Abstract
We have succeeded in culturing human dermal papilla (DP) cell spheroids and developed a three-dimensional (3D) Matrigel (basement membrane matrix) culture technique that can enhance and restore DP cells unique characteristics in vitro. When 1×104 DP cells were cultured on the 96-well plates precoated with Matrigel for 5 days, both passage 2 and passage 8 DP cells formed spheroidal microtissues with a diameter of 150–250 μm in an aggregative and proliferative manner. We transferred and recultured these DP spheroids onto commercial plates. Cells within DP spheres could disaggregate and migrate out, which was similar to primary DP. Moreover, we examined the expression of several genes and proteins associated with hair follicle inductivity of DP cells, such as NCAM, Versican, and α-smooth muscle actin, and confirmed that their expression level was elevated in the spheres compared with the dissociated DP cells. To examine the hair-inducing ability of DP spheres, hair germinal matrix cells (HGMCs) and DP spheres were mixed and cultured on Matrigel. Unlike the dissociated DP cells and HGMCs cocultured in two dimensions, HGMCs can differentiate into hair-like fibers under the induction of the DP spheres made from the high-passage cells (passage 8) in vitro. We are the first to show that passage 3 human HGMCs differentiate into hair-like fibers in the presence of human DP spheroids. These results suggest that the 3D Matrigel culture technique is an ideal culture model for forming DP spheroids and that sphere formation partially models the intact DP, resulting in hair induction, even by high-passage DP cells.
Introduction
Hair is not only a protective accessory structure of the integument, but has aesthetic function for the facial contour. Although alopecia is not life-threatening, it can have a profound impact on social interactions and on psychological well-being,1,2 which in turn increases the demand for treatment options. Current therapeutic treatment includes either medication or hair transplantation, which are effective only for mild—not extensive—hair loss. Both fall short of the ultimate goal of generating new hair follicles (HFs).3,4 With the advent of tissue engineering and regenerative biology, bioengineering for HF neogenesis is a promising potential.3
HFs are ectodermal organs composed of the epithelium of keratinocytes and the mesenchyme of dermal papilla (DP) cells. The reciprocal interactions between the epithelium and mesenchyme are essential for postnatal hair growth and cycling of HFs.5,6 The DP comprises a group of mesenchymal cells at the base of the HF and has a crucial role in HF development and regulation of the postnatal hair growth cycle. DP cells are a specialized cell population, distinguishable from interfollicular dermal fibroblasts by their unique characteristics, such as aggregative behavior, distinct gene expression, and the ability to induce new HF formation.5–8 Cultured DP cells retain the ability to induce neogenesis of HFs in hairless skin.9,10 However, the inductive ability of DP cells tends to be lost during passaging.5,7,8
The development of HFs requires a large number of DP cells, so long-term cultivation is necessary to obtain enough cells for implantation. Thus, how to maintain the inductive potential of cultured human DP cells is a priority in HF bioengineering and regeneration. Various approaches to prevent the loss of inductive potential have been described, including cultivation in the presence of fibroblast growth factor-2 (FGF-2), bone morphogenic proteins, Wnts, among others.10–12 Although this research has not been suitable for clinical application to date, a common characteristic of cultured DP cells is their tendency to aggregate. This has led to strategies for culturing DP cells into three-dimensional (3D) spheres, similar to a natural intercellular organization in vivo, rather than adherence on tissue culture plates.13–16
Although some studies show that DP cells form multicellular microtissues by intercellular collision on plates with low-adhesive surfaces, none mimic the DP cells' niche in vivo.17–19 Physiologically, DP cells are embedded in an extracellular matrix (ECM) rich in basement membrane proteins, including fibronectin, collagen IV, laminin, and hyaluronic acid.20 Although a 2D substrate coating with a thin layer of ECM proteins (usually only collagen or fibronectin) can affect DP cell adhesion, proliferation, and differentiation,14,21 the complexity of the ECM has not been reproduced. Most human cells interact with the neighboring cells and the ECM to establish a unique 3D organization. These cell–cell and cell–ECM interactions form a complex communication network of biochemical and mechanical signals, which are critical for normal cell physiology. As a result, the loss of tissue-specific properties is common for cells grown in cultures.22–24
A simple in vitro system for the ECM is the Matrigel basement membrane matrix, extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, which consists primarily of laminin, collagen IV, heparin sulfate proteoglycans, and growth factors.25 Matrigel has been successfully applied in 3D cell cultures for the purpose of elucidating the establishment of mammary cell morphogenesis and angiogenesis.26,27
To date, the growth of human DP cells in the ECM has rarely been examined. In this study, we sought to develop a culture method with Matrigel that mimics the niche of DP cells in vivo to produce mass human DP spheroids with inductive potential. By using the Matrigel culture system, the dynamics and mechanism of spheroid-forming behavior were investigated. We also investigated the extent to which fibroblasts can be induced to express DP markers and have hair-inducing capacity.
Materials and Methods
The isolation and expansion of human DP cells
Human scalp samples from patients undergoing face lift surgery were collected after obtaining informed consent and after approval by the Medical Ethical Committee of the Southern Medical University. DP cells were isolated and expanded as described by Magerl et al.28 Briefly, dermal papillae were microdissected from the bulbs of dissected HFs, transferred onto plastic dishes, and cultured in the Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 20% (v/v) fetal bovine serum (FBS; Gibco) at 37°C in a humidified 5% CO2 atmosphere. Explants were kept in a medium for 7 days; the medium was changed every 3 days. Once cell outgrowth was subconfluent, cells were harvested with 0.25% (w/v) trypsin-EDTA (Gibco) and subcultured with a split ratio of 1:3. Afterward, DP cells were maintained in the DMEM supplemented with 10% FBS. Cells at passage 8 were used in the following experiments.
Effect of ECM on the growth of DP cells
The Matrigel culture system was prepared according to the manufacturer's protocol. Briefly, a thawed Matrigel solution (BD Biosciences, Baltimore, MD) was added to plates using cooled pipettes and the Matrigel-coated plates were placed at 37°C for 30 min. A 2D or 3D culture system was defined as wells were coated with a thin (50 μL/cm2) or thick gel (150 μL/cm2), respectively. For preparing the 3D culture system with hyaluronan (Restylane, Uppsala, Sweden), the plates were also coated with 150 μL hyaluronan/cm2. Uncoated wells were used as controls. DP cells (1×104 cells/well) were seeded onto wells precoated with Matrigel or hyaluronan or not, respectively, and cultured in a complete medium (DMEM+10% FBS), which was changed every 2 days. The morphology of cells was recorded under a reverse phase-contrast microscope for 5 days.
3D Matrigel culture
To determine the optimal conditions for the formation of DP spheroids in 3D Matrigel culture, different culture conditions were discussed. First, we explored the effects of spatial location on the formation of DP spheroids, that is, DP cells growing on, within, or under a Matrigel, respectively. Correspondingly, we let DP cells grow on wells precoated with Matrigel, or let DP cells mixed with thawed Matrigel grow in culture wells, or let DP cells grow in culture wells, followed by coating with Matrigel for 6 h. Second, to test whether cell density was the main factor affecting DP spheroid formation, DP cells (0.25×104–2×104 cells/well) were seeded onto Matrigel-coated plates. The number of spheroidal microtissues was recorded under a reverse phase-contrast microscope.
After the optimal conditions for DP spheroids were determined, the following experiments were conducted. For gene and protein expression experiments, passage 8 DP cells were incubated on uncoated plates as a negative control, while passage 2 DP cells were inoculated on uncoated plates as a positive control. To test whether 3D culturing can alter gene or protein expression, fibroblasts (1×104 cells/well) inoculated on Matrigel-coated plates were also investigated. All experiments were conducted after cell culture for 5 days. Three-dimensional spheres were recovered from the Matrigel by removing the medium and incubating in 100 μL/well of cell recovery solution (BD Biosciences) at 4°C for 2 h. Spheres were centrifuged at 300 g for 5 min and washed three times with phosphate-buffered saline.
Dynamic process of DP spheroid formation and injection of DP spheroids
To observe the dynamic process of DP spheroid formation, DP cells were seeded onto Matrigel-coated plates and observed using an inverse phase-contrast microscope (Axiovert 200M; Carl Zeiss, Gottingen, Germany). Recording started at time 0 after seeding for 11 days.
To test whether DP spheroids maintained their structure and cell viability after injection for transplantation, DP spheroids formed after 5 days of cultivation were carefully removed from cultured surfaces using a 200-μL pipette (4844, inner diameter=710 μm; Corning Incorporated Life Sciences, Tewksbury, MA). They were then transferred to culture dishes for another 5 days. The morphology and motility of the cells in DP spheroids were recorded under an inverse phase-contrast microscope. Primary DP was used as a positive control.
Quantitative real-time PCR
Total RNA was extracted from DP spheres, adherent DP cells, and fibroblast spheres using an RNAiso Plus reagent (Takara, Dalian, Liaoning Province, China). cDNA was synthesized from 2 μg of total RNA with a SYBR PrimeScriot RT-PCR Kit (Takara). Quantitative RT-PCR (QRT-PCR) was carried out using a SYBR PrimeScriot RT-PCR Kit (Takara) on a Stratagene MX3005P QRT-PCR system (Agilent Technologies, Santa Clara, CA). All the above steps were performed according to the manufacturer's protocol. The primers are listed below. NCAM: 5′-TCCGAGT TCAAGACGCAGCCA-3′ and 5′-GGTGGAGACAATGG AACAGGGGT-3′, Versican: 5′-TGTCCGATTCATAGTC CTGTCC-3′ and 5′-CTCACAGCGATAAGTGCCCTC-3′, α-smooth muscle actin (α-SMA): 5′-GCTTCCCTGAACA CCACCCAGT-3′ and 5′-GCCTTACAGAGCCCAGAGC CAT-3′, β-actin: 5′-AGGCCCAGAGCAAGAGAG-3′ and 5′-GGAGAGCATAGCCCTCGTAG-3′. PCR cycling conditions were as follows: a denaturation step for 10 min at 95°C, followed by 40 cycles of denaturation (95°C for 15 s), annealing (60°C for 20 s), and extension (72°C for 10 s).
Immunofluorescent staining of DP spheroids
Specimens were sectioned for immunostaining according to routine procedures. Briefly, samples were fixed in 4% paraformaldehyde and paraffin embedded. We used the following rabbit monoclonal primary antibodies to characterize DP cells cultured on plastic and on Matrigel: NCAM (Epitomics, Burlingame, CA), Versican (Epitomics), α-SMA (Epitomics), and GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Secondary antibodies were mouse anti-rabbit IgG antibodies (Invitrogen, Carlsbad, CA). Staining procedures were performed according to company guidelines. Images were taken under a fluorescent microscopic system (Axiovert 200M; Carl Zeiss).
Western blotting
Total cell lysates were prepared and 30 μg of protein was subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting analysis. Primary antibodies were incubated at the following dilutions: anti-NCAM monoclonal antibody, 1:500; anti-Versican monoclonal antibody, 1:500; anti-α-SMA monoclonal antibody, 1:500; and anti-GAPDH monoclonal antibody, 1:1000 (Santa Cruz Biotechnology, Inc.). Immune complexes were detected using a western blotting enhanced chemiluminescence (ECL) kit (Santa Cruz Biotechnology, Inc.) and quantified using the analyst/PC densitometry software (Bio-Rad Laboratories, Hercules, CA).
HF induction ability of DP spheroids
To explore the hair-inducing activity of cell spheroids, human cell spheroids and passage 3 hair germinal matrix cells (HGMCs; Science Cell, Canton, MA) were incubated on Matrigel-coated plates. That is, HGMCs were seeded onto Matrigel-coated plates in which DP cells or fibroblasts were precultured for 3–5 days. Meanwhile, DP cells and HGMCs were seeded on uncoated plates as the negative control group 1 and HGMCs were seeded only on Matrigel-coated plates as the negative control group 2. Cells were kept in a medium (DMEM:mesenchymal stem cell medium=1:1) for 10 days and the medium was changed every 2 days. Cell morphology was recorded under a reverse phase-contrast microscope for 10 days. HGMCs and fibroblasts were both seeded at a density of 1×104 cells/well.
Statistical analysis
All experiments are conducted on flat-bottomed 96-well plates. All data are expressed as the mean±SD from three independent experiments. All statistical analysis was done with SPSS statistical software, version 13.0. The independent samples t-test was performed for comparison of microtissues among groups. Differences between RT-PCR or western blot results were evaluated by one-way ANOVA. A p-value of<0.05 was considered statistically significant. All graphs were plotted using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA).
Results
Formation of DP spheroids
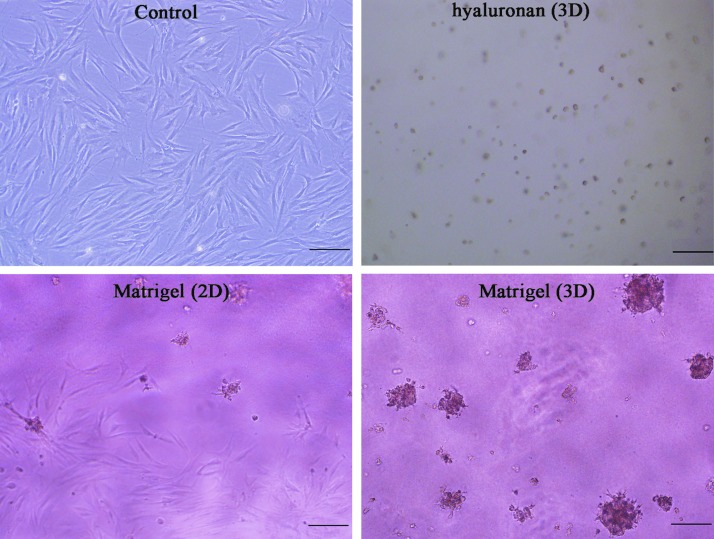
To explore whether the ECM or 3D cultures induce DP spheroid formation, DP cells were seeded on plates precoated with Matrigel or hyaluronan. In the 3D Matrigel group (150 μL/cm2), a large number of spheroids (diameter >150 μm) were found (Fig. 1). Comparatively, DP cells primarily grew in an adherent fashion on the center of the culture well, with only a few spheroidal microtissues with a diameter <50 μm formed on the edge in the 2D Matrigel group (50 μL/cm2; Fig. 1). In the 3D hyaluronan group (150 μL/cm2), DP cells did not attach and show circular nonspreading disassociation on hyaluronan-coated plates (Fig. 1).
FIG. 1.
Dermal papilla (DP) cell morphologies on extracellular matrix-coated or uncoated plates after DP cell seeding for 5 days. DP cells are better spread with a larger surface area on uncoated (control) and Matrigel precoated (2D) plates, in which only a few spheroidal microtissues were found on the edge of the culture well. DP cells did not attach and showed spherical disassociated cells on hyaluronan-coated plates (three-dimensional [3D]). Many multicellular masses were found on the Matrigel-coated (3D) plates. Scale bar: 200 μm.
The optimal conditions for DP spheroids
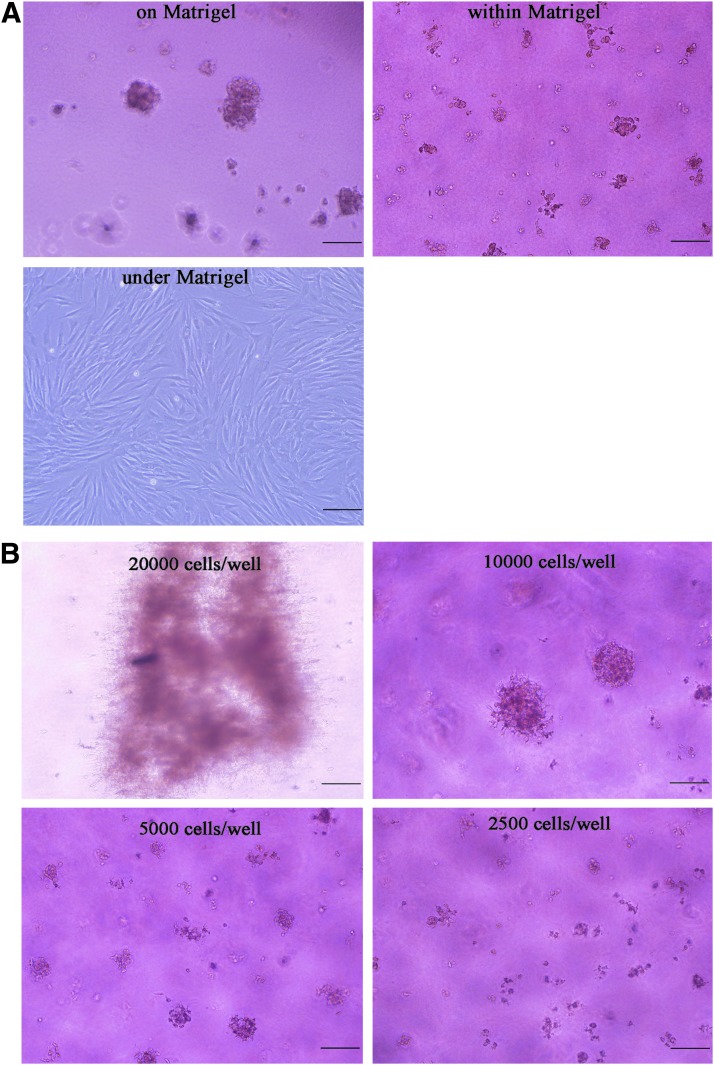
To determine the optimal conditions to yield DP spheroids, the cell spatial location and seeding density were discussed. First, we characterize the number and size distribution of DP spheroids formed on, within, or under the Matrigel after 5 days in culture (Table 1). A large number of DP spheroids were found both on the surface and within the Matrigel. The number of DP spheroids with a diameter ranging from 150 to 250 μm formed on the Matrigel is significantly higher than the number formed within the Matrigel (p<0.05). DP cells were adherent and no cell microtissues were observed under the Matrigel (Fig. 2). Second, the number and size distribution of the DP spheroids formed at different seeding densities after 5 days in culture is characterized in Table 2. When DP cells were seeded at a density of 2×104/well, we did not observe any spheres or spherical cell mass aggregating in an irregular manner (Fig. 2). In the groups of 1×104, 0.5×104, or 0.25×104, several DP spheroids were observed, and the numbers of DP spheroids increased with decreasing amounts of cells and the DP spheroid diameters were inversely proportional (Fig. 2). In group 1×104, the number of cell spheres was the least, but the number of DP spheroids with a diameter ranging between 150 and 250 μm was the most (Fig. 2).
Table 1.
The Effect of DP Cell Spatial Location on the Number and Size Distribution of DP Microtissues
| Number of microtissues | |||
|---|---|---|---|
| Diameter of DP microtissues (μm) | Cells on Matrigel | Cells within Matrigel | Cells under Matrigel |
| >250 | 1 | 0 | 0 |
| 150–250 | 12±4 | 4±2# | 0 |
| 50–150 | 10±6 | 33±8 | 0 |
Only compact spheroidal microtissues with a diameter larger than 50 μm were calculated. The number of spheres with a diameter ranging from 150 to 250 μm formed on Matrigel is significantly higher than the number formed within the Matrigel (#p<0.05).
DP, dermal papilla.
FIG. 2.
The effect of DP cell spatial location and seeding density on the formation of DP microtissues after DP cell seeding for 5 days. (A) Many spheroidal microtissues with a diameter over 150 μm were found on the surface of the Matrigel. Many multicellular masses with a diameter less 50 μm were found within the Matrigel-coated plates. DP cells were better spread with a larger surface under the Matrigel and did not form multicellular masses. (B) DP cells aggregate in an irregular manner at 2×104 cells/well. Many DP microtissues were observed at 1×104, 0.5×104, or 0.25×104 cells/well. The volume of spheroidal microtissues increased with the increase of DP cell seeding density, but the number of microtissues decreased with the increase of DP cell seeding density. Scale bar: 200 μm.
Table 2.
The Effect of Seeding Density of DP Cell on the Number and Size Distribution of DP Microtissues
| Number of microtissues for different cell numbers (cells/well) | ||||
|---|---|---|---|---|
| Diameter of DP microtissues (μm) | 2×104 | 1×104 | 0.5×104 | 0.25×104 |
| >250 | 1 | 0 | 0 | 0 |
| 150–250 | 0 | 13±3 | 5±2# | 0 |
| 50–150 | 0 | 8±3 | 18±7 | 4±3 |
Only compact spheroidal microtissues with a diameter larger than 50 μm were calculated. There is a decreasing trend of microtissue formation as higher seeding cell numbers are used; the size of the DP spheroids was inversely proportional. The number of spheres with diameters ranging from 150 to 250 μm formed at 1×104 is significantly higher than the number formed at 0.5×104 (#p<0.05).
According to the above, the optimal conditions for DP spheroids were found to be 1×104 DP cells/well seeded onto Matrigel-coated (150 μL/cm2) plates.
Characteristics of DP spheroids
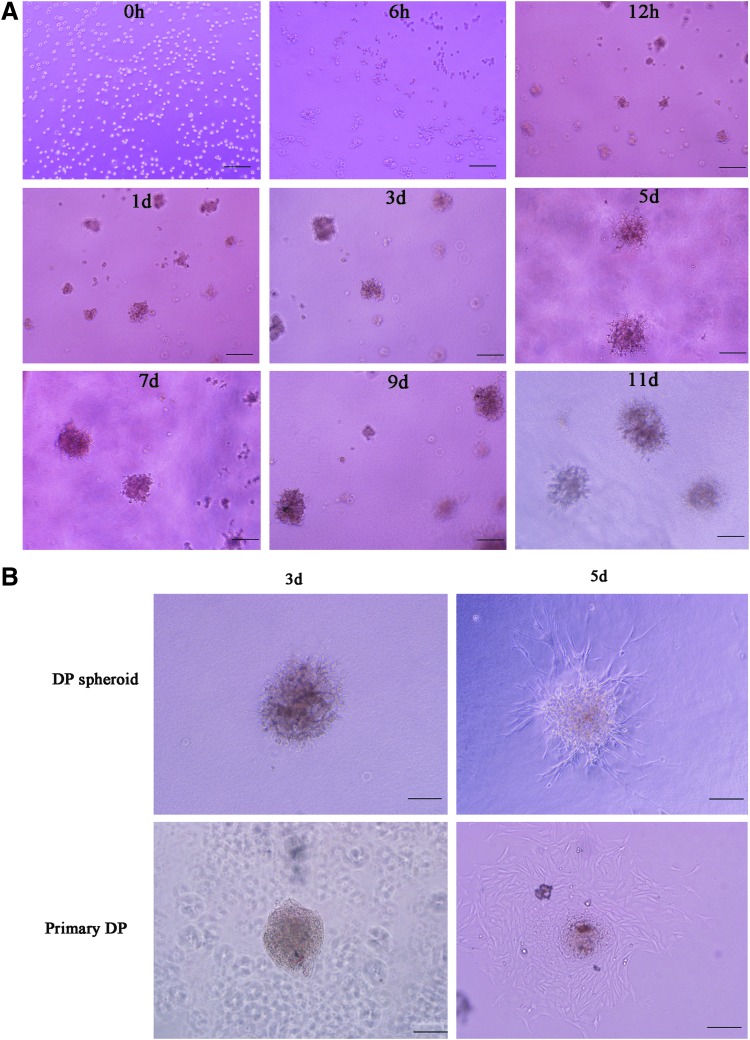
To clarify the formation of DP microtissues on Matrigel to be caused by cell migration or cell expansion, we performed time-lapse recording (Fig. 3). After a 6-h incubation, motile DP cells spontaneously aggregated into multicellular aggregates after intercellular collision. After 12 h, spheroidal microtissues with a diameter of 50 μm can be formed (Fig. 3). Afterward, these small spheroids were rarely motile and the volume of DP spheroids gradually increased as the incubation time progressed. After a 5-day incubation, DP spheres were approximately 150–250 μm in diameter. However, the diameter of the spheroidal microtissues did not significantly increase over the following days (Fig. 3).
FIG. 3.
Characteristics of DP spheroids. (A) Time-lapse microscopic recording of DP cell behavior on Matrigel-coated plates. Shown in the figure are representative images of 1×104 DP cells/well were cultured for up to 11 days. (B) The integrity and viability of DP spheroids after transfer to uncoated culture dishes for 5 days. DP spheroids could maintain a spheroidal structure after through a micropipette and could show cell outgrowth 5 days after culture. Freshly isolated DP was shown as the control. Scale bars: 200 μm.
The integrity and viability of DP spheroids were validated by recapturing the microtissues in new wells. After passing through the micropippete tip, the microtissues remained in an aggregated spheroidal morphology. Similar to primary DP, DP spheroids became attached on the culture plates after 3 days of incubation and showed cell outgrowth after 5 days of incubation (Fig. 3). The results showed that DP spheroids were able to maintain the structural integrity and cellular viability after being injected.
Characterization of protein and gene expression of signature genes in DP spheroids
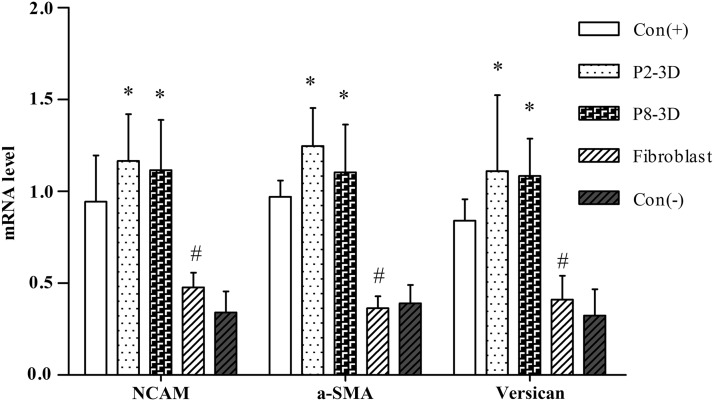
RT-PCR showed that the expression of signature genes, which is associated with HF inductivity of DP cells,6,7,10,17,29,30 was preserved in DP spheroids (Fig. 4). Compared with adherent growth P8 DP cells (negative control), NCAM, Versican, and α-SMA were significantly upregulated during cultivation in sphere-forming conditions for P8 DP cells (Fig. 4). For P2 DP cells, 3D culturing induced gene upregulation compared with 2D culture (positive control), but without significant differences. While we expected fibroblasts to form spheroid microtissues on Matrigel-coated plates, we did not find gene expression to be upregulated.
FIG. 4.
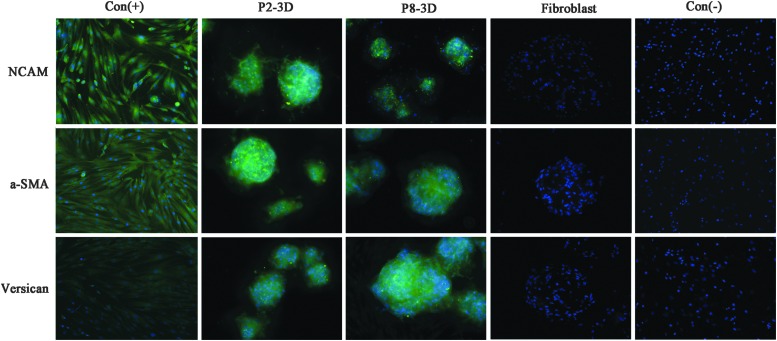
Gene expression of DP spheroids. Expression of DP signature genes, including NCAM, Versican, and α-smooth muscle actin (α-SMA) is well preserved in the spheroidal microtissues. Con (+): positive control, P2 DP cell growth on uncoated plates; Con (−): negative control, P8 DP cell growth on uncoated plates; P8-3D: P8 DP spheroids formed on Matrigel-coated plates; P2-3D: P2 DP spheroids formed on Matrigel-coated plates; fibroblast: fibroblast spheroids formed on Matrigel-coated plates. *p<0.05 compared with the negative control group. #p<0.05 compared with the positive control group.
To observe the change of DP cell signature proteins after culturing, the protein expression of NCAM, Versican, and α-SMA was detected by immunofluorescent staining. Similar to the results of RT-PCR, immunohistochemical studies showed that these sphere-forming P8 DP cells maintained NCAM, Versican, and α-SMA expression (Fig. 5). The fibroblast spheroids did not express NCAM, Versican, and α-SMA (Fig. 5).
FIG. 5.
Immunofluorescent staining of DP spheroids. Shown in the figure are representative images of DP signature protein, including NCAM, Versican, and α-SMA. Compared with adherent DP cells, P spheroids had abundant NCAM, α-SMA, and Versican expression. Green: NCAM, α-SMA, or Versican; blue: nuclei. Con (+) and Con (−): positive and negative control, P2 and P8 DP cell growth on uncoated plates; P8-3D and P2-3D: P8 and P2 DP spheroids formed on Matrigel-coated plates; fibroblast: fibroblast spheroids formed on Matrigel-coated plates.
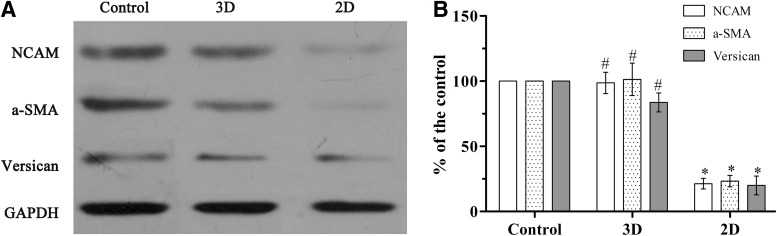
We confirmed that DP spheroids formed on Matrigel can restore expression of markers associated with HF inductivity of DP cells by RT-PCR and immunofluorescence. For a quantitative study of target protein expression, we examined protein expression in response to DP cell culture on Matrigel-coated plates. Immunoblotting studies showed that P8 adherent DP cells cultured on plastic barely expressed NCAM, Versican, and α-SMA proteins, while DP spheroids formed on Matrigel expressed all of them (Fig. 6).
FIG. 6.
The expression of certain proteins in DP spheroids. (A) Passage 8 DP cells were grown on the 3D Matrigel culture system or 2D plates for 5 days and underwent western blot analysis with GAPDH used as the internal control. Passage 2 DP cells were grown on 2D plates as a control. Representative bands are shown. (B) Histograms showing the quantification of western blot bands. *p<0.05 compared with the control group. #p<0.05 compared with 2D culture.
DP spheroid induction of HF
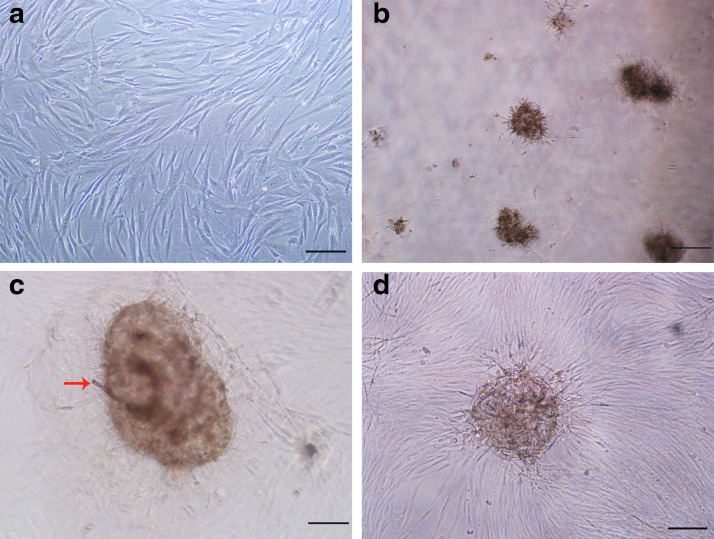
When P8 DP spheroids fabricated on a Matrigel-coated plate mixed with HGMCs were incubated on the Matrigel surface, colorless hair shafts were observed (Fig. 7c). P8 DP cells mixed with HGMCs grew adherently on uncoated plates and did not form hair fibers (Fig. 7a). Although HGMCs also formed cell spheroids on the surface of the Matrigel, they could not be differentiated into hair fibers or hair shafts (Fig. 7b). Similarly, although fibroblasts also formed cell spheroids on the Matrigel surface, they did not induce hair growth (Fig. 7d).
FIG. 7.
Inductivity of DP cell spheres. (a) Negative control group 1: P8 DP cells and hair germinal matrix cells (HGMCs) were seeded on uncoated plates. (b) Negative control group 2: HGMCs were seeded on Matrigel-coated plates. (c) P8 DP spheres and HGMCs were seeded on Matrigel-coated plates; a colorless hair shaft was observed (red arrow). (d) Fibroblast spheres and HGMCs seeded on Matrigel-coated plates. (a, b) Scale bars=200 μm. (c, d) Scale bars=100 μm.
Discussion
The ECM has an important role for the growth and function of cells in vivo. First, the ECM provides not only physical strength to organized cells31,32 but also important key biochemical signals for polarity and growth.32,33 Second, the function of cell populations not only depends on cell–cell interactions but also on cell–ECM interactions. A common feature of DP cells is that they are physiologically in close contact with the ECM. However, the effect of the ECM on the growth of DP cells has not been systemically investigated. To explore whether the ECM benefits the formation of DP spheroids, DP cells were seeded on plates precoated with Matrigel or hyaluronan. As in previous studies,14,24 DP cells cannot form spheroids on the surface of plates precoated with a thin layer of Matrigel (2D). On the contrary, many DP spheroids can be found in 3D Matrigel culture. Our results indicate the importance of 3D culture on the formation of DP spheroids. However, DP spheroids cannot be found in 3D hyaluronan culture. Although the reason for this is not clear, the explanation might be as follows: as a constituent of the ECM, hyaluronan does not provide a suitable microenvironment to cells in the ECM, or our construction of hyaluronan is not suitable for cell growth in vitro.
Previous studies showed that there are two manners in which DP spheroids form. DP cells were cultured on the surface of plates with low-adhesive capacities or within hydrogels. For the former, DP spheroids with varying diameter can be formed on the first day after seeding through the intercellular collision and fusion, and the size of DP spheroids depends on the seeding density of cells, even for 600 μm. However, there is a limited scope for expanding cell numbers.13–16,34 For the latter, DP spheroids also can be formed by the cloning growth of DP cells in hydrogels. The size of DP spheroids depends on the time of cell culture, which usually requires about 2 weeks to form a DP spheroid with a diameter 100 μm, hardly exceeding 200 μm.35 Our results showed that the formation of DP spheroids can be divided into two aspects. In the early stage, DP cells can form spheroids with a diameter of 100 μm, through intercellular collision and fusion. In the later stage, the size of DP spheroids mainly increases through cell proliferation within DP spheroids. Generally, the diameter of DP spheroids formed in the Matrigel can reach to 250 μm, which is similar to normal human scalp DP. Compared with DP spheroids formed on low-adhesive plates, the size and number of DP spheroids is also related to the seeding density of cells. DP cells will aggregate in an irregular manner and cannot form spheres or a spherical cell mass when DP cells are seeded at a density of 2×104/well in 96-well plates. This indicates that the formation of DP spheroids in Matrigel needs optimal conditions. In our experiments, we found that cells within spheres can disaggregate and migrate out from spheres when cultured on plates like primary DP. Previous studies indicated that multicellular spheroids are analogous to avascular tissue, with a diffusion limitation to many molecules and commonly display a layer-like structure comprising a necrotic core, an inner layer of quiescent cells, and an outer layer of proliferating cells when their diameter is above 500 μm.36,37 The migration and growth indicates that cells within the DP spheroid with a diameter of 150–250 μm can obtain enough nutrition and O2 by penetration and can maintain a stable survival status, respectively. Thus, we can deduce that DP spheroids formed in 3D Matrigel culture are similar to primary DP. However, further experiments were necessary to determine whether the migrated cells could form secondary spheres and were as efficient at contributing to DP in vivo as the primary spheres.
In vitro, NCAM, Versican, and α-SMA have been used as major markers for DP cells, with the expression of these markers decreasing in culture, coinciding with the decline of inductive capabilities.6,7 Studies have been presented that detail the maintenance of Versican expression in vitro,29,30 thought to be a way of maintaining the antigen characteristics and inductive capabilities of DP cells.30 As in previous studies,38,39 we also showed that P8 DP cells can restore expression of DP markers after sphere formation. Although the expression of these markers was regarded as an indicator of DP cells in vitro, the hair-inducing ability of DP cells was usually verified by an in vivo experiment.7,10,13–16,35,38,39 As in vivo studies involving labeling transplanted cells with a fluorescence indicator, and not readily to observe the formation of HFs, researchers have been always hoping to find a suitable experimental model in vitro to detect hair-inductivity of DP cells.40–42 We showed that hair fiber-like structures were found using passage 8 DP spheroids and passage 2 HGMCs on Matrigel. Because of the lack of melanophores, only translucent filamentary structures were observed after 10 days in culture. Although hair fiber-like structures did not elongate with longer incubation times and aggregated cells around hair fiber-like structures begin to emigrate after 15 days in culture, this indicated that our current culture conditions were insufficient to maintain continuous growth in vitro. Culture conditions will be optimized in future experiments.
For the first time, we have exploited the Matrigel method to verify the hair-inducing ability of high-passage adult human DP cell in vitro. Compared with in vivo, although there are some disadvantages in vitro, we have constructed hair fiber-like structures using cultured human HF cells. This new method of cell culture can provide not only an opportunity to form DP spheroids but also a model to verify the hair-inducing ability of DP cells or the hair-differentiation ability of HGMCs, even providing a 3D model to explore the interaction of epithelial–mesenchyme in HF formation in vitro.
Previous studies have indicated that sphere-forming multipotent dermal cells possess hair-inducing capacity,38,43,44 raising the question as to whether hair-inducing capacity depends on the identity of DP cells or the process of sphere formation. To address this issue, we investigated whether interfollicle fibroblasts can be induced to form cell spheroids, express DP markers, and possess hair-inducing capacity. We showed that although fibroblasts can form cell spheroids on Matrigel, they barely express DP markers or have hair-inducing capacity. This contrasts with previous studies,38,43,44 in which cells of spheres were derived from multipotent dermal cells. We demonstrated that while cell growth can be regulated by the environment, hair-inducing capacity is only related to cell identity and is an intrinsic property of DP cells.
Our data raise the possibility that DP markers and sphere formation are two major factors on hair-inducing capacity of DP cells, but not determinants. Based on the phenomenon that DP sphere formation in 3D can restore the loss of cell identity in 2D culture, we propose that the intrinsic property of DP cells can be determined by special genes, and the gradual loss of DP cells is caused by the silence, not loss, of these genes due to an unsuitable microenvironment, but these silent genes can be activated under certain conditions. Once this conjecture is confirmed, it means that we can change a few genes to give other cells the hair-inducing capacity, such as fibroblasts, regardless of their cell identity as DP cells. We will verify this hypothesis in the future.
Matrigel recapitulates many in vivo characteristics of the ECM. However, they may contain nonquantified impurities, such as growth factors and intracellular proteins. Major issues are batch-to-batch variations and limited availability. Thus, we will repeat this study to verify our results using a fully synthetic ECM with quantified ingredients in the future.
The data presented here have led us to two major conclusions. First, we have succeeded in developing a 3D Matrigel culture system that mimics the niche of DP cells in vivo, in which adult human later passage (passage 8) DP cells can form spheroids similar to intact DP. Second, these DP spheroids can restore their hair-inductivity lost in adherent growth. Moreover, we are the first to have shown that passage 3 human HGMC differentiate into hair-like fiber in the presence of human DP spheroids in vitro. Our methods are expected to contribute toward the characterization of DP cells and the application of human DP cells to induce HF regeneration.
Acknowledgments
The authors thank Bo-Xin Zhao and Qi-Sheng Wang for their assistance for the immunofluorescent staining portion of this study. The authors thank the Natural Science Foundation of China (Grant No. 31170949) and the Specialized Research Fund for the Doctoral Program of Higher Education, China (Grant No. 20124433110012) for their financial support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cash T.F.The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol 141,398, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S., Fischer T.W., Chren M.M., Strauss B.M., and Elsner P.Strategies of coping and quality of life in women with alopecia. Br J Dermatol 144,1038, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Stenn K.S., and Cotsarelis G.Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol 16,493, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Miteva M., and Tosti A.Treatment options for alopecia: an update, looking to the future. Expert Opin Pharmacother 13,1271, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G., and Paus R.The biology of hair follicles. N Engl J Med 341,491, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Rendl M., Lewis L., and Fuchs E.Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol 3,e331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C.C., and Cotsarelis G.Review of hair follicle dermal cells. J Dermatol Sci 57,2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driskell R.R., Clavel C., Rendl M., and Watt F.M.Hair follicle dermal papilla cells at a glance. J Cell Sci 124,1179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahoda C.A., Reynolds A.J., and Oliver R.F.Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol 101,584, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Osada A., Iwabuchi T., Kishimoto J., Hamazaki T.S., and Okochi H.Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng 13,975, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Rendl M., Polak L., and Fuchs E.BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 22,543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto J., Burgeson R.E., and Morgan B.A.Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14,1181, 2000 [PMC free article] [PubMed] [Google Scholar]
- 13.Young T.H., Lee C.Y., Chiu H.C., Hsu C.J., and Lin S.J.Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly (ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials 29,3521, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Young T.H., Tu H.R., Chan C.C., Huang Y.C., Yen M.H., Cheng N.C., Chiu H.C., and Lin S.J.The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell-substratum adhesivity and cell motility. Biomaterials 30,5031, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C.H., Wang J.L., and Huang Y.Y.Large-scale cultivation of transplantable dermal papilla cellular aggregates using microfabricated PDMS arrays. Acta Biomater 7,315, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y.C., Chan C.C., Lin W.T., Chiu H.Y., Tsai R.Y., Tsai T.H., Chan J.Y., and Lin S.J.Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials 34,442, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Ohyama M., Zheng Y., Paus R., and Stenn K.S.The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol 19,89, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Soma T., Fujiwara S., Shirakata Y., Hashimoto K., and Kishimoto J.Hair-inducing ability of human dermal papilla cells cultured under Wnt/beta-catenin signalling activation. Exp Dermatol 21,307, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds A.J., Lawrence C., Cserhalmi-Friedman P.B., Christiano A.M., and Jahoda C.A.Trans-gender induction of hair follicles. Nature 402,33, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jahoda C.A., Mauger A., Bard S., and Sengel P.Changes in fibronectin, laminin and type IV collagen distribution relate to basement membrane restructuring during the rat vibrissa follicle hair growth cycle. J Anat 181,47, 1992 [PMC free article] [PubMed] [Google Scholar]
- 21.Almond-Roesler B., Schon M., Schon MP., Blume-Peytavi U., Sommer C., Löster K., and Orfanos C.E.Cultured dermal papilla cells of the rat vibrissa follicle. Proliferative activity, adhesion properties and reorganization of the extracellular matrix in vitro. Arch Dermatol Res 289,698, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Griffith L.G., and Swartz M.A.Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7,211, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Pampaloni F., Reynaud E.G., and Stelzer E.H.The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8,839, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Abbott A.Cell culture: biology's new dimension. Nature 424,870, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kleinman H.K., McGarvey M.L., Liotta L.A., Robey P.G., Tryggvason K., and Martin GR.Isolation and characterization of type IV procollagen, Iaminin, and heparin sulfate proteoglycan from the EHS sarcoma. Biochemistry 21,6188, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M.Taking cell-matrix adhesions to the third dimension. Science 294,1708, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lo A.T., Mori H., Mott J., and Bissell M.J.Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia 17,103, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Magerl M., Kauser S., Paus R., and Tobin D.J.Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp Dermatol 11,381, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kim S.R., Cha S.Y., Kim M.K., Kim J.C., and Sung Y.K.Induction of versican by ascorbic acid 2-phosphate in dermal papilla cells. J Dermatol Sci 43,60, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Feng M., Yang G., and Wu J.Versican targeting by RNA interference suppresses aggregative growth of dermal papilla cells. Clin Exp Dermatol 36,77, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Bissell M.J., Radisky D.C., Rizki A., Weaver V.M., and Petersen O.W.The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation 70,537, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghajar C.M., and Bissell M.J.Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol 130,1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickl M., and Ries C.H.Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 28,461, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Higgins C.A., Richardson G.D., Ferdinando D., Westgate G.E., and Jahoda C.A.Modeling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol 19,546, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Driskell R.R., Juneja V.R., Connelly J.T., Kretzschmar K., Tan D.W., and Watt F.M.Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J Invest Dermatol 132,1084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curcio E., Salerno S., Barbieri G., De Bartolo L., Drioli E., and Bader A.Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 28,5487, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Achilli T.M., Meyer J., and Morgan J.R.Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 12,1347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu R., Okabe K., Kubota Y., Nakamura-Ishizu A., Nakajima H., and Kishi K.Sphere formation restores and confers hair-inducing capacity in cultured mesenchymal cells. Exp Dermatol 20,679, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Kang B.M., Kwack M.H., Kim M.K., Kim J.C., and Sung Y.K.Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol 132,237, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Havlickova B., Bíró T., Mescalchin A., Arenberger P., and Paus R.Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial-mesenchymal interactions. Br J Dermatol 151,753, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Havlickova B., Bíró T., Mescalchin A., Tschirschmann M., Mollenkopf H., Bettermann A., Pertile P., Lauster R., Bodó E., and Paus R.A human folliculoid microsphere assay for exploring epithelial-mesenchymal interactions in the human hair follicle. J Invest Dermatol 129,972, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Yen C.M., Chan C.C., and Lin S.J.High-throughput reconstitution of epithelial-mesenchymal interaction in folliculoid microtissues by biomaterial-facilitated self-assembly of dissociated heterotypic adult cells. Biomaterials 31,4341, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Biernaskie J., Paris M., Morozova O., Fagan B.M., Marra M., Pevny L., and Miller F.D.SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5,610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chueh S.C., Lin S.J., Chen C.C., Lei M., Wang L.M., Widelitz R., Hughes M.W., Jiang T.X., and Chuong C.M.Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther 13,377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]