Abstract
Dual-specific protein-tyrosine phosphatases have the common active-site sequence motif HCXXGXXRS(T). The role of the conserved hydroxyl was investigated by changing serine-131 to an alanine (S131A) in the dual-specific protein-tyrosine phosphatase VHR. The pH profile of the kcat/Km value for the S131A mutant is indistinguishable from that of the native enzyme. In contrast, the kcat value for S131A mutant is 100-fold lower than that for the native enzyme, and the shape of the pH profile was perturbed from bell-shaped in the native enzyme to a pH-independent curve over the pH range 4.5-9.0. This evidence, along with results from a previous study, suggests that the S131A mutation alters the rate-limiting step in the catalytic mechanism. Formation of a phosphoenzyme intermediate appears to be rate-limiting with the native enzyme, whereas in the S131A mutant breakdown of the intermediate is rate-limiting. This was confirmed by the appearance of a burst of p-nitrophenol formation when p-nitrophenyl phosphate rapidly reacted with the S131A enzyme in a stopped-flow spectrophotometer. Loss of this hydroxyl group at the active site dramatically diminished the ability of the enzyme to hydrolyze the thiol-phosphate intermediate without exerting any significant change in the steps leading to and including the formation of the intermediate. Consistent with rate-limiting intermediate formation in the native enzyme, the rate of burst in the S131A mutant was 1.5 s-1, which agrees well with the kcat value of 5 s-1 observed for native enzyme. The amplitude of the burst was stoichiometric with final enzyme concentration, and the slow linear rate (0.06 s-1) of p-nitrophenol formation after the burst was in agreement with the steady-state determined value of kcat (0.055 s-1).
Full text
PDF

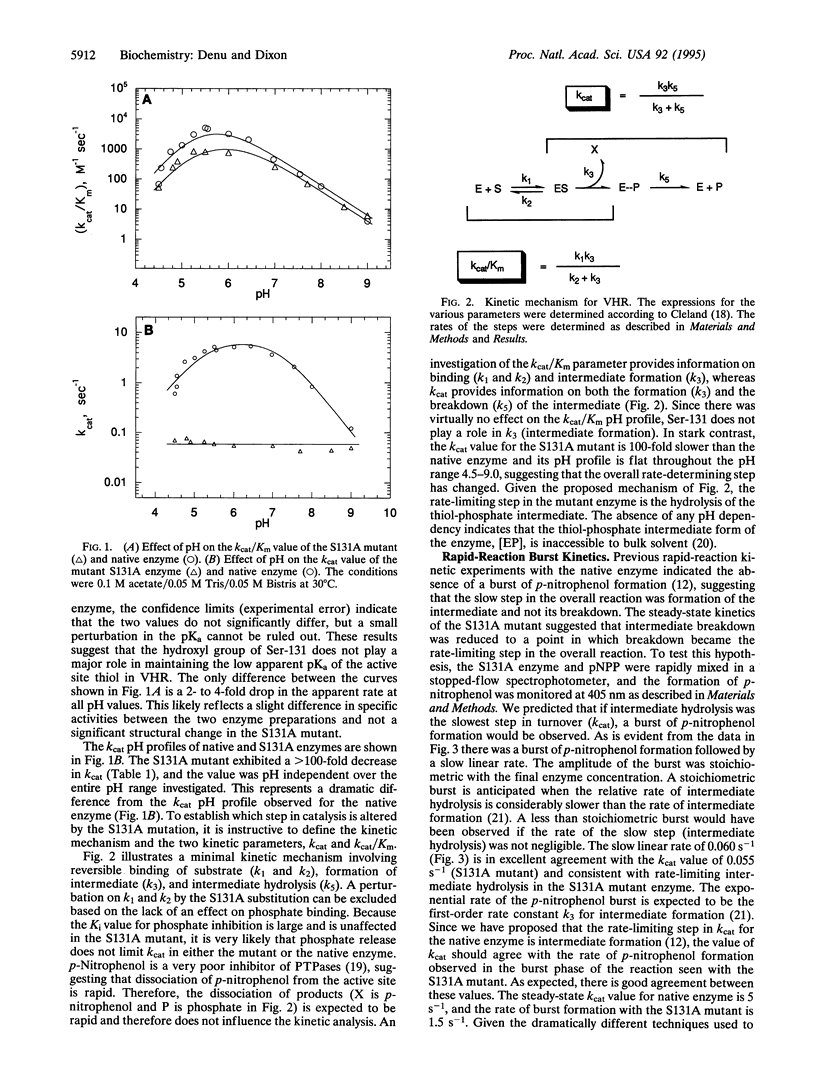
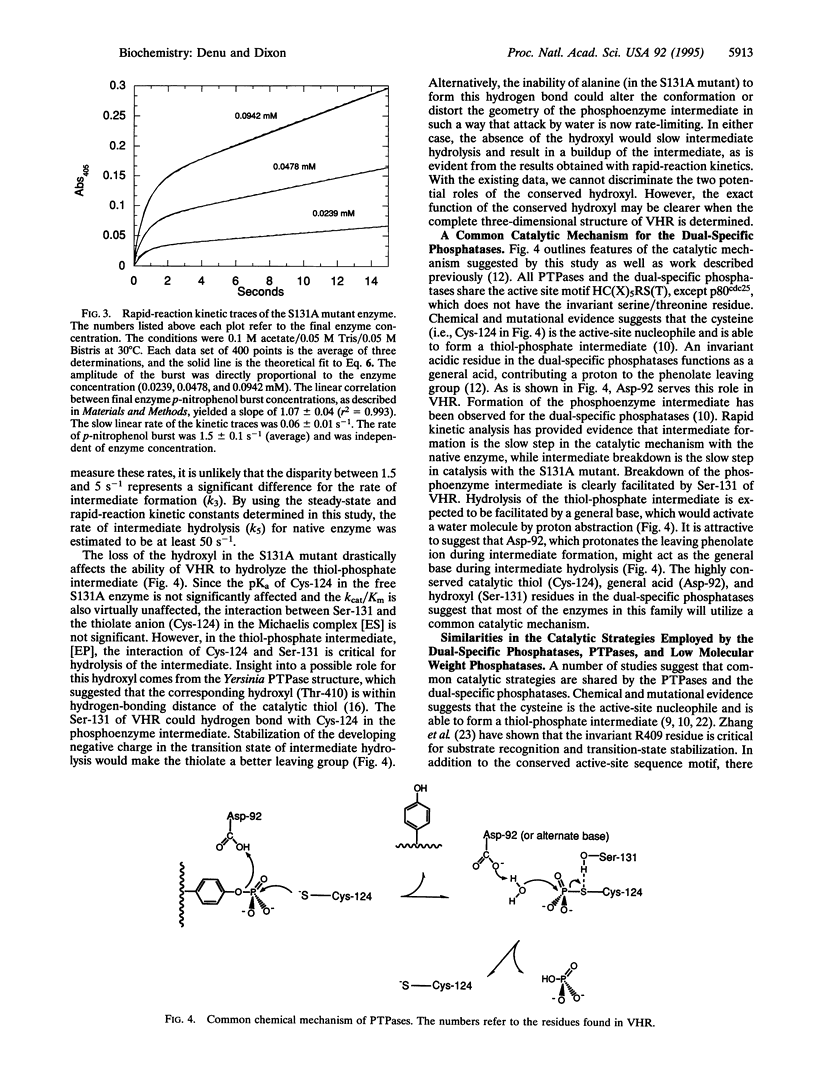

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barford D., Flint A. J., Tonks N. K. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994 Mar 11;263(5152):1397–1404. [PubMed] [Google Scholar]
- Cleland W. W. Partition analysis and the concept of net rate constants as tools in enzyme kinetics. Biochemistry. 1975 Jul 15;14(14):3220–3224. doi: 10.1021/bi00685a029. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Denu J. M., Zhou G., Guo Y., Dixon J. E. The catalytic role of aspartic acid-92 in a human dual-specific protein-tyrosine-phosphatase. Biochemistry. 1995 Mar 14;34(10):3396–3403. doi: 10.1021/bi00010a031. [DOI] [PubMed] [Google Scholar]
- Denu J. M., Zhou G., Wu L., Zhao R., Yuvaniyama J., Saper M. A., Dixon J. E. The purification and characterization of a human dual-specific protein tyrosine phosphatase. J Biol Chem. 1995 Feb 24;270(8):3796–3803. doi: 10.1074/jbc.270.8.3796. [DOI] [PubMed] [Google Scholar]
- Doi K., Gartner A., Ammerer G., Errede B., Shinkawa H., Sugimoto K., Matsumoto K. MSG5, a novel protein phosphatase promotes adaptation to pheromone response in S. cerevisiae. EMBO J. 1994 Jan 1;13(1):61–70. doi: 10.1002/j.1460-2075.1994.tb06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. J., Morrison J. F. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991 Sep 15;266(26):17026–17030. [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991 Sep 15;266(26):17026–17030. [PubMed] [Google Scholar]
- Guan K., Hakes D. J., Wang Y., Park H. D., Cooper T. G., Dixon J. E. A yeast protein phosphatase related to the vaccinia virus VH1 phosphatase is induced by nitrogen starvation. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12175–12179. doi: 10.1073/pnas.89.24.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Bottaro D. P., Chan A., Miki T., Aaronson S. A. Expression cloning of a human dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12170–12174. doi: 10.1073/pnas.89.24.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Stuckey J. A., Schubert H. L., Fauman E. B., Zhang Z. Y., Dixon J. E., Saper M. A. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 A and the complex with tungstate. Nature. 1994 Aug 18;370(6490):571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- Su X. D., Taddei N., Stefani M., Ramponi G., Nordlund P. The crystal structure of a low-molecular-weight phosphotyrosine protein phosphatase. Nature. 1994 Aug 18;370(6490):575–578. doi: 10.1038/370575a0. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Taddei N., Chiarugi P., Cirri P., Fiaschi T., Stefani M., Camici G., Raugei G., Ramponi G. Aspartic-129 is an essential residue in the catalytic mechanism of the low M(r) phosphotyrosine protein phosphatase. FEBS Lett. 1994 Aug 22;350(2-3):328–332. doi: 10.1016/0014-5793(94)00805-1. [DOI] [PubMed] [Google Scholar]
- Ward Y., Gupta S., Jensen P., Wartmann M., Davis R. J., Kelly K. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature. 1994 Feb 17;367(6464):651–654. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- Zhang M., Van Etten R. L., Stauffacher C. V. Crystal structure of bovine heart phosphotyrosyl phosphatase at 2.2-A resolution. Biochemistry. 1994 Sep 20;33(37):11097–11105. doi: 10.1021/bi00203a006. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Malachowski W. P., Van Etten R. L., Dixon J. E. Nature of the rate-determining steps of the reaction catalyzed by the Yersinia protein-tyrosine phosphatase. J Biol Chem. 1994 Mar 18;269(11):8140–8145. [PubMed] [Google Scholar]
- Zhang Z. Y., VanEtten R. L. Pre-steady-state and steady-state kinetic analysis of the low molecular weight phosphotyrosyl protein phosphatase from bovine heart. J Biol Chem. 1991 Jan 25;266(3):1516–1525. [PubMed] [Google Scholar]
- Zhang Z. Y., Wang Y., Dixon J. E. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1624–1627. doi: 10.1073/pnas.91.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y., Wang Y., Wu L., Fauman E. B., Stuckey J. A., Schubert H. L., Saper M. A., Dixon J. E. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry. 1994 Dec 27;33(51):15266–15270. doi: 10.1021/bi00255a007. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Harms E., Van Etten R. L. Asp129 of low molecular weight protein tyrosine phosphatase is involved in leaving group protonation. J Biol Chem. 1994 Oct 21;269(42):25947–25950. [PubMed] [Google Scholar]
- Zhou G., Denu J. M., Wu L., Dixon J. E. The catalytic role of Cys124 in the dual specificity phosphatase VHR. J Biol Chem. 1994 Nov 11;269(45):28084–28090. [PubMed] [Google Scholar]



