Abstract
The aim of this study was to develop and test a controlled delivery system of two adipogenic factors (insulin and dexamethasone [Dex]), to generate stable adipose tissue when mixed with disaggregated human fat. Both drugs were encapsulated in poly(lactic-co-glycolic acid), (PLGA) microspheres (MS) and mixed with human lipoaspirate to induce adipogenesis in vivo. It was hypothesized that the slow release of insulin and Dex would enhance both adipogenesis and angiogenesis, thus retaining the fat graft volume in a nude mouse model. Insulin/Dex-loaded PLGA MS (Insulin/Dex MS) were prepared using both single and double emulsion/solvent extraction techniques. The bioactivity of the drugs was assessed by mixing the MS with human lipoaspirate and injecting subcutaneously into the dorsal aspect of an athymic mouse. Five doses of the drugs were examined and samples were analyzed grossly and histologically after 5 weeks in vivo. Mass and volume of the grafts were measured with the microsphere-containing samples, demonstrating increased mass and volume with increasing drug doses. Histological analysis, including H&E and CD31, indicated increased vascularization within the insulin/Dex MS-containing samples compared with the lipoaspirate-only samples. This study demonstrates that the controlled delivery of adipogenic factors such as insulin and Dex through polymer MS can significantly enhance tissue formation and vascularization, therefore presenting a potentially clinically relevant model of adipose retention.
Introduction
Tissue defects from trauma, tumor resection, or congenital malformations require soft tissue repair. Standard care includes tissue flap transfer or prosthetic components such as silicone or saline implants. Autologous fat grafting is a minimally invasive option in plastic and reconstructive surgery.1 In this technique, the limitations of current restorative and reparative techniques have served as motivation for the development of adipose tissue regeneration as an application area for tissue engineering.
Synthetic materials possess severe limitations, including but not limited to, unpredictable outcome, fibrous capsular contraction, allergic reaction, suboptimal mechanical properties, distortion, migration, and long-term reabsorption.2 Transplanted fat can have a low survival rate, and the adipose tissue can be quickly resorbed and replaced by fibrous tissue and oil cysts.3–5 These issues have greatly impacted the widespread adoption of autologous fat as the ideal soft tissue filler. At present, the exact mechanisms that mediate fat graft survival and resorption remain unclear. One potential mechanism for graft loss is the lack of adequate revascularization within the transplanted fat. Due to the lack of vascularization, ischemia of the tissue occurs, leading to tissue necrosis and graft loss at an early stage.6,7
In this study, we strived to create a predictable and clinically relevant method of soft tissue retention using pharmacologic interventions to improve autologous fat grafting. Our strategy for soft tissue regeneration involves the controlled, long-term, local delivery of adipogenic factors, such as insulin (Ins) and dexamethasone (Dex), within the fat graft.8 This study outlines the design and assessment of encapsulated insulin and Dex in poly(lactic-co-glycolic acid), (PLGA) microspheres (MS) mixed with lipoaspirate, and the effects on both vascularization and fat retention in vivo, using a combined drug therapy approach. We sought to determine whether encapsulation of these adipogenic factors and the subsequent localized delivery within fat grafts, resulted in enhanced adipose retention and vascularization as confirmed by immunohistological analysis of the explanted tissue.
Materials and Methods
Dex encapsulation
The protocol for encapsulating Dex in single-walled PLGA MS has been established in our laboratory.8,9 Dex sodium phosphate PLGA MS (Dex MS) were prepared using a single emulsion/solvent extraction technique. PLGA (75:25) (400 mg) was dissolved in methylene chloride (MC) (4.5 mL). Dexamethasone phosphate (Dex) (20 mg) was dissolved in methanol (0.5 mL), which was added to the polymer solution. After stirring and the addition of a 600 mg of 2% poly(vinyl alcohol) (PVA) solution, the MS were collected by centrifugation, frozen at −20°C, and freeze dried for 12 h (LabConco Freezone 4.5). The loading capacity was determined by using the equation LC=De/Sw, where DE is the amount of drug encapsulated and Sw is the mass of the MS.
Empty MS
Empty MS were prepared and characterized using the same protocol as in the Dex MS, without adding any drugs to the PLGA.
Insulin encapsulation
Our previously established protocol was used to encapsulate insulin in PLGA MS.8 Insulin-loaded PLGA MS (insulin MS) were prepared using a double emulsion/solvent extraction technique. PLGA (75:25) was dissolved in MC (4.5 mL). To form the first emulsion, insulin was dissolved in a phosphate-buffered saline (PBS; 0.2 mL), added to the dissolved PLGA (400 mg), and vortexed to form an emulsion. The first emulsion was added to a stirring 2.0% PVA solution and stirred. After 2 min, water was added and stirred for 3 h at 500 rpm. The MS were collected by centrifugation, frozen at −20°C, and freeze-dried for 12 h. The loading capacity was determined by using the equation LC=De/Sw, where DE is the amount of drug encapsulated and Sw is the mass of the MS.
Dex and insulin microsphere characterization
The morphology of Dex and insulin MS was determined by SEM microscopy. MS were gold coated using a Cressington 108 Auto (Cressington) followed by usage of JSM-6335F SEM (JEOL) operated at 3.0 kV acceleration for morphology characterization. Particle-sized distribution was determined by measuring the diameters of at least 50 MS from SEM images.
Dex and insulin release kinetics
Spectrophotometry was used to determine the release of Dex from PLGA MS, whereas for insulin, a commercial kit, the FlouroProfile Protein Quantification Kit (Sigma Aldrich) was used. For both agents, the MS (10 mg) were placed in a centrifuge containing 1 mL PBS. At the specific time points, MS were vortexed and the supernatant was collected for measuring, followed by replacement of PBS. The concentration of the drug was calculated by comparison to a standard curve.
Dex and insulin dosage
Dex is a synthetic steroid with an anti-inflammatory effect, and is used in different fields of medicine, such as in autoimmune (acquired) disorders of the endocrine system, allergic states, and rheumatologic diseases. Dex can be delivered orally, intramuscularly (I.M.), and in some cases intravenously (I.V.), depending on the severity of symptoms. The official doses used in clinics based on the National Institute of Health (NIH) data, I.V. doses range from 0.5 to 40 mg/kg in life-threatening cases such as unresponsive shock, and the average dose of Dex delivered I.V. or I.M, is typically 10–14 mg/kg, in 24 h.10 The doses in the study used in the animals were designed to be easily translated in human models, with drug doses lower than the doses shown above, when maintaining the ratio, animals to humans.
The concentration of insulin in the insulin-loaded MS was designed in the same fashion. The physiological levels of insulin in the healthy adult are 24–38 units per day or average of 8–11 nlU/mL.11 In this murine study, the dosage of insulin encapsulated in MS is lower than the doses above.
In vivo studies
Dex and insulin alone
The University of Pittsburgh Institutional Animal Care and Use Committee approved all animal studies. Thirty-five female athymic nude mice (5–10 weeks old; Harlan Laboratories) were equally divided into seven groups with five animals in each group: Dex A=40 mg MS, Dex B=80 mg MS, Dex C=150 mg MS, Insulin A=10 mg MS, Insulin B=14 mg MS, Insulin C=28 mg MS, and Insulin D=56 mg MS (Table 1). At the time of surgery, animals were weighed and then anesthetized with 12 mg/kg xylazine followed by 80 mg/kg of ketamine. Human lipoaspirate was processed as approved by the University of Pittsburgh Institutional Review Board, obtained by the patients undergoing elective surgery. Briefly, adipose tissue was gently aspirated using a two-holed, blunt harvesting cannula attached to 10-mL Luer-Lok syringes. The capped 10-mL Luer-Lok syringes then were centrifuged at 1200 g for 3 min. After the upper (oil) and lower (blood and infiltration liquids) layers were removed, the lower one-third of the middle layer (purified and processed lipoaspirate) was transferred through the Luer-Lok connectors to 1-mL syringes, known as the Coleman method.12–13 Immediately after sedation, the processed lipoaspirate (300–1000 mL lipoaspirate) was injected subcutaneously (fan-injection technique) and bilaterally in the dorsal flanks of nude mice using the 16-gauge infiltration cannula: right side lipoaspirate with MS and right side lipoaspirate only. Immediately following injections, photos were captured for analysis of the adipose retention and were photographed weekly throughout the study. After 5 weeks, the animals were sacrificed in the CO2 chamber. Adipose explants were analyzed for mass measurements and volume displacement using an Accupyc II 1340 gas Pycnometer (Micrometrics).
Table 1.
Dexamethasone and Insulin Treatment Groups
| Drug treatment dose | Number of animals | Time points |
|---|---|---|
| Dexamethasone MS/1 mL of human lipoaspirate | ||
| 40 mg MS | 5 | 5 weeks |
| 80 mg MS | 5 | 5 weeks |
| 150 mg MS | 5 | 5 weeks |
| Insulin MS/1 mL of human lipoaspirate | ||
| 10 mg MS | 5 | 5 weeks |
| 14 mg MS | 5 | 5 weeks |
| 28 mg MS | 5 | 5 weeks |
| 56 mg MS | 5 | 5 weeks |
Total number of animals 15, with 5 animals per group.
MS, microspheres.
Combined Dex MS and insulin MS study design
In the subsequent study, both Dex MS and insulin MS were combined and examined in the athymic nude mouse model as described above. Seven groups of mice were used in this part of the experiment, each containing nine animals in each group (powered for eight animals with one extra mouse for persistency) for a total of 63 mice, In addition to control groups, different combinations of Dex MS and insulin MS doses, were examined as follows: Group 1=50 mg Dex+90 mg insulin MS; Group 2=50 mg Dex+10 mg insulin MS; Group 3=27 mg Dex+19 mg insulin MS; Group 4=27 mg Dex+0 mg Insulin; and Group 5=0 mg Dex+10 mg insulin MS. The following two control groups were analyzed: Control 1: Lipoaspirate only and Control 2=Lipoaspirate+100 mg Empty PLGA MS (Table 2). The MS were mixed with 0.3 mL of human lipoaspirate. Animals were sacrificed after 5 weeks and assessed as described above.
Table 2.
Combined Drug Treatment Groups
| Drug treatment dose | Number of animals | Time points |
|---|---|---|
| Combined drug MS/0.3 mL of lipoaspirate | ||
| 50 mg+90 mg | 9 | 5 weeks |
| 50 mg+10 mg | 9 | 5 weeks |
| 27 mg+19 mg | 9 | 5 weeks |
| 27 mg | 9 | 5 weeks |
| 10 mg | 9 | 5 weeks |
| 100 mg Empty | 9 | 5 weeks |
| Lipoaspirate only | 9 | 5 weeks |
The first value on the scale is the Dexamethasone part of MS and the second value is the Insulin, 50 mg of Dex MS+90 mg of insulin MS mixed in 0.3 mL of lipoaspirate. The single drug MS are the 27 mg of Dex MS and 10 mg of insulin MS mixed with 0.3 mL of lipoaspirate and the controls are Empty MS and lipoaspirate only. Treatment groups are labeled with S (sample), whereas the control groups are the C (control).
Histological analysis
The samples were fixed in 4% paraformaldehyde, incubated overnight in 30% sucrose (Sigma-Aldrich), and then embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Inc.), followed by cryosectioning at 18 μm thickness. Hematoxylin (Santa Cruz Biotechnology, Inc.) and eosin (Sigma-Aldrich) (H&E) staining was conducted.
To assess vascularization, horseradish peroxidase-based CD31 antibody staining was performed to confirm the presence of blood vessels. Samples were fixed in 10% buffered formalin for 1 h, processed, and embedded in paraffin. Samples were first deparaffinized and then rehydrated with an ethanol gradient. Antigen retrieval (95°C citrate buffer for human for 20 min) was then performed and slides were washed. Slides were then blocked (sequentially for avidin, biotin, and endogenous enzymes (Dako), proteins (Dako), and 5% rabbit serum (Jackson ImmunoResearch) for human or 3% peroxide and 5% BSA for mouse) and washed with tris-buffered saline between blocking steps. The slides were incubated with the CD31 primary antibody (goat anti-human 1:100 for 1.5 h at room temperature, SC-1506; Santa Cruz Biotechnology or rat anti-mouse 1:100 for 2 h at room temperature, ab56299; Abcam) in PBS. Slides were washed and then incubated with a secondary antibody (rabbit anti-goat 1:200 in PBS with 6% rabbit serum for 30 min at room temperature, BA-5000; Vector Laboratories or rabbit anti-rat 1:100 in PBS for 1 h at room temperature, P0450; Dako). Slides were washed and human slides were incubated with an ABC kit avidin–biotin complex (Vector Laboratories) for 15 min at room temperature and washed.
Image analysis
The slides were imaged using an Olympus Provis microscope (Olympus). Blood vessels were counted using ImageJ (NIH). Cylindrical-shaped structures surrounded with endothelial cells were identified as vascular lumens on H&E-stained slides and were subsequently confirmed to be blood vessels using CD31 staining. Blood vessel lumens were counted using CD31-stained slides, five slides per group, and the average was recorded. In addition, using CD31-stained slides, 20 random cell surface areas were evaluated with ImageJ starting from the center of the slide and counting five cells in four directions for the purpose of setting the difference in cell areas between the control and treatment groups. Ten random tissue sections were quantified for each sample and means were determined for the aforementioned variables.
Statistical analysis
All results are presented as mean±standard deviation. The number of specimens in each group is presented in the above sections. Data were analyzed using Minitab 16 Statistical Software. Paired t-tests, two-sample t-tests, and/or one-factor analysis of variance tests were performed (where applicable) to determine if the differences between groups (control vs. experimental) were statistically different at the α=0.05 significance level.
Results
Dex MS characterization
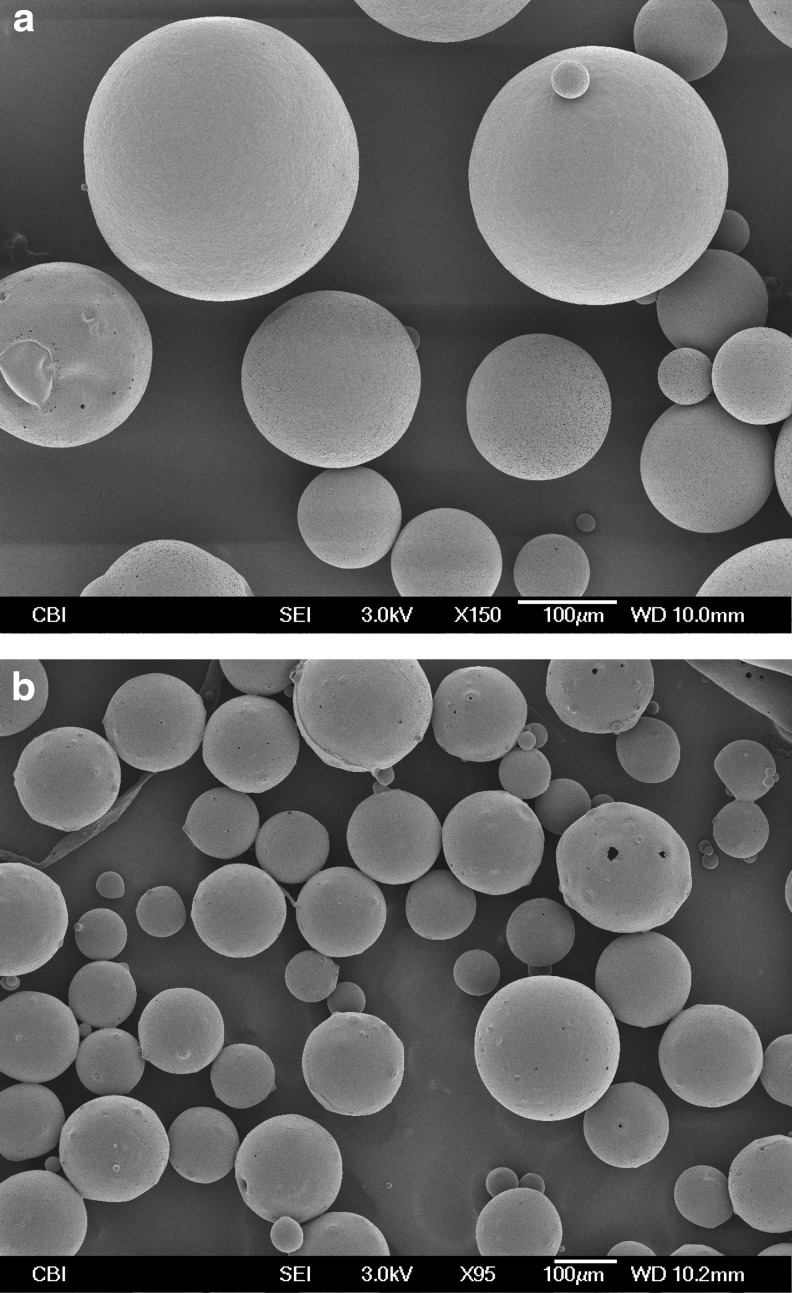
Dex MS were examined by SEM (Fig. 1a). The MS had an average diameter of ∼100–200 μm. The release of Dex occurred over 34 days (Fig. 2a). A controlled release was maintained over this period. After 24 h, 30.4%±4.4% of total Dex was released. A large burst effect (e.g., >80% during the first 24 h) was not observed. After 35 days, 90.0%±1.5% was released. The yield was 68.3%±15.1%. The loading capacity was 7.3±1.5 μg Dex per mg of MS.
FIG. 1.
SEM images of (a) dexamethasone (Dex) poly(lactic-co-glycolic acid) (PLGA) microspheres (MS) and (b) insulin-loaded PLGA MS.
FIG. 2.
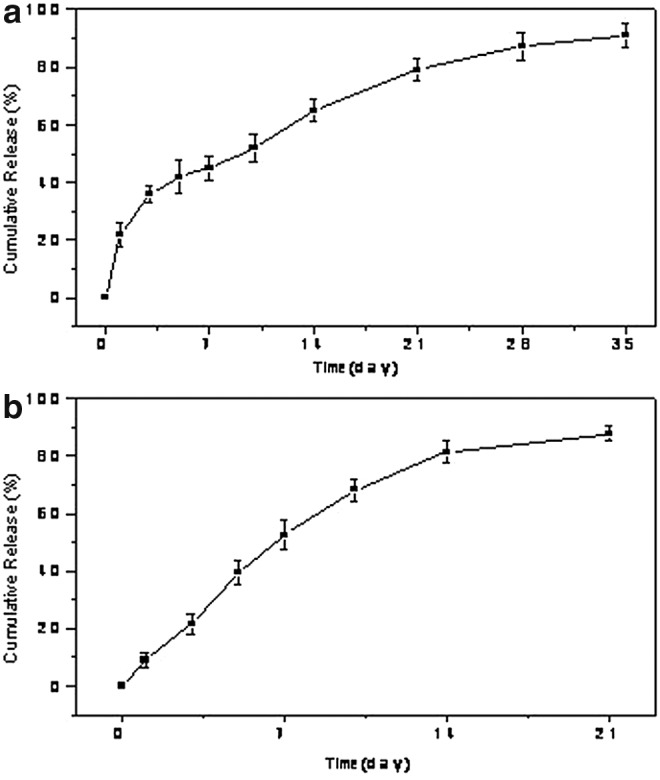
(a) Cumulative in vitro release of Dex from PLGA MS, and (b) cumulative in vitro release of insulin from PLGA MS.
Insulin MS characterization
In the same manner, insulin MS were examined utilizing SEM (Fig. 1b). The average diameter of the insulin MS was∼200–300 μm. The release of insulin was observed over 21 days (Fig. 2b). The insulin microsphere yield was 40.6%±11.3%. The loading capacity 10.7±2.3 μg Ins per mg of MS.
Dex MS and insulin MS in vivo studies
The animals were sacrificed after 5 weeks. Photographs showing the differences between the treatment groups and the control group are depicted in Fig. 3. Dex MS were mixed with l mL of lipoaspirate, whereas the control consisted of 1 mL of human lipoaspirate (C=control without MS and S=sample with Dex MS). Macroscopic differences were observed within the implants that had MS treatment compared with tissues without treatment. The results showed that the fat samples extracted from the nude mice with the Dex MS had increases in mass measurements when compared with lipoaspirate injections only (Fig. 4).
FIG. 3.
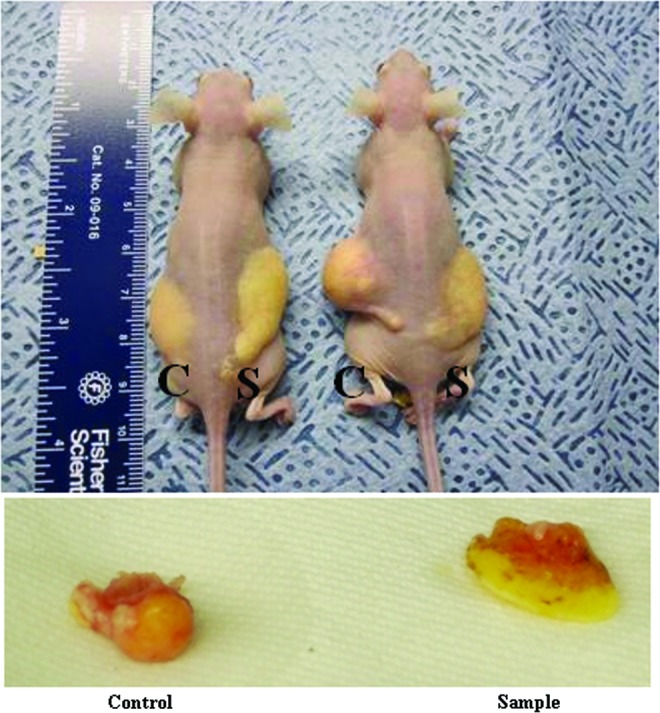
Dex MS effect in adipose tissue enhancement. Gross images were taken at the end of a 5-week time of explantation. In the treatment group, 80 mg of Dex MS was mixed with l mL of lipoaspirate, whereas the control group consisted of 1 mL of only human lipoaspirate (C=control without Dex and S=sample with Dex MS). Color images available online at www.liebertpub.com/tea
FIG. 4.
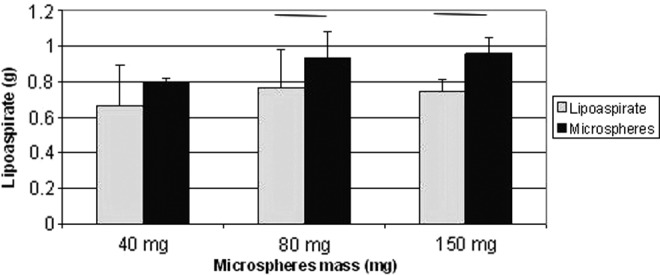
Results from adipose mass analysis of Dex MS-treated animals. Mass of the explanted fat tissue at the end of 5 weeks was increased, as the dose of Dex MS was increased showing significant difference in comparison with the control groups (p>0.05).
Insulin MS were mixed with 1 mL of human lipoaspirate and also compared with lipoaspirate alone (Fig. 5). Results indicate that mass increased with increasing insulin concentrations.
FIG. 5.
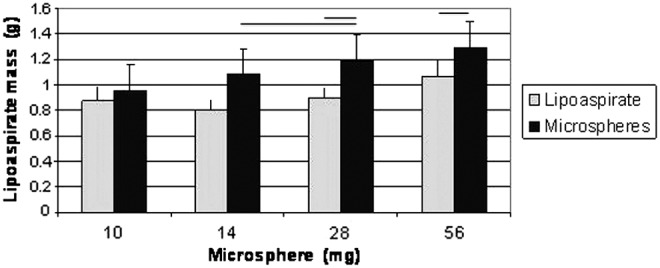
Results from adipose mass analysis of insulin MS-treated animals. Mass of the explanted fat tissue at the end of 5 weeks was increased, as the dose of insulin MS was increased showing significant difference in comparison to the control groups (p>0.05).
Combined drug in vivo studies
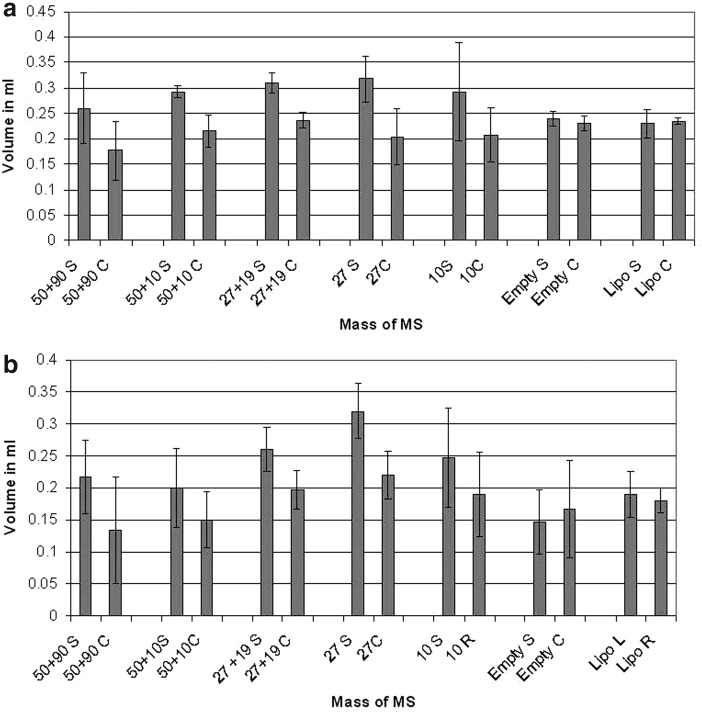
The animals from the Dex MS and insulin MS combined study were sacrificed after 5 weeks in the same way as the single-drug study animals. Fat tissue was extracted, followed by measurements of volume and/or mass. The increase in mass after 5 weeks of MS treatment samples, as well as the decrease in volume for empty MS samples, was statistically significant depending on the dose (Dex MS Δ Volume=0.32±0.043 mL, p=0.002; Empty Δ Volume=−0.14±0.051 mL, p=0.646). Differences were significant when p<0.05. The combined in vivo study of both drugs did not result in a significant difference in volume compared with Dex or Insulin only (Fig. 6).
FIG. 6.
Results from mass (a) and (b) volume analysis of Dex and Insulin combined treated group at 5 weeks. The first numbers on the scale is the Dex part of MS and the second is the Insulin, 50 mg of Dex MS+90 mg of insulin MS mixed in 0.3 mL of lipoaspirate. The single drug MS are the 27 mg of Dex MS and 10 mg of insulin MS mixed with 0.3 mL of lipoaspirate and the controls are Empty MS and lipoaspirate only (L-left side and R- right side). Treatment groups are labeled with S (sample), whereas the control groups are the C (control).
Although the results show a statistical difference compared with controls that were lipoaspirate only, there is a decrease in volume compared with samples that used individual drugs.
Histological and ImageJ analysis
Ten tissue sections were stained with H&E, demonstrating gross architecture of the tissue. Blood vessels were quantified using ImageJ from the CD31 staining (Fig. 7), resulting in a higher number of vessels in the treatment groups. The results shown in Fig. 8 elaborate the difference in vascularization between treatment and control samples in the single-dose study (Fig. 8a) and the combined drug study (8b).
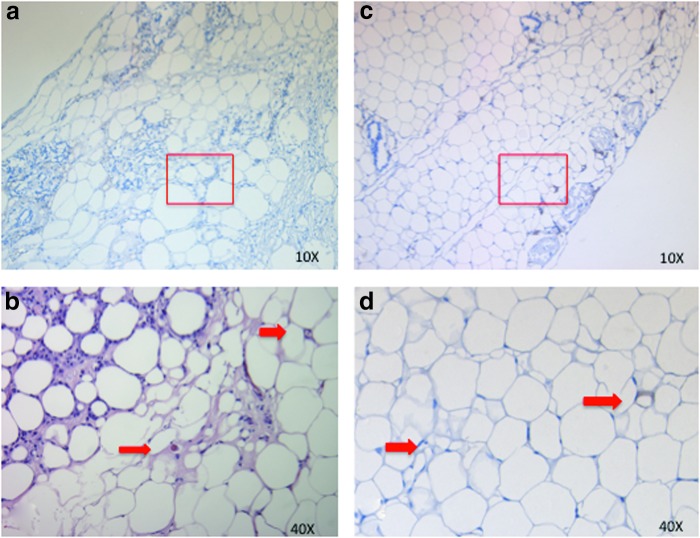
FIG. 7.
Human CD31 staining of Dex-loaded MS and insulin-loaded MS. (a) Dex-loaded microsphere group (80 mg Dex MS), (b) magnified image of the Dex MS (a). (c) Combined drug group CD31 staining. (d) Magnified figure of combined drug study (c). Positive staining is indicated by arrows. Color images available online at www.liebertpub.com/tea
FIG. 8.
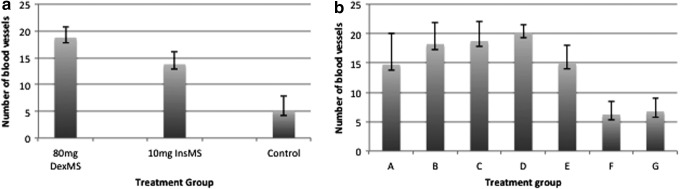
Blood vessel quantification (average per group). Single-dose study (a) containing 80 mg Dex MS, 10 mg insulin MS, and lipoaspirate alone and the combined drug study (b) group A (50 mg Dex MS+90 mg insulin MS), group B (50 mg Dex MS+10 mg insulin MS), group C (27 mg Dex MS+19 mg insulin MS), group D (27 mg Dex MS+0 mg insulin MS), group E (0 mg Dex MS+10 mg insulin MS), group F (empty MS), and group G (lipoaspirate only), show a difference between the control group and the sample group.
Discussion
Resection of tumors in the head and neck, upper and lower extremities, as well as trauma and congenital abnormalities often results in contour defects due to the loss of soft tissue, largely composed of subcutaneous adipose tissue. Adipose tissue is a dynamic and multifunctional tissue that is ubiquitous throughout the human body.14–16 Fat functions as a specialized organ that maintains the energy balance through controlled storage and release. Adipocytes store energy in the form of triglycerides and accumulate or mobilize triacylglycerol in response to the body's energy requirements.16 Adipose tissue is highly plastic and can adapt to facilitate greater storage through the hypertrophic expansion of terminally differentiated mature adipocytes, as well as the hyperplastic growth and differentiation of precursor cells present in the stroma. However, as mature adipose tissue does not transplant with 100% survival, numerous natural, synthetic, and hybrid materials have been used to act as adipose surrogates.
This study outlines a new technology for fat retention during fat transfer using both Dex- and insulin-loaded PLGA MS. The effect of Dex in fat tissue enhancement has been studied before. As a synthetic glucocorticoid, Dex is more potent than the natural hormone cortisol, and its action in the enhancement of fat tissue formation by increasing the expression of C/EBP and PPAR-y has been demonstrated.17–19 Hence, there are a number of cases where Dex is used in medicine and in bioengineering research as an adipogenic catalyst.19,20
Insulin is a hormone that is known for regulation of carbohydrates and fat metabolism in the body. The presence of insulin in tissue stops the process of using fat as an energy resource. When insulin is absent, glucose is not taken up by body cells and the body begins to use fat as an energy source, or by transfer of lipids from adipose tissue to the liver for mobilization as an energy source.21,22 The release of insulin to induce adipogenesis has been demonstrated, using in vitro and in vivo studies, resulting in fat tissue increased weight in a couple of weeks.23,24
Biodegradable drug delivery systems, such as PLGA-polyethylene glycol (PLGA-PEG) MS, have been studied as delivery vehicles for insulin, insulin-like growth factor-1 (IGF-1), and basic fibroblast growth factor. In a subcutaneous rat model, incorporating these growth factors improved autologous free fat graft weight and volume, with the best results observed for either insulin or IGF-1 alone or in combination. PLGA (75:25) foam was also assessed in vivo in combination with IGF-1 and insulin, with fibroelastic tissue formation at the implantation site at 12 weeks.25,26
In this study, we demonstrated that drug-loaded PLGA MS can have an impact on fat tissue enhancement through release of adipogenic drugs. The Dex-loaded MS successfully released the drug in a controlled fashion for a duration of 3–5 weeks. Demonstrated by our in release studies, 70% of drug was released in the first 3 weeks with 100% release in 4–5 weeks (Fig. 2). Based on our in vitro published data,8 we created an experimental design for in vivo studies, using a total three doses of Dex-loaded MS. Results demonstrated that after a 5-week period, there was a significant increase in mass and volume of the samples, which proved to be statistically significant. This observation was also seen with histological analysis (Fig. 7).
Our results were notable when we examined Dex only, which is known to be a synthetic steroid used as a strong anti-inflammatory drug, and also is a highly adipogenic factor,27 increasing the expression of the C/EBP and PPAR-y genes.17 An increase in mass, volume, and vascularization was also observed in the experiments with insulin-MS, which is a known lipogenic drug that enhances the lipid filling, as shown in previous in vitro and in vivo studies.28 Insulin-loaded MS successfully resulted in a controlled release of insulin for a duration of 3–5 weeks, with 70% of insulin released in the first 3 weeks and 100% release in 4–5 weeks. Results demonstrated that after a 5-week period, there was also a significant increase in mass and volume of the samples, but not as striking as Dex alone.
In conclusion, this study demonstrates the use of encapsulated adipogenic drugs, such as insulin and Dex, for stimulating fat tissue enhancement in vivo. Sustained delivery of the drug through PLGA MS, induced enhanced adipose retention, showed by gross and histological differences in fat tissue vascularization compared with lipoaspirate alone.
Acknowledgments
The authors thankfully acknowledge Sun Jung Oh, Harry Nayar, Allen Rakers, and Ryan Nolan for their technical assistance, and the NIH R01CA114246-01A1 (JPR) for funding.
Disclosure Statement
No competing financial interests exist.
References
- 1.Patrick C.W.Engineering adipose tissue for regenerative and reparative therapies. Semin Plastic Surg 19,207, 2005 [Google Scholar]
- 2.Patric J.R.Tissue engineering strategies for adipose tissue repair. Anat Rec 263,361, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kononas T.C., Bucky L.P., and Hurley C.The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg 91,763, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Smahel J.Experimental implantation of adipose tissue fragments. Br J Plast Surg 42,207, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Peer L.A.The neglected free fat graft. Plast Reconstr Surg 18,233, 1956 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen A., Pasyk K.A., Bouvier T.N., et al. . Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg 85,378, 1990 [PubMed] [Google Scholar]
- 7.Billings E., Jr., and May J.W., Jr.Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 83,368, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Rubin J.P., DeFail A., Rajendran N., and Marra K.G.Encapsulation of adipogenic factors to promote differentiation of adipose-derived stem cells. J Drug Target 17,207, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Chung C.W., Marra K.G., Li H., Leung A.S., Ward D.H., Tan H., Kelmendi-Doko A., and Rubin J.P.VEGF microsphere technology to enhance vascularization in fat grafting. Ann Plast Surg 69,213, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Frank E.Clinical observations in shock and management (in: Shields T. F., ed.: Symposium on current concepts and management of shocks), J Maine Med Ass 59,195, 1968 [PubMed] [Google Scholar]
- 11.Iwase H., Kobayashi M., Nakajima M., and Takatori T.The ratio of insulin to C-peptide can be used to make a forensic diagnosis of exogenous insulin overdosage. Forensic Sci Int 115,123, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Coleman S.R.Structural fat grafting: more than a permanent filler. Plast Reconstr Surg 118,108S, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Allen R.J., Jr., Canizares O., Jr., Scharf C., Nguyen P.D., Thanik V., Saadeh P.B., Coleman S.R., and Hazen A.Grading lipoaspirate: is there an optimal density for fat grafting? Plast Reconstr Surg 131,38, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Katz A.J., Llull R., Hedrick M.H., and Futrell J.W.Emerging approaches to the tissue engineering of fat. Clin Plast Surg 26,587, 1999 [PubMed] [Google Scholar]
- 15.Klein J., Permana P.A., Owecki M., Chaldakov G.N., Böhm M., Hausman G., Lapière C.M., Atanassova P., Sowiński J., Fasshauer M., Hausman D.B., Maquoi E., Tonchev A.B., Peneva V.N., Vlachanov K.P., Fiore M., Aloe L., Slominski A., Reardon C.L., Ryan T.J., Pond C.M., and Ryan T.J.What are subcutaneous adipocytes really good for? Exp Dermatol 16,45, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Butterwith S.C.Molecular events in adipocyte development. Pharmacol Ther 61,399, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Malladi P.Xu Y., Yang G.P., and Longaker M.T.Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng 12,2031, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Locklin R.M.Williamson M.C., Beresford J.N., Triffitt J.T., and Owen M.E.In vitro effects of growth factors and dexamethasone on rat marrow stromal cells. Clin Orthop Relat Res 313,27, 1995 [PubMed] [Google Scholar]
- 19.Laferrère B., Fried S.K., Osborne T., and Pi-Sunyer F.X.Effect of one morning meal and a bolus of dexamethasone on 24-hour variation of serum leptin levels in humans. Obes Res 8,481, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding J.E.Body composition in early growth: lessons from domestic animals. Eur J Pediatr 165,1, 2006 [Google Scholar]
- 21.Koschorreck M., and Gilles E.D.Mathematical modeling and analysis of insulin clearance in vivo. BMC Syst Biol 2,43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen R.Pathophysiology of the Endrocrine System: Physiologic Effects of Insulin. Colorado State University Biomedical Hypertextbooks. [Online] August1, 2009
- 23.Yuksel E., Weinfeld A.B., Cleek R., Waugh J.M., Jensen J., Boutros S., Shenaq S.M., and Spira M.De novo adipose tissue generation through long-term, local delivery of insulin and insulin-like growth factor-1 by PLGA/PEG microspheres in an in vivo rat model: a novel concept and capability. Plast Reconstr Surg 105,1721, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Schwarz M.J., Chioléro R., Revelly J.P., Cayeux C., Schneiter P., Jéquier E., Chen T., and Tappy L.Effects of enteral carbohydrates on de novo lipogenesis in critically ill patients. Am J Clin Nutr 72,940, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Shenaq S.M., and Yuksel E.New research in breast reconstruction: adipose tissue engineering. Clin Plast Surg 29,111, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Neubauer M., Hacker M., Bauer-Kreisel P., Weiser B., Fischbach C., Schulz M.B., Goepferich A., and Blunk T.Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng 11,1840, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Fei Z., Bera T.K., Liu X., Xiang L., and Pastan I.Ankrd26 gene disruption enhances adipogenesis of mouse embryonic fibroblasts. J Biol Chem 286,27761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C.Y., Chen P.C., Kuo H.K., Lin L.Y., Lin J.W., and Hwang J.J.Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in the National Health and Nutrition Survey 1999–2002. Nutr Metab Cardiovasc Dis 20,713, 2010 [DOI] [PubMed] [Google Scholar]