to the array slide (HAU/ml) and the y axis is the fluorescent signal. Due to different affinities, the order of binding at 20,000 HAU/ml is somewhat different to that at 10,000 HAU/ml, emphasizing the need to screen multiple concentrations of virus. For clarity, some binding glycans are not shown here and neither are the majority of glycans on the array that remain at baseline at all concentrations.
It is clear from this example that all glycan array experiments should be run at multiple dilutions of HA or virus, and that the concentrations used should be well below saturation of the array. The experiment in Figure 2 was actually done on a single slide, starting with a very dilute sample, washing and reading, then binding the next concentration. The curves are not true binding curves since they are not done at equilibrium because bound virus does not wash off after the slide has been dried for reading, but they do discriminate high affinity from low affinity binding without using multiple array slides.
3.3 How to interpret the binding data at multiple concentrations?
The example above shows the need to assess binding at multiple concentrations, but raises the problem of how to interpret and report the data. It is not possible to extract meaningful Kds from Glycan array data, but Heimburg-Molinaro et al [13] described a method to take into account the differing affinities. At each concentration the glycans are ranked from highest to lowest, then for each glycan an average rank is computed from the three concentrations. A program to do this (“GBP Cross Analysis”) is now available as part of the GlycoPattern suite (http://glycopattern.emory.edu) along with a motif analysis module [8].
3.4 Results for binding of H1N1 (seasonal and pandemic) influenza viruses to the CFG Glycan Array
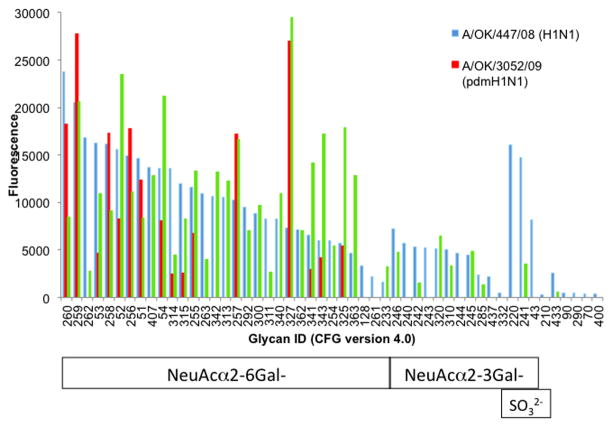
To illustrate the application of the Glycan Array to understanding the receptor specificities of influenza viruses, Figure 3 shows averaged and normalized data for seasonal and pandemic (swine-origin) H1N1 influenza strains obtained with Alexa488 labeled whole virus particles and screened on the CFG Glycan Array v4.0. The raw data for one concentration has been shown previously [13]. The seasonal H1N1 strain A/Oklahoma/447/2008 binds a wide array of α2-6 sialylated glycans with varying affinities and some α2-3 glycans show lower but significant binding. The pdmH1N1 strain shows comparatively narrow specificity, but this is broadened after passage in chicken eggs when some weak binding to α2-3 sialylated glycans appears (Figure 3).
Figure 3.
A seasonal H1N1 virus shows broader binding specificity than pandemic H1N1 virus to the CFG Glycan Array. Results for two concentrations were normalized to 10,000 HAU/ml, averaged, and sorted from high to low binding of the seasonal strain A/Oklahoma/447/2008 to α2-6 linked sialic acid, then α2-3 sialylated and sulfated glycans. The pandemic H1N1 virus A/Oklahoma/3052/2009 passaged only in MDCK cells shows rather restricted binding specificity to a subset of α2-6 sialylated glycans, but after egg passage several additional glycans are seen to bind.
3.4.1
Comparison with other labeling methods and other array platforms: When these results are compared to those of other pdmH1N1 viruses in the literature, there is good agreement (Table 2). CDC scientists use an array that is a subset of the same glycans on the same platform as the CFG array, and they used baculovirus-expressed recombinant HA trimers cross-linked with Alexa488-labeled mouse anti-His5 antibody then with Alexa488-labeled anti-mouse antibody to form oligomers of 4 HA trimers [33]. Four pandemic H1N1 viruses gave rather similar results, and it is notable that the recombinant HA of Texas/5/2009 displayed the same binding profile as our Oklahoma/3052/09 whole virus directly labeled with Alexa488 (Table 2). Our results in Figure 3 show binding by a few additional glycans but these are not on the CDC array, so the results are in excellent accord. Another study using the CFG Glycan Array with directly-labeled whole virus as ligand shows the same results; restricted binding by the pandemic (swine-origin) H1N1 human viruses but much broader specificity of seasonal H1N1 viruses [5]. An independent glycan array containing 17 α2-3 sialosides and 10 α2-6 sialosides but using the same NHS-activated glass slides as the CFG array showed restricted binding by recombinant pandemic H1 HA, and broader binding by seasonal recombinant HA [19]. When whole virus particles of vaccine strains were used with antibody detection, the results were much broader and most sialylated glycans on the array bound, both α2-3 and α2-6 [19]. This broader binding may be a consequence of growth of the vaccine strains in chicken eggs, and possibly the polyclonal antisera used for detection are influencing the results. The primary antibody used in the recombinant HA experiments is monoclonal anti-His tag [27].
Table 2.
Comparison of results in Figure 3 with other studies of binding of pandemic H1N1 viruses.
| Material Array platform | Virus CFG (Fig 3) | HA CDC [33] | Virus CFG [5] | HA NHS-glass [19] | Virus Glycolipid [7] | ||
|---|---|---|---|---|---|---|---|
| Glycan | Rank (binding high to low) | ||||||
| Structure | CFG v4 ID | CDC ID | OK/3052/09 | Tx/5/09 | Tx/15/09 | Calif/07/09 | Calif/04/09 |
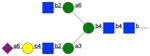
|
|
259 | 57 | 1 | 1 | 2 | np1 | np |
|
|
327 | - | 1 | np | 1 | 1 | np |
|
|
260 | 56 | 3 | 1 | 3 | 2 | 1 (with Glc at posn 5) |
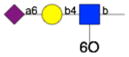
|
256 | 41 | 4 | 4 | 5 | 3 | np |
|
|
257, 258 | 53, 54 | 6 5 |
5, 3 | 4, 6 | 3 | 2 |
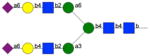
|
51, 52, 54, 53 | -. 47, 46, 48 | 7, 8, 9, 12 | 6, 7, 8 | 7 8 |
np | 4 |
|
|
255 | 55 | 10 | 9 | nb2 | np | np |
|
|
325 | - | 11 | np | 18 | np | np |
|
|
343 | - | 13 | np | nb | np | np |
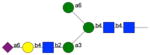
|
341 | - | 14 | np | nb | np | np |
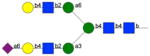
|
314 | 49 | 15 | nb | nb | np | np |
|
315 | 45 | 16 | nb | 11 | np | np |
np = not present on the array
nb = insignificant binding
Using a chemically different, neoglycolipid based array platform, Childs et al found binding of pandemic H1N1 viruses to most α2-6 glycans but some α2-3 sialosides bound at the higher concentrations of virus [7]. Almost all the glycans on this array are different to the CFG array, so the effect of the different array platform cannot be broadly assessed. The few glycans in common with the CFG array bind similarly (Table 2). Overall there is good agreement between all these reports - recombinant HA, whole virus, CFG or glycolipid platform - on the glycans that bind strongly to H1N1. The apparent disagreements arise from (i) lack of consideration of concentration effects and (ii) how much emphasis is placed on weak binding. These results emphasize again the importance of running glycan array screens at multiple concentrations. The array platform and whether recombinant HA or directly labeled virus are used do not seem important, but when antibodies used to detect bound, unpurified virus they need to be carefully screened.
3.5. Specificity of cleavage by influenza neuraminidase (NA)
Another potential use of the glycan array is to determine the substrate specificity of sialidases. There is a considerable literature pertaining to the need for “balance” between HA and NA activities of influenza viruses, the idea being that if the receptor specificity changes with a change in host, then the NA specificity must follow. There are many examples of adjustments to activity levels [14; 21; 24; 30; 32; 34] but the specificity shows only modest changes [2; 15] and the NA always has higher activity on α2-3 than α2-6 sialylated glycans as summarized in Supplementary Table 1 of a recent review [1]. A more recent report [32] shows less activity with MUN substrate for recombinant NA of pdmH1N1 strain A/California/4/2009 (Kcat 3 sec−1, Km 373 μM) than for older strains Japan/57 and Hong Kong/68 (Kcat 29 and 18 sec−1, Km 79 and 20 μM, respectively). Whole virus assays also showed low activity of pdmH1N1 compared to a seasonal H1N1 strain at the same pfu [5]. We find Km of 30, 50 and 60 μM for minimally MDCK-passaged A/OK/3052/09 (pdmH1N1), A/OK/1138/09 (seasonal H1N1) and A/OK/5342/10 (H3N2), respectively.
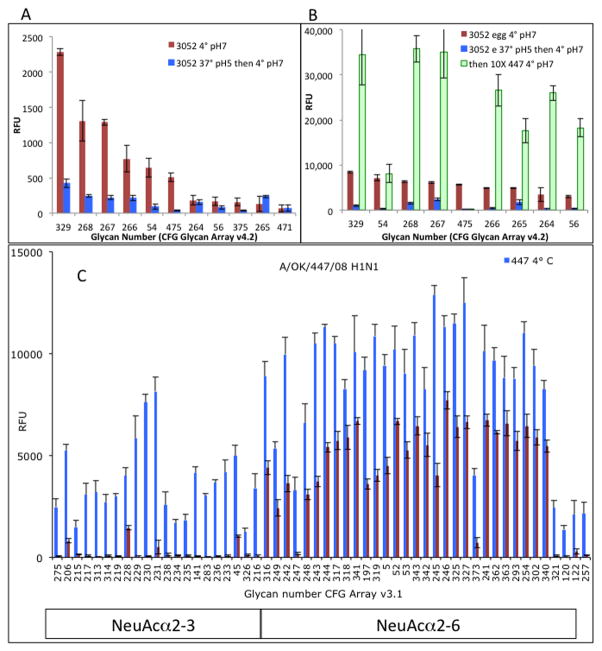
We have used the glycan array as a probe of NA specificity by demonstrating loss of binding activity after treatment with active NA. Array cleavage experiments gave good results for parainfluenza viruses [29], with hPIV1 cleaving almost all α2-3 sialylated glycans on the array but not cleaving any α2-6 sialic acids as measured by binding of a seasonal H1N1 influenza virus after hPIV NA treatment. hPIV2 and 3 cleaved the same α2-3 sialylated glycans and also some α2-6 sialic acid linkages. The NA activity of pdmH1N1 influenza virus reduced binding when the same levels of virus used for NA digestion were used as a probe of residual binding (Figures 4A, 4B) but there was no significant loss of binding when a tenfold higher concentration of a seasonal H1N1 probe virus was used (Figure 4B), indicating incomplete cleavage.. Figure 4C shows that an H3N2 viral NA efficiently cleaves substrates with α2-3 linked sialic acid but has less activity on the α2-6 linked sialic acid, with about 50% of the sialic acid remaining when probed with seasonal H1N1 virus. We did not identify any effect of the downstream sugars; two bi-sialylated biantennary glycans (475, 54) appear to be cleaved more efficiently that linear structures, but the similar structure in glycan 56 is not (Figure 4B). We conclude that influenza viruses cleave the sialic acid from glycans attached to the CFG array, but less efficiently than parainfluenza viruses although the NA activity on soluble substrates is higher for influenza than parainfluenza viruses [1; 29].
Figure 4. Influenza virus NA cleaves sialic acid from glycans on the array, but not completely.
A. Alexa-labeled pdmH1N1 virus grown in MDCK cells was incubated on the array v4.2 at 37°C, pH 5, for 4 hours. The slide was washed and scanned; only background was seen. The slide was then incubated with the same amount of virus at 4°C, pH 7 for 1 hour and the results compared to binding at 4°C without pre-treatment.
B. The same experiment using egg-grown virus, but with a second binding step using a 10-fold higher amount of seasonal H1N1 A/Oklahoma/447/2008. It is now seen that the sialic acid has been only partially removed by the viral NA.
C. Removal of sialic acid from the array by the N2 NA of A/Oklahoma/1123/2008 (H3N2) at 37°C pH 5, 1 hour, probed with seasonal H1N1 strain A/Oklahoma/447/2008. Glycans were divided into α2-3 and α2-6 sialylated, then sorted alphabetically so that similar structures group together. Cleavage of the α2-3 linkage is more efficient than α2-6, in accord with published results from solution assays [1].
To assess the specificity of NA substrate, Xu et al. used the lectin from Erythrina cristagalli (ECL) to probe for newly-exposed Galβ1-4GlcNAc residues on a custom glycan array, although not all desialylated moieties are bound by the lectin. Good signals were obtained from older viruses but only low activity was seen with pdmH1N1 [31].
5. Conclusions
Glycan Array technology has the potential to give very detailed analysis of ligand specificities of influenza viruses. It does not give information on cellular receptors, but shows what glycans or motifs to look for on the cell surface. In the future we hope that cell-specific arrays will become readily available [25]. In assessing ligand specificities, it is important to run a concentration series, not just a single dose point, to distinguish between high and low affinity ligands. Direct fluorescent labeling of well-purified virus particles or of anti-Fc antibody following cross-linking of recombinant HA trimer with a monoclonal anti-His tag antibody have given identical binding profiles for pdmH1N1 viruses. Reliance on polyclonal antiserum against virus for detection is less reliable because the background tends to be high and is different for every virus tested. The influence of the array substrate is yet to be documented; the glycolipid array of Feizi et al contains only a few glycans in common with the CFG array. However, in studying binding of pandemic H1N1 virus, the two common α2-6 sialylated glycans gave robust binding on both platforms.
The array can also be used to probe NA specificity. The NA of the human parainfluenza viruses hPIV1 and hPIV2 is very efficient at removing sialic acid from the arrayed glycans [29], but the NA of influenza virus is less active on the array although solution assays show higher NA activity than hPIV with small substrates. Two detection methods for de-sialylation of the array glycans have been described; ECL lectin to detect newly-exposed Galβ1-4GlcNAc [31], or a virus (or HA) with a broad binding range to measure residual binding (Fig 4). Both methods show incomplete removal of sialic acid by the pdmH1N1 virus or its recombinant NA.
Acknowledgments
This work was supported in part by grant AI 50933 from the National Institute of Allergy and Infectious Diseases. The glycan array experiments were supported by grant GM62116 from the National Institute of General Medical Sciences to the Consortium for Functional Glycomics. The mass spectrometry was done by the Oklahoma Center for Medical Glycobiology and we thank Christa Feasley and Christopher West for their expertise and time for these analyses. We thank Mary Tappert for the initial characterization of A/OK/3052/09 and for assistance with the NA assays, and CFG Core H staff for their excellent skills in running the glycan arrays.
References
- 1.Air GM. Influenza neuraminidase. Influenza Other Respi Viruses. 2012;6:245–256. doi: 10.1111/j.1750-2659.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum LG, Paulson JC. The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology. 1991;180:10–15. doi: 10.1016/0042-6822(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 3.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley KC, Galloway SE, Lasanajak Y, Song X, Heimburg-Molinaro J, Yu H, Chen X, Talekar GR, Smith DF, Cummings RD, Steinhauer DA. Analysis of influenza virus hemagglutinin receptor binding mutants with limited receptor recognition properties and conditional replication characteristics. J Virol. 2011;85:12387–12398. doi: 10.1128/JVI.05570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley KC, Jones CA, Tompkins SM, Tripp RA, Russell RJ, Gramer MR, Heimburg-Molinaro J, Smith DF, Cummings RD, Steinhauer DA. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (Novel 2009 H1N1) Virology. 2011;413:169–182. doi: 10.1016/j.virol.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Chen LM, Rivailler P, Hossain J, Carney P, Balish A, Perry I, Davis CT, Garten R, Shu B, Xu X, Klimov A, Paulson JC, Cox NJ, Swenson S, Stevens J, Vincent A, Gramer M, Donis RO. Receptor specificity of subtype H1 influenza A viruses isolated from swine and humans in the United States. Virology. 2011;412:401–410. doi: 10.1016/j.virol.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs RA, Palma AS, Wharton S, Matrosovich T, Liu Y, Chai W, Campanero-Rhodes MA, Zhang Y, Eickmann M, Kiso M, Hay A, Matrosovich M, Feizi T. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol. 2009;27:797–799. doi: 10.1038/nbt0909-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholleti S, Agravat S, Morris T, Saltz J, Song X, Cummings R, Smith D. Automated motif discovery from glycan array data. OMICS. 2012;16 doi: 10.1089/omi.2012.0013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 10.Eisen MB, Sabesan S, Skehel JJ, Wiley DC. Binding of the Influenza A Virus to Cell-Surface Receptors: Structures of Five Hemagglutinin-Sialyloligosaccharide Complexes Determined by X-Ray Crystallography. Virology. 1997;232:19–31. doi: 10.1006/viro.1997.8526. [DOI] [PubMed] [Google Scholar]
- 11.Gambaryan AS, Tuzikov AB, Piskarev VE, Yamnikova SS, Lvov DK, Robertson JS, Bovin NV, Matrosovich MN. Specification of Receptor-Binding Phenotypes of Influenza Virus Isolates from Different Hosts Using Synthetic Sialylglycopolymers: Non-Egg-Adapted Human H1 and H3 Influenza A and Influenza B Viruses Share a Common High Binding Affinity for 6′-Sialyl(N-acetyllactosamine) Virology. 1997;232:345–350. doi: 10.1006/viro.1997.8572. [DOI] [PubMed] [Google Scholar]
- 12.Gulati S, Smith DF, Air GM. Deletions of neuraminidase and resistance to oseltamivir may be a consequence of restricted receptor specificity in recent H3N2 influenza viruses. Virol J. 2009;6:22. doi: 10.1186/1743-422X-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimburg-Molinaro J, Tappert M, Song X, Lasanajak Y, Air G, Smith DF, Cummings RD. Probing virus-glycan interactions using glycan microarrays. Methods Mol Biol. 2012;808:251–267. doi: 10.1007/978-1-61779-373-8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaverin NV, Gambaryan AS, Bovin NV, Rudneva IA, Shilov AA, Khodova OM, Varich NL, Sinitsin BV, Makarova NV, Kropotkina EA. Postreassortment changes in influenza A virus hemagglutinin restoring HA-NA functional match. Virology. 1998;244:315–321. doi: 10.1006/viro.1998.9119. [DOI] [PubMed] [Google Scholar]
- 15.Kobasa D, Kodihalli S, Luo M, Castrucci MR, Donatelli I, Suzuki Y, Suzuki T, Kawaoka Y. Amino acid residues contributing to the substrate specificity of the influenza A virus neuraminidase. J Virol. 1999;73:6743–6751. doi: 10.1128/jvi.73.8.6743-6751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari K, Gulati S, Smith DF, Gulati U, Cummings RD, Air GM. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J. 2007;4:42. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laver WG, Air GM, Webster RG. Antigenicity of influenza virus hemagglutinin following chemical modification. Virology. 1981;111:538–548. doi: 10.1016/0042-6822(81)90355-x. [DOI] [PubMed] [Google Scholar]
- 18.Laver WG, Webster RG, Air GM. Effect of chemical modification on the antigenic properties of A/Tokyo/67 neuraminidase. Effect of a single sequence change on the thermal stability of A/Tokyo/67 neuraminidase. In: Laver WG, editor. The Origin of Pandemic Influenza Viruses. Elsevier; 1983. pp. 113–120. [Google Scholar]
- 19.Liao HY, Hsu CH, Wang SC, Liang CH, Yen HY, Su CY, Chen CH, Jan JT, Ren CT, Chen CH, Cheng TJR, Wu CY, Wong CH. Differential Receptor Binding Affinities of Influenza Hemagglutinins on Glycan Arrays. J Am Chem Soc. 2010;132:14849–14856. doi: 10.1021/ja104657b. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Eichelberger MC, Compans RW, Air GM. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B, Zhou H, Ye D, Kemble G, Jin H. Improvement of influenza A/Fujian/411/02 (H3N2) virus growth in embryonated chicken eggs by balancing the hemagglutinin and neuraminidase activities, using reverse genetics. The Journal of Virology. 2005;79:6763–6771. doi: 10.1128/JVI.79.11.6763-6771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lugovtsev VY, Smith DF, Weir JP. Changes of the receptor-binding properties of influenza B virus B/Victoria/504/2000 during adaptation in chicken eggs. Virology. 2009;394:218–226. doi: 10.1016/j.virol.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl)-α-D-N-acetylneuraminate substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 24.Richard M, Erny A, Caré B, Traversier A, Barthélémy M, Hay A, Lin YP, Ferraris O, Lina B. Rescue of a H3N2 influenza virus containing a deficient neuraminidase protein by a hemagglutinin with a low receptor-binding affinity. PloS one. 2012;7:e33880. doi: 10.1371/journal.pone.0033880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF. Shotgun glycomics: a microarray strategy for functional glycomics. Nature methods. 2011;8:85–90. doi: 10.1038/nmeth.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Portner A, Scroggs RA, Uchikawa M, Koyama N, Matsuo K, Suzuki Y, Takimoto T. Receptor specificities of human respiroviruses. J Virol. 2001;75:4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tappert MM, Smith DF, Air GM. Fixation of oligosaccharides to a surface may increase the susceptibility to human parainfluenza virus 1, 2, or 3 hemagglutinin-neuraminidase. J Virol. 2011;85:12146–12159. doi: 10.1128/JVI.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- 31.Xu R, Zhu X, McBride R, Nycholat CM, Yu W, Paulson JC, Wilson IA. Functional Balance of the Hemagglutinin and Neuraminidase Activities Accompanies the Emergence of the 2009 H1N1 Influenza Pandemic. J Virol. 2012;86:9221–9232. doi: 10.1128/JVI.00697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R, Zhu X, McBride R, Nycholat CM, Yu W, Paulson JC, Wilson IA. Functional Balance of the Hemagglutinin and Neuraminidase Activities Accompanies the Emergence of the 2009 H1N1 Influenza Pandemic. J Virol. 2012 doi: 10.1128/JVI.00697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Carney P, Stevens J. Structure and Receptor binding properties of a pandemic H1N1 virus hemagglutinin. PLoS currents. 2010;2:RRN1152. doi: 10.1371/currents.RRN1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen HL, Liang CH, Wu CY, Forrest HL, Ferguson A, Choy KT, Jones J, Wong DDY, Cheung PPH, Hsu CH, Li OT, Yuen KM, Chan RWY, Poon LLM, Chan MCW, Nicholls JM, Krauss S, Wong CH, Guan Y, Webster RG, Webby RJ, Peiris M. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc Natl Acad Sci U S A. 2011;108:14264–14269. doi: 10.1073/pnas.1111000108. [DOI] [PMC free article] [PubMed] [Google Scholar]