Abstract
Introduction: Preterm premature rupture of membranes (PPROM) can result in fetal complications such as oligohydramnios. This study aimed to determine the effects of intravenous (IV) fluid bolus on amniotic fluid index (AFI) in pregnant women with PPROM. Methods: 24 women with PPROM during singleton live pregnancy of 28 to 34 weeks whose baseline AFI was ≤ 5cm were randomized into two groups. The study group received one liter intravenous fluid bolus of isotonic Ringer serum during 30-minute period. Reevaluations of amniotic fluid index in both groups were made 90 minutes and 48 hours after baseline measurement. Independent t-test and paired t-test were used to compare the two groups and mean amniotic fluid index before and after treatment, respectively. Results: The results of this study demonstrate that AFI decreased statistically significant in both the control and study groups. AFI decreased in both groups at 48 hours later. This decrease was not statistically significant in any group. The mean change in AFI (90 minutes and baseline) and (48 hours and baseline) between the two groups were not significant. The time between mean baseline measurements and delivery were 196.41 and 140.58 hours in the study and control groups, respectively. This difference was not statistically significant. Conclusion: This study did not find significant impact of hydration On AFI as a prophylactic method on oligohydramnios in pregnant women with PPROM.
Keywords: hydration, amniotic fluid index, preterm premature rupture of fetal membranes
Introduction
Preterm premature rupture of membranes (PPROM) is a frequent obstetrical incident (3-4.5%)”1 which can result in maternal and fetal complications such as sepsis and prematurity 2,3, as well as adverse neurodevelopment outcomes.4
An important outcome of PPROM is decreased amniotic fluid index.5Severe oligohydramnios is a major prognostic factor for neonatal mortality.6,7
Several treatments have been suggested to prevent decreased amniotic fluid volume and there by reduce the perinatal morbidity and mortality associated with PPROM in the second and third trimesters. These treatments include therapeutic transabdominal amnioinfusion,8,9 intra amniotic injection of platelets and cryoprecipitate (amniopatch),10cervical canal occlusion with fibrin gel,11 and receiving supplemental vitamins C and E after PPROM,12 infusion of fluid via transcervical catheter,13 and maternal hydration.14
The mechanisms of amniotic fluid homeos- tasis have been the subject of several recent investigations. Acute maternal hydration with oral or intravenous fluid has been shown to increase amniotic fluid index (AFI) in intact membranes.15-19
Giving these associations, it must be useful to increase amniotic fluid volume, either short term as an adjunct to facilitate amniocentesis or long term to decrease the risk of oligohydramnios such as still birth, non-reassuring fetal heart rate due to cord compression, admission to the neonatal intensive care unit, meconium aspiration syndrome 20and pulmonary hypoplasia.7,21
However, compared to patients with intact membranes, patients with rupture membranes may respond differently to maternal hydration either by increased fluid loss trough the membrane defect or by changes in the normal mechanisms of amniotic fluid homeostasis brought about by the membrane rupture.14
Therefore, the current study was performed to determine the effect of IV hydration with isotonic saline on amniotic fluid index and latency period
Materials and Methods
This single randomized blinded controlled study was conducted from 2010 to 2011 in the Department of Obstetrics and Gynecology at Alzahra Educational Hospital in Tabriz, Iran.The study was approved by the Ethics Committee of Tabriz University of Medical Sciences. (Code: 909)
Singleton Pregnant women with gestational age of 28-34 weeks with detection of PPROM (Preterm premature rupture of membranes before term and before the onset of contractions) and AFI ≤ 5cm that had cephalic presentation, reassuring fetal heart rate on admission, a reactive non-stress test (NST), duration of rupture lower than 48 hours, no pregnancy complications such as (hypertension, vaginal bleeding in the second and third trimesters, intrauterine growth restriction, chorioamnionitis, placental or fetal anomalies), inactive labor were included. Moreover, the participants had no prior history of renal, lung, and heart disease since the use of bolus-fluid therapy is contraindicated in these patients.
These women confined to bed in high risk pregnancy ward from 10/10/jun till 10/15/Sep.
The exclusion criterion of the study was spontaneous onset of labor contraction during hydration.
Gestational age was calculated based on either last menstrual period (LMP) or crown-rump length in ultrasounds taken before 12 weeks. In this study Ruptured membranes were verified by the sterile speculum examination with pooled fluid in the vaginal vault and for more confirmation nitrazine (litmus paper, Machery-Nagel, MN) test was used.
Chorioamnionitis was clinically diagnosed if a maternal fever greater than 38°C, fetal tachycardia (> 160 beats per minute), uterine tenderness, maternal tachycardia (> 120 beats per minute), and foul smelling amniotic fluid were present. The onset of labor was determined by either 4 cm or greater cervical dilatation or regular uterine contractions (> 3 contractions in 10 minutes).
Mean comparison formula was used in order to calculate the sample size. Based on a previous study14, number of participants in each group was determined as 12 with a power of 90%, M1=3.2, M2=5, SD1=0.79, SD2=1.35, α=0.05. Subjects who had the inclusion criteria were given the descriptions about the aims and methods of the study. Written informed consents were obtained from all participants. Then, 24 eligible pregnant women were randomly assigned into the control (A) or study (B) groups. The random allocation of the individuals in the groups was performed by computer-generated random number table provided by the Department of Biostatistics22 through block randomization with 4 block sizes considering allocation concealment. Sealed pockets were handed to the participants with numbers from 1 to 24 (allocation concea- lment). The participants were randomly placed in the two groups of hydration and control.
All women received the regimen of the hospital including an antibiotic prophylaxis (Ampicilline, 2gr, IV, every 6h for 2 days then Amoxicillin 500mg, every 8 h for 5 days) and corticosteroid (12 mg intramuscular injection of betamethasone every 24 hours) and bed rest. Since resting increases the placental blood circulation of the uterus, which is considered to be a confounding factor in this study, it was applied to both case and control groups.
According to the procedure described by Phelan et al,23baseline AFI was measured in all women by summing up the maximum vertical fluid pocket depth in each of the four abdominal quadrants. Portable sonography was performed by a SonoSite machine (SonoSite Inc, USA). Since the blinding of mothers was not possible, only the sonographer was blinded. The sonographer was not informed about the groupings. To exclude inter observer bias, the same sonographer made baseline and follow up measurements.
After AFI measurement, women in the study group received one liter intravenous fluid bolus of isotonic Ringer serum over a 30-minute period. Post hydration AFI was measured at 90 minutes and 48 hours after baseline measurement in both groups.
All subjects were instructed to eat and drink as usual during a 48-hour interval. During this period, fetal heart rate was continuously controlled through external monitoring and pregnancy terminated if fetal distress or chorioamnionitis were observed.
The normal distribution of data was checked through the Kolmogorov-Smirnov test. The average values of baseline amniotic fluid index and the remeasured index (after 90 minutes and 48 h) in the two groups were compared using paired t-test. In addition, the mean difference of amniotic fluid index of the two groups was compared by an indep- endent t-test. Data was analyzed by SPSS version 15 (SPSS Inc., Chicago, IL, USA).
Results
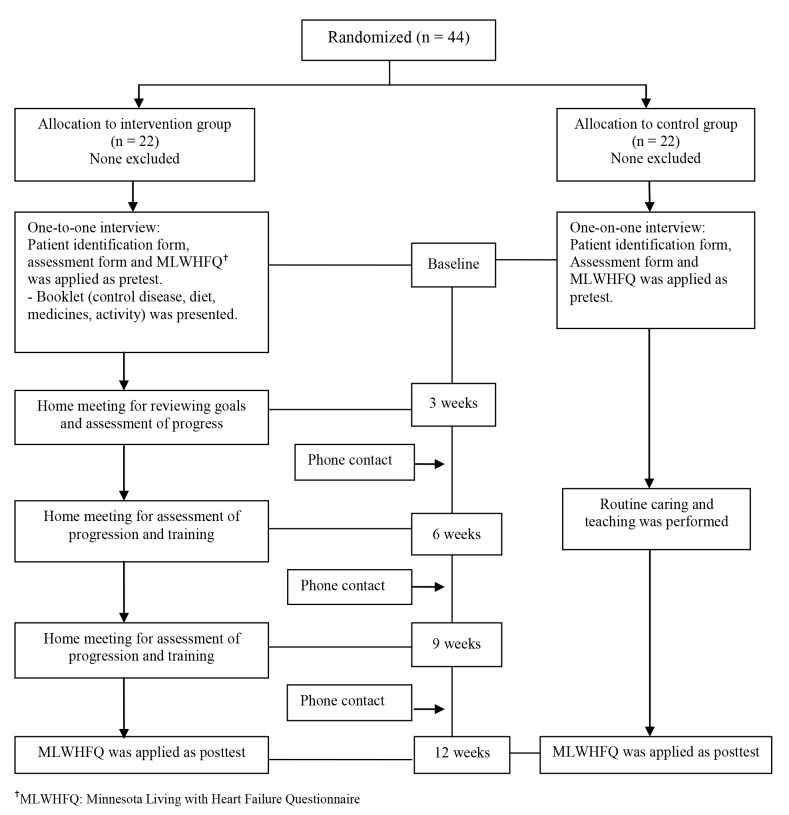
42 women assessed for eligibility.18 women did not meet inclusion criteria. Any one refused to participate. Finally, 24 eligible pregnant women were randomly assigned into the control (A) (n=12) or study (B) (n=12) groups (Fig 1).
Figure 1.
Flow diagram of the progress through the phases of the trial
Tables 1 and 2 summarize demographic characteristics of the patients and the results of the study, respectively.
Table 1. Demographic characteristics of the patien.
| Parameter | Study Group N (%) | control Group N (%) | Statistical Indicators |
| Age (years) | |||
| <20 | 4(33.3) | 0(0) | χ2 = 6.0 P = 0.05 df = 2 |
| 20-30 | 2(16.6) | 6(50) | |
| >30 | 6(50) | 6(50) | |
| gravidity | |||
| 1 | 7(58.3) | 5(41.6) | χ2 = 0.6 P = 0.7 df = 2 |
| 2 | 2(16.6) | 3(25) | |
| > 2 | 3(25) | 4(33.3) | |
| Number of parity | |||
| 0 | 7(58.3) | 9(75) | χ2 = 1.25 P = 0.5 df = 2 |
| 1 | 3(25) | 1(8.3) | |
| >2 | 2(16.6) | 2(16.6) | |
| Gestational age (weeks) | |||
| 28-30 | 4(33.3) | 6(50) | χ2= 0.71 P = 0.7 df = 2 |
| 30-32 | 5(41.6) | 4(33.3) | |
| 32-34 | 3(25) | 2(16.6) | |
| Length of rupture (hours) | |||
| 1-12 | 9(75) | 10(83.3) | χ2 = 5.05 P = 0.8 df = 2 |
| 12-24 | 0(0) | 2(16.6) | |
| 24-48 | 3(25) | 0(0) |
Table 2. Amniotic fluid index (AFI) before and after hydration at 90 min and 48 h in the study and control groups .
| Study group (n = 10) | Control group (n = 10) | Mean difference (CI %95) | statistical Indicator | |
| Baseline AFI | 4.6 (0.3) | 4.4 (0.5) | 0.2(-0.2,0.6) | P=0.31, t=1.03, df=22 |
| AFI at 90 min | 3.5 (1.33) | 2.9 (1.0) | 0.6(-0.4,1.6) | P=0.22, t=1.59, df=22 |
| ΔAFI (base line, 90 min) | -1.12 (1.24) | -1.5 (1.0) | 0.2(-2.9,3.5) | P=0.39, t=0.86, df=22 |
| AFI at 48 h | 3.97 (3.0) | 3.7 (1.56) | 0.4(-0.5,1.35) | P=0.86, t=1.03, df=22 |
| ΔAFI (base line, 48 h) | -0.59 (2.8) | -1.0 (1.45) | 0.4(-2.6,3.4) | P=0.77, t=0.29, df=11 |
All the values are presented in centimeters as mean (SD).
AFI: Amniotic fluid index; ΔAFI: Difference between AFI at the two mentioned times.
Baseline and follow-up measurements at 90 min were not different between the two groups. In the study group, the mean AFI decreased from 4.6 to 3.5 cm. This decrease was statistically significant (P = 0.01). However, in the control group, the mean AFI decreased from 4.4 to 2.9 cm which was statistically significant (P < 0.05).
The mean change in AFI between the two groups at 90 minutes was not statistically significant (P = 0.39). Decreases in AFI at 48 hours were not significant neither in the study group (P = 0.57) nor in the control group (p = 0.2). The mean change in AFI between the two groups at 48 hours was not statistically significant (P = 0.77) either.
We also studied the time between baseline measurements and delivery and found it to be 196.41 hours in the study group (CI %95: 75.9-316.9) and 140.58 hours in the control group (CI%95: 10.2-270.9). However, the difference between the two groups was not statistically significant (P = 0.49).
Discussion
The results of this study demonstrate that AFI decreased statistically significant in both the control and study groups. Dio et al. evaluated AFI in 84 pregnant women in 4 groups. (21: intravenous hydration with isotonic serum, 21: intravenous hydration with hypotonic serum, 21: oral hydration with hypotonic serum and 21: control group. like our study they found that AFI do not increase statistically significant in first group.24Unlike our study, Chelmow et al. evaluated
AFI of 13 pregnant women with PPROM (6 in hydration group and 7 in non-hydration group) at 90 minutes after maternal hydration with one liter intravenous fluid bolus of normal saline over a period of 30 minutes and observed a 5 cm increase in AFI in the hydration group.14
In contrast with the present study, Singala et al. investigated the effects of transabdominal amnioinfusion on AFI in PPROM and oligohy- dramnios patients. They reported increased AFI after the transabdominal amnioinfusion (p < 0.01).8Acute maternal hydration has been shown to increase AFI in women with intact membranes.15-19This increase acts by two mechanisms including the reduction in maternal plasma osmolality which results in increased fetal urine output25,26 and reduced intramembranous amniotic fluid resorption in response to fetal plasma hypoosmolality.17
In the evaluation of long term effects of acute maternal hydration on AFI in this study, an insignificant decreased was observed 48 hours after hydration in both groups.
Malhotra et al. evaluated AFI in 25 pregnant women (10 with normal AFI and 15 with decreased AFI) at 3, 24 and 48 hours after maternal hydration with 2 liters of water over 1 hour. They found that although maternal hydration increased AFI in both groups, the change took at least 24 hours to occure.18
In addition, our results showed hydrated patients tended to have a longer time interval from the fluid bolus to delivery when compared with the control group (196.41 vs. 140.58 hours). However, the difference between the two groups was not statistically significant (p = 0.49).
Chelmow et al. reported similar findings (281.3 vs. 152.3 hours).14Various studies suggested an adequate amniotic fluid volume to be necessary during the latency period to prolong pregnancy.8
The limitation of this study was for the uncertainty about similar background hydration status among the patients of each group. Therefore, future studies are recommended to consider an estimation of the hydration state with urine specific gravity before the hydration process.
The other limitation of this study was relative quantity of the sample size that with this sample size was not possible to identify the low effect of the study.
Conclusion
The complications of oligohydramnios cause the mother and the fetus to suffer from many problems. This study did not find significant impact of hydration On AFI as a prophylactic method on oligohydramnios in pregnant women with PPROM. But Hydration of the mother is a low-cost method with no complications for the fetus and the mother.
So More research with higher number of subjects and in different gestational ages is necessary.
Ethical issues
None to be declared.
Conflict of Interest
The authors declare no conflict of interest in this study.
Acknowledgments
We would like to thank the deputy of research at Tabriz University of Medical Sciences for their financial support. We also appreciate all pregnant mothers participating in this research.
References
- 1.Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clin Prenatal . 2001; 28(4):721–34. doi: 10.1016/s0095-5108(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 2.Tanir HM, Sener T, Tekin N, Aksit A, Ardic N. Preterm premature rupture of membranes and neonatal outcome prior to 34 weeks of gestation. Int J Gynaecol Obstet . 2003; 82(2):167–72. doi: 10.1016/s0020-7292(03)00125-5. [DOI] [PubMed] [Google Scholar]
- 3.Yang LC, Taylor DR, Kaufman HH, Hume R, Calhoun B. Maternal and fetal outcomes of spontaneous preterm premature rupture of membranes. J Am Osteopath Assoc . 2004; 104(12):537–42. [PubMed] [Google Scholar]
- 4.Fukuda S, Yokoi K, Kitajima K, Tsunoda Y, Hayashi N, Shimizu S, Yoshida T, Hamagima N, Watanaba I, Goto H. Influence of premature rupture of membrane on the cerebral blood flow in low-birth-weight infant after the delivery. Brain Dev . 2010; 32(8):631–5. doi: 10.1016/j.braindev.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 5.James DK, Steer PJ, Weiner CP, Gonik B. High risk pregnancy. 3xxsuprdxysup ed. Philadelphia: Elsevier Saunders 2006; 1321-8. [Google Scholar]
- 6.Muris C, Girard B, Creveuil C, Durin L, Herlicoviez M, Dreyfus M. Management of premature rupture of membranes before 25 weeks. Eur J Obstet Gynecol Reprod Biol . 2007; 131(2):163–8. doi: 10.1016/j.ejogrb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Volante E, Gramellini D, Moretti S, Kaihura C, Bevilacqua G. Alteration of the amniotic fluid and neonatal outcome. Acta Biomed . 2004; 75:71–5. [PubMed] [Google Scholar]
- 8.Singla A, Yadav P, Vaid NB, Suneja A, Faridi MMA. Transabdominal amnioinfusion in preterm premature rupture of membranes. International Journal of Gynecology and Obstetrics 2010; 108; 199-202. [DOI] [PubMed] [Google Scholar]
- 9.Turhan NO, Atacan N. Antepartum prophylactic transabdominal amnioinfusion in preterm pregnancies complicated by oligohydramnios. International Journal of Gynecology and Obstetrics . 2002; 76(1):15–21. doi: 10.1016/s0020-7292(01)00504-5. [DOI] [PubMed] [Google Scholar]
- 10.Quintero RA, Morales WJ, Allen M, Bornick PW, Arroyo J, Leparc G. Treatment of iatrogenic previable premature rupture of membranes with intra-amniotic injection of platelets and cryoprecipitate (amniopatch): Preliminary experience. Am J Obstet Gynecol . 1999; 181(3):744–9. doi: 10.1016/s0002-9378(99)70522-3. [DOI] [PubMed] [Google Scholar]
- 11.Baumgarten K, Moser S. The technique of fibrin adhesion for premature rupture of the membranes during pregnancy. J Perinat Med . 1986;14(1):43–9. doi: 10.1515/jpme.1986.14.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Borna S, Borna H, Daneshbodie B. Vitamins C and E in the latency period in women with preterm premature rupture of membranes. Int J Gynaecol Obstet . 2005; 90(1):16–20. doi: 10.1016/j.ijgo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Imanaka M, Ogita S, Sugawa T. Imanaka M, Ogita S, Sugawa TSaline solution amnioinfusion for oligohydramnios after premature rupture of the membranesA preliminary report. Am J Obstet Gynecol . 1989; 161(1):102–6. doi: 10.1016/0002-9378(89)90243-3. [DOI] [PubMed] [Google Scholar]
- 14.Chelmow D, Baker ER, Jones L. Maternal intravenous hydration and amniotic fluid index in patients with preterm ruptured membranes. J Soc Gynecol Investig . 1996; 3(3):127–30. [PubMed] [Google Scholar]
- 15.Shahnazi M, Sayyah Meli M, Hamoony F, Sadrimehr F, Ghatre Samani F, Koshavar H. The effects of intravenous hydration on amniotic fluid volume and pregnancy outcomes in women with term pregnancy and oligohydramnios: A Randomized Clinical Trial. Journal of Caring Sciences . 2012;1(3):123–8. doi: 10.5681/jcs.2012.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magann EF, Doherty DA, Chauhan SP, Barrilleaux SP, Verity LA, Martin JN Jr. Effect of maternal hydration on amniotic fluid volume. Obstet Gynecol . 2003; 101(6):1261–5. doi: 10.1016/s0029-7844(03)00344-2. [DOI] [PubMed] [Google Scholar]
- 17.Umber A, Chohan MA. Intravenous maternal hydration in third trimester oligohydramnios: Effect on amniotic fluid volume. J Coll Physicians Surg Pak . 2007; 17(6):336–9. [PubMed] [Google Scholar]
- 18.Malhotra B, Deka D. Duration of the increase in amniotic fluid index (AFI) after acute maternal hydration. Arch Gynecol Obstet . 2004; 269(3):173–5. doi: 10.1007/s00404-002-0346-z. [DOI] [PubMed] [Google Scholar]
- 19.Al-Salami KhS, Karima AS. Maternal hydration for increasing amniotic fluid volume in oligohydramnios. Basrah Journal of Surgery 2007; 13: 59-62. [Google Scholar]
- 20.Casey BM, McInter DD, Bloom SL, Lucas MJ, Santos R, Twickler DM, Ramus RM, Leveno KJ. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks’ gestation. Am J Obstet Gynecol . 2000; 182(4):909–12. doi: 10.1016/s0002-9378(00)70345-0. [DOI] [PubMed] [Google Scholar]
- 21.Winn HN, Chen M, Amon E, Leet TL, Shumway JB, Mostello D. Neonatal pulmonary hypoplasia and perinatal mortality in patients with midtrimester rupture of amniotic membranes a critical analysis. Am J Obstet Gynecol . 2000; 182:1638–44. doi: 10.1067/mob.2000.107435. [DOI] [PubMed] [Google Scholar]
- 22.True Random Number Service. What's this fuss about true randomness? 2012 [Cited 5 May 2012]; Available from: http://www.random.org/
- 23.Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four-quadrant technique at 36-42 weeks' gestation Amniotic fluid volume assessment with the four-quadrant technique at 36-42 weeks' gestation. J Reprod Med . 1987; 32(7):540–2. [PubMed] [Google Scholar]
- 24.Doi S, Osada H, Seki K, Sekiya S. Effect of maternal hydration on oligohy dramnios: a comparison of three volume expansion methods. Obstet Gynecol . 1998; 92:525–9. doi: 10.1016/s0029-7844(98)00242-7. [DOI] [PubMed] [Google Scholar]
- 25.Oosterhof H, Haak MC, Aarnoudse JG. Acute maternal rehydration increases the urine production rate in the near-term human fetus. AM J Obstet Gynecol . 2000; 183(1):226–9. doi: 10.1067/mob.2000.105817. [DOI] [PubMed] [Google Scholar]
- 26.Robillard JE, Weitzman RE, Burmeister L, Smith FG Jr. Developmental aspects of the renal response to hypoxemia in the lamb fetus. Circ Res . 1981; 48(1):1 28–38. doi: 10.1161/01.res.48.1.128. [DOI] [PubMed] [Google Scholar]