Abstract
OBJECTIVE
To determine whether trophoblast-derived microparticles can induce different inflammatory responses of the peripheral blood mononuclear cells (PBMCs) depending upon the state of trophoblast when the microparticles are generated.
STUDY DESIGN
A trophoblast-derived cell line (ATCC No. CRL-1584) was cultured under normal or hypoxic conditions. Microparticles were isolated from the cell culture supernatants (MP_C: microparticles from normal trophoblast; MP_H: microparticles from hypoxic trophoblast). PBMCs were cultured alone or co-cultured with either MP_C or MP_H.
RESULTS
1) After 48 hours, the PBMCs co-cultured with MP_C released higher concentrations of IL-6 than PBMCs cultured alone; 2) The PBMCs co-cultured with MP_H showed higher concentration of IL-6 and TNF-a than PBMCs co-cultured with PM_C, after 24 hours and 48 hours.
CONCLUSION
More intense and rapid inflammatory response of PBMCs was observed with MP_H than with MP_C. This difference might explain the exaggerated systemic inflammatory response as a result of placental hypoxia in preeclampsia.
Keywords: preeclampsia, microparticles, hypoxia, trophoblast, inflammation
Introduction
Microparticles are small membrane vesicles, which originate from blebbing membranes of either activated cells or cells undergoing apoptosis1, 2. The syncytiotrophoblast forms the maternal-placental interface, and it has been suggested that microparticles from this cell (syncytiotrophoblast microparticles, STBMs) may be the factor which disturbs the maternal endothelium and mediates the inflammatory response3, 4, which is the characteristic feature in preeclampsia5, 6.
However, microparticles also showed immunosuppressive characteristics, and this immunoregulatory function of microparticles has been thought as the explanation of immune tolerance in normal pregnancy7–10. The mechanism of this different action of microparticles (i.e. pro-inflammatory vs. immune-regulatory) in preeclampsia and normal pregnancy is not well determined.
Preeclampsia is initiated by reduced uteroplacental blood flow, resulting in placental hypoxia11, and the experimental evidences and histological feature of placenta in preeclampsia supports this theory12, 13. During normal placentation, the maternal spiral arteries are invaded by trophoblast, resulting in extensive remodeling of spiral arteries to ensure an adequate blood supply to the placenta and fetus. However, placentas of preeclamptic show shallow trophoblast invasion and defects in spiral artery remodeling, resulting in poor perfusion and ischemia14–19. As a result of placental ischemia, it is believed that placenta release a number of soluble factors into maternal circulation, eliciting many of the symptoms of preeclampsia20. In this context, our hypothesis was that the trophoblast in a different condition (i.e. normal vs. hypoxic; which represent normal pregnancy vs. preeclampsia) may shed microparticles which have different characteristics21, resulting in a different systemic inflammatory response to these microparticles.
To this end, we undertook this study to determine whether trophoblast-derived microparticles can induce different inflammatory response, according to the condition of the trophoblast.
Material and Methods
Cell culture
A trophoblast-derived cell line (ATCC No. CRL-1584), which has been used as the model for placenta in previous studies22–25, was obtained from the Global Bioresource Center™ (http://www.atcc.org) and cultured in 10% MEM media (modified Eagle’s medium, GIBCO, USA; with 10% fetal bovine serum and antibiotics). The cultures were maintained at 37°C in an atmosphere of 5% of CO2.
Induction of hypoxic cell death
Two million trophoblast cells were cultured either in normal or hypoxic conditions. For normal condition, the cultures were maintained in an atmosphere of 5% of CO2. For hypoxic condition, cells were cultured in the presence of 100umol/L rotenone (Sigma-Aldrich, St Louis, MO), which induces chemical hypoxia by disruption of the mitochondrial electron transport chain26. To confirm the process of apoptotic cell death after the addition of rotenone, floating cells were collected and adherent cells were detached with trypsin/EDTA, and the cells were pooled with centrifugation (300 × g, at room temperature, for 5 minutes), and were analyzed with flow cytometric analysis (FACS) using the FITC Annexin V Apoptosis Detection kit (BD Biosciences). For assessment, cells were resuspended in 100ul of Binding Buffer at a concentration of 1 × 106 cells/ml, and 5 uL of FITC Annexin V and 5 ul of PI was added. After incubation for 15 minutes at room temperature in the dark, 400ul of Binding Buffer was added and the analysis was performed using FACSCalibur (Becton Dickinson, CA, USA) and the data were evaluated with CellQuest software (Becton Dickinson). In the presence of rotenone, 15 to 40% of the trophoblast cells were stained with Annexin V after 24 hours of culture, and 50 to 80% of cells were stained with Annexin V after 48 hours of culture, although less than 15% of the trophoblast cells were Annexin V positive after 24 and 48 hours of culture in the normal condition (i.e. in the absence of rotenone).
Isolation and quantification of microparticles
After 24 hours of culture in normal or hypoxic condition, the supernatants were collected and microparticles were isolated by differential centrifugation. First, cell culture supernatants were separated from detached cells by two centrifugation steps (300 × g, 5 minutes and 800 × g, 5 minutes)26. Then, the cell-free supernatants were ultra-centrifuged at 100,000 × g at 4°C for 90 minutes. After the removal of the supernatants, the pellet was washed twice with PBS and finally suspended in 10% MEM media.
The number of microparticles was counted by FACS, and adjusted to the concentration of trophoblast cells27. Trophoblast cells were counted with a hemocytometer and 2 × 106 cells/ml of trophoblast cells were used for the experiment. In brief, the number of each microparticles and trophoblast cells were counted for 30 seconds at the “low-flow” modus and the concentration of microparticles was calculated by the following formula, then the concentration of microparticles was adjusted to standard concentration for the following experiment.
The concentration of microparticles = (microparticles count/trophoblast cells count) × (2 × 106) (counts/ml)
Co-culture of peripheral blood mononuclear cells (PBMCs) with microparticles
Peripheral venous blood (40ml) was taken from healthy non-pregnant women (n=4). PBMCs were isolated by density gradient centrifugation using the Ficoll method (Sigma-Aldrich HISTOPAQUE®-1077). The final pellet was re-suspended in 10% MEM, and 2 × 106 cells/ml, 0.2ml aliquots were placed in a 96-well plate.
The PBMCs were cultured alone, or co-cultured with microparticles derived from the trophoblast in normal condition (MP_C, 5 × 106/ml), or co-cultured with microparticles derived from the trophoblast in hypoxic condition (MP_H, 5 × 106/ml), in the presence or absence of T cell mitogen, phytohemagglutinin (PHA, 5 ug/ml) up to 48 hours. Each experiment was done in duplicate. After either 24 or 48 hours of culture, cellular proliferation was measured with the CCK-8 (Cell Counting Kit-8), and the co-culture supernatants were obtained and assayed for interleukin-6 and TNF-a using the commercial ELISA kit (R&D Systems, Minneapolis).
The Institutional Review Board of the Seoul National University Hospital approved this study. The Seoul National University has a Federal Wide Assurance (FWA) with the Office for Human Research Protections (OHRP) of the Department of Health and Human Services (DHHS) of the United States.
Statistical analysis
Comparisons of continuous variables between groups were performed with the Mann-Whitney U test. A p-value <0.05 was considered as statistically significant.
Results
PBMCs co-cultured with microparticles from trophoblast in normal condition
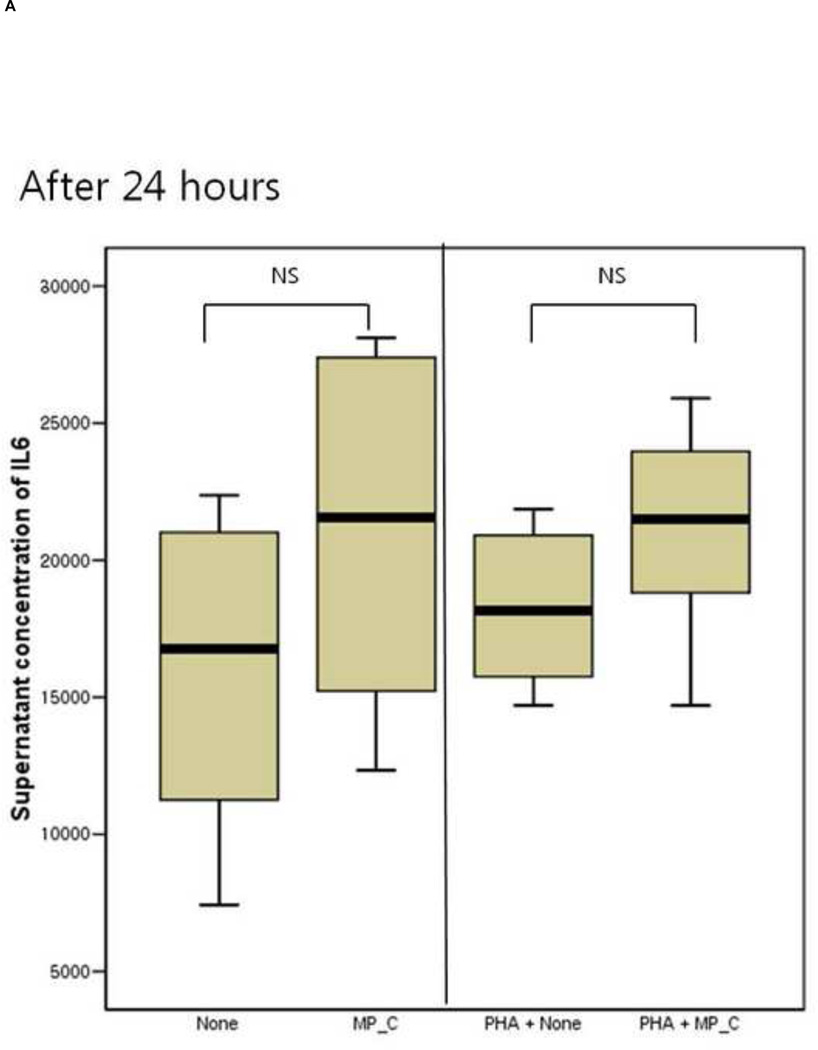
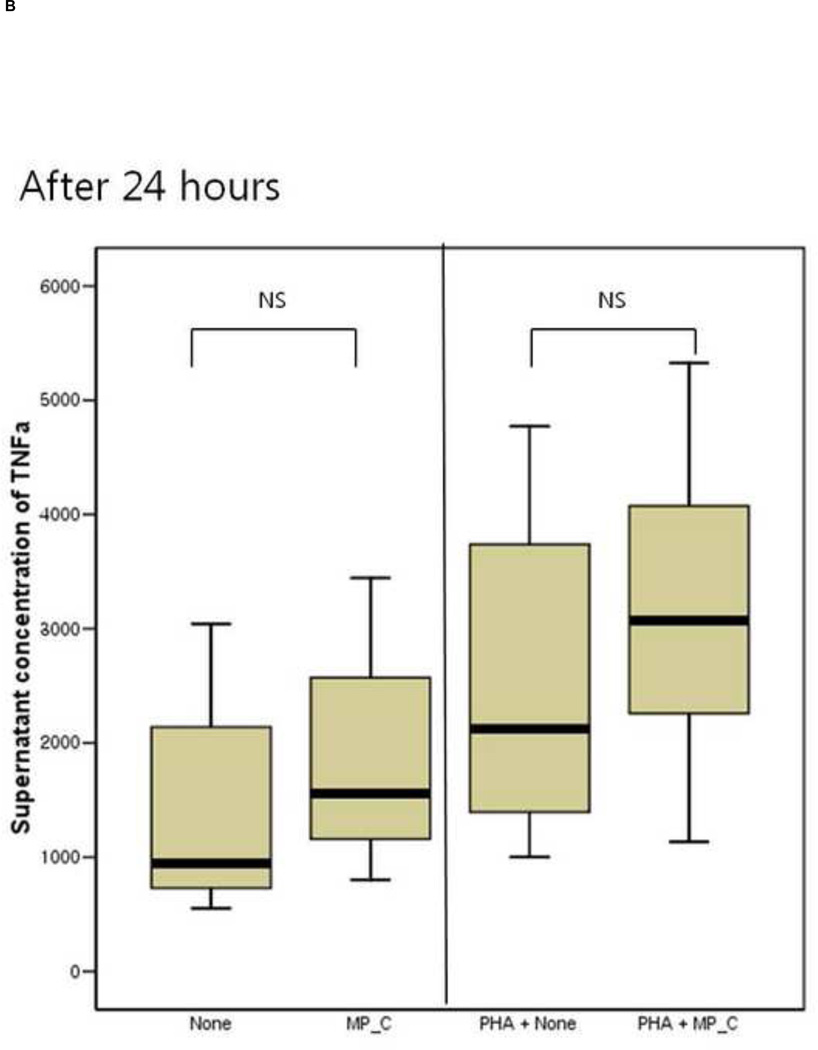
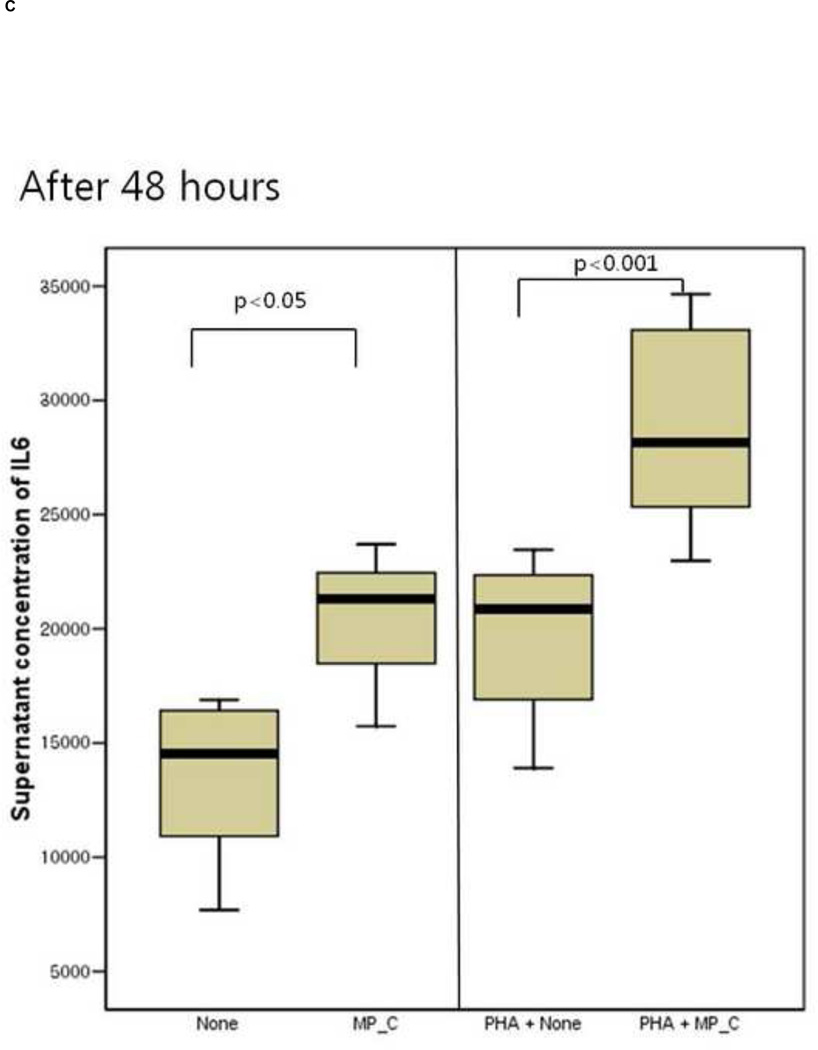
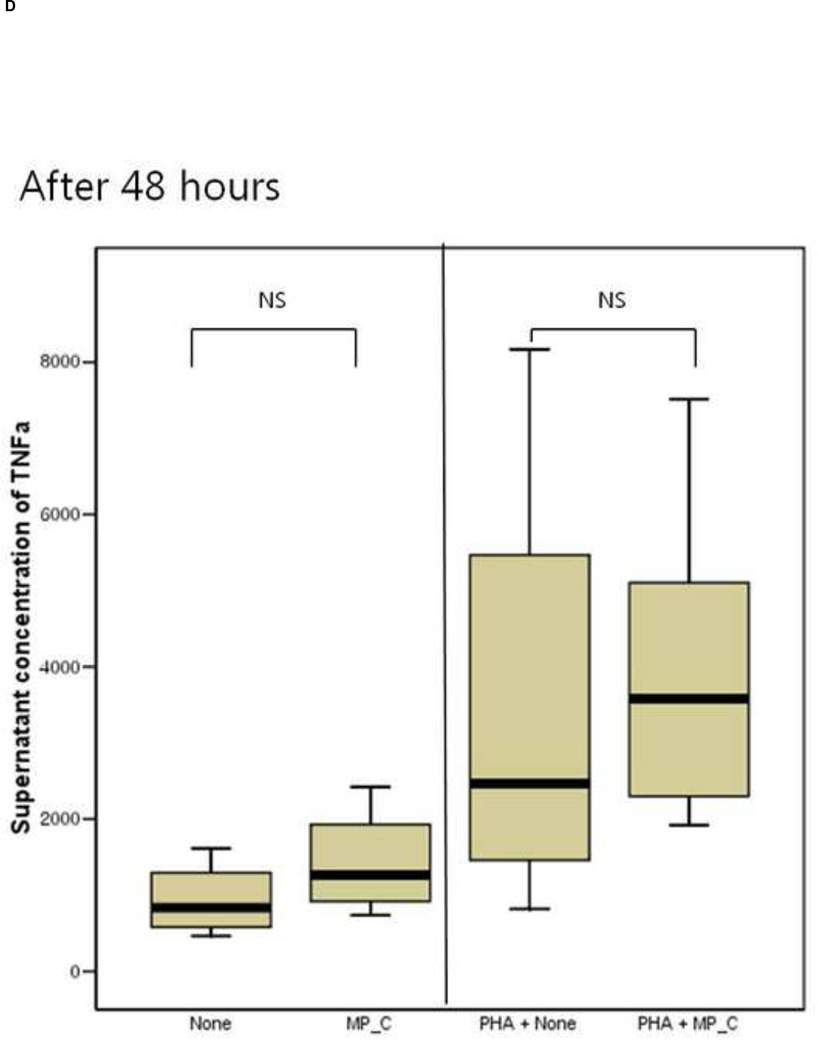
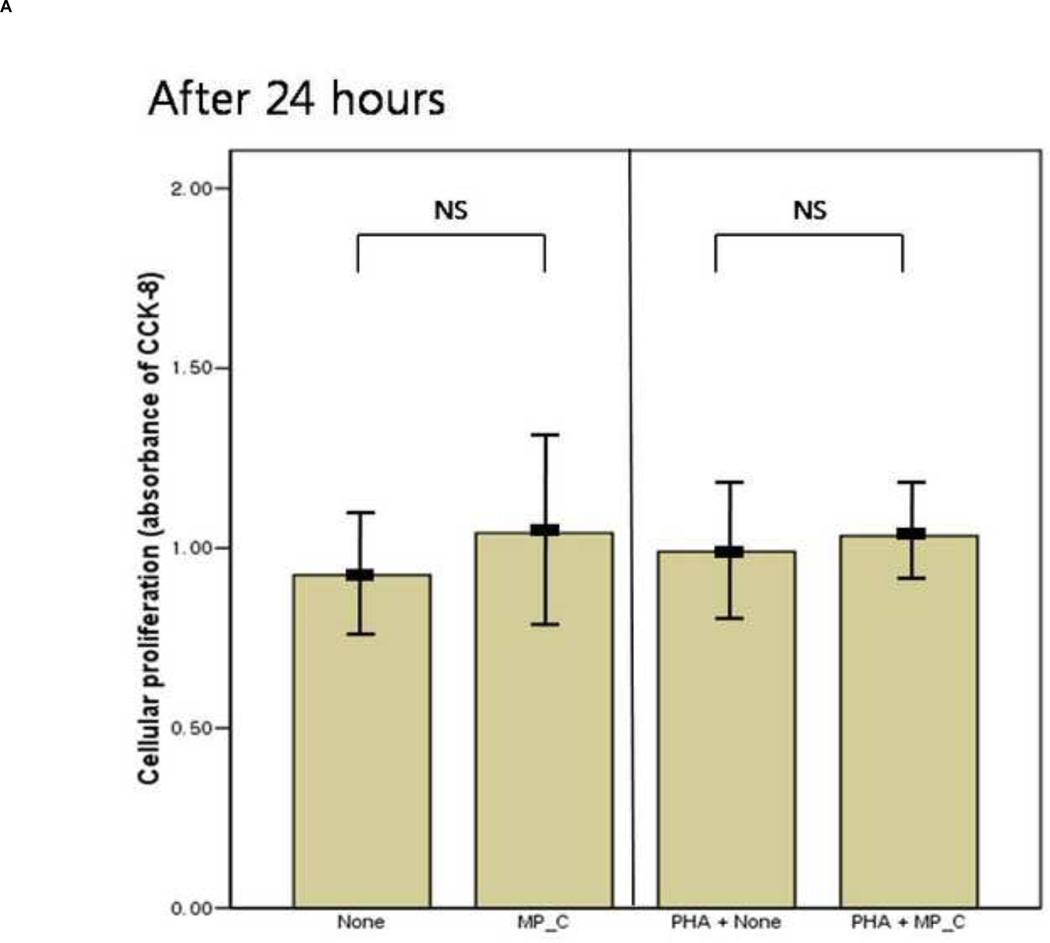
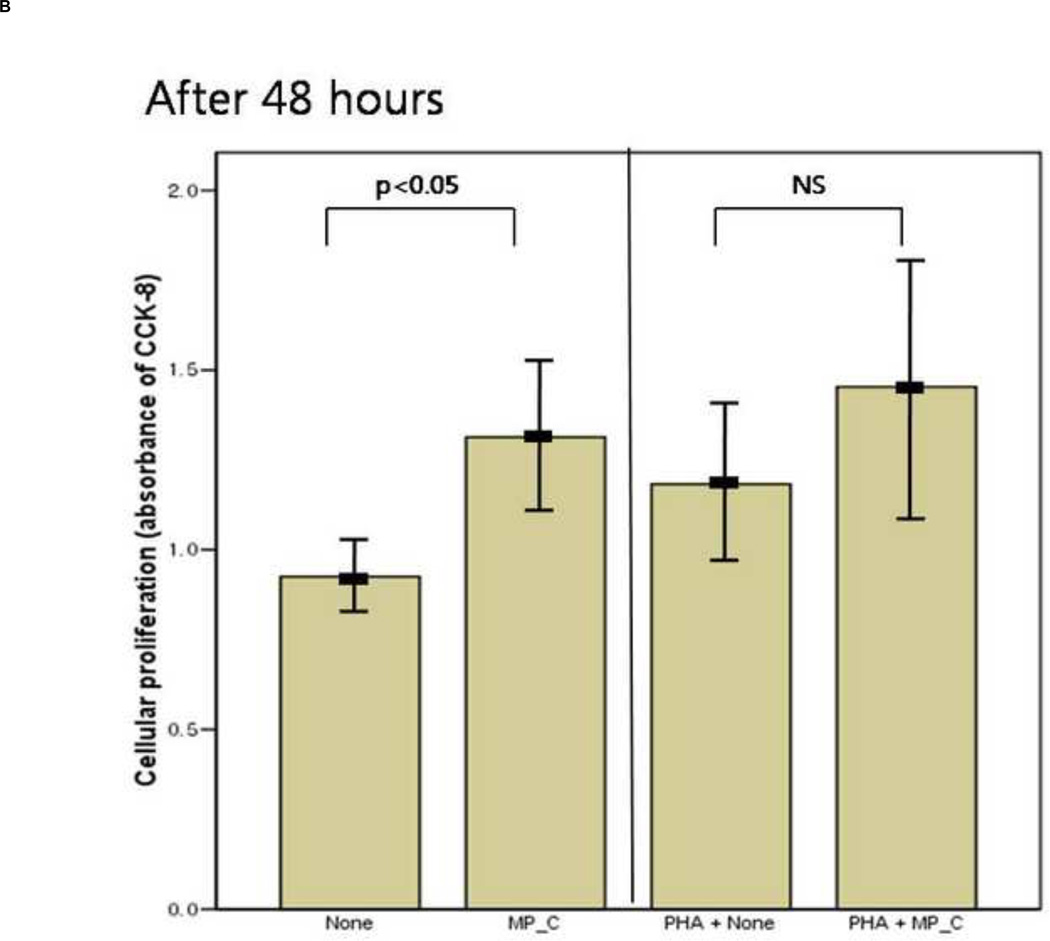
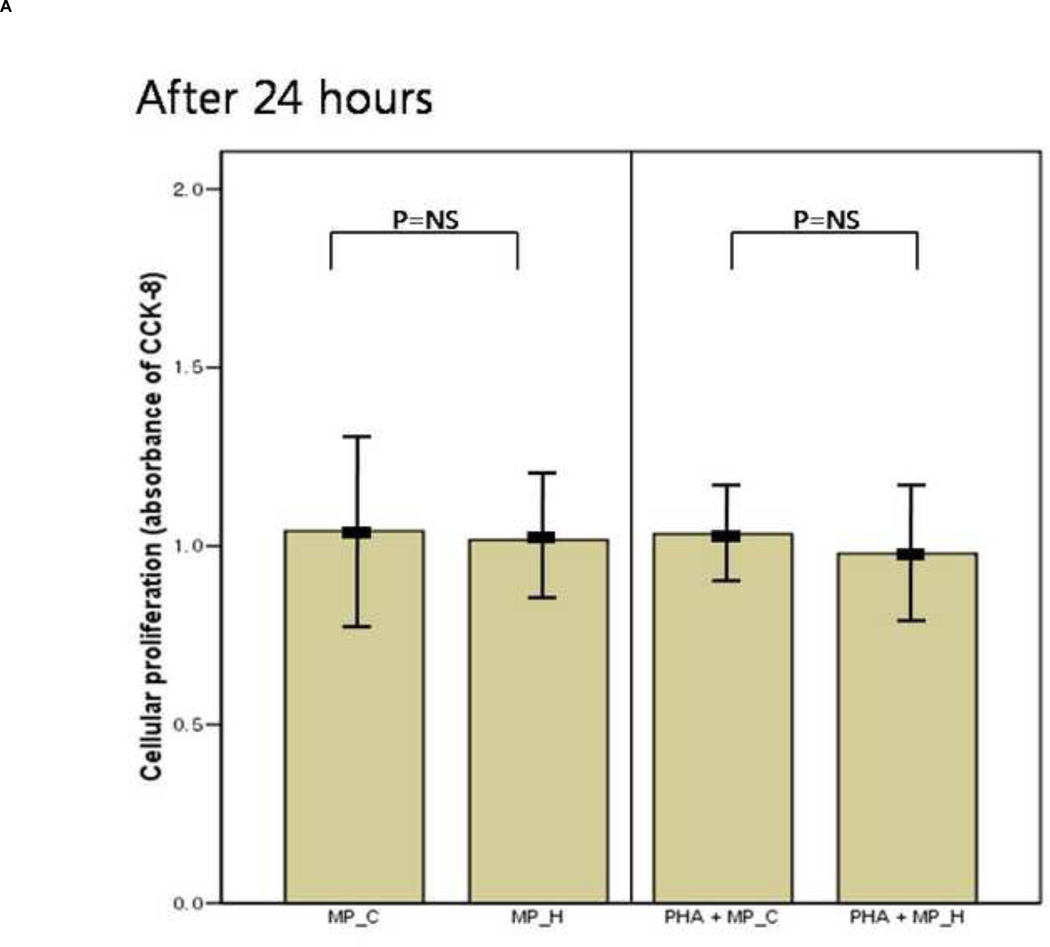
After 24 hours of co-culture, the cytokine (both IL-6 and TNF-a) production and cellular proliferation was not different between PBMCs cultured alone and PBMCs cocultured with MP_C. This was the same in the addition of PHA (Figures 1 and 2). However, after 48 hours of co-culture, the supernatant of PBMCs co-cultured with MP_C had significantly higher concentrations of IL-6 than that of PBMCs cultured alone (Figure 1). Although the supernatant TNF-a concentration of PBMCs co-culture with MP_C was also higher than that of PBMCs cultured alone, this difference did not reach statistical significance. After 48 hours, the cellular proliferation of PBMCs was also increased when co-cultured with MP_C than when cultured alone (Figure 2). In the presence of PHA, the supernatant IL-6 concentration of PBMCs co-cultured with MP_C after 48 hours was also higher, although the cellular proliferation was not increased (Figures 1 and 2).
Figure 1.
The cytokine production of peripheral blood mononuclear cells (PBMCs), after 24-hour and 48-hour culture, in the presence or absence of MP_C, with or without PHA; (A) supernatant concentration of IL-6 after 24-hour culture, (B) supernatant concentration of TNF-a after 24-hour culture, (C) Supernatant concentration of IL-6 after 48-hour culture, and (D) Supernatant concentration of TNF-a after 48-hour culture.
Figure 2.
The mean value of cellular proliferation (absorbance of CCK-8) of peripheral blood mononuclear cells (PBMCs) after 24-hour and 48-hour culture, in the presence or absence of MP_C, with or without PHA; (A) The cellular proliferation after 24-hour culture, and (B) The cellular proliferation after 48-hour culture. Each error bar represents mean±standard deviation.
PBMCs co-cultured with MP_C or MP_H
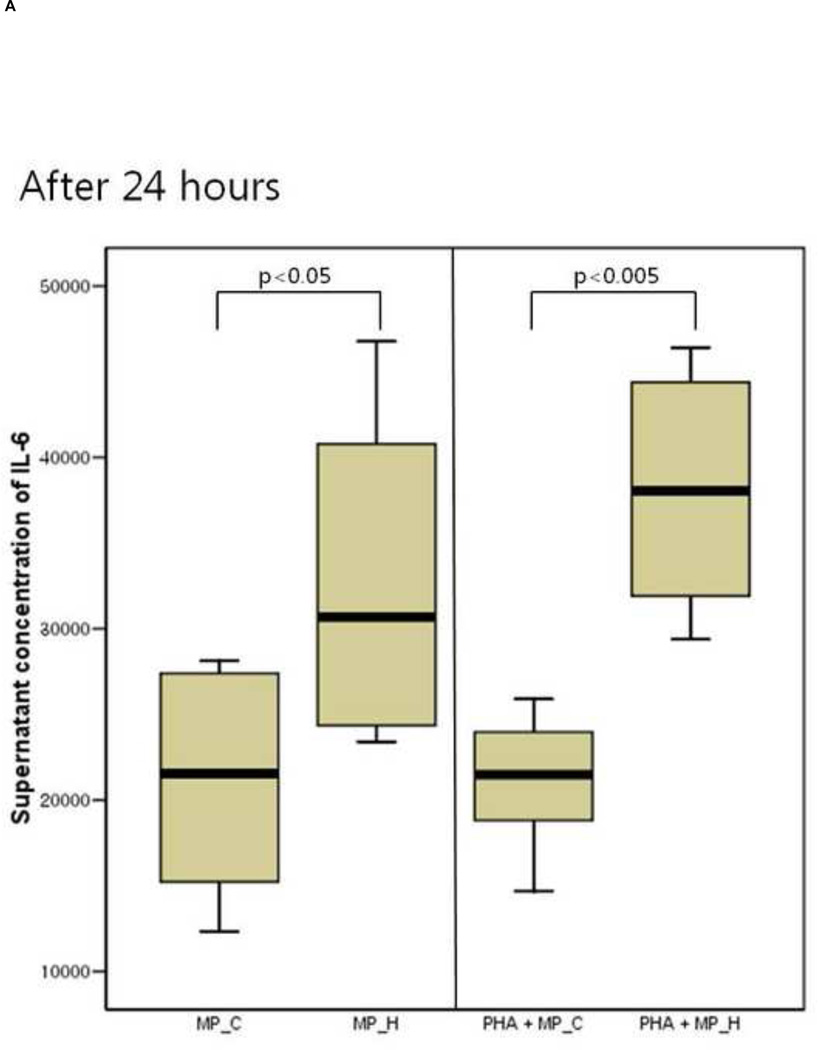
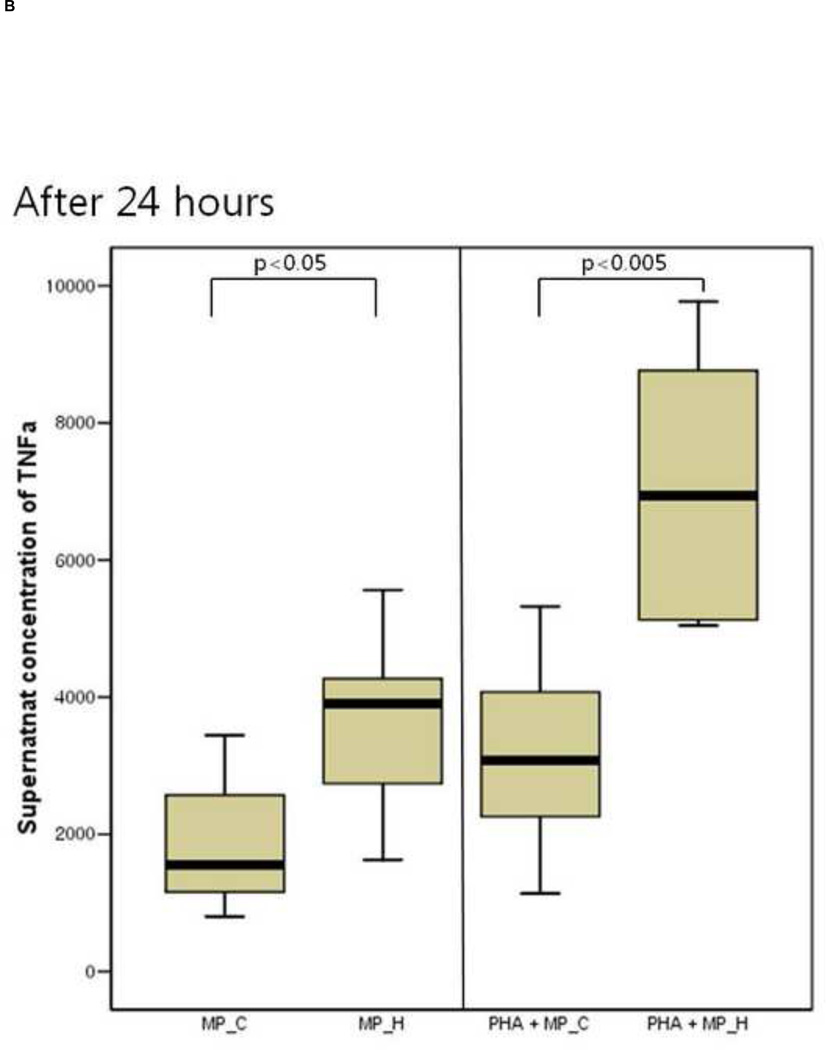
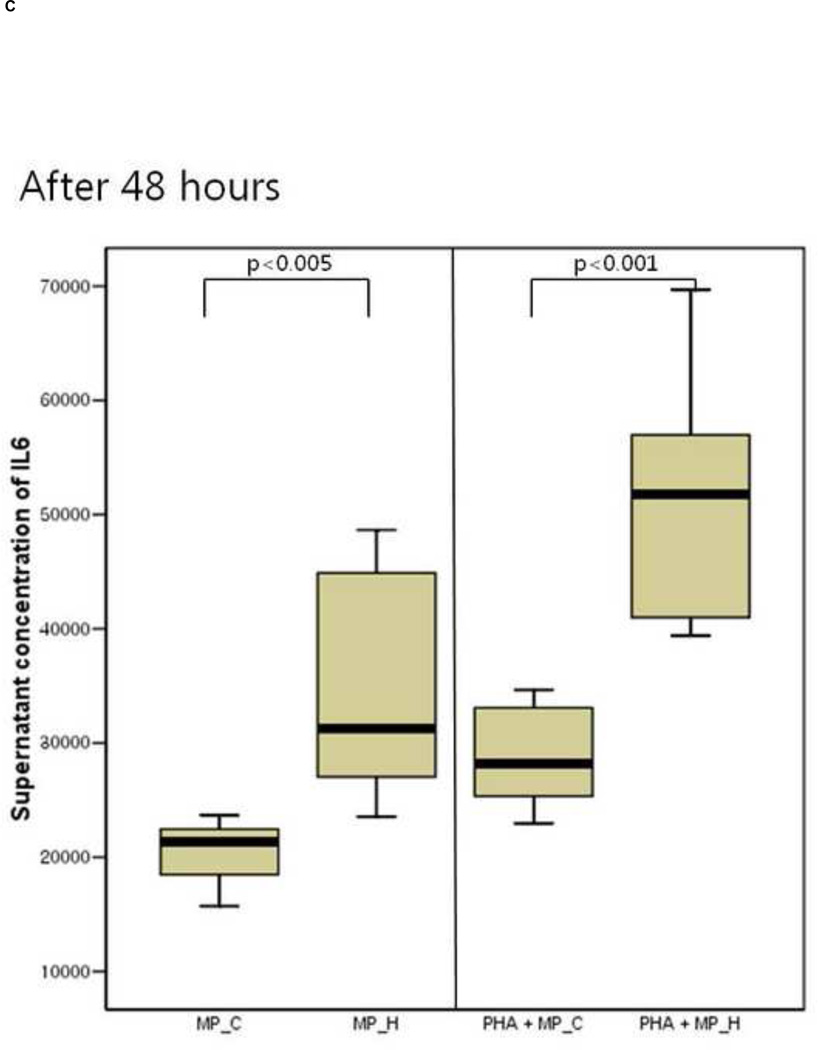
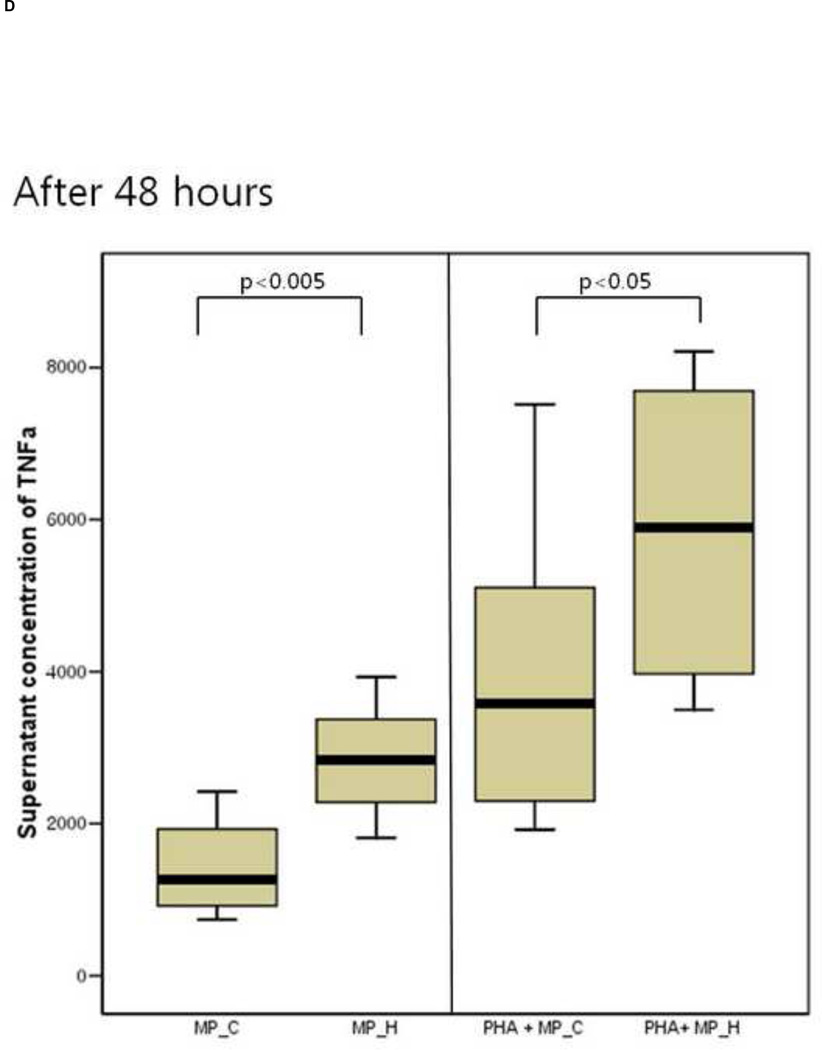
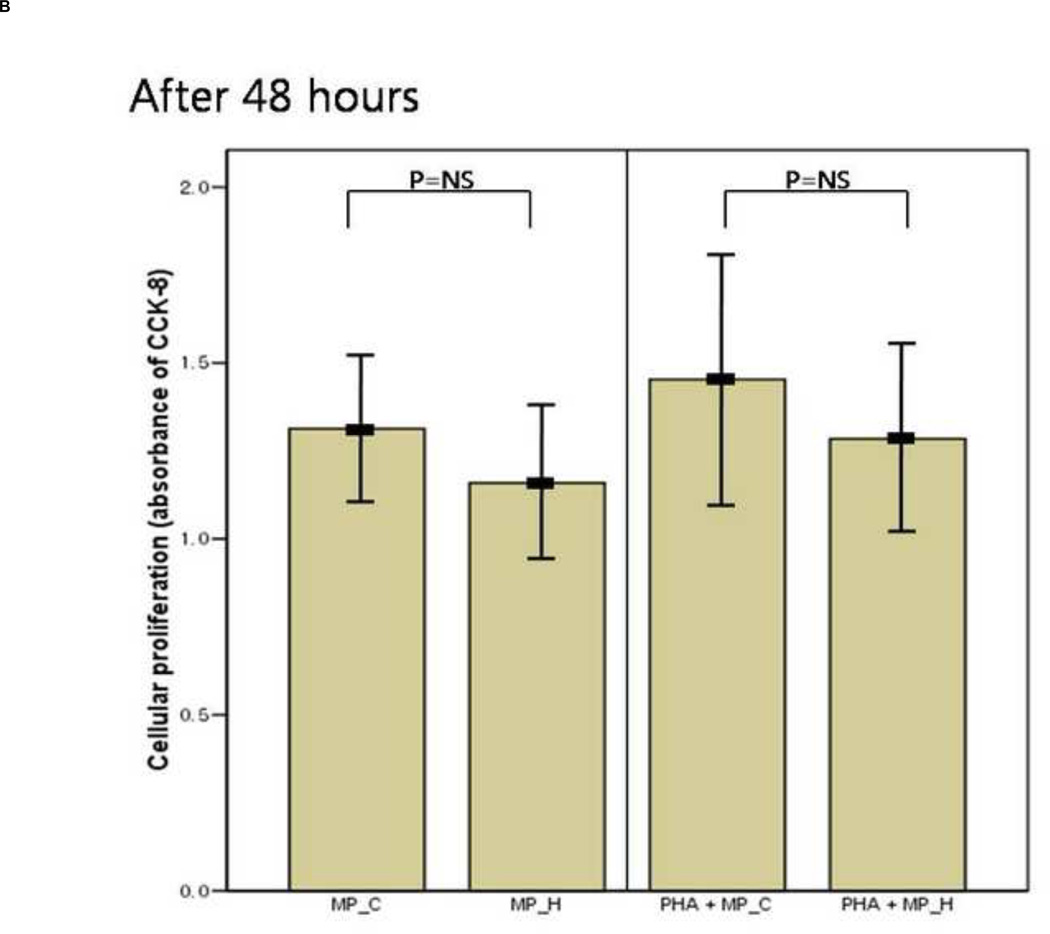
The inflammatory response of PBMCs was compared according to the type of microparticles. The supernatant IL-6 and TNF-a concentration of PBMCs co-cultured with MP_H was significantly higher than that of PBMCs co-culture with MP_C, after both 24 and 48 hours of culture (Figure 3). In the presence of PHA, PBMCs co-cultured with MP_H also showed more intense proinflammatory response. The cellular proliferation was not different between PBMCs cocultured with MP_C and MP_H (Figure 4).
Figure 3.
The cytokine production of peripheral blood mononuclear cells (PBMCs) after 24-hour and 48-hour culture with MP_C or MP_H, with or without PHA; (A) Supernatant concentration of IL-6 after 24-hour culture, (B) Supernatant concentration of TNF-a after 24-hour culture, (C) Supernatant concentration of IL-6 after 48-hour culture, and (D) Supernatant concentration of TNF-a after 48-hour culture.
Figure 4.
The mean value of cellular proliferation (absorbance of CCK-8) of peripheral blood mononuclear cells (PBMCs) after 24-hour and 48-hour culture, with MP_C or MP_H, with or without PHA; (A) The cellular proliferation after 24-hour culture, and (B) The cellular proliferation after 48-hour culture. Each error bar represents mean± standard deviation.
When cultured alone for 24 or 48 hours, neither MP_C nor MP_H in the absence of PBMCs produced any measurable amounts of IL-6 or TNF-a, both in the absence or presence of PHA (data not shown).
Comment
The principal findings of this study
1) The PBMCs co-cultured with MP_C released higher concentrations of IL-6 and showed higher cellular proliferation than PBMCs cultured, alone after 48 hours of culture; 2) The PBMCs co-cultured with MP_H showed more intense inflammatory response (higher concentration of IL-6 and TNF-a) than PBMCs co-cultured with PM_C, and this higher inflammatory response appeared both after 24 hours and 48 hours of co-culture; 3) The cellular proliferation was not different between PBMCs co-cultured with MP_C and MP_H.
Preeclampsia and maternal inflammatory response
The proposed pathogenesis in preeclampsia is the maternal systemic inflammatory response and endothelial activation. It is well known that poor placentation plays an important role in the initiation of this inflammatory process, and it has been postulated that some placental factors are differently released from preeclamptic placenta into maternal circulation20. In this context, several bioactive factors from the syncytiotrophoblast have been shown to be altered in preeclampsia28. These pro-inflammatory placental factors include sFlt-129, endoglin30, placental growth factor31, corticotropin releasing hormone32, Activin-A33, Inhibin A33, Leptin34, and trophoblast microparticles7.
Microparticles in preeclampsia
The finding of the current study indicates that different inflammatory response between microparticles from normal trophoblast and microparticles from hypoxic trophoblast may be the mechanism for the difference between normal pregnancy and preeclampsia. Microparticles from normal trophoblast are pro-inflammatory, but microparticles from hypoxic trophoblast provoke more intense inflammatory response.
This is consistent with the previous suggestions, that normal pregnancy is characterized by a mild systemic inflammatory state, and preeclampsia is associated with more exaggerated systemic inflammatory change35–37. In addition, microparticles from syncytiotrophoblast (STBMs) forming the maternal-placental interface have been thought as candidate for this pro-inflammatory placental factor. Germain et al suggested that STBMs are potential contributors to altered systemic inflammatory responsiveness in pregnancy and preeclampsia, because the inflammatory priming of PBMCs during pregnancy and more enhanced inflammatory response in preeclampsia were confirmed, and STBMs prepared by perfusion of normal placental lobules stimulated production of inflammatory cytokines in PBMCs37. In the study of von Dadelszen et al., endothelial cells co-cultured with STBM (prepared from placenta of normal pregnant women) caused significant activation of peripheral blood leukocytes, in terms of increase in intracellular free ionized calcium, falls in intracellular pH, and release of reactive oxygen species, also suggesting that STBM could activate leukocytes in preeclampsia via endothelial cell activation3. In the study of other investigators38, placental perfusion-derived STBM also significantly induced T cell proliferation and increase in IFNγ release.
But all the above studies did not compare the different stimulatory effect of STBMs between from normal pregnancy and from preeclampsia4, 21. Aly et al examined this difference4, and showed that STBMs isolated from preeclamptic women induced significant greater superoxide production by neutrophils, than STBMs from normal pregnant women. The finding of the current study further indicates that this difference of STBMs from preeclamptic women and normal pregnant women may be attributed to the different characteristics of the trophoblast (normal vs. hypoxic).
In contrast to the current study, other mechanisms for the difference of STBMs between preeclampsia and normal pregnancy have been suggested by other investigators. In the study of Gupta et al., STBMs prepared by different methods (mechanical dissection, villous explants culture, and perfusion of placental cotyledons) showed different effect on both endothelial cells39 and peripheral T lymphocytes38. Placental perfusion-derived STBMs induced enhanced proliferation and IFNγ release of T cell, whereas mechanically- and villous explants-derived STBMs inhibited activation, proliferation, and cytokine release of T lymphocytes38. However, the difference in the mode of preparation of STBMs may not represent the difference between normal pregnancy and preeclampsia.
Elevated concentrations of circulating STBMs in preeclampsia have been observed in several reports, and it has been suggested that this higher concentration may be associated with endothelial dysfunction and maternal inflammatory response in preeclampsia4, 37, 40. However, by flow-cytometric analysis, Lok et al could not find the association between the numbers of microparticles and the severity of preeclampsia, suggesting that the number of microparticles alone does not explain the reported vascular effect of microparticles41. Similarly, in the current study, microparticles from the hypoxic trophoblast induced more intense inflammatory response than microparticles from the normal trophoblast, even in the same concentration of both types of microparticles.
Unanswered questions and further studies
The exact mechanism of the different inflammatory response to microparticles from hypoxic and normal trophoblast was not addressed in the current study. In the study of Pap et al42, among the cells in PBMCs from healthy non-pregnant women, T lymphocytes, but not B lymphocytes or NK cells, were targets of trophoblast-derived microparticles, confirmed by binding of microparticles to T lymphocytes in confocal microscopy. And they also demonstrated that microparticles-lymphocyte interaction induced STAT3 phosphorylation in T cells. The result of the current study also suggests that the targets of hypoxic microparticles might be T lymphocytes, because the pro-inflammatory responses provoked by hypoxic microparticles were more intense in the presence of PHA, which is T cell mitogen. The targets and different mechanism of action of hypoxic microparticles in the pathogenesis of preeclampsia needs further studies.
The endothelial activation is also a key factor in the pathophysiology of preeclampsia3, 43. As previously described, von Dadelzen et al3 showed that human umbilical vein endothelial cells cultured with STBMs activated leukocytes, and suggested that the activation of leukocytes in preeclampsia may be the result of endothelial activation caused by circulating STBMs shed in excess amount from the placenta. The role of hypoxic microparticles in endothelial cell activation also needs to be determined.
We used rotenone to induce chemical hypoxia to represent placental hypoxia in preeclampsia. Huppertz et al21 reported that severe placental hypoxia showed both apoptotic and necrotic characteristics, suggesting aponecrosis. Aponecrosis is a form of cell death with morphologic features of both apoptosis and necrosis in response to hypoxia. And Orozco et al suggested that cells treated with rotenone can mimic this phenomenon26. The type of cell death and characteristics of microparticles also needs to be determined in further studies.
It may be argued that the IL-6 and TNF-a may be also from microparticles besides from PMNC. However, when we cultured microparticles themselves without PMNC in the presence of absence of PHA for 24 and 48 hours, the concentration of supernatant IL-6 and TNF-a from microparticles alone was at negligible level (Data not shown).
In conclusion, microparticles from hypoxic trophoblast induced more intense inflammatory response than those from normal trophoblast, and this difference may be a key mechanism liking placental hypoxia and maternal systemic inflammatory response in preeclampsia.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0000195).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research was presented as a poster at the 32th Annual Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, February 6–11, 2012.
Refernces
- 1.Toth B, Lok CA, Boing A, et al. Microparticles and exosomes: impact on normal and complicated pregnancy. Am J Reprod Immunol. 2007;58:389–402. doi: 10.1111/j.1600-0897.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 2.Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 3.von Dadelszen P, Hurst G, Redman CW. Supernatants from co-cultured endothelial cells and syncytiotrophoblast microvillous membranes activate peripheral blood leukocytes in vitro. Hum Reprod. 1999;14:919–924. doi: 10.1093/humrep/14.4.919. [DOI] [PubMed] [Google Scholar]
- 4.Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol. 2004;190:252–258. doi: 10.1016/j.ajog.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62:191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 7.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and preeclampsia. Placenta. 2008;29(Suppl A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005;11:35–41. doi: 10.1093/molehr/gah129. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DD, Sullivan SA, Eblen AC, Gercel-Taylor C. Modulation of T-cell CD3-zeta chain expression during normal pregnancy. J Reprod Immunol. 2002;54:15–31. doi: 10.1016/s0165-0378(01)00067-5. [DOI] [PubMed] [Google Scholar]
- 10.Gercel-Taylor C, O'Connor SM, Lam GK, Taylor DD. Shed membrane fragment modulation of CD3-zeta during pregnancy: link with induction of apoptosis. J Reprod Immunol. 2002;56:29–44. doi: 10.1016/s0165-0378(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of preeclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 12.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. discussion 23–6. [DOI] [PubMed] [Google Scholar]
- 13.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxiainduced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13:269–275. doi: 10.1007/s11906-011-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 16.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-forgestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe preeclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 19.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppertz B, Kingdom J, Caniggia I, et al. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003;24:181–190. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- 22.Chern SR, Li SH, Chiu CL, Chang HH, Chen CP, Tsuen Chen EI. Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion. Reprod Biol Endocrinol. 2011;9:106. doi: 10.1186/1477-7827-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CP, Chang SC, Vivian Yang WC. High glucose alters proteoglycan expression and the glycosaminoglycan composition in placentas of women with gestational diabetes mellitus and in cultured trophoblasts. Placenta. 2007;28:97–106. doi: 10.1016/j.placenta.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Haase RN, Megnekou R, Lundquist M, Ofori MF, Hviid L, Staalsoe T. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect Immun. 2006;74:3035–3038. doi: 10.1128/IAI.74.5.3035-3038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajashekhar G, Loganath A, Roy AC, Chong SS, Wong YC. Hypoxia up-regulated angiogenin and down-regulated vascular cell adhesion molecule-1 expression and secretion in human placental trophoblasts. J Soc Gynecol Investig. 2005;12:310–319. doi: 10.1016/j.jsgi.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Orozco AF, Jorgez CJ, Horne C, et al. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am J Pathol. 2008;173:1595–1608. doi: 10.2353/ajpath.2008.080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Distler JH, Huber LC, Hueber AJ, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–741. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 28.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 31.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 32.Perkins AV, Linton EA, Eben F, Simpson J, Wolfe CD, Redman CW. Corticotrophin-releasing hormone and corticotrophin-releasing hormone binding protein in normal and pre-eclamptic human pregnancies. Br J Obstet Gynaecol. 1995;102:118–122. doi: 10.1111/j.1471-0528.1995.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 33.Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet. 1997;349:1285–1288. doi: 10.1016/s0140-6736(96)09264-1. [DOI] [PubMed] [Google Scholar]
- 34.Mise H, Sagawa N, Matsumoto T, et al. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998;83:3225–3229. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- 35.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 36.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 37.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 38.Gupta AK, Rusterholz C, Holzgreve W, Hahn S. Syncytiotrophoblast micro-particles do not induce apoptosis in peripheral T lymphocytes, but differ in their activity depending on the mode of preparation. J Reprod Immunol. 2005;68:15–26. doi: 10.1016/j.jri.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Gupta AK, Rusterholz C, Huppertz B, et al. A comparative study of the effect of three different syncytiotrophoblast micro-particles preparations on endothelial cells. Placenta. 2005;26:59–66. doi: 10.1016/j.placenta.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Goswami D, Tannetta DS, Magee LA, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Lok CA, Van Der Post JA, Sargent IL, et al. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:344–360. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 42.Pap E, Pallinger E, Falus A, et al. T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta. 2008;29:826–832. doi: 10.1016/j.placenta.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Vanwijk MJ, Svedas E, Boer K, Nieuwland R, Vanbavel E, Kublickiene KR. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol. 2002;187:1686–1693. doi: 10.1067/mob.2002.127905. [DOI] [PubMed] [Google Scholar]