Acquired severe aplastic anemia (SAA) is a life-threatening hematopoietic stem cell disorder with pancytopenia and a hypocellular BM.1 Allogeneic BMT is the treatment of choice for patients <40 years, who have a human leucocyte antigen (HLA)-matched donor. SAA patients over age 40 with a matched sibling donor and SAA patients of any age without a matched sibling donor are usually treated with immunosuppressive therapy that may include CYA and anti-thymocyte globulin or high dose of cyclophosphamide (CY).2,3 Unfortunately, 30–50% of SAA patients treated with immunosuppressive therapy, do not respond to treatment, relapse, or develop secondary clonal diseases. For these patients, BMT (BMT) offers the best chance for cure. Patient age and the type of allograft (HLA-matched sibling, unrelated or mismatched donors) are the most important factors influencing the outcome. The risk of GVHD steadily increases with age, leading to reduced survival, especially in patients over the age of 40. TRM approaches 50% in patients over 40-years old.4–6 Unrelated donors and mismatched transplants have almost twice the TRM and risk of GVHD as matched sibling donor transplants.7 We have previously shown that high-dose CY (50 mg/kg/day) on days 3 and 4 following HLA-matched sibling, unrelated and haplo-identical BMT can facilitate engraftment and mitigate GVHD in hematological malignancies.8,9 Here, we show that GVHD and rejection can be prevented in SAA patients over the age of 40 with post-transplant CY.
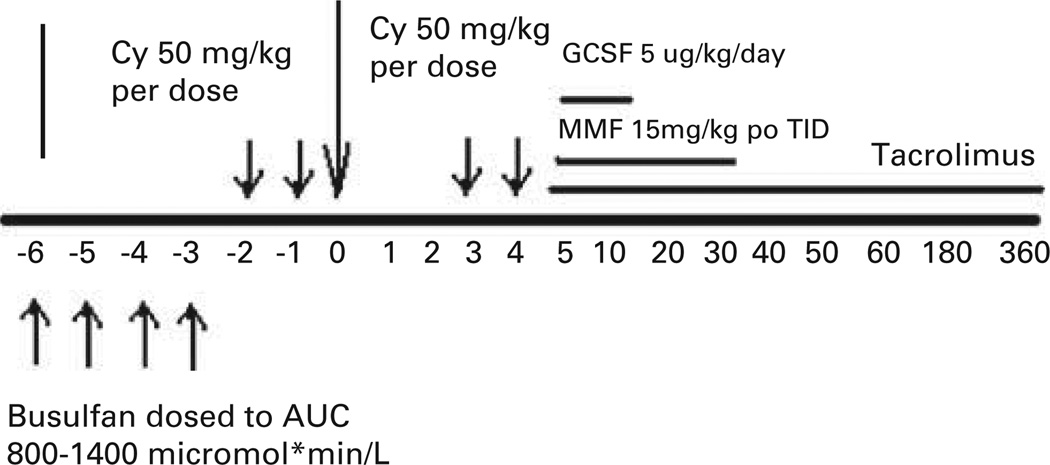
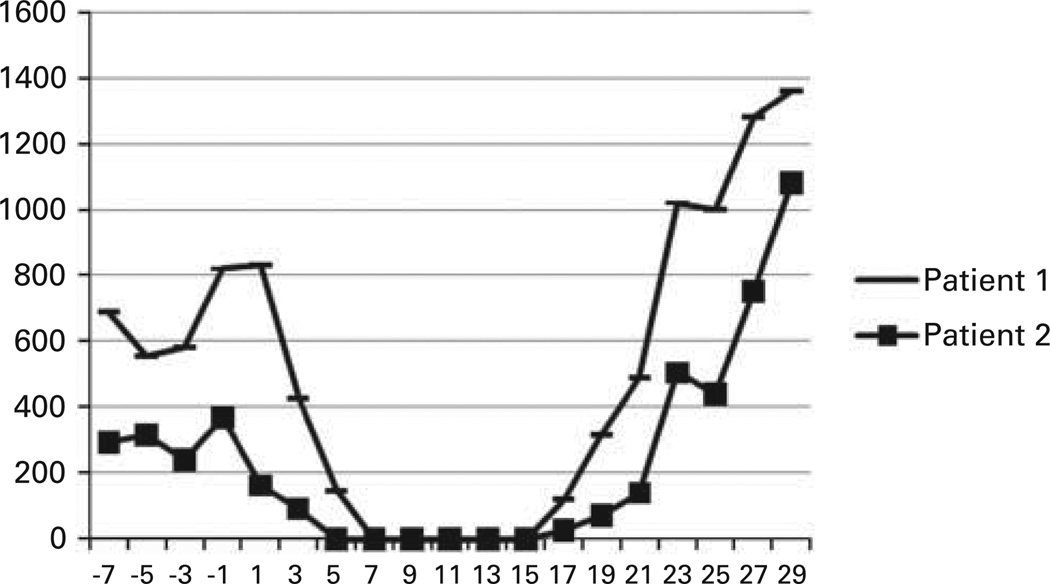
Patient 1, a 55-year-old female diagnosed with pancytopenia from myelodysplastic syndrome since 1994 presented to our institution. She was reevaluated by BM biopsy and found to have SAA with paroxysmal nocturnal hemoglobinuria clone. In May 2006, she was treated with high dose of CY. She did not respond, remaining dependent on red cell and platelet transfusions. She received an HLA-matched BMT from her brother 30 months after her initial therapy. Patient 2, a 54-year-old female was diagnosed with SAA 15 months before her BMT. She was also treated with high dose of CY, but showed no evidence of response at 6 months. She received an HLA-matched BMT from her sister. Both patients received conditioning therapy and post-transplantation GVHD prophylaxis as previously described (Figure 1). Both received oral BU on days −6 to −2, followed by CY 50 mg/kg/day given i.v. with Mesna on days −2 and −1. The rationale for using myeloablative BU is that these patients were older, heavily pretreated and transfusion dependent; we wanted to shift the balance toward engraftment as much as possible. On days 3 and 4 after BMT, 50 mg/kg CY was administered over 90 min together with Mesna (80% of CY dose in four divided doses over 8 h) by i.v. infusion. Both patients received mycophenolate mofetil 15 mg/kg orally (per os), every 8 h from days 4 to 35 and tacrolimus from day 4 to day 180 or 360. Tacrolimus was initiated at a dose of 1 mg i.v. daily, adjusted to achieve a therapeutic level of 5–15 ng/mL, and then converted to oral form until discontinuation. Patient 1 was electively admitted for her BMT, and following a 26-day hospital stay (day 20 after BMT), she was discharged. Her course was complicated by hemorrhagic cystitis while inpatient as well as a presumed herpes simplex virus infection, which responded quickly to treatment with valacyclovir. She experienced rapid hematological recovery (Figure 2) and achieved packed red blood cell and platelet transfusion independence on days 13 and 12, respectively. Full-donor chimerism was documented on days 30 and 60, and at 6- and 12-month follow-up after BMT. She had no GVHD and has discontinued tacrolimus. She is doing well currently, transfusion independent with 100% donor chimerism and a Karnofsky performance score of 100. Patient 2 was electively admitted for her BMT, and following a 27-day hospital stay (day 21 after BMT), she was discharged. During her preparative regimen, she developed Grade 1 mucositis. She experienced rapid hematological recovery (Figure 2) and achieved red blood cell and platelet transfusion independence on days 16 and 9 after transplantation, respectively. Full-donor chimerism was documented on day 30, 60 and at 6- and 12-month follow-up after BMT. She has no evidence of GVHD and has also discontinued tacrolimus; she has a Karnofsky performance score of 100 and also a 100% donor chimerism.
Figure 1.
Treatment Schema. CY = cyclophosphamide; MMF = mycophenolate mofetil.
Figure 2.
ANC by date.
Increasing age has been shown to adversely influence the survival of SAA patients who undergo BMT. In the years 1976–1989, the European Group for Blood and Marrow Transplantation reported an actuarial survival of SAA patients aged <16, 17–40 and >40 of 60, 53 and 27%, respectively.7 In the years 1990–1998, survival improved to 77, 68 and 54%, respectively. Survival is even less for adult SAA patients undergoing unrelated BMT mainly owing to the complications of graft failure and GVHD.10,11 Thus, novel regimens that reduce the risk of graft failure and GVHD are needed for adult SAA patients who are unresponsive to immunosuppressive therapy. Here, we demonstrate the feasibility and potential efficacy of post-transplantation CY in two patients over 50 years with refractory SAA, who received a BMT from their HLA-identical siblings following our protocol. Clearly, additional experience is required to characterize further efficacy of high dose, post-transplantation CY. We chose BU/CY as the myeloablative preparatory regimen with the rationale that these patients were older, highly pretreated and heavily transfused. It is possible that a non-myeloablative approach would have allowed engraftment; however, we have previously shown high engraftment rates using this BU/CY conditioning regimen in patients with high-risk hematological malignancies.12 Thus, our goal was to maximize the probability of engraftment and minimize the likelihood of GVHD as there is no need for a graft-versus-tumor effect in SAA. Given the high risk of GVHD following BMT in patients >40 years, and the high risk of GVHD in patients receiving a BMT from mismatched or unrelated BMT donors, these data suggest that the addition of post-transplantation CY to traditional immunosuppression deserves consideration as GVHD prophylaxis in SAA patients at high risk for GVHD.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy—The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000;37:69–80. doi: 10.1016/s0037-1963(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000;37:30–42. doi: 10.1016/s0037-1963(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136–2141. doi: 10.1182/blood-2009-06-225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doney K, Leisenring W, Storb R, Appelbaum FR. Primary treatment of acquired aplastic anemia: outcomes with bone marrow transplantation and immunosuppressive therapy. Ann Intern Med. 1997;126:107–115. doi: 10.7326/0003-4819-126-2-199701150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103:19–25. doi: 10.1159/000041000. [DOI] [PubMed] [Google Scholar]
- 8.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodsky RA, Luznik L, Bolanos-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42:523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeg HJ, Seidel K, Casper J, Anasetti C, Davies S, Gajeweski JL, et al. Marrow transplantation from unrelated donors for patients with severe aplastic anemia who have failed immunosuppressive therapy. Biol Blood Marrow Transplant. 1999;5:243–252. doi: 10.1053/bbmt.1999.v5.pm10465104. [DOI] [PubMed] [Google Scholar]
- 11.Peinemann F, Grouven U, Kroger N, Pittler M, Zschorlich B, Lange S. Unrelated donor stem cell transplantation in acquired severe aplastic anemia: a systematic review. Haematologica. 2009;94:1732–1742. doi: 10.3324/haematol.2009.007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]