Abstract
Melanoma is the most aggressive form of skin cancer and until recently, it was extremely resistant to radio-, immuno-, and chemotherapy. Despite the latest success of BRAF V600E-targeted therapies, responses are typically short lived and relapse is all but certain. Furthermore, a percentage (40%) of melanoma cells is BRAF wild type. Emerging evidence suggests a role for normal host cells in the occurrence of drug resistance. In the current study, we compared a variety of cell culture models with an organotypic incomplete skin culture model (the “dermal equivalent”) to investigate the role of the tissue microenvironment in the response of melanoma cells to the chemotherapeutic agent doxorubicin (Dox). In the dermal equivalent model, consisting of fibroblasts embedded in type I collagen matrix, melanoma cells showed a decreased cytotoxic response when compared with less complex culture conditions, such as seeding on plastic cell culture plate (as monolayers cultures) or on collagen gel. We further investigated the role of the microenvironment in p53 induction and caspase 3 and 9 cleavage. Melanoma cell lines cultured on dermal equivalent showed decreased expression of p53 after Dox treatment, and this outcome was accompanied by induction of interleukin IL-6, IL-8, and matrix metalloproteinases 2 and 9. Here, we show that the growth of melanoma cells in the dermal equivalent model inflects drug responses by recapitulating important pro-survival features of the tumor microenvironment. These studies indicate that the presence of stroma enhances the drug resistance of melanoma in vitro, more closely mirroring the in vivo phenotype. Our data, thus, demonstrate the utility of organotypic cell culture models in providing essential context-dependent information critical for the development of new therapeutic strategies for melanoma. We believe that the organotypic model represents an improved screening platform to investigate novel anti-cancer agents, as it provides important insights into tumor-stromal interactions, thus assisting in the elucidation of chemoresistance mechanisms.
Introduction
Although the incidence of melanoma is low relative to the more common basal cell carcinoma and squamous cell carcinoma, its lethality is high and it is known to account for 80% of all skin cancer deaths.1,2 If detected early, melanoma is readily curable through surgery. However, once disseminated, the potential for curative therapy is minimum. The recent years have seen many notable breakthroughs in the management of advanced melanoma with the anti-CTLA4 antibody ipilimumab and the BRAF kinase inhibitor vemurafenib gaining FDA-approval in 2011. Despite these successes, response rates to ipilimumab are low, and long-term responses to BRAF inhibitors have provided elusion for the majority of patients.3,4
Resistance to chemotherapy is a major factor in the failure of many forms of treatments in cancer, specifically in melanoma. Tumors usually consist of heterogeneous populations of malignant cells, some of which are drug-sensitive while others are drug-resistant. Chemotherapy kills drug-sensitive cells, leaving behind a higher proportion of drug-resistant cells. As the tumor begins to grow again, chemotherapy now fails because the remaining tumor cells are resistant. Both intrinsic and acquired resistance results from the numerous genetic and epigenetic changes occurring in cancer cells.5
Furthermore, metastatic melanoma cells are highly plastic and can accommodate new and reorganized microenvironments, consisting of a rich milieu of stromal cells and extracellular molecules. Therefore, the tumor is not only composed of cancer cells, but it also contains different types of stromal cells, which may play important roles in tumor initiation, progression, metastasis, and resistance to treatments.6 Thus, it is possible that the microenvironment contributes to chemoresistance and decreased drug uptake in tumors, thus regulating tumor sensitivity to a variety of chemotherapies. In fact, carcinoma-associated fibroblasts contribute directly to carcinogenesis7 through their secretion of multiple growth factors and cytokines.8 In turn, the tumor cells alter the extracellular matrix (ECM) by modulating the stromal metabolism and releasing growth factors, cytokines, and proteases such as matrix metalloproteinases (MMPs). This cross-talk between host and tumor leads to the formation of a permissive stroma that faciliates tumor progression as well as chemoresistance.9,10 To date, a few studies have accounted for the role of the tumor microenvironment in determining therapeutic outcome, and, therefore, experiments performed are often not predictive of drug responses in patients.11,12
Here, we have employed a variety of cell culture models, including plating melanoma cells on (i) plastic (monolayer culture); (ii) type I collagen; and (iii) in an organotypic skin culture model (the “dermal equivalent,” containing collagen and fibroblasts), to investigate the role of the tissue microenvironment in the response to chemotherapeutic agents (e.g., doxorubicin [Dox]). Our data demonstrate that the effect of cytotoxic agents on melanoma cells is not equivalent under differing culture conditions. The dermal equivalent modulates melanoma cell growth and affects drug responses by recapitulating important pro-survival features of the tumor microenvironment, thus preventing efficient induction of cell death. We believe this organotypic model may provide important new insights to further elucidate mechanisms of melanoma chemoresistance.
Materials and Methods
Cell culture
The melanoma cell lines used were SK-Mel-19 (BRAF mutant V600E), -103, and -147 (both BRAF wild-type), and, also, primary cultures of normal human dermal fibroblasts (NHDF, used until the 15th passage). Cells were grown at 37°C in Dulbecco's Modified Eagle's Medium (GIBCO, #12100-060; Life Technologies, Grand Island, NY), containing 4 mM of L-glutamine and supplemented with 10% fetal bovine serum (FBS; Life Technologies, South America), 25 μg/mL ampicillin, and 100 μg/mL streptomycin.
Generation of dermal equivalents with melanoma cells (adapted from Brohem et al.13)
The dermal equivalent was generated by resuspension of NHDF in a collagen mixture consisting of 750 μL/mL of 2.5 mg/mL of type I collagen (BD Biosciences, San Jose, CA), 50 μL/mL of FBS (Life Technologies), 100 μL/mL of Reconstitution Buffer 10×(0.05 M NaOH, 2.2% NaHCO3, and 200 mM HEPES), and 100 μL/mL of HAM-F12 medium 10×(GIBCO, Life Technologies). For melanoma cells culture in the dermal equivalent, 1.5×105/well fibroblasts were firstly resuspended in the collagen mixture and transferred to 24-well plates (1 mL/well). After solidification at 37°C in a 5% CO2 atmosphere, culture medium was added (1 mL/well) containing metastatic human melanoma cells SK-Mel-103 (105 cells/well), with subsequent incubation in 5% CO2 at 37°C. For experiments utilizing dermal equivalents, or collagen gel coating, cells were seeded 5 to 15 days before Dox treatment with culture medium replaced every 2 days.
For additional analysis, including cell viability, DNA fragmentation, protein imunoblotting, or enzyme-linked immuno sorbent assay (ELISA) studies, fibroblasts (106 cell/600 μL/60 mm cell culture plate) were resuspended in type I collagen mixture as described earlier, with subsequent plating of 5.5×105 melanoma cells/3 mL/60 mm cell culture plate and cultured for 1 day before treatment.
For histological analysis of dermal equivalents, samples were washed once with phosphate buffered saline (PBS), fixed with formalin 10% overnight at 4°C, and then stored in 70% ethanol at 4°C, until further processing and embedding in paraffin. Histological sections (5 μm thick) were stained with hematoxylin and eosin (H&E) and analyzed by light microscopy.
Dox treatment
To evaluate inhibition of melanoma cells grown on different substrates, 0 to 2.8 μM of Dox (Doxorubicin, Adriamycin; Thermo Fisher Scientific, Waltham, MA) were used, as previously determined.14–17 Additional tests were performed with Dox IC50 (doxorubicin concentration that inhibits 50% of the cells) for melanoma cell lines cultured on dermal equivalents, which correspond to 1.2 μM. For histological studies, SK-Mel-103 melanoma cells were cultured as described earlier and treated with Dox for 48 h.
Experimental conditions
In order to analyze melanoma responses to Dox on different substrates, the following conditions were used: (i) melanoma cells cultured on plastic in monolayer (M): cells were seeded on plastic cell culture plates; (ii) melanoma cells cultured on collagen type I (M+Col): cells were seeded on type I collagen gel after a 1 h solidification period; (iii) melanoma cells cultured on a dermal equivalent (M+Eq): cells were seeded on a dermal equivalent consisting of fibroblasts embedded in type I collagen matrix after a 1 h solidification period; and (iv) dermal equivalent (Eq): human fibroblasts without melanoma cells were embedded in type I collagen matrix as described earlier and used as a microenvironment control.
Digestion of the collagen matrix and cell collection
In order to collect cells grown on type I collagen or dermal equivalents, collagen digestion was performed using collagenase type I (Sigma-Aldrich, Saint Louis, MO) at a final concentration of 1 mg/mL. After the addition of collagenase, cultures were placed at 37°C for 20–30 min, followed by cell collection.
Cell viability by Trypan Blue exclusion assay
Cell viability was assessed by standard Trypan Blue dye-exclusion assay using 0.4% Trypan Blue in PBS.18 For experiments involving melanoma cells cultured on dermal equivalents, cell counting was performed without separation or discrimination between cell types. The assay was performed in biological triplicates from three different experiments.
DNA fragmentation by fluorescence-activated cell sorter
To differentiate between fibroblasts and melanoma cells present in the dermal equivalent, we used a fluorescence-activated cell sorter (FACS)-dependent DNA fragmentation assay. This assay was performed using SK-Mel-103 cells labeled with green fluorescent protein (GFP) by infection with lentiviral vector pLV-GFP-LC3II, and also NHDF cells with no vector. After Dox treatment, cells were collected, washed twice with PBS, and incubated for 30 min in the dark with a solution of 100 μg/mL propidium iodide (PI) in PBS. Samples were analyzed immediately by flow cytometry. DNA fragmentation was assessed by the incorporation of PI. The channels selected were PerCP (emission 675 nm) for PI staining and FITC (emission 533 nm) for GFP-labeled cells. Data analysis was performed using FlowJoTM software (version 10.0.6; Tree Star, Ashland, OR). The assay was performed in biological duplicates from three different experiments.
Protein immunoblotting
At the end of treatment, cultures were collected by centrifugation after trypsinization and treatment with collagenase I (1 mg/mL). Laemmili lysis buffer (62.5 mM Tris-base pH 6.8, 2% SDS, 10% glycerol, and 5% 2-mercaptoethanol), 70–100 μL was added to the pellet. Protein was quantified by standard Bradford method using Bio-Rad Protein Assay dye reagent, separated on 4–20% gradient SDS-polyacrylamide gels, and transferred to Immobilon-P membranes. The antibodies used included anti-human p53 (Vector Laboratories, Burlingame, CA), anti-human caspase 3 (Cell Signaling, Danvers, MA), anti-human caspase-9 (Cell Signaling), and anti-human β-actin (Cell Signaling). The assay was performed in experimental triplicate.
Gelatin zymography
Gelatin-substrate gel electrophoresis was used to measure metalloproteinase activity as described by da Silva Cardeal et al.,19 with minor modifications. Cells were cultured as described earlier in “Generation of dermal equivalents with melanoma cells.” At the end of the treatment, conditioned culture medium was collected and centrifuged twice at 3000 rpm for 5 min at 4°C. Protein concentration was determined by the Folin method.20 Supernatant protein (25 μg) from each sample was separated by electrophoresis on a 10% acrylamide gel containing 0.5 mg/mL gelatin at 100 V. The gel was washed twice with 2.5% Triton X-100 for 15 min at 37°C, and once with reaction buffer (0.05 M Tris–HCl (pH 8), 5 mM CaCl2, 5 mM ZnCl2). The gel was then incubated overnight in the same reaction buffer at 37°C. Gels were stained with Coomassie solution (0.5% Coomassie Brilliant Blue R-250 in 10% methanol and 10% acetic acid) for 30 min and destained in 10% methanol+10% acetic acid solution. Clear zones of gelatin lysis (bands) against a blue background indicated the presence of active MMPs. Each lysed band within a given sample lane was analyzed by scanning densitometry (450 d.p.i. in GS-700; Bio-Rad, Hercules, CA). MMP-2 and MMP-9 bands were measured using ImageJ 1.47 software (U.S. National Institutes of Health, Bethesda, MD). The assay was performed in biological triplicates from three different experiments.
Gelatinase activity
SK-Mel-103 melanoma cells were cultured on dermal equivalents as described earlier, and the conditioned culture medium was collected after Dox treatment and incubated with DQ™ gelatin (gelatin conjugated to FITC; Invitrogen, Life Technologies) for 6 h at room temperature in the dark, according to the manufacturer's guidelines. The fluorescence was measured at 495 nm (excitation) and 515 nm (emission). The assay was performed in biological triplicates from three different experiments.
MMP-2 activity
For MMP-2 activity quantification, melanoma cells were cultured as described earlier. Conditioned culture medium was collected after Dox treatment and centrifuged at 3000 rpm for 5 min at 4°C. Protein concentration was determined by the Folin method20 and the supernatant used to perform the MMP-2 activity assay using a standard colorimetric kit, Biotrak MMP-2 Activity (Amersham, GE Healthcare Biosciences, Pittsburgh, PA), which provides a colorimetric quantitative determination of pro- and active- enzyme after incubation with a fluorescent substrate. The kit was used according to the manufacturer's instructions. The assay was performed in biological triplicates from three different experiments.
Interleukin quantification by ELISA
Measurements of interleukin (IL)-6 and IL-8 secretion were performed for the different experimental conditions, as described earlier. The conditioned culture medium from melanoma or fibroblasts cell cultures was collected after Dox treatment, centrifuged at 3000 rpm for 5 min at 4°C, and further used to perform the ELISA assay (DuoSet; RandD System, Minneapolis, MN), according to supplier instructions and to Lequin.21 Culture medium was replaced by conditioned medium 1 day after plating followed by treatment with Dox. The last conditioned medium was collected at the end of the treatment, and it was used to perform the ELISA assay, as described earlier. The assay was performed in biological quintuplicates from three different experiments.
H&E staining and immunohistochemistry
SK-Mel-103 melanoma cells were cultured as described in “Generation of dermal equivalents with melanoma cells” item. Five to 15 days after plating, melanoma cells were treated with Dox for 48 h. The collagen or dermal equivalent were washed once with PBS, fixed with formaldehyde 3.7% in PBS at room temperature for 1 h, kept in ethanol 70% at 4°C, and further included in paraffin. For histological analysis, tissue sample slides were stained with H&E. For immunohistochemical analysis of Vimentin, slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's protocol. Briefly, slides were deparaffinized on the automated system with EZ Prep solution (Ventana). Heat-induced antigen retrieval method was used in Cell Conditioning 1(Ventana). The mouse monoclonal antibody that reacts to Vimentin (#760-2917; Ventana) was used at a predilute concentration and incubated for 12 min. The Ventana OmniMap Anti-mouse secondary Antibody was used for 16 min. The detection system used was the Ventana ChromoMap kit, and slides were then counterstained with Hematoxylin. Slides were then dehydrated and coverslipped as per normal laboratory protocol. The assay was performed in experimental duplicate.
Statistical analysis
Results were expressed as mean±standard deviation and statistically analyzed using the software GraphPad Instat™ (GraphPad Software, La Jolla, CA), two-way analysis of variance (p-value<0.05).
Results
Inhibition of cell viability by Dox
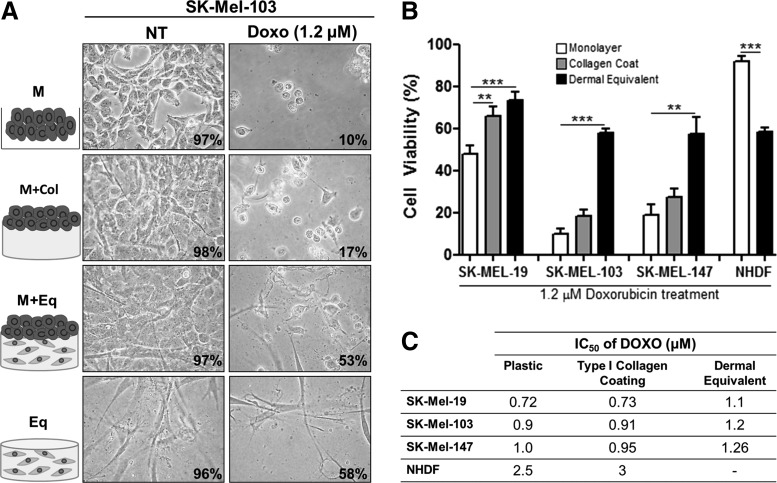
Three melanoma cell lines (SK-Mel-19, -103, and -147) were treated with Dox and analyzed by cytotoxicity assays. The cells were cultivated on different substrates (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea) as follows: monolayer (M); melanoma on type I collagen gel (M+Col); and melanoma on a dermal equivalent (M+Eq), composed of fibroblasts in type I collagen gel. A dermal equivalent with no melanoma cells (Eq) served as a control. As shown in Figure 1A and B, melanoma cells cultured on the dermal equivalent (M+Eq) showed reduced cytotoxicity when treated with Dox IC50 in comparison with the other culture substrates. For SK-Mel-103, cell viability was 50% in M+Eq culture condition, while cells growing on monolayer (M) and collagen coating (M+Col) exhibited 10 and 17% viable cells, respectively. Figure 1A shows the microphotographs for the representative phenotype. It should be noted that the only difference between NHDF in the monolayer and in the dermal equivalent was the use of collagenase I to collect those cells from the dermal equivalent (Fig. 1B). Interestingly, the IC50 is higher in the dermal equivalent (Eq) when compared with melanoma cells growing in monolayers or on type I collagen coating in all investigated cell lines (Fig. 1C). Since M+Eq is a co-culture, cell viability for that condition is very similar to that of Eq.
FIG. 1.
Dox-mediated inhibition of cell viability in melanoma cells grown on different substrates. (A) Cell morphology of SK-Mel-103 cells grown on dermal equivalents after treatment with Dox IC50 (1.2 μM) for 48 h. The panel represents all the culture conditions assessed. Magnification: 100×. (B) Cell viability of human metastatic melanoma cells lines SK-Mel-19, SK-Mel-103, and SK-Mel-147 treated with 1.2 μM of Dox (IC50 to melanoma on dermal equivalent) or not treated (NT). (C) IC50 of Dox under all the evaluated conditions. Treatment conditions were melanoma cells seeded as a monolayer on plastic (M); on type I collagen (M+Col); on dermal equivalents (M+Eq); and the dermal equivalent control with no melanoma cells (Eq). **p<0.01 and ***p<0.001. NHDF, normal human dermal fibroblasts; Dox, doxorubicin; NT, not treated.
Inhibition of cell death pathways in melanoma cells
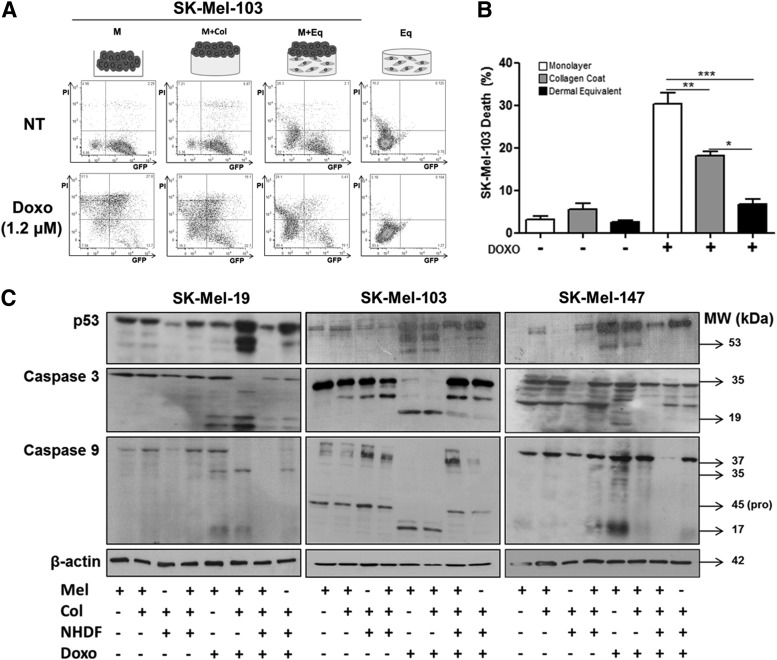
Given that it is not possible to distinguish the relative contribution of melanoma cells and fibroblasts in this dermal equivalent model, it is plausible that the drug-induced decreased cytotoxicity seen in this condition could be a specific response from fibroblasts in the dermal equivalent. To address this concern, we performed a co-culture experiment in which melanoma cells were labeled with GFP and co-cultured with unlabeled fibroblasts. After Dox treatment, DNA fragmentation was assessed by the incorporation of PI, and analyzed by flow cytometry (Fig. 2A). In this assay, cells in late apoptosis or necrosis enable cell membrane permeation and labeling with PI, while viable cells have intact cell membranes and cannot incorporate the fluorophore.22 Thus, GFP-positive cells represent viable melanoma cells; unlabeled cells represent the population of viable fibroblasts; double-labeled cells represent nonviable GFP-melanoma cells; and PI-positive cells represent either nonviable GFP-melanoma cells that lost GFP labeling or nonviable fibroblasts. Our data indicate that SK-Mel-103 melanoma cells grown on dermal equivalents exhibit a decrease in both GFP fluorescence and PI staining (Fig. 2A, B) as shown by FITC and PerCP channels, indicating a lower percentage of nonviable melanoma cells on the dermal equivalent substrate compared with melanoma cells on plastic or on collagen coating.
FIG. 2.
Inhibition of cell death by Dox in melanoma cells grown on dermal equivalent. (A) Characterization of selective cell death of GFP-SK-Mel-103 metastatic melanoma cells cultured in different conditions. Data are expressed as a percentage of fluorescent cells in 10,000 events—live melanoma cells labeled with GFP, dead melanoma cells double labeled with propidium iodide (PI) and GFP (PI/GFP), live fibroblast cells unlabeled, and dead fibroblast cells labeled with PI. (B) Cell death data, plotted from (A). (C) Profile of p53 and caspases 3 and 9 in melanoma cell lines SK-Mel-19, SK-Mel-103, and SK-Mel-147, as modulated by treatment with Dox. Melanoma cells were treated with 1.2 μM of Dox (IC50) or NT. Experimental conditions for melanoma growth are previously described. *p<0.05, **p<0.01, and ***p<0.001. GFP, green fluorescent protein.
Therefore, melanoma cells on the dermal equivalent maintain a lower number of dead cells, compared with the other culture conditions as shown by quantitative analysis in Figure 2B. Considering that fibroblasts are known to be Dox resistant, it is most likely that the poor fibroblast survival found on the dermal equivalent is due to the use of collagenase, which may be cytotoxic for these cells.23–25
We further investigated the role of the microenvironment in p53 induction and caspase 3 and 9 cleavage. Figure 2C indicates that melanoma cell lines cultured on dermal equivalent showed decreased expression of p53 after Dox treatment. Furthermore, the occurrence of cleaved caspase 3 and 9 as seen in monolayer or collagen-coated culture conditions was completely abolished when melanoma cells were grown on the dermal equivalent, suggesting that the apoptotic pathway is compromised when melanoma cells are grown in a tumor microenvironment.
Induction of MMPs after Dox treatment
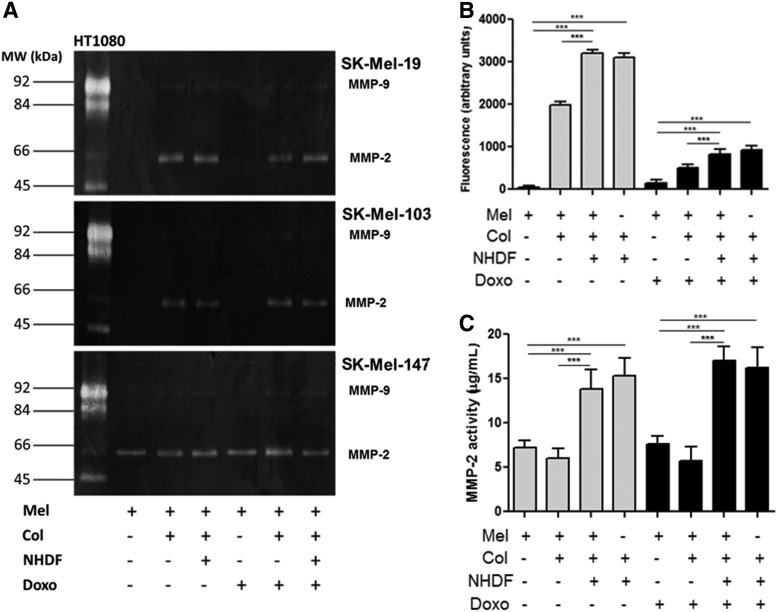
To assess the modulation of metalloproteinases by treatment with Dox under the different culture conditions, a zymogram was generated to evaluate the level of gelatinases activity by gelatin digestion (Fig. 3A). Culture on a collagen substrate coating stimulated the activity of MMP-2 in SK-Mel-19, -103, and -147 compared with culture on plastic. Little variation in the levels of active MMP-9 was observed after Dox treatment under the different culture conditions. In order to evaluate this with greater confidence, we utilized a specific fluorescent substrate for gelatinases showing that dermal equivalent with or without melanoma exhibited the higher levels of fluorescence, which represents increased gelatin digestion by MMPs 2 and 9 (Fig. 3B). In the Biotrak assay, we verified the same high levels of MMP-2 activity (Fig. 3C).
FIG. 3.
Metalloprotease activity is inhibited by Dox-treated melanoma cells grown on different substrates. (A) Zymography assay of human melanoma cell lines SK-Mel-19, SK-Mel-103, and SK-Mel-147 treated with 1.2 μM of Dox (IC50) or NT. (B) Gelatinolytic activity of MMP-2 and -9 in human melanoma cell line SK-Mel-103 (C) Inhibition of MMP-2 activity of by Biotrak® assay in human melanoma cell line SK-Mel-103, whose culture medium was incubated with a fluorescent substrate. Treatment conditions were melanoma cells seeded on plastic, in monolayer (M); on type I collagen (M+Col); on dermal equivalent (M+Eq); and the dermal equivalent control with no melanoma cells (Eq). ***p<0.001. MMP, matrix metalloproteinase.
Interleukin production is favored by fibroblast presence on tumoral microenvironment
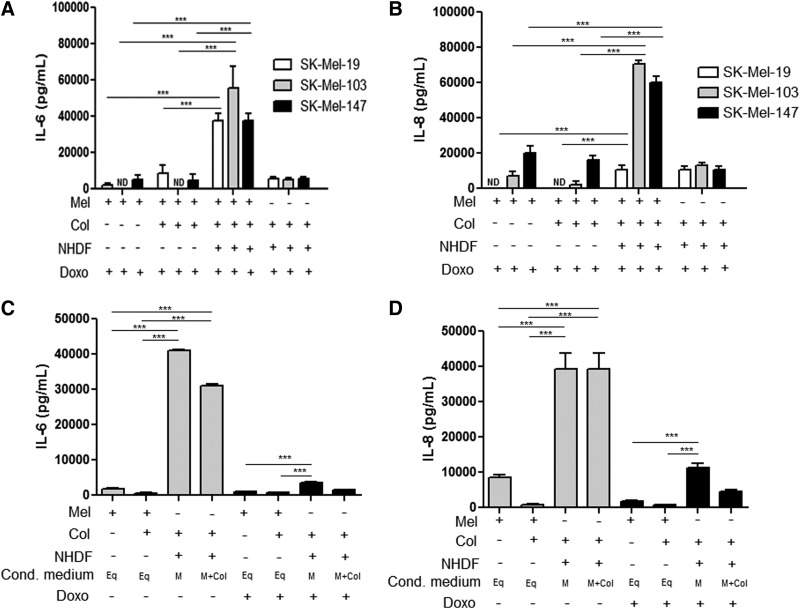
In melanoma, increased expression of IL-6 has been correlated with poor prognosis26; for this reason, we assessed whether Dox treatment induced cytokine production in the dermal equivalent model. As shown in Figure 4A–B, a higher induction of IL-6 and IL-8 secretion was observed when Dox-treated melanoma cells were cultured on dermal equivalents compared with other culture conditions. These results provide some insight on the importance of the co-culture of melanoma cells and fibroblasts. In order to assess which cell type was responsible for IL-6 and IL-8 production, we separately stimulated SK-Mel-103 melanoma cells and dermal equivalents to conditioned media as shown in Figure 4C and D. The dermal equivalent stimulated with conditioned medium from melanoma cells was able to secrete higher levels of IL-6 and IL-8, even after drug treatment, demonstrating the critical role of fibroblasts in the production of immunomodulatory cytokines.
FIG. 4.
Pro-tumoral interleukins activity after drug treatment, which were stimulated by dermal equivalent with melanoma. (A) Interleukin (IL)-6 and (B) IL-8 secretion in human melanoma cell lines SK-Mel-19, SK-Mel-103, and SK-Mel-147 treated with 1.2 μM of Dox (IC50) at 48 h or NT. (C) Conditioned media assessed to identify cells type responsible to IL-6 secretion. (D) Conditioned media assessed to identify cells type responsible to IL-8 secretion. Treatment conditions were melanoma cells seeded on plastic, in monolayer (M); on type I collagen (M+Col); on dermal equivalent (M+Eq); and the dermal equivalent control with no melanoma cells (Eq). ***p<0.001. ND, non detectable.
Melanoma cells grown on dermal equivalents sustain growth on Dox treatment
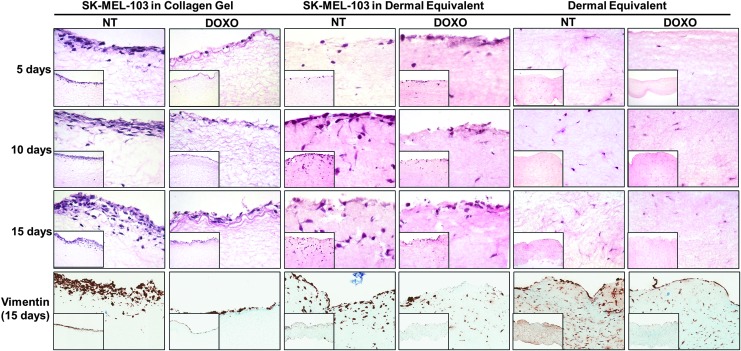
In order to investigate SK-Mel-103 proliferation and growth in the biomimetic environment of the dermal equivalent, tissue samples were submitted to histological evaluation (Fig. 5). H&E staining indicates melanoma proliferation is progressive in the nontreated collagen gel; however, Dox treatment completely inhibits this process (Fig. 5, first three panels from left). Dermal equivalents depict melanoma proliferation and invasion deep into the dermal layer, which is partially halted on treatment with Dox (Fig. 5, first three panels from middle). In this culture condition, while there is a notable decrease in the proliferation of melanoma cells, there is a persistent presence of rounded nucleated cells in the dermal layer, which are morphologically characteristic of melanoma cells and have little resemblance with the spindle-like cells present in the dermal equivalent with no melanoma (right panels). Nevertheless, we have tried to discriminate cell types using a specific antibody for melanoma detection such as S-100 and HMB45; however, the cell line SK-Mel 103 is well known as an amelanotic cell line and for this reason, melanin was undetectable (data not showed). These findings suggest sustained invasion of melanoma cells in the dermal equivalent, even after 15 days of Dox treatment. Furthermore, Dox treatment does not seem to interfere with fibroblasts proliferation.
FIG. 5.
Melanoma cells growth into dermal equivalent under Dox treatment. Human melanoma cell line SK-Mel-103 was seeded on type I collagen gel or dermal equivalent and 5 to 15 days after plating, the cultures were treated with Dox. The histological slides were seen by H&E staining. Also, vimentin staining of SK-Mel-103 seeded on type I collagen gel or dermal equivalent for 15 days, after treatment with Dox. Dermal equivalent with no melanoma cells was used as a control. Magnification: 100× (right bottom smaller figures) and 400× (larger figures). H&E, hematoxylin and eosin.
On evaluating the Vimentin expression in the organotypic models used in this study (Fig. 5), we observed that both melanoma cell lines and fibroblasts express that protein, even though Vimentin is a filament protein which is specifically expressed by mesenchymal cells (Fig. 5, bottom panels). In fact, it has been reported that Vimentin may be overexpressed in several epithelial metastatic cancer cells, including melanoma.27
Discussion
The use of organotypic cultures, such as artificial human skin, enables reproducing cell interactions with the ECM and with other cell types with greater fidelity.28 For this reason, studies exploring the effects of drugs on tumor cells cultured in a biomimetic environment in vitro can better assist in unraveling the therapeutic action on tumor cells in a situation more closely resembling what occurs in vivo. In addition, monolayer cell cultures do not accurately reflect the changes that occur in the early stages of tumor progression. In the current study, we evaluated the role of the microenvironment in the drug responses of human metastatic melanoma cell lines grown on plastic as well as in type I collagen or dermal equivalent culture conditions. We demonstrated that human metastatic melanoma cell lines cultured in a dermal equivalent containing type I collagen and dermal fibroblasts more closely mimic the dermal microenvironment.
As noted in the viability assay, NHDF cultured in monolayer present higher cell survival when compared with fibroblasts in three-dimensional structure (dermal equivalent). This finding can be explained by the cytotoxic effect of collagenase that was used to collect the fibroblasts from its substrate, which may have caused some cells to be discarded along with the collagen matrix, thus negatively interfering with the calculation of cell viability. Furthermore, it is well known that fibroblasts cultured in three-dimensional collagen gels have lower proliferation rates than in monolayer cultures.29,30
The present study shows that Dox cytotoxicity was higher in monolayers, reaching 75% of cell death, compared with culture on collagen where 67% cell death was observed. When the same cell lines were grown under biomimetic environmental conditions, we observed significantly lower (17%) cell death rate among the combined cell populations (melanoma cells and fibroblasts). Cell viability assays on all three melanoma cell lines showed that Dox had a higher IC50 for cells grown on the dermal equivalent, suggesting the need for a higher concentration of drug treatment in this condition. Furthermore, our data indicate that the dermal equivalent protects melanoma cells against Dox cytotoxicity. The protective effect of the dermal equivalent on tumor cell viability was further confirmed by flow cytometric assays, which we showed a decrease in SK-Mel-103 cell death, from either apoptosis or necrosis. Moreover, the protection conferred to melanoma cells was shown to be dependent on the presence of both fibroblasts and the ECM. It is worth noting that under the same conditions, minimal induction of cell death was observed in the fibroblast compartment.
Although Dox is known to be able to induce apoptosis in melanoma cells in vitro,14 we found that induction of p53 and cleavage of caspases 3 and 9 was decreased when melanoma cells were grown on a dermal equivalent, suggesting once more a strong protective role for the stroma. Accordingly, the literature reports that cells in the microenvironment, such as fibroblasts, can assist melanoma chemoresistance via the secretion of growth factors, cytokines, and MMPs which favor tumor growth and induce the production of proteins that evade the normal mechanisms of cell death.31–33 Alt-Holland et al.34 and Rasanen and Vaheri33 have considered not only the matrix as the predominant factor simulating the tumor environment, but also the presence of stromal cells as critical for reliable reconstruction of the processes involved in cancer promotion.
In the process of cellular invasion and metastasis, matrix remodeling occurs by enzymatic action of MMPs, which represent a family of more than twenty endopeptidases that can be synthesized by tumor cells or by stromal cells.35,36 Type I collagen is the main constituent of the dermis and, therefore, consists of an important substrate for MMPs. Ntayi et al.37 demonstrated that MMP-2 levels were not changed when cultured melanoma cells and fibroblasts were physically isolated, whereas the expression of MMP-2 increases on co-culture. Although those authors did not recreate the three-dimensional environment presented by our study, our results are in agreement with their findings, as we also observed higher activity of MMP-2 when melanoma cells were grown in the presence of the dermal equivalent. According to Hofmann et al.,38 the expression of MMP-1, -2, and -13 is increased in advanced stages of melanoma; whereas the expression of MMP-9 is limited to early stages of progression, reducing the evolution of the tumor. Likewise, this enzyme showed low activity levels in our zymograms with metastatic melanoma cell lines.
In this study, we observed the gradual growth of melanoma tumor cells into the dermal equivalent from the 5th day of culture and also the progressive invasion of these cells into the substrate up to the 15th day of culture. Interestingly, the dermal equivalent appeared to provide a protective environment for the melanoma cells, as there was a decreased response to Dox treatment, enabling a higher percentage of viable melanoma cells in this culture condition when compared with monolayer culture on plastic or on collagen type 1.
Cancer cells are known to produce cytokines that attract inflammatory cells, which, in turn, also express a wide array of cytokines, proteases, and pro-invasive ECM proteins.39 Proinflammatory cytokines such as those belonging to the IL-6 family are frequently observed in the tumor microenvironment.26,40–42 IL-6 is a pleiotropic cytokine with numerous biological activities. It can also be produced by normal components of the skin, including epidermal cells, dermal fibroblasts, and endothelial cells.43 In many studies, the presence of this cytokine has been associated with malignant cells and it is a candidate for a prognostic marker for melanoma.44 We observed dramatic increases in production of the cytokine IL-6 in all three SK-Mel-19, -103, and -147 melanoma cell lines grown on dermal equivalents when compared to the same melanoma cells grown on plastic or collagen coated plates. In addition, we showed a higher production of IL-8 in SK-Mel-103 and -147, when melanoma cells were cultured in the dermal equivalent, compared to other culture conditions. These findings suggest that a direct interaction between melanoma cells and the stroma cells was necessary to increase production of those cytokines and provide further evidence that the microenvironment is capable of modulating melanoma growth.
In parallel, Vimentin is the major filament protein expressed in the cytoskeleton of mesenchymal cells. For this reason, this protein is often used as a marker of mesenchymally derived cells or cells undergoing epithelial-to-mesenchymal transition (EMT) during both normal development and metastatic tumor progression.45 Its overexpression in several epithelial cancers, including prostate cancer, gastrointestinal tumors, tumors of the central nervous system, breast cancer, lung cancer, and malignant melanoma, correlates with accelerated tumor growth, invasion, and poor prognosis.27 In our study, we found high Vimentin expression in melanoma cells cultured on collagen gel or dermal equivalent even after Dox treatment.
Altogether, our results indicate that the dermal equivalent protects melanoma cells against Dox cytotoxicity, inhibits cell death, and induces the secretion of MMPs and pro-tumoral interleukins, possibly supporting melanoma survival and promoting invasion. Given that our findings demonstrated distinct drug responses for the same cell types grown under different culture conditions, we conclude that the microenvironment is critical and should be considered a platform for further evaluation of drugs in anti-cancer therapeutic strategies.
Supplementary Material
Acknowledgments
The authors are especially grateful to Renato R. Massaro, MSc (Department of Clinical Chemistry and Toxicology, Faculty of Pharmaceutical Sciences, University of São Paulo, Brazil) for generating the GPF-melanoma cells and Renata C. Albuquerque, MSc (Department of Clinical Chemistry and Toxicology, Faculty of Pharmaceutical Sciences, University of São Paulo, Brazil) for the FACS analyses. Bianca Ferrucio, MSc., (Department of Clinical Chemistry and Toxicology, Faculty of Pharmaceutical Sciences, University of São Paulo, Brazil), for English review. This study was supported by FAPESP (2010/15919-1, and 2011/19045-9), CNPq, INCT-if, CAPES, and PRP-USP.
Disclosure Statement
No competing financial interests exist.
References
- 1.Fidler I.J.The pathogenesis of cancer metastasis: the ‘seed and soil'hypothesis revisited. Nat Rev Cancer 3,453, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Naishadham D., and Jemal A.Cancer statistics. CA Cancer J Clin 62,10, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., Akerley W., van den Eertwegh A.J., Lutzky J., Lorigan P., Vaubel J.M., Linette G.P., Hogg D., Ottensmeier C.H., Lebbe C., Peschel C., Quirt I., Clark J.I., Wolchok J.D., Weber J.S., Tian J., Yellin M.J., Nichol G.M., Hoos A., and Urba W.J.Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363,711, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding J.J., Pulitzer M., and Chapman P.B.Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med 366,866, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Loeffler M., Kruger J.A., Niethammer A.G., and Reisfeld R.A.Targeting tumorassociated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 116,1955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank A., David V., Aurelie T.R., Florent G., William H., and Philippe B.Regulation of MMPs during melanoma progression: from genetic to epigenetic. Anticancer Agents Med Chem 12,773, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Olumi A.F., Grossfeld G.D., Hayward S.W., Carroll P.R., Tlsty T.D., and Cunha G.R.Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59,5002, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R., and Zeisberg M.Fibroblasts in cancer. Nat Rev Cancer 6,392, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Mueller M.M., and Fusenig N.E.Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4,839, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Zigrino P., Loffek S., and Mauch C.Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie 87,321, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Giordano F.J., and Johnson R.S.Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev 11,35, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Minchinton A.I., and Tannock I.F.Drug penetration in solid tumours. Nat Rev Cancer 6,583, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Brohem C.A., Massaro R.R., Tiago M., Marinho C.E., Jasiulionis M.G., de Almeida R.L., Rivelli D.P., Albuquerque R.C., de Oliveira T.F., Melo Loureiro A.P., Okada S., Soengas M.S., Moraes Barros S.B., and Maria-Engler S.S.Proteasome inhibition and ROS generation by 4-nerolidylcatechol induces melanoma cell death. Pigment Cell Melanoma Res 25,354, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Benbow U., Maitra R., Hamilton J.W., and Brinckerhoff C.E.Selective modulation of collagenase 1 gene expression by the chemotherapeutic agent doxorubicin. Clin Cancer Res 5,203, 1999 [PubMed] [Google Scholar]
- 15.Yamada T., Shinohara K., Takeda K., Kameda N., Katsuki K., Ariyoshi K., and Kamei T.Second lung adenocarcinoma after combination chemotherapy in two patients with primary non-Hodgkin's lymphoma. Jpn J Clin Oncol 29,226, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Albright C.F., Graciani N., Han W., Yue E., Stein R., Lai Z., Diamond M., Dowling R., Grimminger L., Zhang S.Y., Behrens D., Musselman A., Bruckner R., Zhang M., Jiang X., Hu D., Higley A., Dimeo S., Rafalski M., Mandlekar S., Car B., Yeleswaram S., Stern A., Copeland R.A., Combs A., Seitz S.P., Trainor G.L., Taub R., Huang P., and Oliff A.Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth better than doxorubicin with less toxicity. Mol Cancer Ther 4,751, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Injac R., and Strukelj B.Recent advances in protection against doxorubicin induced toxicity. Technol Cancer Res Treat 7,497, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Freshney R.Culture of Animal Cells: A Manual of Basic Technique. New York: Alan R. Liss, Inc., 1,177, 1987 [Google Scholar]
- 19.da Silva Cardeal L.B., Brohem C.A., Correa T.C., Winnischofer S.M., Nakano F., Boccardo E., Villa L.L., Sogayar M.C., and Maria-Engler S.S.Higher expression and activity of metalloproteinases in human cervical carcinoma cell lines is associated with HPV presence. Biochem Cell Biol 84,713, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosebrough N.J., Farr A.L., and Randall R.J.Protein measurement with the Folin phenol reagent. J Biol Chem 193,265, 1951 [PubMed] [Google Scholar]
- 21.Lequin R.M.Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem 51,2415, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Givan A.L.Flow Cytometry: First Principles, 2nd edition. Wilmington: Wiley-Liss, Inc., 2001, pp. 123–125 [Google Scholar]
- 23.Anuszewska E.L., and Gruber B.DNA damage and repair in normal and neoplastic cells treated with Adriamycin. Acta Biochim Pol 41,385, 1994 [PubMed] [Google Scholar]
- 24.Flatt P.M., Price J.O., Shaw A., and Pietenpol J.A.Differential cell cycle checkpoint response in normal human keratinocytes and fibroblasts. Cell Growth Differ 9,535, 1998 [PubMed] [Google Scholar]
- 25.Siu W.Y., Arooz T., and Poon R.Y.Differential responses of proliferating versus quiescent cells to adriamycin. Exp Cell Res 250,131, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Melnikova V.O., and Bar-Eli M.Inflammation and melanoma metastasis. Pigment Cell Melanoma Res 22,257, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Satelli A., and Li S.Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 18,3033, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berking C., and Herlyn M.Human skin reconstructs models: a new application for studies of melanocyte and melanoma biology. Histol Histopathol 16,669, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Croce M.A., Silvestri C., Guerra D., Carnevali E., Boraldi F., Tiozzo R., Parma B.Adhesion and proliferation of human dermal fibroblasts on collagen matrix. J Biomater Appl 18,209, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Rittié L., and Fisher G.J.Isolation and culture of skin fibroblasts. Methods Mol Med 177,83, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Burdela L.G., Komarova E.A., Hill J.E., Browder T., Tararova N.D., Mavrakis L., Dicorleto P.E., Folkman J., and Gudkov A.V.Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res 66,9356, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ahmed F., Steele J.C., Herbert J.M., Steven N.M., and Bicknell R.Tumor stroma as a target in cancer. Curr Cancer Drug Targets 8,447, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Rasanen K., and Vaheri A.Activation of fibroblasts in cancer stroma. Exp Cell Res 316,2713, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Alt-Holland A., Shamis Y., Riley K.N., Desrochers T.M., Fusenig N.E., Herman I.M., and Garlick J.A.E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. J Invest Dermatol 128,2498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermarck J., and Kahari V.M.Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13,781, 1999 [PubMed] [Google Scholar]
- 36.Bornstein P., and Sage E.H.Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14,608, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ntayi C., Hornebeck W., and Bernard P.Involvement of matrix metalloproteinases (MMPs) in cutaneous melanoma progression. Pathol Biol 52,154, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Hofmann U.B., Houben R., Brocker E.B., and Becker J.C.Role of matrix metalloproteinases in melanoma cell invasion. Biochimie 87,307, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Coussens L.M., and Werb Z.Inflammation and cancer. Nature 420,860, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose-John S., Waetzig G.H., Scheller J., Grötzinger J., and Seegert D.The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 11,613, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Erez N., Truitt M, Olson P., Arron S.T., and Hanahan D.Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17,135, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Kim Y., Wallace J., Li F., Ostrowski M., and Friedman A.Transformed epithelial cells and fibroblasts/myofibroblasts interaction in breast tumor: a mathematical model and experiments. J Math Biol 61,401, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Paquet P., and Pierard G.E.Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med 1,459, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Molnár E.L., Hegyesi H., Toth S., Darvas Z., Laszlo V., Szalai C., and Falus A.Biosynthesis of interleukin-6, an autocrine growth factor for melanoma, is regulated by melanoma-derived histamine. Semin Cancer Biol 10,25, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Savagner P.The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol 21,89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofmann U.B., Westphal J.R., Waas E.T., Zendman A.J., Cornelissen I.M., Ruiter D.J., and Van Muijen G.N.Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer 81,774, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Rocca G., Pucci-Minafra I., Marrazzo A., Taormina P., and Minafra S.Zymographic detection and clinical correlation of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer 90,1414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nabeshima K., Inoue T., Shimao Y., and Sameshima T.Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int 52,255, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.