Abstract
Background
Infection is common following stroke and adversely affects outcome. Previous studies suggest that interleukin-1 receptor antagonist (IL-1ra) and single nucleotide polymorphisms (SNPs) in the IL1RN gene might influence the risk of post-stroke infection and outcome. In this study, we addressed the effects of the rs4251961 SNP in IL1RN on infection risk and outcome.
Methods
Subjects with acute ischemic stroke were enrolled within 72 hours of symptom onset and followed up to one year. Plasma IL-1ra was measured at multiple time points and outcome assessed at 1, 3, 6, and 12 months. Active surveillance for infection occurred while subjects were hospitalized. Subjects were genotyped for the IL1RN rs4251961 polymorphism.
Results
In this population of 113 subjects, those with the minor C allele of rs4251961 polymorphism in IL1RN were more likely to be Caucasian, hypertensive and to have coronary heart disease. Higher plasma IL-1ra was associated with an increased risk of infection (other than pneumonia) and the minor C allele of rs4251961 was independently associated with a decreased risk of infection (other than pneumonia). Initial plasma IL-1ra was not predictive of long term outcome, but patients with the minor C allele of rs4251961 were more likely to experience good (modified Rankin Score <2) long-term outcome.
Conclusions
These data indicate that IL-1ra and IL1RN may influence the risk of infection after stroke, but this influence seems limited to infections other than pneumonia. Further studies are needed to better understand the complexities of immune regulation on infection and outcome after stroke.
Keywords: IL-1ra, IL1RN, stroke, infection, outcome
Interleukin-1 receptor antagonist (IL-1ra) is an endogenous immunomodulatory cytokine encoded by IL1RN on chromosome 2 that inhibits the actions of IL-1α and IL-1β [1, 2]. IL-1ra is secreted by cells of the immune system as well as a variety of other cell types; it is also considered to be an acute phase reactant and is secreted by the liver [3]. In animal studies, exogenous administration of IL-1ra or over expression of IL-1ra improves outcome from experimental stroke [4]. In a clinical study, we found that elevated plasma IL-1ra early after stroke independently predicted infection within the first 15 days after stroke [5]. Importantly, infection is an independent predictor of poor outcome after stroke [6, 7]. Polymorphisms in IL1RN that affect the production of IL-1ra thus have the potential to influence infection risk and stroke outcome. The minor IL1RN*2 allele of the variable number tandem repeat (VNTR) of IL1RN, for instance, is reported to be associated with increased IL-1ra production in most studies [8–11]. There are some studies, however, in which IL1RN*2 is associated with decreased IL-1ra production, an increase in inflammation and inflammatory diseases, as well as cancer [12–17]. These observations suggest that the genetic regulation of IL-1ra and inflammation is thus complex and likely context dependent.
In a study of 391patients with ischemic stroke, those that were homozygous for IL1RN*2 had better neurological outcomes, but the allele was associated with an increased risk of early death [18]. The authors hypothesized that IL1RN*2 was associated with increased IL-1ra (although IL-1ra was not assessed), and IL-1ra is neuroprotective experimental stroke. Further, the increase in early death was attributed to infection, although not infections were explicitly tracked. The minor C allele of the single nucleotide polymorphism (SNP) rs4251961 SNP in IL1RN is associated with lower concentrations of plasma IL-1ra and increased IL-1β and C-reactive protein (CRP) [19, 20]. In this study we sought to determine whether plasma IL-1ra concentrations after stroke were predictive of outcome and whether the rs4251961 polymorphism in IL1RN influenced the risk of post-stroke infection risk or stroke outcome.
Materials and Methods
Research Subjects
Patients with ischemic stroke admitted to Harborview Medical Center from 9/2005 through 5/2009 who were at least 18 years of age were enrolled within 72 hours of symptom onset. Individuals with ongoing therapy for malignancy, known history of HIV, hepatitis B or C, history of brain tumor, anemia (hematocrit<35 on admission), and those taking immunomodulatory medications were excluded. Blood was drawn as soon as possible after stroke onset and at 3, 7, 30, 90, 180 and 365 days. Blood was also drawn from 40 volunteers to determine normative data for IL-1ra. All aspects of this study were approved by the University of Washington Institutional Review Board; subjects with stroke or their surrogates as well as control subjects provided informed consent.
Clinical Data
Clinical and demographic data were collected on all subjects with stroke. Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score and outcome by the modified Rankin Scale (mRS). There was active ascertainment of infection in patients. Infection was defined as clinical symptoms of an infection (including fever as well as pyuria for urinary tract infection [UTI], productive cough and radiographic evidence of consolidation for pneumonia [PNA]) and positive culture data). The date of infection onset was considered to be the date of symptom onset. Antibiotic therapy (ABX) was used as appropriate to treat infections (prophylactic ABX therapy was not used). MRI was done as part of usual clinical care (generally within 24 hours of admission). Total infarct volume on diffusion weighted MRI imaging was calculated by the ABC/2 method [21]. Outcome was assessed in person at 1, 3, 6 and 12 months after stroke.
Laboratory Studies
Plasma was frozen at −80° within an hour after blood draw; the concentration of circulating IL-1ra was measured using a cytometric bead-based system (Fluorokine MAP®; R&D Systems). The sensitivity of the assay was 2.23 pg/mL.
IL1RN SNP Genotyping
DNA was extracted from blood plasma samples using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, California) per manufacturer’s protocols. Genotyping for the IL1RN rs4251961 was carried out using TaqMan SNP Genotyping Assay Sets and Master Mix (Applied Biosystems, Carlsbad, California). In brief, 2 ng of sample DNA was genotyped per manufacturer’s protocols on StepOnePlus™ Real-Time PCR System (Applied Biosystems) under the following cycling conditions: 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. An allelic discrimination plot was then generated using StepOne Software v2.0 (Applied Biosystems). All samples were processed in triplicate. The reproducibility of the plasma-based PCR genotyping method was confirmed by carrying out identical PCR-based genotyping on DNA extracted from isolated leukocytes in a subset (n=42) of subjects. In these 42 subjects there was 100% concordance between the plasma-based and leukocyte-based samples.
Statistics
Descriptive data for continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR). Data for categorical variables are presented as percentages. Group comparisons are performed using analysis of variance (ANOVA) and the t-tests for parametric data and the Kruskal-Wallis H test or Mann-Whitney U test for non-parametric data. Categorical data are compared using the χ2 test statistic. Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI) for the effect of plasma IL-1ra and the rs4251961 SNP in IL1RN on infection risk and outcome. Good outcome was defined as mRS<2. Significance was set at P<0.05.
Results
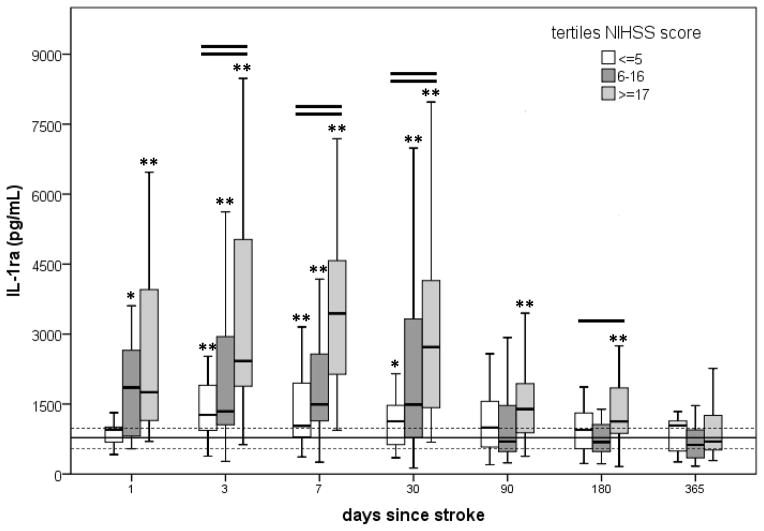
Genotyping was possible in 113 of the 114 subjects enrolled in this study. Among these 113 subjects there was a rapid and sustained elevation of IL-1ra after stroke (Figure 1). Subjects with the most severe strokes (NIHSS≥17) had the highest plasma IL-1ra. IL-1ra concentrations were the highest on day 7 after stroke, but remained elevated in comparison to the control population until at least 180 days after stroke. The concentrations of IL-1ra in plasma were more highly correlated to clinical stroke severity (NIHSS score) than infarct volume at all-time points; at 3 days after stroke onset, the correlation between IL-1ra and NIHSS score was ρ =0.399, P<0.001 and the correlation between IL-1ra and infarct volume was ρ =0.265, P<0.010. Subsequent models were thus corrected for the NIHSS score.
Figure 1.
Changes in plasma IL-1ra over time as a function of stroke severity. Values are depicted as the median and interquartile range. The solid horizontal line represents the median value for the control population, the dashed lines represent the interquartile range. One horizontal line over the box plots at a given time point indicates that the groups differ from each other at P<0.05; two horizontal lines indicates that the groups differ from each other at P<0.01 (Kruskal-Wallis H test). An asterisk (*) indicates that the group differs from controls at P<0.05; ** indicates that the group differs from controls at P<0.01 (Mann-Whitney U test).
The IL1RN allele frequencies for rs4251961 did not differ from the Hardy-Weinberg equilibrium among our subject population: TT (49%), TC (36%), CC (15%). The characteristics of these subjects are detailed in Table 1. Subjects with the minor (C) allele of the rs4251961 SNP in IL1RN were more likely to be Caucasian, have coronary heart disease (CHD) and hypertension (HTN). The data suggest increased rates of PNA in subjects with the minor C allele, but this increase was not statistically significant. There were no differences in the concentrations of plasma IL-1ra at any time point among subjects with different genotypes (data not shown).
Table 1.
Characteristics of patients with and without the minor (C) allele of the rs4251961 SNP in IL1RN.
| TT 55/113 49% |
TC 41/113 36% |
CC 17/113 15% |
P | TT 55/113 49% |
TC or CC 58/113 51% |
P | |
|---|---|---|---|---|---|---|---|
| patient characteristics | |||||||
| age | 55 (57, 67) | 54 (45, 65) | 65 (52, 71) | NS | 55 (57, 67) | 56 (46, 66) | NS |
| Caucasian | 46/55 (84%) | 39/41 (95%) | 17/17 (100%) | 0.06 | 46/55 (84%) | 56/58 (97%) | 0.02 |
| gender (female) | 16/55 (29%) | 15/41 (37%) | 7/17 (41%) | NS | 16/55 (29%) | 22/58 (38%) | NS |
| prior stroke | 18/55 (27%) | 15/41 (37%) | 7/17 (41%) | NS | 18/55 (27%) | 22/58 (38%) | NS |
| stroke characteristics | |||||||
| NIHSS | 10 (3, 18) | 12 (4, 20) | 10 (4, 22) | NS | 10 (3, 18) | 12 (5, 20) | NS |
| infarct volume (cc) | 6.1 (0.5, 64.2) | 19.8 (4.3, 147.9) | 14.6 (2.5, 47.7) | NS | 6.1 (0.5, 64.2) | 18.6 (3.5, 129.3) | 0.11 |
| stroke risk factors | |||||||
| AF | 9/55 (16%) | 4/41 (10%) | 3/17 (18%) | NS | 9/55 (16%) | 7/58 (12%) | NS |
| CHD | 8/55 (14%) | 12/41 (29%) | 7/17 (41%) | 0.05 | 8/55 (14%) | 19/58 (33%) | 0.02 |
| DM | 11/55 (20%) | 8/41 (20%) | 8/17 (47%) | 0.05 | 11/55 (20%) | 16/58 (28%) | NS |
| HTN | 23/55 (42%) | 24/41 (58%) | 13/17 (76%) | 0.03 | 23/55 (42%) | 37/58 (64%) | 0.02 |
| smoker | 23/55 (42%) | 15/41 (37%) | 4/17 (24%) | NS | 23/55 (42%) | 19/58 (33%) | NS |
| infection by day 15* | |||||||
| any | 14/55 (25%) | 11/40 (28%) | 3/16 (19%) | NS | 14/55 (25%) | 14/56 (25%) | NS |
| PNA | 2/55 (4%) | 5/40 (12%) | 2/16 (12%) | NS | 2/55 (4%) | 7/56 (12%) | 0.09 |
| any but PNA | 12/55 (22%) | 7/40 (18%) | 1/16 (6%) | NS | 12/55 (22%) | 8/56 (14%) | NS |
NIHSS = National Institutes of Health Stroke Scale, AF = atrial fibrillation, CHD = coronary heart disease, DM = diabetes mellitus, HTN = hypertension, PNA=pneumonia.
some patients had more than one infection, which is why the number of PNAs and other infections exceeds any infection; NS=not significant and indicates P≥0.200.
We previously showed that an early elevation in IL-1ra was an independent risk factor for infection in the first 15 days after stroke [5]. The median time to PNA was 5 days; the mean time to any infection was 8 days. The effect of IL-1ra on infection risk was true only for infections other than PNA (Table 2). Despite the lack of difference in IL-1ra concentrations among subjects with different genotypes, we built logistic regression models to test the association between infection and the minor C allele of rs4251961 SNP. The model was controlled for stroke severity (using the NIHSS) as well as those characteristics that differed (P<0.100) among subjects with the different genotypes at baseline (Table 1). The presence of a C allele did not affect the overall risk of infection, but was associated with a decrease in the risk of infections other than PNA (Table 3).
Table 2.
Risk of infection* by day 15 based on plasma IL-1ra concentration at 72 hours after stroke onset. Patients are excluded stepwise based on their time to infection in order to avoid confounding by infection related increases in IL-1ra.
| infection by day 15: | any infection | PNA | any infection but PNA | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| all patients | N=28 | N=9 | N=20 | |||
| IL-1ra (per 1000 pg/mL) | 1.25 (1.06–1.48) | 0.01 | 1.07 (0.92–1.24) | NS | 1.21 (1.05–1.39) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.18 (1.02–1.38) | 0.03 | 0.97 (0.79–1.19) | NS | 1.17 (1.02–1.34) | 0.03 |
| excluding patients with infection to day 1 | N=27 | N=9 | N=19 | |||
| IL-1ra (per 1000 pg/mL) | 1.22 (1.03–1.45) | 0.02 | 1.08 (0.93–1.25) | NS | 1.18 (1.03–1.36) | 0.02 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.17 (1.00–1.36) | 0.04 | 0.98 (0.80–1.22) | NS | 1.15 (1.00–1.32) | 0.05 |
| excluding patients with infection to day 2 | N=23 | N=9 | N=15 | |||
| IL-1ra (per 1000 pg/mL) | 1.26 (1.05–1.51) | 0.01 | 1.08 (0.93–1.25) | NS | 1.23 (1.05–1.41) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.22 (1.04–1.45) | 0.02 | 0.98 (0.79–1.21) | NS | 1.19 (1.02–1.39) | 0.02 |
| excluding patients with infection to day 3 | N=22 | N=8 | N=14 | |||
| IL-1ra (per 1000 pg/mL) | 1.25 (1.05–1.49) | 0.01 | 1.07 (0.92–1.25) | NS | 1.21 (1.05–1.40) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.22 (1.04–1.44) | 0.02 | 0.97 (0.78–1.21) | NS | 1.19 (1.02–1.38) | 0.02 |
| excluding patients with infection to day 4 | N=20 | N=6 | N=14 | |||
| IL-1ra (per 1000 pg/mL) | 1.22 (1.03–1.43) | 0.02 | 1.03 (0.84–1.26) | NS | 1.22 (1.05–1.42) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.20 (1.02–1.42) | 0.02 | 0.89 (0.68–1.18) | NS | 1.20 (1.03–1.41) | 0.02 |
| excluding patients with infection to day 5 | N=16 | N=2 | N=14 | |||
| IL-1ra (per 1000 pg/mL) | 1.22 (1.04–1.44) | 0.01 | 1.06 (0.80–1.40) | NS | 1.22 (1.05–1.42) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.21 (1.03–1.43) | 0.02 | 0.91 (0.64–1.30) | NS | 1.20 (1.02–1.41) | 0.02 |
| excluding patients with infection to day 6 | N=16 | N=2 | N=14 | |||
| IL-1ra (per 1000 pg/mL) | 1.22 (1.04–1.44) | 0.01 | 1.06 (0.80–1.40) | NS | 1.22 (1.05–1.42) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.21 (1.03–1.43) | 0.02 | 0.91 (0.64–1.30) | NS | 1.20 (1.02–1.41) | 0.02 |
| excluding patients with infection to day 7 | N=15 | N=2 | N=13 | |||
| IL-1ra (per 1000 pg/mL) | 1.24 (1.05–1.46) | 0.01 | 1.06 (0.80–1.40) | NS | 1.23 (1.05–1.44) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.25 (1.05–1.49) | 0.01 | 0.91 (0.64–1.30) | NS | 1.23 (1.04–1.45) | 0.02 |
| excluding patients with infection to day 8 | N=14 | N=1 | N=13 | |||
| IL-1ra (per 1000 pg/mL) | 1.23 (1.05–1.45) | 0.01 | 1.04 (0.68–1.60) | NS | 1.23 (1.05–1.44) | 0.01 |
| IL-1ra (per 1000 pg/mL) + NIHSS | 1.24 (1.04–1.48) | 0.02 | NC | NC | 1.23 (1.04–1.46) | 0.02 |
2 patients were excluded from analyses – 1 was infected at the time of stroke onset and the other died before day 15.
OR = odds ratio, CI = confidence interval, IL-1ra = interleukin-1 receptor antagonist, PNA = pneumonia, NIHSS = National Institutes of Health Stroke Scale, NS = not significant (P≥0.200), NC = not calculable.
Table 3.
Risk of infection* by day 15 after stroke for each C allele of the rs4251961 SNP in IL1RN.
| infection by day 15: | any infection N=28 |
PNA N=9 |
infection (not PNA) N=20 |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| C allele | 0.90 (0.49–1.64) | NS | 1.72 (0.78–3.81) | 0.18 | 0.54 (0.24–1.24) | 0.15 |
| C allele + NIHSS | 0.76 (0.36–1.60) | NS | 2.12 (0.70–6.45) | 0.19 | 0.46 (0.19–1.14) | 0.09 |
| C allele + NIHSS + race | 0.72 (0.34–1.53) | NS | 2.02 (0.66–6.18) | NS | 0.48 (0.19–1.19) | 0.11 |
| C allele + NIHSS + race + CHD | 0.57 (0.25–1.31) | 0.19 | 1.87 (0.57–6.08) | NS | 0.38 (0.14–1.00) | 0.05 |
| C allele + NIHSS + race + CHD + HTN | 0.59 (0.25–1.37) | NS | 1.83 (0.56–5.97) | NS | 0.39 (0.14–1.06) | 0.06 |
| C allele + NIHSS + race + CHD + HTN + IL-1ra† | 0.50 (0.21–1.22) | 0.13 | 1.60 (0.49–5.22) | NS | 0.32 (0.11–0.97) | 0.04 |
2 patients were excluded from analyses – 1 was infected at the time of stroke onset and the other died before day 15.
SNP = single nucleotide polymorphism, OR = odds ratio, CI = confidence interval, IL-1ra = interleukin-1 receptor antagonist, NIHSS = National Institutes of Health Stroke Scale, CHD = coronary heart disease, HTN = hypertension, NS = not significant (P≥0.200);
indicates the plasma concentration of IL-1ra at 72 hours after stroke onset.
No effect of early plasma IL-1ra on stroke outcome at any time point was seen using a logistic regression model controlled for known predictors of stroke outcome (age, stroke severity and infection) as well as for characteristics that differed between subjects with different IL1RN genotypes (race, CHD, HTN). The effect of the IL1RN genotype on outcome after stroke was also explored controlling for these same variables (Table 4). At one year after stroke onset, those individuals with a C allele were nearly 8 times more likely (when controlled for initial NIHSS) to experience a good outcome (mRS<2).
Table 4.
The predictive value of the minor C allele of the IL1RN SNP rs4251961 on good outcome (mRS<2) after stroke. N=the number of patients with a good outcome (total number of patients available for assessment at that time point).
| outcome at: | 30 days N=22 (104) |
90 days N=32 (103) |
180 days N=34 (92) |
365 days N=36 (81) |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| C allele | 1.48 (0.57–3.85) | NS | 1.13 (0.49–2.62) | NS | 1.05 (0.45–2.45) | NS | 1.22 (0.51–2.97) | NS |
| C allele + NIHSS | 2.72 (0.85–8.68) | 0.09 | 2.28 (0.71–7.29) | 0.16 | 2.13 (0.63–7.25) | NS | 3.59 (0.88–14.74) | 0.08 |
| C allele + NIHSS + age | 2.78 (0.86–8.99) | 0.09 | 2.31 (0.72–7.45) | 0.16 | 2.14 (0.63–7.27) | NS | 3.62 (0.88–14.83) | 0.07 |
| C allele + NIHSS + age + race | 2.70 (0.82–8.82) | 0.10 | 2.46 (0.74–8.13) | 0.14 | 2.19 (0.63–7.61) | NS | 3.14 (0.73–13.46) | 0.12 |
| C allele + NIHSS + age + race + CHD | 2.16 (0.62–7.46) | NS | 2.12 (0.62–7.23) | NS | 1.66 (0.45–6.04) | NS | 3.00 (0.67–13.37) | 0.15 |
| C allele + NIHSS + age + race + CHD + HTN | 2.49 (0.69–9.02) | 0.17 | 2.50 (0.70–9.00) | 0.16 | 1.86 (0.48–7.13) | NS | 6.52 (1.07–39.83) | 0.04 |
| C allele + NIHSS + age + race + CHD + HTN + infection* | 2.33 (0.63–8.63) | NS | 2.34 (0.62–8.83) | NS | 1.68 (0.43–6.61) | NS | 7.67 (1.02–57.46) | <0.05 |
SNP = single nucleotide polymorphism, mRS = modified Rankin Scale, OR = odds ratio, CI = confidence interval, NIHSS = National Institutes of Health Stroke Scale, CHD = coronary heart disease, HTN = hypertension, NS = not significant (P≥0.200),
denotes infection by day 15 after stroke
Conclusions
In this cohort of 113 subjects with ischemic stroke, we found that those with the minor C allele of the rs4251961 SNP in IL1RN were more likely to be Caucasian and more likely to have CHD and HTN. This observation needs to be confirmed in a larger population, but it is intriguing to speculate that the minor allele of this SNP could contribute to vascular risk factors and vascular damage. In fact, mice that lack IL1RN develop arterial inflammation [22]. The C allele of rs4251961 is associated with decreased ex vivo cellular production of IL-1ra, decreased plasma IL-1ra and increased plasma IL-1β and C-reactive protein in healthy individuals [20, 23]. These observations suggest that the C allele of the rs4251961 SNP is associated with chronic inflammation and may predispose to vascular disease. The fact that we did not detect differences in plasma IL-1ra among subjects with different alleles of the rs4251961 SNP in this study may be related to the relatively small sample size, the possibility that changes in IL-1ra production induced by the acute stroke may have overwhelmed any potential effect of the SNPs, or that genetic regulation of IL-1ra is complex and context dependent.
We previously showed that plasma IL-1ra was an independent predictor of post-stroke infection. We now extend those analyses and show that the increased risk applies only to infections other than PNA. Based on prior reports showing a decrease in IL-1ra in subjects with the C allele of rs4251961, we anticipated that subjects with this allele would experience a decreased risk of infection. The data, however, show no association between the C allele and overall infection risk. Interestingly, similar to the data for IL-1ra, there appears to be a differential effect of the C allele on infection risk depending on the type of infection in question; the C allele was associated with a decrease in the risk of infections other than PNA. Since the risk of PNA is so closely linked to stroke severity and the ability to protect the airway (including the level of consciousness and the degree of dysphagia/risk for aspiration), these observations suggest that the effect of cytokines/genes on infection risk (ie. PNA) might be overpowered by other factors (ie. dysphagia and the risk of aspiration).
There are strong preclinical data suggesting a neuroprotective role for IL-1ra in cerebral ischemia; both overexpression of IL-1ra and exogenous administration of IL-1ra are associated with improved outcome from experimental stroke [4]. Our data show a marked increase in endogenous levels of IL-1ra after stroke onset with an associate increase in infection independent of stroke severity. Exogenous administration of IL-1ra and medications that increase IL-1ra production also appear to be associated with an increased risk of infection [24–26]. In the study by Gromadzka and colleagues, they suggest that the IL1RN*2 allele of the VNTR polymorphism is associated with increased IL-1ra production and suggest that this allele may be associated with better long term outcome because of the neuroprotective properties of IL-1ra [18]. They further suggest that the early increase in mortality associated with the IL1RN*2 allele of the VNTR polymorphism may have been related to infection, although no data about infections was provided. While most publications suggest that the IL1RN*2 allele of the VNTR polymorphism is associated with increased IL-1ra [8–11], there is not universal agreement on this point. The regulation of IL-1ra is likely dependent on a complement of genes as well as environmental context; since IL-1ra was not assessed in this study, the actual mechanism by which IL1RN*2 affected outcome is unclear. We hypothesized that the C allele of rs4251961, which is associated with decreased ex vivo cellular IL-1ra production and decreased plasma IL-1ra in healthy individuals [19, 20], would be associated with worse stroke outcome. On the contrary, subjects with this allele were more likely to experience good outcomes after stroke, although the results were not significant at each time point.
To our knowledge, this is the first study to examine the effects of the rs4251961 SNP in IL1RN on post-stroke infection and stroke outcome. The complexities of understanding how the immune response following stroke modulates the risk of infection and stroke outcome are highlighted. Previously it was shown that endogenous increases in IL-1ra were associated with increased risk of infection and that SNPs in IL1RN that lead to increased IL-1ra production are associated with better outcome in those who remain infection free [5, 18]. In the current study, we showed that the C allele of rs4251961 that leads to decreased IL-1ra production was associated with decreased risk of infection (other than PNA) as predicted, but counter to what was predicted, this SNP was also associated with good outcome. Because the study is relatively small (N=113) and the number of variables entered into the logistic regression models relatively large, the findings will need to be replicated in a larger population. The preliminary data presented here, however, suggest that the genetic profile of individuals may affect the risk of post-stroke infection and influence stroke outcome.
Acknowledgments
We thank all of the individuals who volunteered for participation in this study.
Funding
This research was funded by NINDS 5R01NS049197.
Footnotes
Competing Interests
There are no competing interests to disclose.
References
- 1.Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991;88:1445–51. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinkasserer A, Spurr NK, Cox S, Jeggo P, Sim RB. The human IL-1 receptor antagonist gene (IL1RN) maps to chromosome 2q14-q21, in the region of the IL-1 alpha and IL-1 beta loci. Genomics. 1992;13:654–7. doi: 10.1016/0888-7543(92)90137-h. [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunological reviews. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 4.Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2009;18:269–76. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Tanzi P, Cain K, Kalil A, et al. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14:244–52. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 8.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–10. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28:2598–602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scandinavian journal of immunology. 1998;47:195–8. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Candiotti KA, Yang Z, Morris R, et al. Polymorphism in the interleukin-1 receptor antagonist gene is associated with serum interleukin-1 receptor antagonist concentrations and postoperative opioid consumption. Anesthesiology. 2011;114:1162–8. doi: 10.1097/ALN.0b013e318216e9cb. [DOI] [PubMed] [Google Scholar]
- 12.Tountas NA, Casini-Raggi V, Yang H, et al. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999;117:806–13. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- 13.Rocha AM, De Souza C, Rocha GA, et al. IL1RN VNTR and IL2-330 polymorphic genes are independently associated with chronic immune thrombocytopenia. British journal of haematology. 2010;150:679–84. doi: 10.1111/j.1365-2141.2010.08318.x. [DOI] [PubMed] [Google Scholar]
- 14.Smolonska J, Wijmenga C, Postma DS, Boezen HM. Meta-analyses on suspected chronic obstructive pulmonary disease genes: a summary of 20 years’ research. American journal of respiratory and critical care medicine. 2009;180:618–31. doi: 10.1164/rccm.200905-0722OC. [DOI] [PubMed] [Google Scholar]
- 15.Queiroz DM, Oliveira AG, Saraiva IE, et al. Immune response and gene polymorphism profiles in Crohn’s disease and ulcerative colitis. Inflammatory bowel diseases. 2009;15:353–8. doi: 10.1002/ibd.20757. [DOI] [PubMed] [Google Scholar]
- 16.Vencovsky J, Jarosova K, Ruzickova S, et al. Higher frequency of allele 2 of the interleukin-1 receptor antagonist gene in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:2387–91. doi: 10.1002/1529-0131(200110)44:10<2387::aid-art403>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. Journal of gastroenterology and hepatology. 2010;25:1604–17. doi: 10.1111/j.1440-1746.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 18.Gromadzka G, Sarzynska-Dlugosz I, Czlonkowska A. IL1RN intron 2 polymorphism caused by variable number tandem repeats is associated with 1-year outcome in patients with ischaemic stroke. J Neurol Neurosurg Psychiatry. 2007;78:183–6. doi: 10.1136/jnnp.2006.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafiq S, Stevens K, Hurst AJ, et al. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun. 2007;8:344–51. doi: 10.1038/sj.gene.6364393. [DOI] [PubMed] [Google Scholar]
- 20.Reiner AP, Wurfel MM, Lange LA, et al. Polymorphisms of the IL1-receptor antagonist gene (IL1RN) are associated with multiple markers of systemic inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1407–12. doi: 10.1161/ATVBAHA.108.167437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. The Journal of experimental medicine. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafiq S, Melzer D, Weedon MN, et al. Gene variants influencing measures of inflammation or predisposing to autoimmune and inflammatory diseases are not associated with the risk of type 2 diabetes. Diabetologia. 2008;51:2205–13. doi: 10.1007/s00125-008-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker K, Tanzi P, Kalil A, Shibata D, Cain K. Early statin use is associated with increased risk of infection after stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22:66–71. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol. 2008;15:82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 26.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–72. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]