Abstract
Objective
To develop a constitutively active K+ leak channel using TREK-1 (TWIK-related potassium channel 1; TREK-M) that is resistant to compensatory down-regulation by second messenger cascades, and to validate the ability of TREK-M to silence hyperactive neurons using cultured hippocampal neurons. To test if adenoassociated viral (AAV) delivery of TREK-M could reduce the duration of status epilepticus and reduce neuronal death induced by lithium-pilocarpine administration.
Methods
Molecular cloning techniques were used to engineer novel vectors to deliver TREK–M via plasmids, lentivirus, and AAV using a cytomegalovirus (CMV)-enhanced GABRA4 promoter. Electrophysiology was used to characterize the activity and regulation of TREK–M in human embryonic kidney (HEK-293) cells, and the ability to reduce spontaneous activity in cultured hippocampal neurons. Adult male rats were injected bilaterally with self-complementary AAV particles composed of serotype 5 capsid into the hippocampus and entorhinal cortex. Lithium-pilocarpine was used to induce status epilepticus. Seizures were monitored using continuous video–electroencephalography (EEG) monitoring. Neuronal death was measured using Fluoro-Jade C staining of para-formaldehyde-fixed brain slices.
Results
TREK-M inhibited neuronal firing by hyperpolarizing the resting membrane potential and decreasing input resistance. AAV delivery of TREK-M decreased the duration of status epilepticus by 50%. Concomitantly it reduced neuronal death in areas targeted by the AAV injection.
Significance
These findings demonstrate that TREK-M can silence hyperexcitable neurons in the brain of epileptic rats and treat acute seizures. This study paves the way for an alternative gene therapy treatment of status epilepticus, and provides the rationale for studies of AAV-TREK-M’s effect on spontaneous seizures in chronic models of temporal lobe epilepsy.
Keywords: Potassium currents, Gene therapy, Hippocampus, Status epilepticus, T-type calcium channel, Temporal lobe epilepsy
In 1964, the International League Against Epilepsy (ILAE) defined status epilepticus (SE) as a seizure that “persists for a sufficient length of time or is repeated frequently enough to produce a fixed and enduring epileptic condition.”1 The Neurocritical Care Society now defines SE as continuous electrographic seizure activity that lasts longer than 5 min.2 Three classes of SE are now defined as: (1) convulsive SE, characterized by generalized tonic–clonic seizures and impairment of mental status; (2) nonconvulsive SE, or subtle status, characterized by little or no motor component; and (3) refractory SE, characterized by recurring, pharmacoresistant episodes of SE. Early estimates of the cumulative incidence of SE was 4 in 1,000, with most cases occurring in ages <1 and >60.3 Mortality is high, especially in the elderly, as 30% of patients who have SE die within 30 days.4 In addition to the disruption caused during the seizure, SE is associated with a high degree of morbidity due to disruption of cognitive function, as well as pulmonary congestion and edema, cardiac arrhythmias, and rhabdomyolysis.5
SE has been studied extensively in animal models due to its similarity to human SE in terms of induction, behavioral manifestations, evolution of electroencephalography (EEG) patterns, pharmacology, and pathology.6–8 It is notable that many of the treatments that induce SE in animals, such as pilocarpine, reliably produce a chronic model of temporal lobe epilepsy (TLE) with spontaneous seizures and hippocampal sclerosis.9 The hippocampus is prone to seizures because of the presence of reciprocal connections between hippocampal and parahippocampal subregions.10 A simplified circuit begins with the entorhinal cortex (EC), sending perforant path inputs into the dentate gyrus (DG) and temporoammonic path inputs to the CA1 and subiculum. The perforant path initiates a trisynaptic circuit leading from the DG to CA3 via the mossy fiber pathway, and then from CA3 to CA1 via Schaffer collaterals. CA1 then projects to both the subiculum and EC to complete the recurrent circuit. Therefore, the EC plays a dominant role in modulating hippocampal activity, making it an interesting target for molecular therapy.
The goal of these studies was to validate the anticonvulsant effects of a novel gene therapy using a rapid and commonly used assay: pilocarpine-induced SE. We hypothesized that a leak K+ current would reduce firing of neurons in the epileptic circuit. TREK–1 (KCNK2) is a two-pore domain K+ channel that is highly expressed in the nervous system and plays important roles in modulating neuronal excitability.11,12 Because of this important role, the channel is highly regulated, including inhibition by protein kinase A and C phosphorylation.13,14 The resting activity of TREK-1 channels drives the membrane potential closer to the K+ equilibrium potential (−80 mV), and therefore hyperpolarizes neurons.12 Because Trek–1−/− knockout mice are more sensitive to kainate-induced seizures,15 increasing TREK-1 activity might reduce pilocarpine-induced seizures.
In this study we developed a TREK-1 mutant (TREK-M) with enhanced constitutive activity and resistance to compensatory down-regulation by protein kinases. We characterize the ability of TREK-M to hyperpolarize cells and reduce spontaneous firing of cultured hippocampal neurons. We then developed recombinant adenoassociated virus (AAV)–mediated delivery of TREK-M using an enhanced human GABRA4 promoter. AAV has emerged as the top choice for gene therapy due to its safety and efficacy, and recently entered European clinics—Glybera, uniQure.16 We used a self-complementary (sc) AAV. since it infects 10 times more neurons than the commonly used single-stranded AAV.17 Our approach was to inject AAV-GABRA4-TREK-M into both EC and CA3 regions and then test its efficacy on SE. We show injection of AAV-TREK-Minto–naive rats before pilocarpine injection reduced the duration of SE compared to controls. In addition, we show that AAV-TREK-M prevented neuronal death in the brain regions where it was injected. This study identifies a novel gene therapy treatment of SE, and provides the rationale for studies of AAV-TREK-M’s effect spontaneous seizures in chronic models of TLE.
Methods
Cloning of TREK-M and viral constructs
A mouse TREK-1 Mutant (TREK-M) was generated by combining the mutations that remove phosphorylation sites S333A and S300A14 with the mutation that removes pH and arachidonic acid sensitivity, E306A.12 The S300A and E306A mutations were introduced using the single overlap extension method into the S333A mutant.13,14 TREK-M was subcloned into three different plasmids as described in the Data S1. One plasmid was used for electrophysiologic characterization of TREK-M K+ currents in cultured cells (VTREK2). The second plasmid was used for production of lentivirus (pLGG-TREK) using the ViraPower kit (Life Technologies, Grand Island, NY, U.S.A.). The third plasmid (GTAAVE) was used for production of AAV serotype 5 particles by the University of North Carolina (UNC) Vector Core. Control scAAV5-CMV-GFP was also purchased from the UNC core.
Electrophysiology
Coverslips containing transfected cells were placed in a recording chamber (RC-26G, Warner Instruments, Ham-den, CT, U.S.A.) that was steadily superfused at a rate of 2 ml/min with recording solution containing (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.6 MgCl2, 0.6 NaH2PO4, 1 NaH-CO3, 5.5 glucose, and 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), adjusted to pH of 7.4 (NaOH). Intracellular solution for voltage-clamp and current clamp and cell-attached recordings contained (in mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 ATP, 0.3 GTP-Tris, pH 7.2. Recording pipettes were pulled from thin-wall borosilicate glass (G150T-3, Warner Instruments) to a final tip resistance of 2–4 MΩ for HEK-293 cells or 3–5 MΩ for hippocampal neurons. Transfected cells were visualized by fluorescence microscopy (BX50WI microscope, Olympus, Center Valley, PA, U.S.A.). In hippocampal neuronal cultures, pyramidal neurons were identified according to their morphologic appearance and used for experiments allowing at least 4 days after transfection. To isolate intrinsic activity of transfected neurons, the following blockers of synaptic transmission were added to the external solution: 2-amino-5-phosphonovaleric acid (30 μM); 6-cyno-7-nitroquinoxaline-2, 3-dione disodium salt (10 μM); saclofen (30 μM); and bicuculline methiodide (20 μM). Currents were recorded using a Multiclamp 700B amplifier, computer (Dell, Plano, TX, U.S.A.), Digi-data 1322A A/D converter, and CLAMPEX 9.2 software (Molecular Devices, Sunnyvale, CA, U.S.A.). Data were filtered at 2 kHz and digitized at 5 kHz. Series resistance and cell capacitance were compensated to the maximal possible extent. All recordings were performed at room temperature. The measured liquid junction potential in voltage-clamp and current clamp experiments was 10 mV (n = 3) and corrected offline. Input resistance and resting membrane potential measurements were performed in current clamp mode shortly after stabilization of whole-cell configuration. Cell-attached recording was used to characterize the intrinsic spike frequency from individual neurons without disturbing the intracellular milieu.18 Seal resistance was typically 0.3–2 GΩ. For inside-out experiments, the bath solution contained (in mM): 150 KCl, 3 MgCl2, 5 EGTA, and 10 HEPES, pH 7.2. The pipette solution contained (in mM): 150 NaCl, 5 KCl, 3 MgCl2, 1 CaCl2, and 10 HEPES, pH 7.4.19 For acidic (pH 5.0) bath solutions, HEPES was substituted with 2-(N-morpholino) ethanesulfonic acid) (MES).
Stereotaxic brain injections
We used 50-day-old male Sprague-Dawley rats obtained from either Taconic or Charles River rats. Animals were acclimated for at least 48 h before any procedure. These were anesthetized with isoflurane (Sigma, St. Louis, MO, U.S.A.), given eye lubricant, and placed on a heated pad inside a stereotactic frame (David Kopf Instruments, Tujunga, CA, U.S.A.; Model 940 with digital display). The rats were continuously anesthetized with isoflurane (EZ-B800, World Precision Instruments, Inc Sarasota, FL, U.S.A.) and monitored for depth of anesthesia every 10 min. An incision was made on the skull, then all the muscles and underlying tissues were cleared to expose the bony skull for brain injections and headset mounting. Four burr holes were drilled bilaterally for injection of AAV2/5 particles. The rationale for choosing the following injection coordinates is provided in Figure S4 and validation of proper targeting is provided in Figure S5. The injection coordinates were (mm from bregma): entorhinal cortex, lateral, 5, posterior, −8.3; and depth from −6.6 to −5.6; CA3, lateral 4.6, posterior −4.4, and depth from −6.6 to −5.1. To increase accuracy, we used a mounted stereotaxic drill (Model 1474, David Kopf Instruments), zeroed the display while touching the ear bars, and used interaural coordinates.20 Seven additional burr holes were drilled into the skull. Four of these were used for screws to anchor the EEG headset, and three were used to place two cortical electrodes and one depth electrode. The depth electrode was made from perfluoroalkoxy-insulated silver wire (0.008” diameter, A-M Systems, Sequim, WA, U.S.A.) and placed in the piriform cortex at the following coordinates (mm from bregma): lateral, 5, posterior, −0.3 posterior, and depth −9.5. Three microliters of virus—serotype 5; titer 7.5 × 1012 particles/ml—was injected into each site using a 10 μl Hamilton syringe and a 26 gauge needle at a rate of 20 nl/sec. The syringe was mounted on a UMP3 microsyringe injector and infusion was controlled by a Micro4 controller (World Precision Instruments). To minimize viral diffusion up the needle track, the injector was left in place for 2 min after virus infusion. The EEG recoding set was fixed to the head by applying dental cranioplast. The skin was sutured once the cranioplast dried and set. The rat was given an intra-peritoneal injection of 0.1 ml ketoprofen for analgesia and a local application of lidocaine around the suture. The rat was allowed to recover on a heated pad after surgery and then returned to the vivarium once it was fully awake.
Induction of status epilepticus
We used the lithium-pilocarpine method to induce status, because lithium potentiates the seizure-inducing action of pilocarpine, resulting in fewer deaths.21 Rats were weighed to calibrate injection volumes. They were preinjected with lithium (3 mmol/kg) 20 h, and with scopolamine (1 mg/kg) 40 min in advance of pilocarpine (50 mg/kg). The rats were injected with diazepam (10 mg/kg) (Sigma) after 2 h of pilocarpine injection in order to stop the convulsions and reduce mortality. All drugs were injected intraperitoneally.
Electroencephalography analysis
Rats were monitored with video-EEG for at least 24 h after pilocarpine injections. The EEG patterns were scored using a modified Lothman scale.22 Electrographic status was defined by stages 3 and 4, which are characterized by uninterrupted spikes with a frequency >1.5 Hz (stage 3) or when this pattern is interrupted with periodic epileptic discharges (PEDS; stage 4). We defined the end of status when the interspike frequency declined to 1 Hz and the interspike interval returned to baseline levels. Discrete seizures were measured up to 20 h after pilocarpine injection. The duration of these seizures was added to the time spent in status and reported as total seizure duration. The EEG was read independently by two investigators who were blinded to the treatment group.
Fluoro-Jade C staining and image analysis
Rats were deeply anesthetized with 0.5 ml of pentobarbital and perfused through the ascending aorta with 100 ml of saline followed by 400 ml of freshly prepared 4% paraformaldehyde. Brains were removed and kept in 4% paraformaldehyde for 24 h at 4°C for postfixation. Brains were sliced the following day. The brains were embedded in agarose, and thin slices (30–50 μm) were cut with Leica VT 1000S Vibratome. Horizontal brain slices were stored in cryoprotectant (30% glycerol, 30% ethylene glycol, 0.2 M phosphate buffer) using 12 well plates where each well had 4–5 slices. In this manner, selecting slices from a given well reproducibly corresponded to a certain depth. Slices taken from depths of −5.0 to −7.0 from bregma were mounted on gelatin-coated slides (Lab Scientific, Highlands, NJ, U.S.A.) and then stained with Fluoro-Jade C and 4′,6-diami-dino-2-phenylindole (DAPI) following the manufacturer’s instructions (Histochem, Jefferson, AR, U.S.A.). Images were collected on an Olympus BX61WI microscope equipped with appropriate fluorescent filter sets, various objectives, and a Cooke Sensicam QE camera. IPLAB 4.0 software (BioVision, Exton, PA, U.S.A.) was used for acquisition and analysis. To increase reproducibility, FJC+ cells were counted using autosegmentation using the following parameters: triangle threshold algorithm, split distance contour, and a minimum area of 2 mm.
Ethical considerations
Experiments were done in accordance with guidelines from National Institute of Neurological Disorders and Stroke (NINDS) and Animal Research: Reporting of In Vivo Experiments (ARRIVE), the Basel declaration, and 3R concepts. Protocols were pre-approved by the University of Virginia Animal Care and Use Committee. Data are presented as mean ± standard deviation (SD), and p-values were determined with two-tailed tests. Nonparametric tests were applied when appropriate. All statistical tests were performed with Prism software (v.6, Graphpad, La Jolla, CA, U.S.A.). Experiments on animals were acquired and analyzed by experimenters who were blinded to the treatment group.
Results
Generation of a constitutively active potassium leak channel
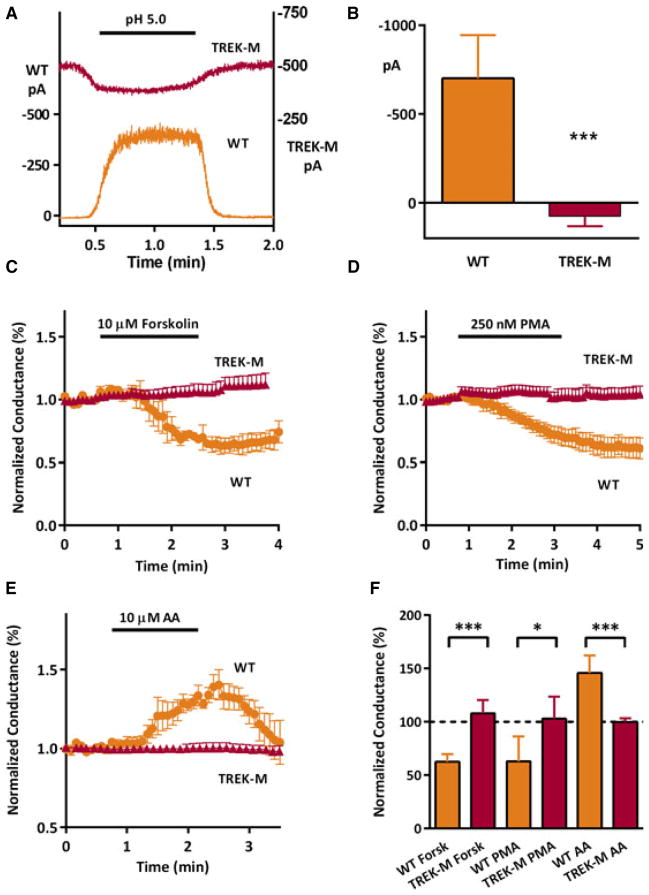
TREK-1 plays important roles in modulating neuronal activity and network oscillations, and its activity is regulated by many second messenger cascades.23 Our goal was to develop a constitutively- active version of TREK-1 that would resist homeostatic regulation. To this end we combined three different mutations that had been shown to play critical roles in channel regulation: S333A and S300A that remove phosphorylation sites,14 and E406A that removes pH and arachidonic acid sensitivity.12 The resulting mutant, TREK-M, was expressed in HEK-293 cells, and its activity was tested in various electrophysiologic assays (Fig. 1). Using inside-out patch recording, we show that TREK-M is no longer stimulated by acidic pH as observed in wild-type (WT) TREK-1 (Fig. 1A). Averaged results show that acidic pH slightly lowered TREK-M, while causing a large increase in WT currents (Fig. 1B). This decrease is likely caused by the reported decrease in single-channel conductance at intracellular acidic pH.19 Using whole-cell voltage-clamp recordings, we show TREK-M is no longer inhibited by either the adenylyl cyclase activator forskolin (Fig. 1C) or the protein kinase C activator PMA (Fig. 1D). Control experiments showed that these treatments could inhibit WT channels by ~40% (Fig. 1F). We also show that TREK-M is no longer stimulated by arachidonic acid, which stimulated WT TREK-1 50% in a readily reversible manner (Fig. 1E, F).
Figure 1.
TREK-M is not regulated by intracellular acidosis or second-messenger signaling cascades. Ionic currents were measured using the cell excised, inside-out patch configuration at a holding potential of 0 mV and a physiologically relevant K+ gradient (A, B). Decreasing the pH of the bathing solution from 7.2 to pH 5.0 opens TREK-1WT channels in a reversible manner. In contrast, changing the pH to 5.0 reduces TREK-M current amplitude. (B) Average current change induced by acidification inWT(n = 4) and TREK-M (n = 4). Whole-cell voltageclamp recording of voltage ramps (−140 to +10 mV) were used to measure changes in slope conductance induced by various secondmessenger cascades (C–F). (C) Average time course after bath application of 10 μM forskolin (horizontal bar, n = 4 for both WT and TREK-M). (D) Bath application of 250 nM phorbol 12-myristate 13-acetate (PMA) also decreased WT TREK-1 currents but had no effect on TREK-M currents (WT, n = 7; TREK-M, n = 5). (E) In contrast, perfusion with 10 μM arachidonic acid (AA) induced a transient stimulation of WT TREK-1 currents (n = 7), but had no effect on TREK-M (n = 4). Peak effects and statistical analysis of the forskolin, PMA, and AA experiments are shown in panel. (F) Statistical significance estimated with Student’s t-test is indicated with a single asterisk if p < 0.05, and if p < 0.005 with a triple asterisk.
Epilepsia ILAE
Mutation of TREK-1 enhances its ability to alter basic membrane properties
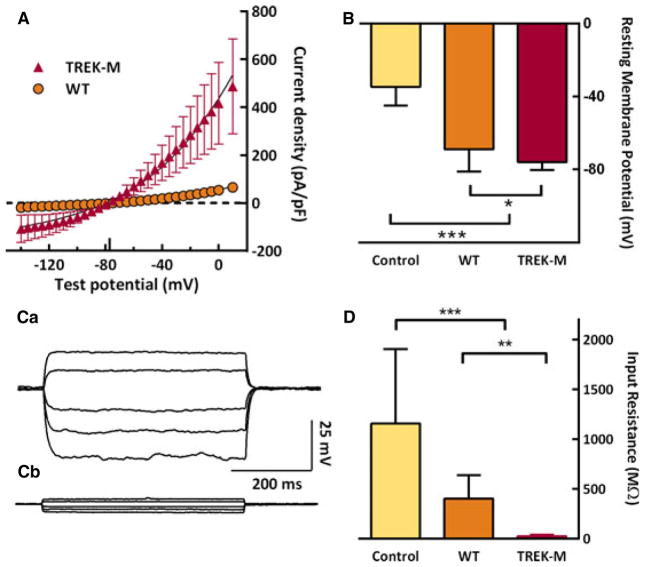
Whole-cell voltage-clamp recordings also show that TREK-M generates much larger currents than WT channels (Fig. 2A), corresponding to a sevenfold increase in conductance. These currents reversed direction at −77 mV, as expected for a highly selective K+ channel. Using current-clamp recordings, we compared the ability of the TREK channels to alter both the resting membrane potential and input resistance. Under our recording conditions, HEK-293 cells displayed a resting membrane potential of −35 mV. Transfection with WT TREK-1 significantly lowered the membrane potential to −69 mV; whole TREK-M lowered it even further, to −76 mV (Fig. 2B). A similar result was observed in the neuroblastoma cell line ND7/23 (results not shown). Hyperpolarization of spontaneously active neurons would reduce firing by moving the membrane potential away from the threshold of voltage-gated Na+ and Ca2+ channels. Excitatory postsynaptic potentials induced by synaptic transmission cause neuronal firing by depolarizing the membrane, so we next tested the ability of TREK-M to dampen membrane voltage changes. Injection of current pulses produced large changes in membrane potential in control cells, smaller changes in cells expressing TREK-1 WT, and very little changes in cells expressing TREK-M (Fig. 2C). Input resistance was calculated from fits to these data, and the average is presented in Figure 2D.
Figure 2.
Mutations in TREK-1 increase K+ leak currents, lower the resting membrane potential, and decrease input resistance. (A) Expression of WT and TREK-M channel in HEK-293 cells measured by whole-cell voltage-clamp recording. Ionic current was normalized to the capacitance of the cell (pA/pF) and then averaged. Currents were evoked during step pulses to the indicated potentials from a holding potential of −80 mV. The smooth lines represent fits to the data with the Goldman-Hodgkin-Katz current equation.38 Ionic flow reversed direction at the calculated reversal potential of potassium (Erev, −77 mV) and is shown as a tick on the x-axis. TREK-M had a sevenfold higher conductance (in nS/pF): TREK-M, 5.3 ± 2.2, n = 15; WT, 0.7 ± 0.7, n = 15; p < 0.0001). (B) Both WT and TREK-M expression significantly altered the resting membrane potential (in mV): Control, −35 ± 10, n = 12; WT, −69 ± 21, n = 18; and TREK-M, −76 ± 4, n = 17. Control cells were transfected with empty vector (pcDNA3). (C) Representative current traces (Ca, WT; Cb, TREK-M) recorded at the resting membrane potential and injecting either 100, 50, −50, −100, or −150 pA current. Input resistance was calculated by fitting the resulting change in voltage with linear regression. (D) Both WT and TREK-M also significantly reduced input resistance (in MΩ): Control, 1155 ± 716, n = 12; WT, 399 ± 227, n = 18; and TREK-M, 22 ± 12, n = 17). Statistical significance estimated with Student’s t-test is indicated with a single asterisk if p < 0.05, and if p < 0.005 with a triple asterisk.
Epilepsia ILAE
TREK-M inhibits spontaneously active neurons
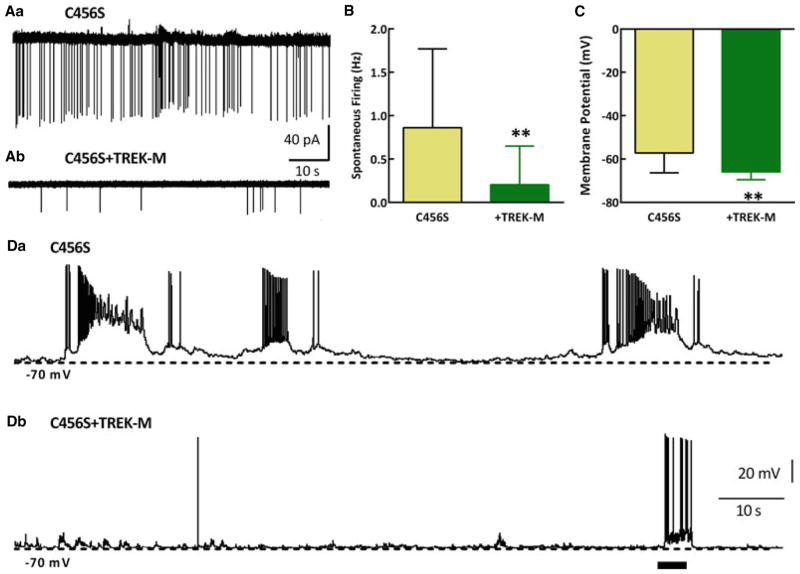
Our previous studies focused on the mechanisms that mutations in the gene encoding the Cav3.2 T-type Ca2+ channel might increase seizure susceptibility in patients with idiopathic epilepsies.24 Expression of the C456S mutation in cultured hippocampal neurons increased T–currents, rebound firing, and spontaneous activity. Therefore, we used C456S transfected neurons as an assay for the ability of TREK-M to silence hyperactive neurons. We transfected mature hippocampal neuronal cultures with plasmids and recorded activity 4–9 days later. The loose cell-attached patch recording configuration was used to characterize firing of neurons without disturbing the intra-cellular milieu.18 TREK-M produced a fourfold decrease in action potential firing compared to C456S alone (Fig. 3B). Using whole-cell current-clamp recording we measured the resting membrane potential. As observed in HEK-293 cells, the resting membrane potential was driven closer to the potassium reversal potential (Erev), showing a 9 mV shift to more negative membrane potentials (Fig 3C). Voltage-clamp recordings confirmed expression of TREK-M in hippocampal neurons (results not shown). In whole-cell current-clamp recordings, spontaneous activity was observed in 90% of the neurons expressing C456S, but only in 40% of those expressing both C456S + TREK-M. Representative traces are shown in Figure 3D. As a control, injection of current did produce a burst of firing, even in the presence of TREK-M. A similar inhibition of neuronal firing was obtained using virally delivered TREK-M (Fig. S2). These in vitro results provide strong support for the hypothesis that TREK-M is a powerful tool to inhibit hyperactive neurons, so next we studied its effect in vivo.
Figure 3.
TREK-M reduces spontaneous firing of hyperactive hippocampal pyramidal neurons. The loose cell-attached patch recording technique was used to measure spontaneous activity of mature cultures of hippocampal neurons transfected with the Cav3.2 epilepsy mutant C456S ± TREK-M. To measure spontaneous firing without cell dialysis, we used the loose cell-attached patch recording mode (A, B). Representative traces of spontaneous activity measured in loose cell-attached patch recording mode from neurons transfected with either C456S (Aa) or C456S + TREK-M (Ab) are presented. Scale bars are shown at the ends of the traces. Note, the observed current amplitude is largely a function of access resistance. (B) Average spontaneous firing recorded in loose cell-attached patch configuration (n is shown on the graph). (C) Expression of TREK-M hyperpolarized the neurons about 9 mV from −57 to −66 mV. The input resistance was reduced by TREK-M about 35% compared to C456S (in MΩ): C456S, 188 ± 60, n = 11; and TREK-M, 121 ± 26, n = 5 (p < 0.05). Representative traces in current-clamp mode for C456S and C456S + TREK-M are shown in panels Da and Db, respectively. Horizontal bar in Db represents a + 100 pA current injection. Action potential firing was readily induced by this pulse, showing that the neuron was still capable of firing.
Epilepsia ILAE
Viral delivery of TREK-M ameliorates status epilepticus
Because our long-term goal was to use viral delivery to cure seizures in chronic models of temporal lobe epilepsy (TLE), we decided to use the GABRA4 promoter fragment, the use of which had been validated in epilepsy models.25 Notably, expression from the promoter is enhanced in epileptic neurons.26 We first tested the activity of this promoter to drive expression of fluorescent protein, mVenus, in ND7/ 23 neuroblastoma cells (construct abbreviated GY). Surprisingly, very little fluorescence could be observed in transfected cells (see Fig. S3 for diagram of constructs and results). This GABRA4 promoter fragment lacks a canonical TATA box near the transcription start site. Addition of this sequence and the initiation site from CMV minimal promoter [CMVm27] enhanced expression fivefold (construct abbreviated MGY). We also tested the effect of adding the CMV immediate-early enhancer [CMVe28] to the 5′ side of the promoter, and this increased expression 10-fold (construct abbreviated EGY). Addition of both CMV elements dramatically increased expression 50-fold (EMGY), bringing it to within 50% of the full CMV promoter (Fig. S3). Previous studies using the CMV enhancer found that it in some cases, but not all, it affects neuronal specificity.29 To address this issue, we tested the activity of the base and the doubly enhanced promoter (GY and EMGY) in human embryonic kidney cells, finding little or no expression (Fig. S3D). We conclude that the CMVe-GABRA4-CMVm promoter selectively drives robust expression in cells of neuronal lineage.
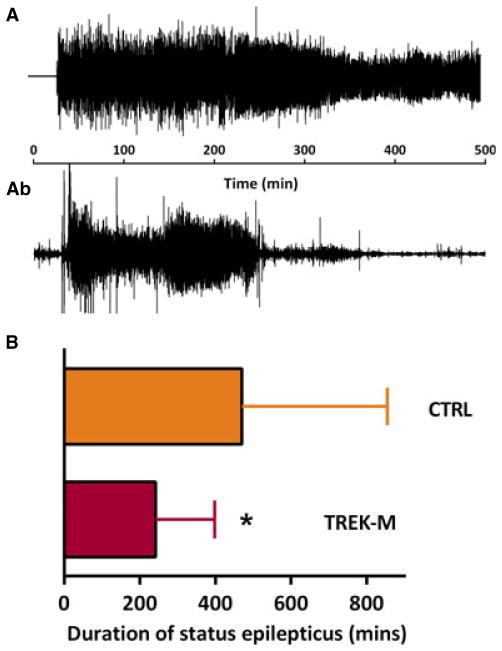
Anticonvulsant activity of novel compounds is routinely tested in animals after administration of chemoconvulsants such as picrotoxin, kainate, or pilocarpine. We hypothesized that expression of TREK-M in hippocampal circuits would decrease pilocarpine-induced SE. To disrupt the hippocampal trisynaptic circuit, we targeted both the hippocampus itself, centering our injections to the CA3 cell layer, and the entorhinal cortex, which provides the major input and output paths of the hippocampus.10 A detailed description of the injection strategy is provided in the Data S1 and Figure. S4. We validated our ability to target these sites using the control (CTRL) self-complementary AAV expressing the green fluorescent protein (GFP) (Fig. S5). We then engineered a novel scAAV targeting plasmid expressing TREK-M under control of the CMV-enhanced GABRA4 promoter (Data S1). CTRL and TREK-M scAAV particles composed of serotype 5 capsid were injected into young adult male rats and an EEG headset was implanted. We waited 4 weeks to allow for expression, and then induced status as detailed in the Methods. We scored both the behavior and electrographic sequelae. Representative EEG traces are shown in Figure 4A. TREK-M significantly reduced the duration of SE by 50% (p < 0.05, Fig. 4B). We also measured the total time spent in seizures for the first 24 h after pilocarpine injection and found a similar reduction (p < 0.05). Onset of SE and behavioral score were not significantly altered (results not shown).
Figure 4.
Adenoassociated viral (AAV) delivery of TREK-M reduces time spent in SE induced by pilocarpine. (Aa) Representative EEG trace recorded during SE from a rat injected with an scAAV serotype 5 construct with an inactive promoter. (Aa) An EEG trace from an animal preinjected with AAV-TREK-M. (B) Average duration of SE was significantly decreased in rats preinjected with AAV-TREK-M (242 ± 153 min, n = 15) compared to the control virus (470 ± 375 min, n = 18). For control we injected similar scAAV5 constructs that expressed fluorescent proteins. This experiment was performed three times. Statistical analysis used the Mann- Whitney test and p < 0.05 is shown with a single asterisk.
Epilepsia ILAE
TREK-M reduced neuronal death
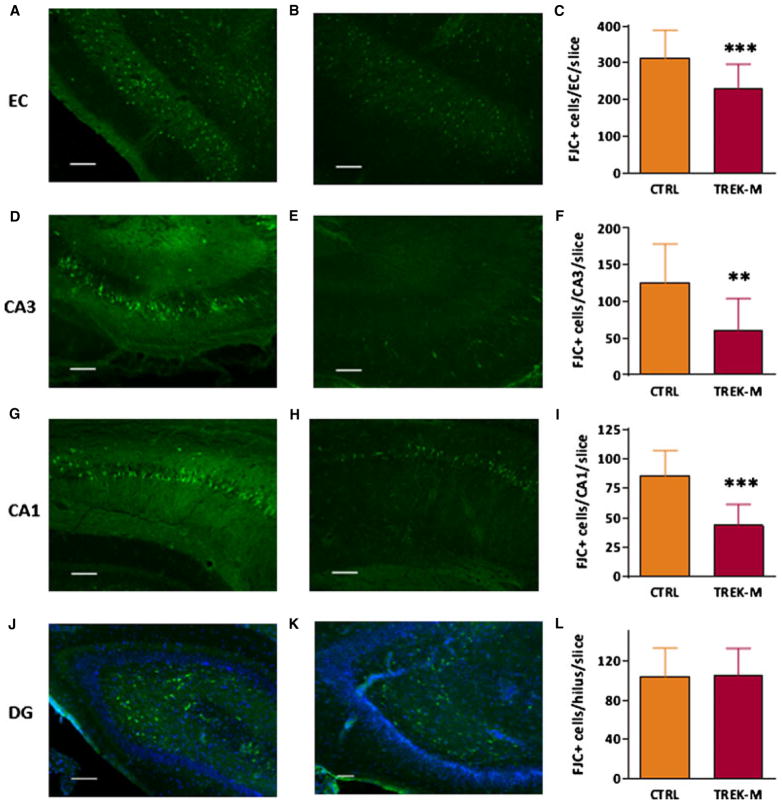
We also hypothesized that the ability of TREK-M to reduce firing would reduce the excitotoxic death induced by pilocarpine. We measured neuronal death using Fluoro-Jade C (FJC), a Nissl stain that has proven to be a reliable marker for neuronal degeneration.30 Forty-eight hours after induction of SE we began preparation of brain slices from control and TREK-M animals (n = 4 each). Staining was performed on horizontal slices obtained near the center of the injection track. Representative images of FJC-stained brain slices are presented in the Figure 5. We found that rats preinjected with TREK-M had significantly less FJC+ cells in the entorhinal cortex, CA3, and CA1 regions compared to control (Fig. 5C, F, and I). However, no reduction was observed in FJC+ cells in the dentate gyrus, where neuronal death occurs predominantly in the hilus as we and others have observed previously.30–32 These results indicate that TREK-M reduced neuronal death in regions where the AAV was injected and extending into CA1.
Figure 5.
Adenoassociated viral (AAV) delivery of TREK-M ameliorates neuronal death induced by pilocarpine. Typical examples of Fluoro-Jade C labeling in the hippocampus and EC. Images taken from AAV control injected rats (n = 4) are for the following brain regions: EC (A), CA3 (D), CA1 (G), and DG (J). Images taken from AAV-TREK-M injected rats (n = 4) from the same brain regions are in panels B, E, H, and K, respectively. Average number of Fluoro-Jade C–positive (FJC+) neurons and statistical analysis is shown in panels C (EC), F (CA3), I (CA1), and L dentate gyrus (DG). Most of the FJC+ cells in the DG were found in the hilar region. Blue fluorescent signal corresponds to DAPI labeling of nuclei in the DG cell layer. Due to their size and the ability to resolve individual neurons, FJC+ cells in the hilus of the dentate gyrus and entorhinal cortex were analyzed from 10 × images. However, counting of FJC+ cells in the CA subfields required higher resolution, 25 × . The exposure was fixed at 500 msec for images acquired at 10 × and 100 msec for those at 25 × (oil immersion lens). Results are from four animals per experimental group, and analysis was performed on four slices per animal taken near the center of the injection site (−6.1 mm depth from bregma; Fig. S4). Calibration bar corresponds to 100 μm. Statistical analysis used the Mann-Whitney test and p < 0.05 is shown with a single asterisk, p < 0.01 with two asterisks, and p < 0.001 with three asterisks.
Epilepsia ILAE
Discussion
In this study we engineered a new triple mutant of TREK-1 and showed that it potently lowers the resting membrane potential and decreases input resistance. These findings were replicated in HEK-293 cells, ND7/23 neuroblastoma cells, and in cultured hippocampal neurons. We induced hyperactivity in cultured neurons by expression of a Cav3.2 T-type channel mutant found in patients with absence epilepsy, and then showed that TREK-M could reduce spontaneous firing of cultured neurons. This important in vitro result was replicated using viral delivery. We then showed that scAAV5 delivery of GABRA4-driven TREK-M reduced the duration of SE and neuronal damage caused by lithium-pilocarpine.
TREK-1 channel
TREK-1 is a member of the two-pore–domain potassium channel family. It is highly expressed in the nervous system, where it can exert a dominant role in setting the resting membrane potential.11,12 In addition, by decreasing the membrane input resistance, TREK-1 channels shunt the changes induced by synaptic inputs. Previous studies have shown that TREK-1 current is activated by intracellular acidosis, polyunsaturated fatty acids, and volatile anesthetics.23 In contrast, activation of protein kinase A and protein kinase C pathways leads to down-regulation of TREK-1 activity.14,33 To reduce homeostatic regulation of the channel, we generated the triply mutated channel TREK-M that lacks amino acid residues phosphorylated by protein kinases and the intracellular pH sensor, E30614,33 We show that TREK-M induces large leak K+ currents, which are insensitive to intracellular acidosis, arachidonic acid, and inhibition by protein kinase A and C activation.
TREK-M effect on hippocampal pyramidal neurons
In our studies on the mechanisms by which mutations in Cav3.2 channels increase seizure susceptibility, we found that the absence epilepsy mutant C456S could increase spontaneous firing activity of cultured hippocampal neurons.24 This provided an assay to test the effect of TREK-M on neuronal activity. Indeed, expression of TREK-M markedly reduced spontaneous firing of C456S-expressing neurons. We validated the ability of TREK-M to lower neuronal resting membrane potential and input resistance, thereby providing a likely mechanism for its action.
TREK-M is anticonvulsant and reduces neuronal death
Our in vivo studies show that AAV delivery of TREK-M reduced the duration of electrographic SE by 50% after lithium-pilocarpine induction. Although preliminary studies suggested TREK-M could reduce the duration of tonic–clonic seizures, this finding was not replicated in subsequent experiments (we report the average of three independent experiments). We also saw no effect on seizure latency. To provide an independent measure of TREK-M’s effect, we also measured its effect on neuronal death. We found significant reductions in Fluoro-Jade–positive neurons in the injected areas (EC and CA3) as well as CA1. In contrast, cell loss was not prevented in the hilus of the dentate gyrus. Loss of hilar neurons is a common finding in animal models of SE and human TLE.32,34
Enhancing K+ currents to treat epilepsy is the mechanism of action of retigabine [ezogabine; Potiga35]. Recent studies in a tetanus toxin animal model of focal epilepsy also support the hypothesis that overexpression of K+ channels might provide a therapeutic effect.36 We conclude that AAV-TREK-M might provide a novel gene therapy treatment for refractory SE, and that further preclinical studies are needed to study its effect on spontaneous seizures in chronic models of TLE. Future studies will also need to compare the various gene therapy treatments that have been developed so far [see review37].
Supplementary Material
Acknowledgments
We thank Deborah Perez-Reyes for technical assistance. We thank Aleksandr Shcheglovitov and Roman Lazarenko for electrophysiologic advice, and Thurl Harris for advice on lentiviral production. We thank the following for plasmids: Douglas Bayliss for providing us with both TREK-1 wild-type and S333A mutant; Nikolai Soldatov for mVenus; and Didier Trono for lentiviral packaging constructs (Addgene plasmids 11650, 12259, and 12260). We thank Paula Barrett and Patrice Guyenet for encouraging discussions. This work was supported by an NIH grant NS040337 to JK and a CURE Challenge grant to JK and EPR.
Footnotes
Disclosure
The authors do not have any conflicts to declare. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Supporting Information may be found in the online version of this article:
Data S1. Drugs and chemicals.
Figure S1. Maps of the plasmids engineered for this study.
Figure S2. Validating viral delivery of TREK-M into hippocampal neurons.
Figure S3. Enhancing expression from the human GABRA4 promoter with elements from CMV.
Figure S4. Rationale for the brain coordinates used for AAV injection.
Figure S5. Validation of adenoassociated viral (AAV) expression after intraparenchymal injection into the hippocampal CA3 layer and entorhinal cortex (EC).
References
- 1.Arnautova EN, Nesmeianova TN. A proposed international classification of epileptic seizures. Epilepsia. 1964;5:297–306. doi: 10.1111/j.1528-1157.1964.tb03337.x. [DOI] [PubMed] [Google Scholar]
- 2.Brophy G, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Logroscino G, Cascino G, et al. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50:735–741. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- 4.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 5.Joshi S, Kapur J. GABAA Receptor plasticity during status epilepticus. In: Noebels J, Avoli M, Rogawski MA, et al., editors. Jasper’s basic mechanisms of the epilepsies. 4. National Center for Biotechnology Information (US); Bethesda, MD: 2012. [PubMed] [Google Scholar]
- 6.Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004;54:473–485. [PubMed] [Google Scholar]
- 7.Andre V, Ferrandon A, Marescaux C, et al. The lesional and epileptogenic consequences of lithium-pilocarpine-induced status epilepticus are affected by previous exposure to isolated seizures: effects of amygdala kindling and maximal electroshocks. Neuroscience. 2000;99:469–481. doi: 10.1016/s0306-4522(00)00209-8. [DOI] [PubMed] [Google Scholar]
- 8.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Lothman EW, Bertram EH, Kapur J, et al. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990;6:110–118. doi: 10.1016/0920-1211(90)90085-a. [DOI] [PubMed] [Google Scholar]
- 10.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 11.Talley EM, Solorzano G, Lei Q, et al. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honoré E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 13.Horton RM, Ho SN, Pullen JK, et al. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- 14.Murbartián J, Lei Q, Sando JJ, et al. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- 15.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudet D, Methot J, Dery S, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 18.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maingret F, Patel AJ, Lesage F, et al. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C, Pennisi M, et al. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 21.Jope RS, Morrisett RA, Snead OC. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp Neurol. 1986;91:471–480. doi: 10.1016/0014-4886(86)90045-2. [DOI] [PubMed] [Google Scholar]
- 22.Lothman EW, Bertram EH, Bekenstein JW, et al. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 23.Dedman A, Sharif-Naeini R, Folgering JH, et al. The mechano-gated K2P channel TREK-1. Eur Biophys J. 2009;38:293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- 24.Eckle V-S, Shcheglovitov A, Vitko I, et al. Mechanisms by which CACNA1H mutations found in epilepsy patients increase seizure susceptibility. J Physiol. doi: 10.1113/jphysiol.2013.264176. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raol YH, Lund IV, Bandyopadhyay S, et al. Enhancing GABAA receptor a1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts DS, Raol YH, Bandyopadhyay S, et al. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up- regulation of GABAA receptor a4 subunit expression. Proc Natl Acad Sci USA. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loew R, Heinz N, Hampf M, et al. Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 2010;10:81. doi: 10.1186/1472-6750-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruh I, Wunderlich S, Winkler M, et al. Human CMV immediate-early enhancer: a useful tool to enhance cell-type-specific expression from lentiviral vectors. J Gene Med. 2008;10:21–32. doi: 10.1002/jgm.1122. [DOI] [PubMed] [Google Scholar]
- 29.Hioki H, Kameda H, Nakamura H, et al. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 2007;14:872–882. doi: 10.1038/sj.gt.3302924. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Liu YH, Huang YG, et al. Time-course of neuronal death in the mouse pilocarpine model of chronic epilepsy using Fluoro-Jade C staining. Brain Res. 2008;1241:157–167. doi: 10.1016/j.brainres.2008.07.097. [DOI] [PubMed] [Google Scholar]
- 31.Motte J, Fernandes MJ, Baram TZ, et al. Spatial and temporal evolution of neuronal activation, stress and injury in lithium-pilocarpine seizures in adult rats. Brain Res. 1998;793:61–72. doi: 10.1016/s0006-8993(98)00135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C, Mtchedlishvili Z, Bertram EH, et al. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honoré E, Maingret F, Lazdunski M, et al. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathern GW, Babb TL, Pretorius JK, et al. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 36.Wykes RC, Heeroma JH, Mantoan L, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4:161ra52. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg MS, McCown TJ. Current prospects and challenges for epilepsy gene therapy. Exp Neurol. 2013;244:27–35. doi: 10.1016/j.expneurol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hille B. Ionic channels of excitable membranes. 3. Sunderland, MA: Sinauer Associates, Inc; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.