Abstract
Increasing interest in the role of brain activity in insect motor control requires that we be able to monitor neural activity while insects perform natural behavior. We previously developed a technique for implanting tetrode wires into the central complex of cockroach brains that allowed us to record activity from multiple neurons simultaneously while a tethered cockroach turned or altered walking speed. While a major advance, tethered preparations provide access to limited behaviors and often lack feedback processes that occur in freely moving animals. We now present a modified version of that technique that allows us to record from the central complex of freely moving cockroaches as they walk in an arena and deal with barriers by turning, climbing or tunneling. Coupled with high speed video and cluster cutting, we can now relate brain activity to various parameters of the movement of freely behaving insects.
Keywords: Neuroscience, Issue 86, Central complex, Free walking, Climbing, Brain recording, Tetrode, Fan-shaped body
Introduction
This article describes a successful system for recording from neurons within the central complex (CC) of the cockroach, Blaberus discoidalis, as the insect walks in an arena and deals with objects that cause it to turn around, tunnel under, or climb over obstacles. The wires can also be connected to a stimulator to evoke activity in the surrounding neuropil with consequent behavioral changes.
Over the last decade considerable attention has been directed at the roles played by various brain regions in controlling insect behavior. Much of this focus has been directed toward the midline brain neuropils that are collectively referred to as the central complex (CC). Progress has been made as a result of wide varieties of techniques targeting questions about the role of the CC in behavior. Those techniques range from neurogenetic manipulations, primarily in Drosophila, coupled with behavioral analysis1-3, to electrophysiological techniques that monitor neural activity within the CC and attempt to relate that activity to behaviorally relevant parameters.
Electrophysiological techniques include intracellular recording from individual identified neurons4-9 and extracellular recording, often with multi channel probes10,11. These two techniques are complimentary. Intracellular recording with sharp electrodes or whole cell patch provides very detailed data on identified neurons, but is limited to one or two cells at once, requires limited or no movement, and can be maintained for relatively short periods of time. Extracellular recordings can be easily set up, do not require restraint, and can be maintained for hours. With multi channel tetrodes and cluster cutting, fairly large populations of neurons can be analyzed simultaneously9,12. While whole cell patch has been successfully used in tethered insects13, we feel that there is also a need for techniques that allow us to record neural activity in the brain for long periods of time in freely behaving insects as they deal with barriers to forward movement.
The need to record as the insect moves and bounces up and down pushed us toward extracellular recording methods. We have had good success recording in restrained preparations with commercially available 16 channels silicon probes11, however the small size of even large cockroaches means that the probes have to be mounted off the body. That, coupled with the delicacy of the probe tines, made them inappropriate for a free walking preparation. In two previous projects, we used bundles of fine wires forming a tetrode to accomplish similar recording properties but in a more robust arrangement. These tetrode bundles allowed us to record from tethered cockroaches and relate CC unit activity to changes in walking speed14 and turning behavior resulting from antennal contact with a rod10.
As useful as these tethered preparations have been and will continue to be, they do present some limitations. First, the behaviors that the insect can perform are limited to one plane. That is, we could readily evoke changes in walking speed or turning, but climbing and tunneling actions were not possible, at least with the typical tether arrangement. Second, our tethered preparations are “open loop”. That is, they do not allow for normal movement related feedback to the system. Thus, as the cockroach turned on our tether, its visual world was not altered accordingly. It is possible to build closed loop tether systems to introduce this kind of feedback. However, they are limited by the complexity of the programming and hardware of the simulated visual environment. Nevertheless, we felt that we could improve upon our existing tethered recording methods by recording from the animal as it walked freely in an arena or track and encountered objects as it would in its natural surroundings.
Although wireless systems for recording brain activity15 would be ideal, current systems have limitations in the number of recording channels, time of data acquisition, battery life and weight. We, therefore, opted to try to adapt our tethered recording system for use in freely moving preparations. As better wireless systems become available, this technique can be readily adapted to such devices. The system that is described in this article is light weight, works very well and appears to have little deleterious effect on the cockroach’s behavior. With an inexpensive high speed camera and cluster cutting software, activity in individual brain neurons can be related to movement. Here we describe the preparation of the tetrode wires and their implantation into the insect’s brain as well as recording techniques for electrical activity and motion and how those data can be brought together for subsequent analysis.
Protocol
1. Preparation of Tetrode Wires
Pull out a very thin nichrome wire (12 μm diameter, PAC coating) of about 1.1 m length. Attach a tape tag to each end. Hang the wire over a horizontal threaded rod such that the two ends are at the same height near the benchtop.
Repeat step 1.1 for a second wire, making two more ends for a total of 4, and place it next to the first wire (about 1 cm in between).
Stick the four ends together with a tape tag and attach the tag to a motorized rotating winding device. This device can be made from an inexpensive dc motor.
Wind the tetrode in one direction for 2 min (60 rpm) and unwind it in the opposite direction for 30 sec.
Use a heat gun to fuse the wires together. Do not touch the wires with the gun. Use three up and down passes from alternating directions, with each pass taking about 10 sec.
Cut the top and the bottom of the wound wires. The four wires are twisted and fused together at one end but separate at the other.
Add the supporting tube. Cut a 30 cm length of polyethylene tubing (diameter: inside 0.28 mm, outside 0.61 mm). Thread the tetrode very slowly and carefully into the supporting tube so that it does not kink.
Once the fused end appears out the other side, pull it through so that there is an equal length of wire at both ends of the guide tube.
Grab the separate end of each wire with a forceps. Using the base of the flame of a gas burner, carefully burn the insulation off of the last 2 or 3 mm of each wire. Heat the wire until it glows, but does not curl.
Connect the tetrode with a male-female IC socket adaptor that fits your recording device. Put the deinsulated end of each wire into a different socket of the adaptor with a forceps. Stabilize the wire in the socket with a small brass pin. Use a fine point soldering iron and fill the socket with the melted solder. Be careful to not contact the fragile wire with the soldering iron.
- Check the impedance of each wire and the inter impedance of each pair of wires.
- Place the fused, twisted end into a container of saline and connect a copper wire conductor from the saline to the ohm meter.
- Connect the other end of the meter to the socket pin containing the wire. The impedance of each wire should be below 3 MΩ.
- If the above values are not attained, reattempt the solder connections.
- Remove the wires from the saline, rinse the tips with water, and test the inter wire impedance for each pairing (n=6). The inter impedance should be above 5 MΩ.
- If the above values are not attained, slice a small amount of the tip off at the fused end and retest.
- Discard any wire set that does not meet both of the impedance requirements for all of the wires.
- Secure the tetrode.
- Fold a small rectangular paper box slightly larger than the socket adaptor.
- Transfer the adaptor into the box with the male side at the bottom. Penetrate the box such that all the pins of the male side are outside the box while the rest of the adaptor is inside the box.
- Tape the corners of the box on the outside. Use small pieces of double sided sticky tape on the inside of the box to stabilize any individual strands of wire. The wire should be fused as it exits the box.
- Mix fast set 2 part epoxy and pour into the box to secure the adaptor and all the wires.
- Attach the near end of the guide tubing to one side of the box with dental wax but leave the tubing open such that the tetrode can be pulled through freely at both ends.
- Sharpen the tetrode.
- Before each experiment, cut the tip of the tetrode with a sharp scalpel blade, not scissors. This prevents crushing and splaying of the wire ends while providing a clean flat edge for the next step.
- Use a small rotary tool mounted vertically with medium and fine grit sanding disks (these can be combined on one platform) to polish the tetrode and remove some tip insulation. Hold the bundle near its end with forceps. Tilt the wire set end to a 45° angle relative to the sanding disk and gently touch it to the moderate speed spinning disk for about 1 or 2 sec each on the medium and then the fine grits. Repeat this three more times, axially rotating the bundle 90° each time. It is critical that the direction of spin of the sanding disks is away from the shallow angle of the wire ends, otherwise separation of the wires may occur.
- The desired result transforms the bundle end from a straight edge to a pointed tip with small amounts of insulation removed from the end of each wire. Verify the point using a dissecting microscope before plating the tetrode. If any fraying occurs at the tip, recut and repolish.
- If impedance testing during the subsequent plating step shows extremely low inter wire values (less than 4 MΩ), it indicates too much material removed during the polishing step. Recut and repolish the tetrode.
- Plate the tetrode. Put the tip of the tetrode into a saturated copper sulfate solution (85 ml water, 5 ml sulfuric acid, 50 g copper sulfate). Plate each wire with a current of 2.5 μA with a stimulus isolator. Inject the current for 1 sec, pause for 1 sec and repeat this process 4x.
- Check the impedance of each wire and the interimpedance of each pair of wires. The impedance of each wire should be between 0.5-1 MΩ and the inter impedance should be above 4 MΩ.
- Mount the adaptor onto the headstage of a multichannel recording system.
- Attach a bent insect pin to a micromanipulator. Attach the tip of the tetrode to the insect pin with dental wax
2. Animal Preparation
Anesthetize the cockroach with ice.
After the cockroach stops moving, restrain the cockroach vertically against a flat cork surface with large saddle pins that straddle the insect but do not penetrate any part of its body.
Transfer the preparation into a plastic container and place ice around the animal to minimize blood flow and body movements.
Position a plastic collar at the neck to support the head and place dental wax around the head to stabilize it.
Cut a small window between the ocelli with a razor blade and remove the cuticle from the head.
Remove connective tissues and fat with a forceps to expose the brain.
Place some cockroach saline into the head capsule to cover the brain tissue.
To desheath the brain, use a fine forceps to gently grab the sheath on top of the brain and use another fine forceps to tear the sheath apart in the wire implanted area.
Open a small hole in the head capsule anterior to the brain with an insect pin. Insert a braid of three larger diameters (56 µm) insulated copper wires into the hole to serve as a reference/ground electrode.
Lower the tip of the tetrode to the brain surface with the micromanipulator and position it near the brain region of interest.
Carefully place two small pieces of thin acetate sheet (2 mm x 1 mm), slightly larger than the hole in the head capsule, anterior and posterior to the tetrode.
Turn on the recording system.
Slowly lower the tetrode 150-250 µm below the brain surface depending on the recording quality.
Turn off the recording system.
Move the two pieces of acetate sheet as close to the tetrode as possible without touching it (Figure 1A).
Heat a small spatula or flattened hypodermic needle and put it into dental wax such that there is liquid wax at the tip of the spatula. Carefully touch the far end of each piece of acetate sheet from the tetrode with the spatula so that liquid wax can flow onto each piece and seal the gap between it and the head cuticle.
Repeat step 2.16. Drop a small amount of liquid wax onto the acetate sheet each time. Start the process far away from the tetrode and move gradually towards it. Eventually the tetrode will be anchored by dental wax. Avoid getting hot wax into the cavity and onto the brain.
Use the same method as steps 2.16 and 2.17 to anchor the reference/ground electrode with wax.
Heat the wax that attaches the tetrode to the micromanipulator to release the tetrode from it.
Loop the tetrode into the dental wax on the head to provide a strain relief (Figure 1B).
Cover the strain relief loop with dental wax (Figure 1C).
Carefully remove the constraints and transfer the preparation onto a Petri dish. Restrain the preparation dorsal side up with large saddle pins.
Attach a rod to the pronotum using a glue gun. This is a wooden stick which extends from the pronotum over the abdomen.
Attach the tip of the tetrode tubing to the posterior end of the rod with dental wax.
Anchor the tetrode and the reference/ground electrode to the anterior end of the rod with dental wax.
Pull the tetrode from the socket end of the tubing as much as possible, but do not tug on it, in order to eliminate the chance that the animal may damage the portion of the tetrode outside the tubing (Figure 1D).
Remove all the constraints. Attach the reference/ground electrode to the tetrode tubing with dental wax.
Wait at least 60 min for the animal to recover from the ice anesthesia before any experiment.
3. Experimental Procedures
Connect a PC with both the recording system and an LED light using a USB to serial port cable.
Start neural recordings.
Start video recordings at 20 frames per second for walking experiments using the Motmot image acquisition package16 or 120 fps for climbing experiments using a high speed camera.
Place the cockroach into a 40 cm x 40 cm Plexiglas arena for walking experiments or a 58 cm long, 5 cm wide, and 5 cm high arena for climbing experiments. The walking arena has a transparent barrier extending from the middle of the right wall to the center of the arena, above which the headstage is located. The barrier is used to prevent animals from walking in areas where the camera view is blocked by the headstage. The climbing arena has an acrylic block (either 1.2 cm or 1.8 cm high, and 5 cm wide) or a shelf located at a comparable height in the center.
Generate a TTL pulse from the PC using a customized MATLAB command. (s=serial('COM4'); fopen(s); s.RequestToSend='off'/s.RequestToSend='on'/; fclose(s); delete(s);). The TTL pulse generates a timestamp for the recording system and either turns on or turns off the LED light.
Allow the cockroach to explore the arena until it stops moving for more than 30 seconds for walking experiments. Allow the cockroach to either climb over the block/shelf or tunnel through the shelf for climbing experiments.
Stop video recordings.
Stop neural recordings.
Write down the timestamp generated by the TTL pulse.
Remove the cockroach from the arena and wait at least 3 min.
Repeat steps 3.2-3.10 for the next trial.
Once all recordings have been completed, pass 5 sec of 5 µA DC current through one of the wire tips (anode) and the reference electrode (cathode) to deposit copper into the brain at the wire tip.
4. Offline Analysis
Synchronize video and neural data by linking the frame where the LED light is switched and the timestamp recorded by the recording system at that moment.
Mark wire tip locations. Use Timms intensification procedures to precipitate and observe the copper in 12 µm serial sections17. Prominent deposits should be visible in 3-8 adjacent sections (about 18-48% of the length of the dorsal ventral plane of the area we record from) (Figure 2).
Correlate specific electrical impulses to the activity of single neurons. Follow spike sorting procedures laid out in detail elsewhere10,14,18. Use the program KlustaKwik (version 1.5, author K. Harris, Rutgers University) to generate initial, automated clustering. Import them into the program MClust (version 3.5, authors A.D. Redish et al., University of Minnesota) for further refinement and analysis (Figure 3).
Track the cockroach’s movements. For walking experiments, extract the position of the cockroach’s (visual) center of mass and its body orientation in each frame of the video recordings using the Caltech Multiple Fly Tracker (version 0.1.5.6; http://ctrax.sourceforge.net/) and the associated FixErrors toolbox for MATLAB19. For climbing experiments, extract the position of the block and the cockroach’s head and pronotum in each frame of the video using motion analysis software package.
Representative Results
We recorded the neural activity of 50 units from the CC in 27 preparations for walking experiments. For 15 of those preparations (23 units), climbing experiments were also performed. Individual units are named according to preparation and unit numbers (e.g. ʻunit 1-2ʼ indicates preparation 1, unit 2).
Snapshots of the video of one climbing trial are shown in Figure 4. The entire video is available in supplemental Video 1 (The sound is from unit 1-2). The recording was made in the right fan-shaped body (FB). The cockroach stopped walking when it encountered the block and used its antennae to assess the block (Figures 4A-C). Then the cockroach raised the front of its body, changing the body substrate angle (Figures 4D-E), before it swung its leg toward the top of the block and climbed over it (Figures 4F-I). The speed and height of the cockroach as well as the instantaneous firing rate of the two sorted units from the first to the current frame are shown above each frame. The instantaneous firing rate was calculated by smoothing spike times of each unit using a Gaussian kernel with a width of 50 msec. The firing rate of unit 1-1 increased during climbing and the increase of firing rate preceded the increase of speed (Figure 4I). Unit 1-2 was silent before climbing but started to fire after climbing was initiated (Figure 4I). The spikes of the two sorted units within 1 sec of the current frame are displayed below each frame. The orange line indicates the time covered by each frame and the blue rectangle indicates twice the width of the kernel that was used to calculate the instantaneous firing rate for the current frame.
One snapshot of the video of one arena exploration trial is shown in Figure 5A. The entire video is available in supplemental Video 2 (The sound is from unit 2-1). The recording was made in the middle FB. The position of the cockroach and its body orientation in each frame were extracted using Ctrax and used to calculate forward and heading speed as well as instantaneous firing rate. The trajectory of the cockroach in the entire video is shown in Figure 5B. Each black dot indicates the position of the cockroach in each frame and the path is color coded with the instantaneous firing rate of unit 2-1. As we recorded each trial at a constant frame rate (i.e. 20 fps), the longer the distance between two dots, the faster the speed at that time. The firing rate of unit 2-1 increased when the cockroach started to walk and was correlated with walking speed. In order to examine the tuning of individual units to the animal’s locomotion state (i.e. speed and direction), we constructed firing rate maps based on forward walking speed and turning speed for each unit. For many CC units, increased firing rate was restricted to specific locomotion states. For instance, unit 2-1 was tuned to forward walking irrespective of turning speed (Figure 5C).
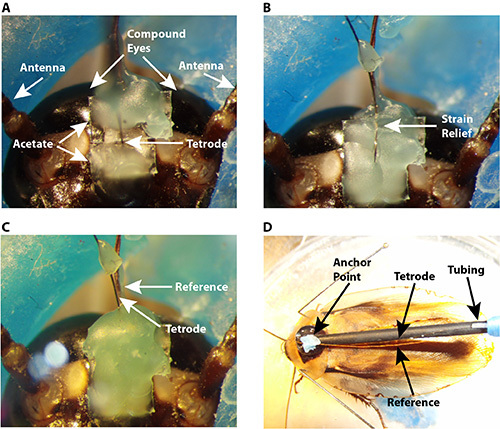 Figure 1. Photos of animal preparation. A-C Frontal view of the cockroach head capsule. A. Two pieces of acetate sheet were placed close to the tetrode to provide the base for wax. B. A strain relief was create d by bending the tetrode into wax. C. The tetrode was fully covered by dental wax. D. Dorsal view of the cockroach body. A wooden rod was attached to the animal’s pronotum and the tetrode tubing was attached to the rod. The tetrode and the reference/ground electrode were further secured by attaching them to the anterior of the rod. Click here to view larger image.
Figure 1. Photos of animal preparation. A-C Frontal view of the cockroach head capsule. A. Two pieces of acetate sheet were placed close to the tetrode to provide the base for wax. B. A strain relief was create d by bending the tetrode into wax. C. The tetrode was fully covered by dental wax. D. Dorsal view of the cockroach body. A wooden rod was attached to the animal’s pronotum and the tetrode tubing was attached to the rod. The tetrode and the reference/ground electrode were further secured by attaching them to the anterior of the rod. Click here to view larger image.
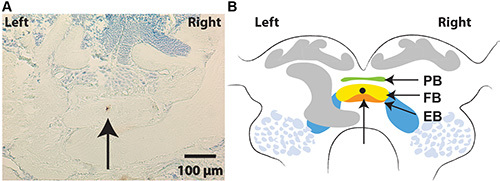 Figure 2. Mark wire tip locations. A. A section of the brain of preparation no 2, showing one brown copper deposition site in the fan shaped body (FB). B. Schematic drawing of the CC and the wire tip location. PB, protocerebral bridge; FB, fan-shaped body; EB, ellipsoid body. Click here to view larger image.
Figure 2. Mark wire tip locations. A. A section of the brain of preparation no 2, showing one brown copper deposition site in the fan shaped body (FB). B. Schematic drawing of the CC and the wire tip location. PB, protocerebral bridge; FB, fan-shaped body; EB, ellipsoid body. Click here to view larger image.
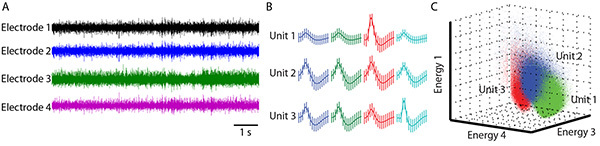 Figure 3. A typical tetrode recording.A. Raw voltage traces from single electrodes within one tetrode bundle. Note the difference of the voltage traces among different electrodes. B. Three units were sorted using MClust. C. 3-dimensional view of the waveform energy as recorded on three of the four electrodes. Each dot is a single threshold event, color coded by the cluster it was ultimately assigned to. Click here to view larger image.
Figure 3. A typical tetrode recording.A. Raw voltage traces from single electrodes within one tetrode bundle. Note the difference of the voltage traces among different electrodes. B. Three units were sorted using MClust. C. 3-dimensional view of the waveform energy as recorded on three of the four electrodes. Each dot is a single threshold event, color coded by the cluster it was ultimately assigned to. Click here to view larger image.
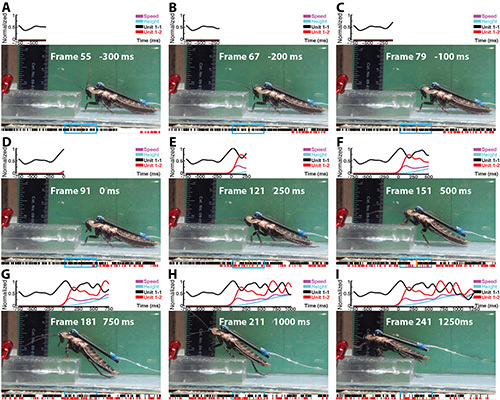 Figure 4. Snapshots of the video of one climbing trial. Above each frame: normalized speed, height of the cockroach as well as instantaneous firing rate of the two sorted units from the first to the current frame. Time 0 indicates the onset of climbing. Firing rate was normalized from 0-1, and speed and height were normalized from 0-0.5 for display purposes. Below each frame: the spikes of the two sorted units within 1 sec of the current frame. The orange line indicates the time covered by each frame and the blue rectangle indicates twice the width of the kernel that was used to calculate the instantaneous firing rate for the current frame. Individual units were named according to preparation and unit numbers (e.g. “unit 1-2” indicates preparation 1, unit 2). Click here to view larger image.
Figure 4. Snapshots of the video of one climbing trial. Above each frame: normalized speed, height of the cockroach as well as instantaneous firing rate of the two sorted units from the first to the current frame. Time 0 indicates the onset of climbing. Firing rate was normalized from 0-1, and speed and height were normalized from 0-0.5 for display purposes. Below each frame: the spikes of the two sorted units within 1 sec of the current frame. The orange line indicates the time covered by each frame and the blue rectangle indicates twice the width of the kernel that was used to calculate the instantaneous firing rate for the current frame. Individual units were named according to preparation and unit numbers (e.g. “unit 1-2” indicates preparation 1, unit 2). Click here to view larger image.
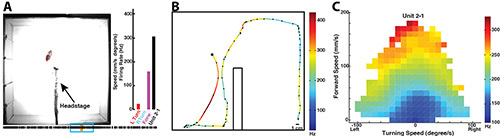 Figure 5. One snapshot of the video of one arena exploration trial A. The red oval line indicates the shape of the cockroach in that frame and the red dashed line indicates the position of the cockroach’s center of mass in the previous 10 frames. Right: turning and forward walking speed as well as the instantaneous firing rate of unit 2-1 at that frame. Below: the spikes of unit 2-1 within 4 sec of the current frame. As in Figure 4, the orange line indicates the time covered by each frame and the blue rectangle indicates twice the width of the kernel that was used to calculate the instantaneous firing rate for the current frame. B. The trajectory of the cockroach in the entire video. The large black dot indicates the starting point of the cockroach and each small black dot indicates the position of the cockroach in each frame. The trajectory was color coded with the instantaneous firing rate of unit 2-1, from blue (low) to red (high). C. The firing rate map of unit 2-1. For the entire experiment, forward and turning speed as well as spike times were smoothed using a Gaussian kernel with a width of 150 msec and were divided into nonoverlapping 50 msec long sections. For each divided section, a velocity vector was generated by averaging forward and turning speed within that period respectively. Firing rate for each velocity vector was also calculated. All velocity vectors were binned (10 mm/sec for forward walking speed and 10 degree/sec for turning speed) and a firing rate map was generated by overlaying the averaged firing rate for each bin obtained by averaging all the firing rates whose corresponding velocity vectors fell into that bin. X axis is the turning speed and y axis is the forward walking speed. Positive turning speed indicates right turning and negative turning speed indicates left turning. Click here to view larger image.
Figure 5. One snapshot of the video of one arena exploration trial A. The red oval line indicates the shape of the cockroach in that frame and the red dashed line indicates the position of the cockroach’s center of mass in the previous 10 frames. Right: turning and forward walking speed as well as the instantaneous firing rate of unit 2-1 at that frame. Below: the spikes of unit 2-1 within 4 sec of the current frame. As in Figure 4, the orange line indicates the time covered by each frame and the blue rectangle indicates twice the width of the kernel that was used to calculate the instantaneous firing rate for the current frame. B. The trajectory of the cockroach in the entire video. The large black dot indicates the starting point of the cockroach and each small black dot indicates the position of the cockroach in each frame. The trajectory was color coded with the instantaneous firing rate of unit 2-1, from blue (low) to red (high). C. The firing rate map of unit 2-1. For the entire experiment, forward and turning speed as well as spike times were smoothed using a Gaussian kernel with a width of 150 msec and were divided into nonoverlapping 50 msec long sections. For each divided section, a velocity vector was generated by averaging forward and turning speed within that period respectively. Firing rate for each velocity vector was also calculated. All velocity vectors were binned (10 mm/sec for forward walking speed and 10 degree/sec for turning speed) and a firing rate map was generated by overlaying the averaged firing rate for each bin obtained by averaging all the firing rates whose corresponding velocity vectors fell into that bin. X axis is the turning speed and y axis is the forward walking speed. Positive turning speed indicates right turning and negative turning speed indicates left turning. Click here to view larger image.
Discussion
While previous electrophysiological studies on the CC or other regions of the insect brain have provided us with insights into the central control of behavior, most of them were performed in either restrained preparations9,11 or tethered ones10,14. As a result, the animal’s sensory experience and physiological state could be very different from those in a natural setting. Furthermore, the behavioral tasks that the animal can perform are limited to one plane under those situations. Here we presented a method to record from the CC in freely behaving cockroaches. Hopefully, we have provided you with all the necessary information you will need to capture electrophysiological recordings in freely behaving insects in your own laboratory. We presented the procedures for the systems that we use (Neuralynx, MClust, WinAnalzye, and Ctrax), but once the recoding electrodes are implanted, recording setup can be readily adapted to other systems.
We have performed 27 preparations, and as of yet none of the experiments was terminated because the cockroach damaged the wire sets. We have not observed any attempt by the animal to clean or remove the wire sets, wax, or rod. The implanted cockroaches walked in a normal gait. They were able to explore the arena and perform climbing tasks just as well as intact ones. Our experiments usually lasted 2-4 hr after the tetrode was implanted. Occasionally some units disappeared or their activity diminished throughout time, but most recordings were very stable throughout the whole experiment. We have also isolated some subjects and returned to recording and stimulation the following day. This method appears reliable for extended periods of extracellular recordings in freely behaving insects.
One point of emphasis is the fragile nature of the wire sets. They are easily damaged if great care is not taken during construction and implantation. Always move the wires and any dissecting instruments near them slowly, being careful not to bump or tear them. Wires may be carefully pulled from the preparation after the experiment and lesioning are completed, allowing for two or three uses. Be sure to retest, repolish, and replate before each use.
The key to a successful preparation is to keep the wire sets away from the cockroach. We use a long rod extending from the pronotum to above the abdomen and attach the tetrode tubing to the posterior end of the rod. Consequently, the tetrode tubing is always behind the cockroach when it is moving around in an arena such that the insect cannot reach the tubing with its antennae or legs. Placing the wire sets behind the cockroach also provides clearance over the animal’s body. This improves the video quality of our arena experiments because the camera is positioned above the arena. Leave no excess wires between the animal’s head and the tetrode tubing. If the insect can reach the wires with its antennae or legs, it will break them. In this method, the tubing slides freely over the wire, allowing us to draw excess wire up and secure it near the headstage.
One potential limitation of our method is the size of the arena where the cockroach can explore. The tetrode is 40 cm in length which is enough to provide access to the entire 40 x 40 cm2 arena. We have not encountered problems such as noise and tetrode quality. However, such problems could appear as we make longer tetrodes for a larger arena. Another potential problem with a longer tetrode is the weight of the tetrode. Our tetrode and rod weigh about 0.25 g which apparently does not impede a 2-3 g cockroach. We observed intact cockroaches exploring the same arena used for electrophysiology experiments. The walking activity and overall speed were similar between cockroaches carrying a rod and tetrode and unencumbered animals. However, we have not tested the limit of the load that a cockroach can carry before its performance drops. One solution to the limitations of a longer wire is to build a motorized platform for the headstage and the camera. Under such a system, the camera can track the cockroach’s movements in real time and output to the motor such that the platform can move accordingly. Therefore, a relatively short tetrode would be sufficient for a large arena because the headstage would remain directly above the animal.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Nick Kathman for suggestions and help at preparing for the manuscript. This technique was developed in conjunction with work supported by the AFOSR under grant FA9550-10-1-0054 and the National Science Foundation under Grant No. IOS-1120305 to RER.
References
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr. Opin. Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr. Biol. 2005;15:1473–1478. doi: 10.1016/j.cub.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr. Biol. 2010;20:663–668. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. J. Neuorosci. 2009;29:11783–11793. doi: 10.1523/JNEUROSCI.1870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: Intrinsic and columnar neurons. J. Comp. Neurol. 2008;511:454–478. doi: 10.1002/cne.21842. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J. Neurosci. 2009;29:4911–4921. doi: 10.1523/JNEUROSCI.0332-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron. 2011;69:345–358. doi: 10.1016/j.neuron.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Brill MF, et al. Parallel processing via a dual olfactory pathway in the honeybee. J Neurosci. 2013;33:2443–2456. doi: 10.1523/JNEUROSCI.4268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Ritzmann RE. Neural activity in the central complex of the cockroach brain is linked to turning behaviors. J. Exp. Biol. 2013;216:992–1002. doi: 10.1242/jeb.080473. [DOI] [PubMed] [Google Scholar]
- Ritzmann RE, Ridgel AL, Pollack AJ. Multi-unit recording of antennal mechanosensitive units in the central complex of the cockroach, Blaberus discoidalis. J. Comp. Physiol. A. 2008;194:341–360. doi: 10.1007/s00359-007-0310-2. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nature Neurosci. 2004;7.5:446–445. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Huston SJ, Jayaraman V. Studying sensorimotor integration in insects. Curr. Opin. Neurobiol. 2011;21:527–534. doi: 10.1016/j.conb.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr. Biol. 2010;20:921–926. doi: 10.1016/j.cub.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Harrison RR, et al. Wireless Neural/EMG telemetry systems for small freely moving animals. IEEE. 2011;5:103–111. doi: 10.1109/TBCAS.2011.2131140. [DOI] [PubMed] [Google Scholar]
- Straw AD, Dickinson MH. Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol. Med. 2009;4:5. doi: 10.1186/1751-0473-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer NM, Shaw MK, Altman JS. Strausfeld NJ, Miller TA. Neuroanatomical Techniques. Insect Nervous System. Springer Verlag; 1980. [Google Scholar]
- Daly K, Wright G, Smith B. Molecular features of odorants systematically influence slow temporal responses across clusters of coordinated antennal lobe units in the moth, Manduca sexta. J. Neurophsyiol. 2004;92:236–254. doi: 10.1152/jn.01132.2003. [DOI] [PubMed] [Google Scholar]
- Branson K, Robie A, Bender J, Perona P, Dickinson M. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]


