Abstract
Background
Migraine is a common, disabling condition and a burden for the individual, health services and society. Many sufferers choose not to, or are unable to, seek professional help and rely on over-the-counter analgesics. Co-therapy with an antiemetic should help to reduce nausea and vomiting commonly associated with migraine.
Objectives
To determine the efficacy and tolerability of paracetamol (acetaminophen), alone or in combination with an antiemetic, compared to placebo and other active interventions in the treatment of acute migraine in adults.
Search methods
We searched Cochrane CENTRAL, MEDLINE, EMBASE and the Oxford Pain Relief Database for studies through 4 October 2010.
Selection criteria
We included randomised, double-blind, placebo- or active-controlled studies using self-administered paracetamol to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Numbers of participants achieving each outcome were used to calculate relative risk and numbers needed to treat (NNT) or harm (NNH) compared to placebo or other active treatment.
Main results
Ten studies (2769 participants, 4062 attacks) compared paracetamol 1000 mg, alone or in combination with an antiemetic, with placebo or other active comparators, mainly sumatriptan 100 mg. For all efficacy outcomes paracetamol was superior to placebo, with NNTs of 12, 5.2 and 5.0 for 2-hour pain-free and 1- and 2-hour headache relief, respectively, when medication was taken for moderate to severe pain. Nausea, photophobia and phonophobia were reduced more with paracetamol than with placebo at 2 hours (NNTs of 7 to 11); more individuals were free of any functional disability at 2 hours with paracetamol (NNT 10); and fewer participants needed rescue medication over 6 hours (NNT 6).
Paracetamol 1000 mg plus metoclopramide 10 mg was not significantly different from oral sumatriptan 100 mg for 2-hour headache relief; there were no 2-hour pain-free data. There was no significant difference between the paracetamol plus metoclopramide combination and sumatriptan for relief of “light/noise sensitivity” at 2 hours, but slightly more individuals needed rescue medication over 24 hours with the combination therapy (NNT 17).
Adverse event rates were similar between paracetamol and placebo, and between paracetamol plus metoclopramide and sumatriptan. No serious adverse events occurred with paracetamol alone, but more “major” adverse events occurred with sumatriptan than with the combination therapy (NNH 32).
Authors’ conclusions
Paracetamol 1000 mg alone is an effective treatment for acute migraine headaches, and the addition of 10 mg metoclopramide gives short-term efficacy equivalent to oral sumatriptan 100 mg. Adverse events with paracetamol did not differ from placebo; “major” adverse events were slightly more common with sumatriptan than with paracetamol plus metoclopramide.
Medical Subject Headings (MeSH): Acetaminophen [adverse effects; * therapeutic use]; Analgesics, Non-Narcotic [adverse effects; * therapeutic use]; Antiemetics [adverse effects; * therapeutic use]; Drug Therapy, Combination; Hyperacusis [drug therapy]; Metoclopramide [adverse effects; therapeutic use]; Migraine Disorders [* drug therapy]; Photophobia [drug therapy]; Randomized Controlled Trials as Topic; Sumatriptan [adverse effects; therapeutic use]
MeSH check words: Adult, Humans
BACKGROUND
Description of the condition
Migraine is a common, disabling headache disorder, affecting about 12% of Western populations, and with considerable social and economic impact. It is more prevalent in women than men (on the order of 18% versus 6% 1-year prevalence), and in the age range 30 to 50 years (Hazard 2009; Lipton 2007, Moens 2007). The International Headache Society (IHS) classifies two major subtypes (IHS 2004). Migraine without aura is the most common, and usually more disabling, subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity and associated with nausea and/or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of 5 to 20 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether.
A recent large prevalence study in the US found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one-third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over-the-counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol (acetaminophen) and paracetamol with caffeine (Bigal 2008, Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009). Since 2006 sumatriptan 50 mg has been available OTC in the UK, and naratriptan 2.5 mg in Germany.
The significant impact of migraine with regard to pain, disability, social functioning, quality of relationships, emotional well-being and general health (Edmeads 1993; Osterhaus 1994; Solomon 1997) results in a huge burden for the individual, health services and society (Clarke 1996; Ferrari 1998; Hu 1999; Solomon 1997). The annual US economic burden relating to migraine, including missed days of work and lost productivity, is US$14 billion (Hu 1999). Thus successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health-related quality of life, but also has the potential to reduce the need for healthcare resources and increases economic productivity (Jhingran 1996; Lofland 1999).
Description of the intervention
Paracetamol (acetaminophen) was first identified as the active metabolite of two older antipyretic drugs, acetanilide and phenacetin, in the late nineteenth century. It became available in the UK on prescription in 1956, and OTC in 1963 (PIC 2009). Since then it has become one of the most popular antipyretic and analgesic drugs worldwide, and is often also used in combination with other drugs. OTC medications are less expensive, more accessible and have favourable safety profiles relative to many prescription treatments.
Despite a low incidence of adverse effects, paracetamol has a recognised potential for hepatotoxicity and is thought to be responsible for approximately half of all cases of liver failure in the UK (Hawton 2001), and about 40% in the US (Norris 2008). Acute paracetamol hepatotoxicity at therapeutic doses is extremely unlikely despite reports of so-called therapeutic misadventure (Prescott 2000). In recent years legislative changes restricting pack sizes and the maximum number of tablets permitted in OTC sales were introduced in the UK (CSM 1997) on the basis of evidence that poisoning is lower in countries that restrict availability (Gunnell 1997; Hawton 2001). The contribution of these changes, which are inconvenient and costly (particularly to chronic pain sufferers), to any observed reductions in incidence of liver failure or death, remains uncertain (Hawkins 2007). There have been concerns over the safety of paracetamol in patients with compromised hepatic function (those with severe alcoholism, cirrhosis or hepatitis), but these have not been substantiated (Dart 2000; PIC 2009).
Oral paracetamol, used appropriately, has the potential to reduce unnecessary pain in a variety of conditions. Paracetamol is the analgesic of choice for adult patients in whom salicylates or other NSAIDs are contraindicated. Such patients include about one in five asthmatics, those with salicylate allergies and those with a history of peptic ulcer.
In order to establish whether paracetamol is an effective analgesic at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
The lack of significant anti-inflammatory activity of paracetamol implies a mode of action distinct from that of NSAIDs; yet, despite years of use and research, the mechanisms of action of paracetamol are not fully understood. NSAIDs act by inhibiting the activity of cyclooxygenase (COX), now recognised to consist of two isoforms (COX-1 and COX-2), which catalyses the production of prostaglandins responsible for pain and inflammation. Paracetamol has previously been shown to have no significant effects on COX-1 or COX-2 (Schwab 2003), but is now being considered as a selective COX-2 inhibitor (Hinz 2008). Significant paracetamol-induced inhibition of prostaglandin production has been demonstrated in tissues in the brain, spleen and lung (Botting 2000; Flower 1972). A ‘COX-3 hypothesis’, wherein the efficacy of paracetamol is attributed to its specific inhibition of a third cyclooxygenase isoform enzyme, COX-3 (Botting 2000; Chandrasekharan 2002), now has little credibility, and a central mode action of paracetamol is thought to be likely (Graham 2005). The efficacy of oral medications is reduced in many migraineurs because of impaired gastrointestinal motility, which is associated with nausea, and because of non-absorption of the drug due to vomiting (Volans 1974). The addition of an antiemetic may improve outcomes by alleviating the often incapacitating symptoms of nausea and vomiting, and (at least potentially) by enhancing the bioavailability of the co-administered analgesic. In particular, prokinetic antiemetics such as metoclopramide, which stimulate gastric emptying, may improve outcomes by increasing absorption of the analgesic. This has been investigated for metoclopramide and aspirin (Ross-Lee 1983; Volans 1975). It has been claimed that treatment with intravenous metoclopramide alone can reduce pain in severe migraine (Friedman 2005; Salazar-Tortolero 2008),but this claim requires further investigation, since metoclopramide has not been shown to be an analgesic in classical pain studies. The present review will seek to determine whether treatment of acute migraine attacks with paracetamol plus an antiemetic is in any way superior to treatment with paracetamol alone.
Why it is important to do this review
Population surveys show that paracetamol is frequently used to treat migraine headaches, but we could find no systematic review of the efficacy of this intervention in adults. It is important to know where this widely available and inexpensive drug fits in the range of therapeutic options for migraine therapy. For many migraineurs, non-prescription therapies offer convenience, and may be the only ones available or affordable.
OBJECTIVES
The objective of the review will be to determine the efficacy and tolerability of paracetamol, alone or in combination with an antiemetic, compared to placebo and other active interventions in the treatment of acute migraine headaches in adults.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised, double blind, placebo- or active-controlled studies using paracetamol to treat a migraine headache episode were included. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified below. Studies reporting treatment of consecutive headache episodes were accepted if outcomes for the first, or each, episode were reported separately. Cross-over studies were accepted if there was adequate washout (≥48 hours) between treatments.
Types of participants
Studies included adults (at least 18 years of age) with migraine. The diagnosis of migraine specified by the International Headache Society (IHS 1988; IHS 2004) was used, although other definitions were considered if they conformed in general to IHS diagnostic criteria. There were no restrictions on migraine frequency, duration or type (with or without aura). Participants taking stable prophylactic therapy to reduce the frequency of migraine attacks were accepted; details on any prophylactic therapy prescribed or allowed is provided in the Characteristics of included studies table.
Types of interventions
Included studies had to use either a single dose of paracetamol to treat a migraine headache episode when pain was of moderate to severe intensity, or investigate different dosing strategies and/or timing of the first dose in relation to headache intensity. There was no restriction on dose or route of administration, provided the medication was self-administered.
Included studies could use either paracetamol alone, or paracetamol plus an antiemetic. The antiemetic had to be taken either combined with paracetamol in a single formulation, or separately not more than 30 minutes before paracetamol, and had to be self-administered.
A placebo comparator is essential to demonstrate that paracetamol is effective in this condition. Active-controlled trials without a placebo were considered as secondary evidence. Studies to demonstrate prophylactic efficacy in reducing the frequency of migraine attacks were not included.
Types of outcome measures
Primary outcomes
The choice of main outcome measures for this review was made by taking into consideration scientific rigour, availability of data and patient preferences (Lipton 1999). Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), the main outcomes to be considered were:
Pain-free at 2 hours, without the use of rescue medication;
Reduction in headache pain (‘headache relief’) at 1 and 2 hours (pain reduced from moderate or severe to none or mild without the use of rescue medication);
Sustained pain-free over 24 hours (pain-free within 2 hours, with no use of rescue medication or recurrence within 24 hours);
Sustained pain reduction over 24 hours (headache relief at 2 hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication).
Pain intensity or pain relief had to be measured by the patient (not the investigator or carer). Pain measures accepted for the primary outcomes were:
Pain intensity (PI): 4-point categorical scale, with wording equivalent to none, mild, moderate and severe; or 100 mm VAS;
Pain relief (PR): 5-point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS
Only data obtained directly from the patient were considered.
Secondary outcomes
Secondary outcomes considered included:
Participants with any adverse event over 24 hours post dose;
Participants with particular adverse events over 24 hours post dose;
Withdrawals due to adverse events over 24 hours post dose;
Relief of headache-associated symptoms;
Functional disability.
Search methods for identification of studies
Electronic searches
The following databases were searched:
Cochrane CENTRAL, most recent search 4 October 2010.
MEDLINE (via OVID), most recent search 4 October 2010.
EMBASE (via OVID), most recent search 4 October 2010.
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 1 for the search strategy for MEDLINE (via OVID), Appendix 2 for the search strategy for EMBASE (via OVID), and Appendix 3 for the search strategy for CENTRAL. There were no language restrictions.
Searching other resources
Reference lists of retrieved studies and review articles were searched for additional studies, as were two clinical trials databases (www.clinicaltrials.gov and www.gsk-clinicalstudyregister.com). Grey literature and abstracts were not searched. Glaxo-Smith Kline provided additional detail relating to two studies for which a summary of results has been published on-line.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. Titles and abstracts of all studies identified by electronic searches were viewed on screen, and any that clearly did not satisfy inclusion criteria excluded. Full copies of the remaining studies were read to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. Data were entered into RevMan 5.0 by one author.
Assessment of risk of bias in included studies
Methodological quality was assessed using the Oxford Quality Score (Jadad 1996b).
The scale is used as follows:
Is the study randomised? If yes, give one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point; if no, deduct one point.
Is the study double blind? If yes, add one point.
Is the double blind method reported and is it appropriate? If yes, add one point; if no deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The scores for each study are reported in the Characteristics of included studies table.
A risk of bias table was also be completed, using assessments of randomisation, allocation concealment and blinding.
Measures of treatment effect
Relative risks (or ‘risk ratios’, RR) were used to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
The following terms are used to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occur with paracetamol than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occur with paracetamol compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to individual patient only.
Dealing with missing data
The source of most missing data was cross-over studies where only participants who completed all treatment phases were included in analyses. It was our intention, in such circumstances, to analyse first-period data only, but these data were not provided. Data for participants completing all phases were extracted, together with attrition rates, where available.
Assessment of heterogeneity
Heterogeneity of studies was assessed visually (L’Abbe 1987).
Data synthesis
Studies using a single dose of paracetamol in established pain of at least moderate intensity were analysed separately from studies in which medication was taken before pain was well established or in which a second dose of medication was permitted.
Effect sizes were calculated and data combined for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). In case only one study on relevant outcomes in at least 200 participants was available, prohibiting combining of data for analysis, a summary of data on relevant outcomes is provided. Relative risk of benefit or harm was calculated with 95% confidence intervals (CIs) using a fixed-effect model (Morris 1995). NNT, NNTp and NNH with 95% CIs were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995) when there was a statistically significant difference from control. A statistically significant difference from control was assumed when the 95% CI of the relative risk of benefit or harm did not include the number one. Significant differences between NNT, NNTp and NNH for different doses of active treatment, or between groups in the sensitivity analyses, would be determined using the z test (Tramer 1997).
Subgroup analysis and investigation of heterogeneity
Issues for subgroup analysis were dose, monotherapy or combination with an antiemetic, formulation, and route of administration. For combined treatment with an antiemetic, different antiemetics would be compared if there were sufficient data.
Sensitivity analysis
Sensitivity analysis was anticipated for study quality (Oxford Quality Score of 2 versus 3 or more), and for migraine type (with aura versus without aura). A minimum of two studies, with 200 participants in total, had to be available for any sensitivity analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Included studies
Ten studies fulfilled the inclusion criteria for this review; eight were published in full in peer-reviewed journals (Dexter 1985; Dowson 2000; Freitag 2008; Hoernecke 1993; Lipton 2000; MacGregor 1993; Norrelund 1989; Prior 2010), and two were available as Results Summaries on the manufacturer’s web site (GL/MIG/001/92; GL/MIG/001A/92). No further details were provided by the manufacturer for these two studies.
All 10 studies recruited participants between 18 and 70 years of age (mean ages ranging from 33 to 49 years; one study (Hoernecke 1993) included one participant aged 75 years). Six studies used IHS criteria (IHS 1988; IHS 2004) to diagnose migraine or other criteria which we considered compatible (Ad Hoc 1962; Soyka 1988); four studies (Dexter 1985; Dowson 2000;GL/MIG/001/92; GL/MIG/001A/92) did not report criteria for diagnosis, and we planned to include them, with a sensitivity analysis to determine their effect on results. In fact Dexter 1985 did not provide any usable efficacy data, and the others could not be compared with studies evaluating the same treatment comparisons that did report diagnostic criteria. Most studies reported a history at study entry of migraine headaches for at least 6 months, 1 year or 2 years, with between one attack every 2 months to eight attacks per month of moderate to severe intensity if untreated. Participants were most commonly recruited through attendance at specialised migraine clinics or through primary care practices, although Lipton 2000 used telephone recruitment, and Prior 2010 used a combination of clinic patients and advertising.
Three studies excluded people who had recently used prophylactic medication (Dexter 1985; GL/MIG/001/92; GL/MIG/001A/92), and two permitted stable prophylactic medication, provided it remained constant (Dowson 2000; Prior 2010). The implication in other studies was that prophylactic medication was not permitted. Lipton 2000 and Prior 2010 excluded anyone who needed bed rest in at least 50% of attacks, or experienced vomiting in at least 20% of attacks.
The 10 studies reported on 12 different treatment comparisons:
Four studies compared paracetamol 1000 mg alone with placebo (Freitag 2008; Hoernecke 1993; Lipton 2000; Prior 2010; 1293 participants in the comparison);
One study compared paracetamol 500 mg plus the antiemetic metoclopramide 5 mg with placebo, but did not provide any usable efficacy data (Dexter 1985; 47 participants).
One study compared paracetamol 1000 mg plus a different antiemetic, domperidone 20 mg or 30 mg, with paracetamol 1000 mg alone (MacGregor 1993; 46 participants in a cross-over study).
Two studies compared paracetamol 1000 mg plus metoclopramide 10 mg with sumatriptan 100 mg (GL/MIG/001/92; GL/MIG/001A/92; 721 participants).
One study compared paracetamol 1000 mg plus domperidone 20 mg with sumatriptan 50 mg (Dowson 2000; 120 participants in a cross-over study).
One study compared paracetamol 1000 mg plus dihydroergotamine 2 mg with paracetamol 1000 mg alone and with dihydroergotamine 2 mg alone (Hoernecke 1993; 288 participants in a cross-over study).
One study compared paracetamol 1000 mg plus rizatriptan 10 mg with paracetamol 1000 mg alone and rizatriptan 10 mg alone (Freitag 2008; 173 participants).
One study compared paracetamol 1000 mg with tolfenamic acid 400 mg (Norrelund 1989; 116 participants in a cross-over study).
All treatments were administered orally, either at the onset of an attack when pain intensity was usually still mild (Dexter 1985;Hoernecke 1993; MacGregor 1993; Norrelund 1989), or when pain intensity was moderate or severe (Dowson 2000; Freitag 2008; GL/MIG/001/92; GL/MIG/001A/92; Lipton 2000; Prior 2010). Six studies used a parallel-group design (Dexter 1985;Freitag 2008; GL/MIG/001/92; GL/MIG/001A/92; Lipton 2000; Prior 2010) and the remainder a cross-over design. None of the cross-over studies reported data for the first attack only, and in three (Dowson 2000; MacGregor 1993; Norrelund 1989) the attrition rate was 20% to 25%, while in the fourth (Hoernecke 1993) only the number completing all four attacks was reported. Some participants would have been excluded because they did not experience the requisite number of qualifying headaches in the specified study period; none of the studies contributed to meta-analyses of efficacy data.
Most studies used a particular medication to treat a single attack (participants in cross-over studies treated each attack with a different medication), but three studies treated three (GL/MIG/001/92; GL/MIG/001A/92) or four (Dexter 1985) attacks with the same medication. GL/MIG/001/92 and GL/MIG/001A/92 reported data separately for each attack, and first attack data is used for the primary analysis. Dexter 1985 did not provide any usable data for the primary analysis.
Excluded studies
Three studies were excluded after reading the full paper (Diamond 1976; Diener 2005; Larsen 1990). Details are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
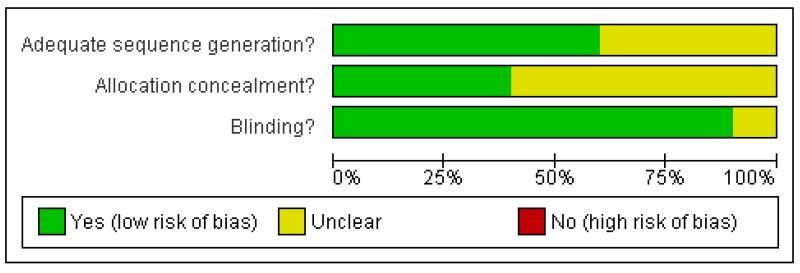
Studies were of good methodological quality, with six scoring 5/5, three scoring 4/5, and one scoring 3/5 on the Oxford Quality Scale. Points were lost due to failure to adequately describe the methods of randomisation and blinding.
A Risk of bias table was completed for randomisation, allocation concealment and blinding. No studies were at high risk of bias, but none reported on allocation concealment (Figure 1).
Figure 1. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Four studies used a cross-over design. None reported data for the first phase separately, and three (Dowson 2000; Hoernecke 1993;Norrelund 1989) reported only on participants who took each of the study medications. Two cross-over studies excluded significant (> 10%) numbers of participants because they did not treat attacks with each study medication; Dowson 2000 excluded 41/161 (25%) participants, and Hoernecke 1993 excluded 186/474 (39%) participants, mostly because they did not report four separate attacks. While this is a significant loss of data from these studies, there is no reason to think that there was any systematic bias involved. Neither study contributed data to any pooled analysis. MacGregor 1993 did not exclude two participants who had invalid data for two of three treatments.
Effects of interventions
Although 10 studies were identified for inclusion in this review, few compared paracetamol, with or without an antiemetic, with either placebo or the same active comparator, when taken either at onset of pain (while pain intensity was usually mild) or once pain intensity was moderate or severe. Consequently, few studies could be combined, and there were few data available for meta-analysis. We have reported on all comparisons with at least 200 participants and have analysed quantitatively all those involving at least two studies and 200 participants or treated attacks. Details of outcomes in individual studies are provided in Appendix 4 (efficacy), Appendix 5 (migraine-associated symptoms and functional disability) and Appendix 6 (adverse events and withdrawals).
Pain-free at 2 hours
Paracetamol 1000 mg versus placebo
Three studies (717 participants) provided data for pain-free response at 2 hours when medication was taken for moderate to severe pain (Freitag 2008; Lipton 2000; Prior 2010).
The proportion of participants pain-free at 2 hours with paracetamol 1000 mg was 19% (68/367; range 14% to 26%).
The proportion of participants pain-free at 2 hours with placebo was 10% (36/350; range 8% to 15%).
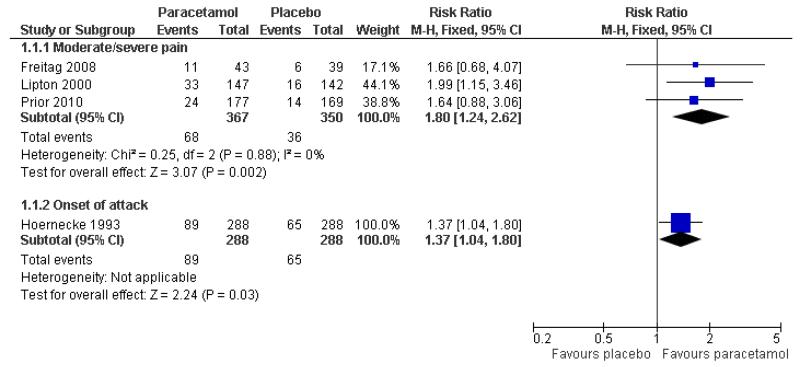
The relative benefit of treatment compared with placebo was 1.8 (1.2 to 2.6; Figure 2), giving an NNT for pain-free at 2 hours of 12 (7.5 to 32; Summary of results A).
Figure 2. Forest plot of comparison: 1 Paracetamol 1000 mg versus placebo, outcome: 1.1 Pain-free at 2 hours.
One other study (576 attacks) provided data equivalent to pain-free response at 2 hours (pain 0 or 1 on scale 0 to 9) when medication was taken at the onset of attack and the pain not necessarily moderate or severe (Hoernecke 1993). Thirty-one percent (31%; 89/288) of participants were pain-free at 2 hours with paracetamol 1000 mg compared with 19% (65/288) with placebo (Summary of results A [also in Figure 2 = Analysis 1.1]).
Paracetamol 1000 mg versus dihydroergotamine 2 mg
In addition to providing data for comparison of paracetamol 1000 mg with placebo (above), Hoernecke 1993 also provided data for comparison with dihydroergotamine 2 mg, with medication taken at the onset of attack and the pain not necessarily moderate or severe. Thirty-one percent (31%; 89/288) of participants were pain-free at 2 hours with paracetamol 1000 mg compared with 25% (72/288) with dihydroergotamine 2 mg (Summary of results A).
Headache relief at 1 hour
Paracetamol 1000 mg versus placebo
Two studies (635 participants) provided data for headache relief at 1 hour when medication was taken for moderate to severe pain (Lipton 2000; Prior 2010).
The proportion of participants with headache relief at 1 hour with paracetamol 1000 mg was 39% (127/324; range 37% to 42%).
The proportion of participants with headache relief at 1 hour with placebo was 20% (62/311; range 17% to 23%).
The relative benefit of treatment compared with placebo was 2.0 (1.5 to 2.6; Analysis 1.2), giving an NNT for headache relief at 1 hour of 5.2 (3.8 to 8.1; Summary of results A).
No other studies reported this outcome.
Headache relief at 2 hours
Paracetamol 1000 mg versus placebo
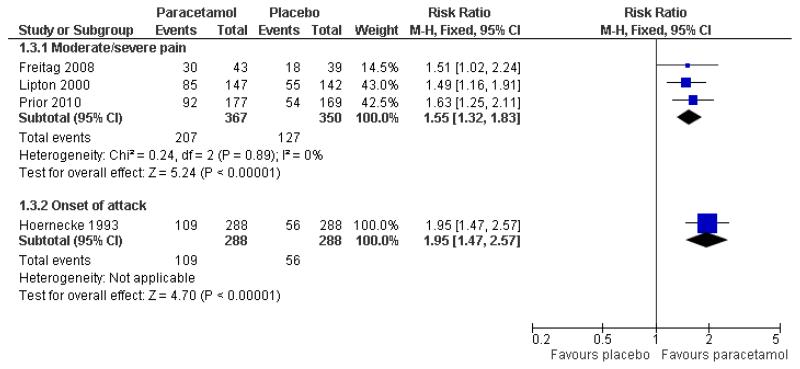
Three studies (717 participants) provided data for headache relief at 2 hours when medication was taken for moderate to severe pain (Freitag 2008; Lipton 2000; Prior 2010).
The proportion of participants with headache relief at 2 hours with paracetamol 1000 mg was 56% (207/367; range 52% to 70%).
The proportion of participants with headache relief at 2 hours with placebo was 36% (127/350; range 32% to 46%).
The relative benefit of treatment compared with placebo was 1.6 (1.3 to 1.8; Figure 3), giving an NNT for headache relief at 2 hours of 5.0 (3.7 to 7.7; Summary of results A).
Figure 3. Forest plot of comparison: 1 Paracetamol 1000 mg versus placebo, outcome: 1.3 Headache relief at 2 hours.
One other study (Hoernecke 1993) treated at onset of attack when pain was not necessarily moderate or severe. At 2 hours, 109/288 (38%) had mild or no pain with paracetamol 1000 mg and 56/288 (20%) with placebo (Summary of results A [also in Figure 3 = Analsyis 1.3]). This cross-over study reported efficacy data only for participants who treated all four attacks, each with a different intervention. The attrition rate over the course of the study is not known.
Paracetamol 1000 mg versus dihydroergotamine 2 mg
One study (Hoernecke 1993) treated at onset of attack when pain was not necessarily moderate or severe. At 2 hours, 109/288 (38%) had mild or no pain with paracetamol 1000 mg and 82/288 (28%) with dihydroergotamine 2 mg (Summary of results A). This crossover study reported efficacy data only for participants who treated all four attacks, each with a different intervention. The attrition rate over the course of the study is not known.
Paracetamol 500 mg plus domperidone 20 mg versus sumatriptan 50 mg
Only one study provided data for this comparison, with medication taken for moderate to severe pain (Dowson 2000): 44/120 (36%) of participants had headache relief at 2 hours with paracetamol 500 mg plus domperidone 20 mg, compared with 40/120 (33%) with sumatriptan 50 mg (Summary of results A). Twenty five percent of study participants did not treat both attacks and are not included in any analysis. This study did not have a placebo control group for internal sensitivity and did not report the criteria used for diagnosis.
Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg
Two studies (1140 participants) provided data for these treatments, with medication taken for moderate to severe pain (GL/MIG/001/92; GL/MIG/001A/92). Data for the first of three attacks treated are used for this analysis. These studies did not have placebo control groups for internal sensitivity and did not report the criteria used for diagnosis.
The proportion of participants with headache relief at 2 hours with paracetamol 1000 mg plus metoclopramide 10 mg was 39% (225/580; range 36% to 41%).
The proportion of participants with headache relief at 2 hours with sumatriptan 100 mg was 42% (233/560; range 41% to 42%).
The relative benefit of the combination compared with sumatriptan was 0.93 (0.81 to 1.1; Analysis 2.1). The NNT was not calculated (Summary of results A).
24-hour sustained relief and sustained pain-free
Only one study (Freitag 2008), with 173 participants, reported these outcomes. There were insufficient data for analysis.
Subgroup analyses
All included studies used paracetamol at a dose of 1000 mg, but different comparator doses have been analysed separately. There were no data to compare paracetamol with and without an antiemetic, and insufficient data to compare different antiemetics (metoclopramide and domperidone). All medication was administered orally as standard formulations (e.g. not soluble, effervescent). Studies treating at onset of a migraine attack have been analysed separately from those treating once pain is moderate or severe; there were insufficient data for any pooled analysis of studies treating at onset. There were insufficient data for analysis of studies using multiple dosing strategies.
Sensitivity analyses
No sensitivity analysis could be carried out for methodological quality or migraine subtype, as all studies scored 3 or more on the Oxford Quality Scale, and no study analysed migraine subtypes separately.
Summary of results A: Pain-free and headache relief.
| Baseline pain intensity |
Studies | Participants or attacks treated | Treatment (%) | Placebo or comparator (%) | RR (95% CI) | NNT (95% CI) | |
|---|---|---|---|---|---|---|---|
| Pain-free at 2 hours | |||||||
| Paracetamol 1000 mg versus placebo | Moderate/severe | 3 | 717 | 19 | 10 | 1.8 (1.2 to 2.6) | 12 (7.5 to 32) |
| Paracetamol 1000 mg versus placebo | Onset | 1 | 576 | 31 | 19 | - | - |
| Paracetamol 1000 mg versus dihydroergotamine 2mg | Onset | 1 | 576 | 31 | 25 | - | - |
| Headache relief at 1 hour | |||||||
| Paracetamol 1000 mg versus placebo | Moderate/severe | 2 | 635 | 39 | 20 | 2.0 (1.5 to 2.6) | 5.2 (3.8 to 8.1) |
| Headache relief at 2 hours | |||||||
| Paracetamol 1000 mg versus placebo | Moderate/severe | 3 | 717 | 56 | 36 | 1.6 (1.3 to 1.8) | 5.0 (3.7 to 7.7) |
| Paracetamol 1000 mg versus placebo | Onset | 1 | 576 | 38 | 20 | - | - |
| Paracetamol 1000 mg versus dihydroergotamine 2 mg | Onset | 1 | 576 | 28 | 20 | - | - |
| Paracetamol 1000 mg + domperidone 20 mg versus sumatriptan 50 mg | Moderate/severe | 1 | 240 | 36 | 33 | - | - |
| Paracetamol 1000 mg + metoclopramide 10 mg versus sumatriptan 100 mg | Moderate/severe | 2 | 1140 | 39 | 42 | 0.93 (0.81 to 1.1) | Not calculated |
Relief of migraine-associated symptoms
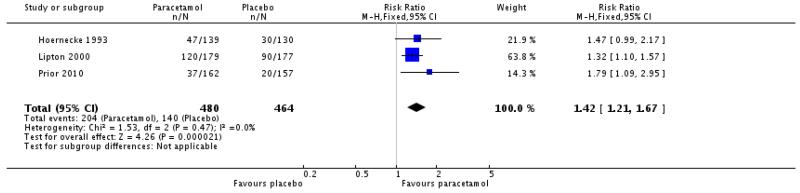
Reporting of migraine-associated symptoms such as nausea, vomiting, phonophobia and photophobia was inconsistent, with few studies contributing data for analysis (Appendix 5). In the five studies that reported on vomiting (Dowson 2000; GL/MIG/001/92; Hoernecke 1993; Lipton 2000; Prior 2010), too few attacks had vomiting at baseline to allow any analysis. Information about other symptoms is summarised below (Summary of results B). We combined data from Hoernecke 1993, in which treatment was taken at the onset of an attack, with data from studies in which treatment was taken when pain intensity was moderate or severe. The response rate in the study where medication was taken at the onset of an attack was higher (in both active and placebo groups) than in the study where it was taken when pain intensity was moderate or severe. Overall, paracetamol 1000 mg was better than placebo for relief of nausea, photophobia and phonophobia, with an additional 10% to 15% of participants experiencing relief at 2 hours compared with placebo, giving NNTs of 7 to 9 (Analysis 1.4; Analysis 1.5; Analysis 1.6; Summary of results B). There was no clear difference between paracetamol 1000 mg plus metoclopramide 10 mg and sumatriptan 100 mg, taken when pain intensity was moderate or severe, for relief of light and noise sensitivity during the first attack (Analysis 2.2). When data from all three attacks were combined, this outcome just reached statistical significance in favour of sumatriptan over paracetamol plus metoclopramide.
Summary of results B: Relief of associated symptoms 2 hours after taking study medication.
| Intervention | Studies | Attacks with symptom present | Treatment (%) | Placebo (%) | Relative risk (95%CI) | NNT/NNH |
|---|---|---|---|---|---|---|
| Nausea | ||||||
| Paracetamol 1000 mg versus placebo (Hoernecke 1993; Prior 2010) | 2 | 536 | 59 | 44 | 1.4 (1.2 to 1.6) | 6.7 (4.3 to 15) |
| Paracetamol 1000 mg versus dihydroergotamine 2 mg (Hoernecke 1993) | 1 | 341 | 70 | 69 | - | - |
| Photophobia | ||||||
| Paracetamol 1000 mg versus placebo (Hoernecke 1993; Lipton 2000; Prior 2010) | 3 | 985 | 41 | 30 | 1.4 (1.2 to 1.6) | 9.2 (6.0 to 20) |
| Paracetamol 1000 mg versus placebo (Lipton 2000; Prior 2010), treatment when pain moderate or severe | 2 | 609 | 25 | 16 | 1.6 (1.1 to 2.1) | 11 (6.5 to 39) |
| Photophobia | ||||||
| Paracetamol 1000 mg versus placebo (Hoernecke 1993; Lipton 2000; Prior 2010) | 3 | 944 | 43 | 30 | 1.4 (1.2 to 1.7) | 8.1 (5.4 to 16) |
| Paracetamol 1000 mg versus placebo (Lipton 2000; Prior 2010), treatment when pain moderate or severe | 2 | 588 | 28 | 17 | 1.6 (1.2 to 2.2) | 9.5 (5.8 to 26) |
| Combined “light/noise sensitivity” | ||||||
| Paracetamol + metoclopramide 1000 + 10 mg versus sumatriptan 100 mg (1st attack, GL/MIG/001/92; GL/MIG/001A/92) | 2 | 1001 | 32 | 32 | 1.0 (0.84 to 1.2) | not calculated |
| Paracetamol + metoclopramide 1000 + 10 mg versus sumatriptan 100 mg (all 3 attacks, GL/MIG/001/92; GL/MIG/001A/92) | 2 | 2617 | 28 | 33 | 0.87 (0.77 to 0.98) | 22 (12 to 91) |
Data from these studies were also analysed according to the persistence of associated symptoms 2 hours after treatment, and NNTps calculated (Appendix 7). About 10% fewer participants had nausea, photophobia or phonophobia 2 hours after taking medication with paracetamol 1000 mg than with placebo, giving an NNTp of 10. Once again, there was no clear difference between paracetamol 1000 mg plus metoclopramide 10 mg and sumatriptan 100 mg for persistence of light and noise sensitivity at 2 hours.
Functional disability
Three studies reported on the presence of any degree of functional disability at 2 hours (Freitag 2008; Lipton 2000; Prior 2010; 717 participants). All compared paracetamol 1000 mg with placebo.
The proportion of participants with functional disability at 2 hours following paracetamol 1000 mg was 72% (263/367; range 51% to 78%).
The proportion of participants with functional disability at 2 hours following placebo was 82% (288/350; range 59% to 85%).
The relative risk with treatment compared with placebo was 0.87 (0.81 to 0.94; Analysis 1.7), giving an NNTp of 9.4 (6.0 to 22).
Two of these studies (Lipton 2000; Prior 2010, 610 participants) also reported numbers of participants with functional disability at baseline, from which the numbers free of disability following treatment were calculated.
The proportion of participants with functional disability at baseline who were free of disability at 2 hours following paracetamol 1000 mg was 24% (74/309; range 18% to 31%).
The proportion of participants with functional disability at baseline who were free of disability at 2 hours following placebo was 14% (41/301; range 12% to 15%).
The relative risk with treatment compared with placebo was 1.8 (1.2 to 2.5; Analysis 1.8), giving an NNT for improvement of 9.7 (6.1 to 24).
Use of rescue medication
Use of rescue medication was permitted after 2 hours in most studies using single dosing regimens, after 1 to 4 hours in those allowing repeat dose(s), and not reported in GL/MIG/001/92; GL/MIG/001A/92. The time post medication at which use was reported varied between studies, further limiting analysis.
Two studies (635 participants) comparing paracetamol 1000 mg with placebo, with medication taken for moderate to severe pain, permitted use of rescue medication after 2 hours and reported on use within 6 hours (Lipton 2000, Prior 2010).
The proportion of participants using rescue medication within 6 hours with paracetamol 1000 mg was 24% (79/324; range 14% to 33%).
The proportion of participants using rescue medication within 6 hours with placebo was 41% (129/311; range 32% to 49%).
The relative risk of treatment compared with placebo was 0.59 (0.47 to 0.74; Analysis 1.9), giving an NNT to prevent use of rescue medication at 6 hours of 5.9 (4.1 to 10).
A further two studies (1243 participants) comparing paracetamol 1000 mg plus metoclopramide 10 mg with sumatriptan 100 mg, with medication taken for moderate to severe pain, reported on use within 24 hours (GL/MIG/001/92; GL/MIG/001A/92).
The proportion of participants using rescue medication within 24 hours with paracetamol 1000 mg plus metoclopramide 10 mg was 38% (245/637; range 32% to 44%).
The proportion of participants using rescue medication within 24 hours with sumatriptan 100 mg was 33% (198/606; range 27% to 37%).
The relative risk of treatment with paracetamol plus metoclopramide compared with sumatriptan was 1.2 (1.0 to 1.4; Analysis 2.3), giving an NNH for use of rescue medication at 24 hours of 17 (9.0 to 210) compared with sumatriptan 100 mg.
Adverse events
Any adverse event
All included studies made some mention of adverse events, but did not always report the numbers of participants in each treatment group who experienced at least one adverse event. The incidence of adverse events varied considerably between studies, and probably reflects whether all events were recorded, or just those that were treatment-emergent, not part of the migraine attack (i.e. not migraine-associated symptoms) or considered drug-related. Differences in the methods used to collect data (e.g. diary vs. spontaneous reporting) may also influence recorded incidence. In addition, it was not always stated whether data continued to be collected after use of rescue medication, which may cause its own adverse events. Where reported, adverse events were described as mostly of mild or moderate intensity, and transient.
One study comparing paracetamol 1000 mg with placebo reported the number of participants with at least one adverse event before use of rescue medication and within 24 hours (Freitag 2008), two reported within 6 hours (Lipton 2000; Prior 2010), and one within 2 hours (Hoernecke 1993). These studies were combined for analysis (1293 participants).
The proportion of participants experiencing adverse events with paracetamol 1000 mg was 18% (117/655; range 1% to 34%).
The proportion of participants experiencing adverse events with placebo was 23% (144/638; range 3% to 46%).
The relative risk with treatment compared with placebo was 0.78 (0.64 to 0.95; Analysis 1.10). There were more adverse events with placebo than with paracetamol, giving an NNTp of 21 (11 to 300) for paracetamol compared with placebo.
In the two studies with high rates of adverse events (Lipton 2000;Prior 2010), most were migraine-associated symptoms.
One study comparing paracetamol 1000 mg plus metoclopramide 10 mg with sumatriptan 100 mg reported the numbers of participants with “major” and “minor” adverse events (GL/MIG/001/92), and a similar study reported numbers with any adverse events (GL/MIG/001A/92). We assumed for analysis that major and minor categories are mutually exclusive (which may slightly overestimate the event rate) and combined the studies for analysis.
The proportion of participants experiencing adverse events with paracetamol 1000 mg plus metoclopramide 10 mg was 28% (191/675; range 25% to 32%).
The proportion of participants experiencing adverse events with sumatriptan 100 mg was 47% (304/653; range 41% to 53%).
The relative risk with paracetamol plus metoclopramide compared with sumatriptan was 0.61 (0.53 to 0.71; Analysis 2.4), giving an NNTp of 5.5 (4.3 to 7.6) for paracetamol plus metoclopramide compared with sumatriptan.
There were insufficient data for analysis of other comparisons (Appendix 6).
Specific adverse events
Not all studies reported on numbers of participants with specific adverse events. Some reported the most common events, or those occurring in a given percentage of any treatment arm, while others reported “drug-related adverse events” or “side effects”. Most reported events affected the digestive system (abdominal pain, nausea, vomiting), special senses (sensitivity to stimuli) or the central nervous system (dizziness), and many were likely to be symptoms associated with the migraine attack itself, rather than an adverse effect of the medication. There were too few events for detailed analysis.
Serious adverse events
One study (Norrelund 1989) did not report on serious adverse events. No study reported any serious adverse events in participants treated with paracetamol 1000 mg alone or paracetamol 1000 mg plus domperidone 20 mg. Two studies (GL/MIG/001/92; GL/MIG/001A/92) reported on “major” adverse events, which were defined as “serious adverse events or clinical abnormalities that led to withdrawal”, and appear to include severe (a measure of intensity) adverse events necessitating withdrawal in addition to any serious events (usually defined as having significant medical consequences such as death, prolonged hospitalisation, permanent disability, or congenital anomaly).
Two studies (GL/MIG/001/92; GL/MIG/001A/92) reported numbers of participants with “major” adverse events (defined as serious adverse events or clinical abnormalities that led to withdrawal, presumably over 48 hours) in participants treated with paracetamol 1000 mg plus metoclopramide 10 mg or sumatriptan 100 mg.
The proportion of participants experiencing major adverse events with paracetamol 1000 mg plus metoclopramide 10 mg was 3% (21/675).
The proportion of participants experiencing major adverse events with sumatriptan 100 mg was 6% (41/653; range 5% to 8%).
The relative risk with paracetamol plus metoclopramide compared with sumatriptan was 0.50 (0.30 to 0.83; Analysis 2.5), giving an NNH of 32 (18 to 112).
In GL/MIG/001/92 only two types of “major” adverse events were reported by more than 1% of participants: 5/305 reported dizziness and 4/305 reported nausea with sumatriptan 100 mg. One participant reported chest pain with sumatriptan 100 mg. In GL/MIG/001A/92 only one “major” adverse event was reported by more than 1% of participants: 4/348 reported chest pain with sumatriptan 100 mg. There were no “major” adverse events occurring in more than 1% of participants with paracetamol plus metoclopramide in either study.
Withdrawals
Adverse event withdrawals
Eight studies reported on withdrawals due to adverse events. In four there were no withdrawals in any treatment arm (Freitag 2008;Hoernecke 1993; Lipton 2000; MacGregor 1993). In Dexter 1985 there were two withdrawals due to nausea with paracetamol plus metoclopramide, and in Prior 2010 there were two withdrawals with paracetamol and one with placebo, all due to migraine-associated symptoms. In GL/MIG/001/92 and GL/MIG/001A/92 there were five and eight withdrawals, respectively, with paracetamol plus metoclopramide, and 18 and 13, respectively, with sumatriptan 100 mg, with no reasons for withdrawal given.
Other withdrawals and exclusions
Some participants were excluded form analyses because they vomited within 30 minutes of taking study medication, were lost to follow-up or had major protocol violations. Generally these numbers were small and equally distributed between treatment arms. One four-period cross-over study (Hoernecke 1993) and three studies treating more than one attack with the same medication (Dexter 1985; GL/MIG/001/92; GL/MIG/001A/92) reported small numbers of participants who withdrew between treatment periods due to lack of efficacy. Lack of efficacy during a single attack should be captured by use of rescue medication (above), although there were limited data reported for this outcome.
DISCUSSION
Summary of main results
This review included 10 randomised, double-blind, controlled studies with 2769 participants (4062 attacks). Medication was taken either at the onset of an attack or once pain intensity was moderate or severe, and most studies investigated the effects of a single dose of medication for an attack. Only one dose of paracetamol, 1000 mg, was used. Four different active comparators were evaluated (sumatriptan 50 mg and 100 mg, dihydroergotamine 2 mg, rizatriptan 25 mg, and tolfenamic acid 400 mg), plus two in combination with paracetamol (dihydroergotamine and rizatriptan), and two different antiemetics were combined with paracetamol (metoclopramide 10 mg and domperidone 20 and 30 mg), but there were sufficient data to compare only paracetamol with placebo, and paracetamol plus metoclopramide with sumatriptan. Outcomes were not consistently reported, and only one small study reported on 24-hour sustained efficacy. There were insufficient data to compare treating an attack at onset with treating once pain has become moderate or severe, or to compare single with multiple dosing regimens.
For the IHS preferred outcome of pain-free at 2 hours, paracetamol 1000 mg was better than placebo, giving an NNT of 12, when baseline was pain was moderate or severe (three studies; 717 participants). Around 1 in 5 participants achieved this outcome with paracetamol compared with 1 in 10 with placebo. For headache relief at 1 hour and 2 hours, paracetamol was also better than placebo, giving NNTs of 5, when baseline pain was moderate or severe. Over half of participants achieved relief at 2 hours with paracetamol, compared with about one in three with placebo. There were no data for pain-free at 2 hours for the combination of paracetamol 1000 mg and metoclopramide 10 mg versus sumatriptan 100 mg, but for headache relief at 2 hours, there was no significant difference between the two treatments when baseline pain was moderate or severe (two studies; 1140 participants). About two in five participants achieved this outcome in these studies. For relief of migraine-associated symptoms of nausea, photophobia and phonophobia, about 10% to 15% more participants achieved relief with 2 hours with paracetamol than with placebo, giving NNTs of 7 to 11. There was no significant difference between paracetamol 1000 mg plus metoclopramide 10 mg and sumatriptan 100 mg for relief of “light/noise sensitivity” at 2 hours. In the three studies that reported functional disability, most participants were experiencing some degree of disability when they took study medication, and significantly more were free of disability at 2 hours with paracetamol than with placebo, giving an NNT of 10. About one in four of those with disability at the time of treatment were free of disability at 2 hours with paracetamol compared with one in seven with placebo.
Fewer participants needed to use rescue medication over 6 hours with paracetamol than with placebo (NNT 6), but more needed it over 24 hours with paracetamol than with sumatriptan (NNH 17).
Adverse events were poorly reported, but there was no evidence of an increase in the number of participants experiencing any adverse events with paracetamol 1000 mg compared with placebo, and no serious adverse events were reported with paracetamol alone. Significantly fewer participants experienced any adverse event with the combination of paracetamol plus metoclopramide compared with sumatriptan 1000 mg, and there were more “major” adverse events with sumatriptan (6% versus 3%).
Overall completeness and applicability of evidence
Included studies did not always report our prespecified outcomes of interest, and for some outcomes (such as use of rescue medication and adverse events) data were not reported consistently, making it difficult to combine data for analysis. There were insufficient data for most drug comparisons or treatment protocols to allow analysis, so that evidence is limited to the comparison of a single dose of paracetamol (1000 mg) with placebo, and a single dose of the combination of paracetamol plus metoclopramide (1000/10 mg) with sumatriptan 100 mg, both taken when pain intensity was moderate or severe. Even for these comparisons, not all of the primary outcomes were reported. Of particular note is that only one small study reported on 24-hour sustained response (relief or pain-free), so that no conclusions can be drawn about whether paracetamol can prevent recurrence of the attack.
Participants in most studies had a diagnosis of migraine according to IHS or comparable criteria, but four studies did not report criteria. Of these four, Dexter 1985 and Dowson 2000 did not contribute to any efficacy analyses; data for the other two studies (GL/MIG/001/92; GL/MIG/001A/92) were taken from a manufacturer’s Summary of Results sheet, which did not report details of methods, but it is very likely that IHS diagnostic criteria from 1988 were used in these trials. These two studies did provide data for analysis, but it was not possible to compare their results with those from studies that reported diagnostic criteria.
Participants were mostly recruited from migraine clinics or primary care, which might lead to under-representation of individuals with milder headaches. Lipton 2000 and Prior 2010 excluded those who usually required bedrest or who vomited during more in than 20% of attacks, and Prior 2010 included only those who had also previously used OTC medications. These two studies contributed almost 90% of the data for the primary outcomes for paracetamol compared with placebo. Some individuals with very severe or difficult-to-treat migraine headaches may have been excluded in these two studies, and limits on the frequency of attacks would exclude those with very frequent attacks, but otherwise the population in this review was probably representative, in terms of migraine headaches, of those who seek help for the condition. Most studies specified that participants were required to be “in good general health” or excluded those with significant co-morbidities (including, for example uncontrolled hypertension, renal or hepatic disease, cardio- and cerebrovascular disease). This may mean that the population studied may differ from the general public who choose to self-medicate with OTC paracetamol.
The amount of information for active comparators was small, so that direct comparisons could not be made except for paracetamol plus metoclopramide versus sumatriptan, and there were insufficient data to compare paracetamol plus an antiemetic with placebo.
Individual studies are underpowered to determine differences between treatments for adverse events, and even pooling studies may not provide adequate numbers of events to demonstrate differences or allow confidence in the size of the effect. Analysis of adverse events in these studies was further compromised by incomplete and inconsistent reporting, but there was no evidence of increased numbers of adverse events, or any serious adverse events, with paracetamol 1000 mg alone or in combination with metoclopramide 10 mg.
Quality of the evidence
Included studies were of adequate or good methodological quality and validity; reporting of details of the methods of randomisation and allocation concealment tended to be better in more recent studies.
Potential biases in the review process
There were no potential biases in the review process, other than the fact that the numbers of participants were small and divided between many different comparators.
Agreements and disagreements with other studies or reviews
A systematic review of interventions for acute migraine with a literature search to 2000 found no trials using paracetamol that satisfied inclusion criteria and provided primary efficacy data (Oldman 2002). A review of OTC drugs for acute migraine with a search up to 2002 (Wenzel 2003) found that paracetamol was more effective than placebo for headache relief at 2 hours, and recommended OTC products (paracetamol, aspirin, ibuprofen and combination products) as “a feasible option” for those who experience disability with fewer than 50% of attacks and/or vomiting with fewer than 20% of attacks. Earlier reviews have also recommended OTC products, together with an antiemetic, for mild migraine attacks (e.g. Diener 1998). Recent guidelines on the drug treatment of migraine headaches (Evers 2009) acknowledge that paracetamol has shown efficacy in acute treatment in one RCT. This review includes new studies, presenting more robust estimates of efficacy for a number of standard and validated outcomes.
AUTHORS’ CONCLUSIONS
Implications for practice
Paracetamol 1000 mg alone may be a useful first-line treatment for individuals with migraine headaches that do not cause severe disability and, when combined with metoclopramide, may offer similar efficacy to oral sumatriptan 100 mg, but with fewer adverse events.
Implications for research
Studies are needed to investigate further whether the addition of an antiemetic, such as metoclopramide, to paracetamol can improve either pain relief or migraine-associated symptoms, and also to investigate potential benefits of different dosing strategies such as treating when pain is still mild or multiple dosing regimens. Studies should assess whether efficacy at early time points is sustained. Head-to-head studies with active comparators, particularly other OTC medications, would allow direct comparison between treatments. Ideally these studies would include a placebo comparator for internal validity.
PLAIN LANGUAGE SUMMARY.
Paracetamol (acetaminophen) with or without an antiemetic for acute migraine in adults
A single oral dose of paracetamol 1000 mg is effective in relieving migraine headache pain and associated symptoms of nausea, photophobia and phonophobia. Pain will be reduced from moderate or severe to no pain by 2 hours in 1 in 5 people (19%) taking paracetamol, compared with 1 in 10 (10%) taking placebo. Pain will be reduced from moderate or severe to no worse than mild pain by 2 hours in about 1 in 2 people (56%) taking paracetamol, compared with about 1 in 3 (36%) taking placebo. Too few data were available to assess efficacy beyond 2 hours.
Paracetamol 1000 mg plus metoclopramide 10 mg and oral sumatriptan 100 mg provide similar levels of headache relief and relief of sensitivity to light and noise at 2 hours. There was insufficient information to compare paracetamol, alone or in combination, with other active treatments.
Adverse events do not differ significantly between paracetamol and placebo. Slightly more major adverse events occur with sumatriptan 100 mg than with paracetamol 1000 mg plus metoclopramide 10 mg.
ACKNOWLEDGEMENTS
Sebastian Straube translated the German study (Hoernecke 1993). GlaxoSmithKline provided further details of methods used in the two studies published as Results Summaries on the manufacturer’s web site.
SOURCES OF SUPPORT
Internal sources
Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
NIHR Biomedical Research Centre Programme, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | R, DB, PC, parallel-group Four attacks treated. Medication (2 tablets) taken at onset of attack, repeated after 1 and 4 h if necessary (maximum 6 tablets in 24 h). Rescue medication (aspirin 600 mg) after 4 h if necessary |
|
| Participants | Common or classical migraine (≥ 2 years). Aged 20-50 years. Frequency 2 to 8 per month. Excluded: prophylactic treatment within 4 weeks N = 49 (42 completed 4 attacks) M 16, F 33 Mean age 33 years |
|
| Interventions | Paracetamol + metoclopramide 2 × 500/5 mg Placebo |
|
| Outcomes | Severity and duration of attacks Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “matched placebo” |
| Methods | R, DB, DD, AC, cross-over Two attacks treated with single doses of each of two study medications. Medication taken when pain was moderate or severe. Rescue medication after 4 h if necessary Severity assessed at 0, 1, 2, 4, 24, 48, 72 h |
|
| Participants | Migraine ± aura (diagnostic criteria not reported). Aged 18 to 65 years. Duration of condition > 1 year. Frequency ≥ 2 every 12 weeks. Prophylactic therapy (pizotifen, clonidine, β-blockers, calcium channel blockers) allowed if stable for ≥ 3 months and kept constant N = 161 (120 took both treatments and analysed for efficacy) M 9, F 111 Mean age 43 years |
|
| Interventions | Paracetamol + domperidone 1000/20 mg + placebo Sumatriptan 50 mg + placebo |
|
| Outcomes | Headache relief at 2 h Presence of nausea and vomiting Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “matching placebos” |
| Methods | Multicentre, R, DD, AC and PC, parallel-group Single attack treated with a single dose of study medication. Medication taken when pain was moderate or severe. Rescue medication after 2 h if necessary Assessments at 0, 0.5, 1, 1.5, 2, 3, 4, 24 h |
|
| Participants | Migraine ± aura (IHS 2004). Aged ≥ 18 years. Frequency 0.5 to 6 per month. Untreated severity ≥ moderate. Excluded: >10 headache days per month or used analgesics regularly > 3 days per week N = 173 M 21, F 152 Mean age 43 years |
|
| Interventions | Paracetamol 1000 mg, n = 43 Rizatriptan 10 mg, n = 43 Rizatriptan + paracetamol 10/1000 mg, n = 48 Placebo, n = 39 |
|
| Outcomes | Pain-free at 2 h Headache relief at 2 h 24 h sustained relief 24 h sustained pain-free Relief of nausea, photophobia, phonophobia, functional disability Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “computer-generated” |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Double-dummy, “matched placebos” |
| Methods | Multicentre, R, DB, parallel-group Three attacks treated with same medication. Second dose of sumatriptan or placebo paracetamol + domperidone allowed after 2 h if necessary. Rescue medication after 4 hours if necessary |
|
| Participants | Migraine ± aura (diagnostic criteria not reported). Aged 20 to 65 years. Duration of condition ≥ 1 year. Frequency ≥ 1 every 8 weeks. Excluded: prophylactic treatment within 2 weeks, IHD, chronic analgesic use for other condition N = 607 M 98, F 509 Mean age 39 years |
|
| Interventions | Paracetamol + metoclopramide 1000/10 mg, n = 302 Sumatriptan 100 mg, n = 305 |
|
| Outcomes | Headache relief at 2 h 24 h sustained relief Relief of nausea, vomiting, photophobia/phonophobia Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated |
| Allocation concealment? | Yes | Investigators provided with a sealed envelope for each patient - to be returned unopened at the end of the trial, or patient discontinued from trial |
| Blinding? All outcomes |
Yes | Each active treatment had a matched placebo tablet (triple dummy) |
| Methods | Multicentre, R, DB, parallel-group Three attacks treated with single doses of the same medication. Rescue medication after 4 hours if necessary |
|
| Participants | Migraine ± aura (diagnostic criteria not reported). Aged 20 to 65 years. Duration of condition > 1 year. Frequency ≥ 1 every 8 weeks. Excluded: prophylactic treatment within 2 weeks, IHD, chronic analgesic use for other condition N = 721 M 124, F 597 Mean age 40 years |
|
| Interventions | Paracetamol + metoclopramide 1000/10 mg, n = 373 Sumatriptan 100 mg, n = 348 |
|
| Outcomes | Headache relief at 2 h 24 h sustained relief Relief of photophobia/phonophobia Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated |
| Allocation concealment? | Yes | Investigators provided with a sealed envelope for each patient - to be returned unopened at the end of the trial, or patient discontinued from trial |
| Blinding? All outcomes |
Yes | Each active treatment had a matched placebo tablet (triple dummy) |
| Methods | Multicentre, R, DB, PC and AC, four-way cross-over Four consecutive attacks treated with single doses of each test medication |
|
| Participants | “Simple” migraine (criteria of Soyka). Aged 18-70 years (included one patient aged 75 years). Prior attacks uniform with regard to headache intensity and duration N = 288 (completed 4 attacks M 55, F 233 Mean age 42 years |
|
| Interventions | Paracetamol 1000 mg Dihydroergotamine 2 mg Paracetamol + dihydroergotamine 1000/2 mg Placebo |
|
| Outcomes | Pain-free at 2 h Headache relief at 1 h and 2 h ≤ 50% pain relief at 1 h and 2 h Presence of nausea, vomiting, photophobia, phonophobia Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “randomised order in the form of a Latin square” |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Unclear | Not described |
| Methods | Multicentre, R, DB, PC, parallel-group Single attack treated with a single dose of study medication. Medication taken when pain was moderate or severe. Rescue medication after 2 h if necessary Assessments at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6 h |
|
| Participants | Migraine ± aura (IHS 1988). Aged ≥ 18 years. Frequency 0.5 to 6 per month. Untreated severity ≥ moderate. Excluded: require bedrest for >50%, or vomiting with >2 0% of attacks N = 289 M 58, F 231 Mean age 37 years 15% with aura |
|
| Interventions | Paracetamol 1000 mg, n = 147 Placebo, n = 142 |
|
| Outcomes | Pain-free at 2 h Headache relief at 1 h and 2 h Relief of nausea, photophobia, phonophobia, functional disability Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “computer-generated randomization schedule” |
| Allocation concealment? | Yes | “all information kept in a blinded form until after database was locked” |
| Blinding? All outcomes |
Yes | “identical appearing placebo tablets” |
| Methods | R, DB, PC, cross-over Three attacks treated, each with a single dose of each of three study medications. Medication taken at onset of attack, repeated every 4 h (maximum four doses). Rescue medication after 1 h if necessary Severity assessed at 0, 0.5, 1, 2, 4, 8, 24 h |
|
| Participants | Migraine ± aura (IHS 1988). Aged 18 to 70 years N= 58 M8, F 50 Median age 43 years Aura: 43% |
|
| Interventions | Paracetamol + domperidone 1000/20 mg, n = 44 Paracetamol + domperidone 1000/30 mg, n = 46 Paracetamol 1000 mg + placebo, n = 44 |
|
| Outcomes | Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “predetermined randomized code” |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “matched placebo” |
| Methods | R, DB, AC, cross-over Two attacks treated, each with a single dose of each study medication. Mediaction taken at onset. Rescue medication (usual) after 3 h if necessary |
|
| Participants | Migraine (Ad Hoc criteria). Aged 18 to 70 years. Frequency 1 to 6 attacks per month N = 149 (116 used both treatments) M 16, F 100 Mean age 45 years |
|
| Interventions | Paracetamol 1000 mg, n = 116 Tolfenamic acid 400 mg, n = 116 |
|
| Outcomes | Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “identical sealed packages containing two capsules of each drug” |
| Methods | Multicentre, R, DB, PC, parallel-group Single attack treated with a single dose of study medication. Medication taken when pain was moderate or severe. Rescue medication after 2 h if necessary Assessments at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6 h |
|
| Participants | Episodic migraine ± aura (IHS 2004). Age ≥ 18 years. History of 0.5 to 6 attacks/month in past year and previous treatment with OTC medication. Untreated severity ≥ moderate. Excluded: require bedrest for > 50%, or vomiting with > 20% of attacks N = 346 M 58, F 288 Mean age 39 years 22% with aura |
|
| Interventions | Paracetamol 1000 mg, n = 177 Placebo, n = 169 Prophylactic medication continued unchanged |
|
| Outcomes | Pain-free at 2 h Headache relief at 1 h and 2 h Relief of nausea, photophobia, phonophobia, functional disability Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “computer-generated randomization code” |
| Allocation concealment? | Yes | “blister cards with winged 2-piece code label including treatment assignment according to sponsor-generated randomization code” |
| Blinding? All outcomes |
Yes | “tablets identical in shape, size and color” |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Diamond 1976 | No usable single dose data. No diagnostic criteria. |
| Diener 2005 | Mixed migraine and tension headache. |
| Larsen 1990 | Up to 8 attacks treated/participant, but total number of attacks/treatment not reported, so denominator unknown |
DATA AND ANALYSES
Comparison 1. Paracetamol 1000 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain-free at 2 hours | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1. 1 Moderate/severe pain | 3 | 717 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [1.24, 2.62] |
| 1.2 Onset of attack | 1 | 576 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [1.04, 1.80] |
| 2 Headache relief at 1 hour | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate/severe pain | 2 | 635 | Risk Ratio (M-H, Fixed, 95% CI) | 1.97 [1.52, 2.55] |
| 3 Headache relief at 2 hours | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Moderate/severe pain | 3 | 717 | Risk Ratio (M-H, Fixed, 95% CI) | 1.55 [1.32, 1.83] |
| 3.2 Onset of attack | 1 | 576 | Risk Ratio (M-H, Fixed, 95% CI) | 1.95 [1.47, 2.57] |
| 4 Relief of nausea at 2 hours | 2 | 536 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [1.17, 1.61] |
| 5 Relief of photophobia at 2 hours | 3 | 985 | Risk Ratio (M-H, Fixed, 95% CI) | 1.40 [1.19, 1.64] |
| 6 Relief of phonophobia at 2 hours | 3 | 944 | Risk Ratio (M-H, Fixed, 95% CI) | 1.42 [1.21, 1.67] |
| 7 Any functional disability at 2 hours | 3 | 717 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 8 Relief of functional disability at 2 hours | 2 | 610 | Risk Ratio (M-H, Fixed, 95% CI) | 1.76 [1.24, 2.48] |
| 9 Use of rescue medication at 6 h | 2 | 635 | Risk Ratio (M-H, Fixed, 95% CI) | 0.59 [0.47, 0.74] |
| 10 Any adverse event | 4 | 1293 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.64, 0.95] |
Comparison 2. Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 hours | 2 | 1140 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 2 Relief of light/noise sensitivity at 2 hours | 2 | 1001 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.85, 1.21] |
| 3 Use of rescue medication at 24 h | 2 | 1243 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [1.01, 1.36] |
| 4 Any adverse event | 2 | 1328 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.53, 0.71] |
| 5 Major adverse event | 2 | 1328 | Risk Ratio (M-H, Fixed, 95% CI) | 0.50 [0.30, 0.83] |
Analysis 1.1. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 1 Pain-free at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 1 Pain-free at 2 hours
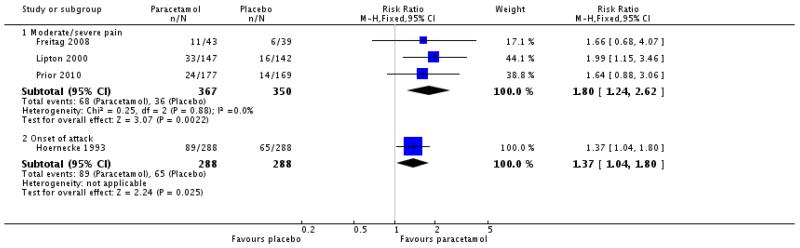
|
Analysis 1.2. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 2 Headache relief at 1 hour.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 2 Headache relief at 1 hour
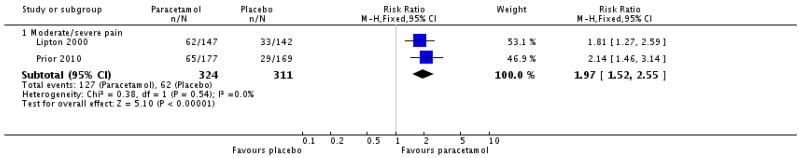
|
Analysis 1.3. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 3 Headache relief at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 3 Headache relief at 2 hours
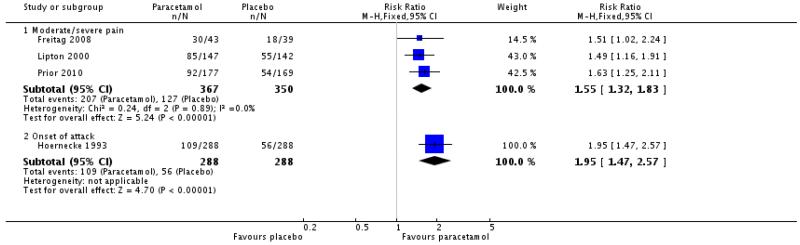
|
Analysis 1.4. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 4 Relief of nausea at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adult
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 4 Relief of nausea at 2 hours
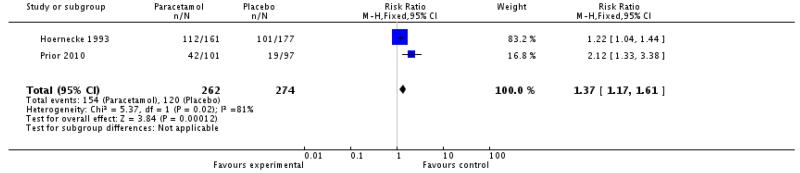
|
Analysis 1.5. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 5 Relief of photophobia at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 5 Relief of photophobia at 2 hours
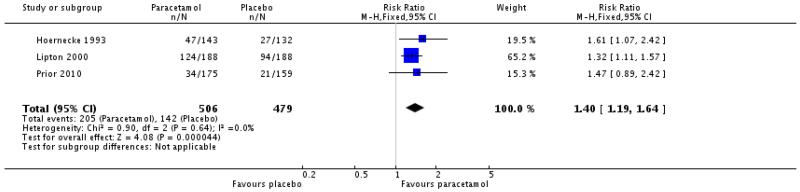
|
Analysis 1.6. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 6 Relief of phonophobia at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 6 Relief of phonophobia at 2 hours
|
Analysis 1.7. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 7 Any functional disability at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache; in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 7 Any functional disability at 2 hours
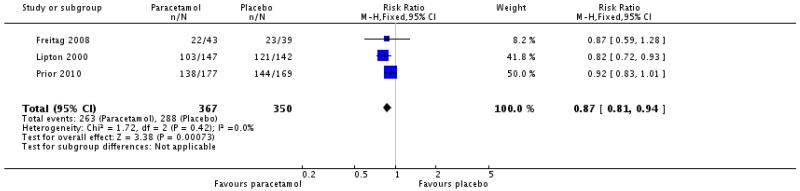
|
Analysis 1.8. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 8 Relief of functional disability at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: B Relief of functional disability at 2 hours
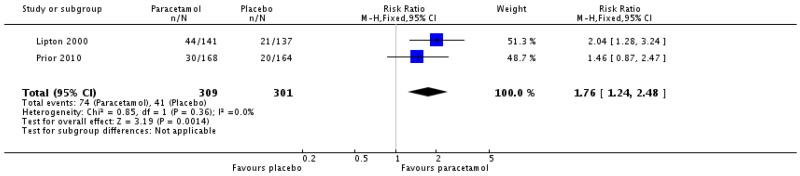
|
Analysis 1.9. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 9 Use of rescue medication at 6 h.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headache in adults
Comparison: Paracetamol 1000 mg versus placebo
Outcome: 9 Use of rescue medication at 6 h
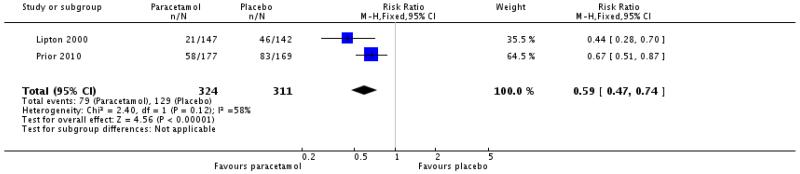
|
Analysis 1.10. Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 10 Any adverse event.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 1 Paracetamol 1000 mg versus placebo
Outcome: 10 Any adverse event
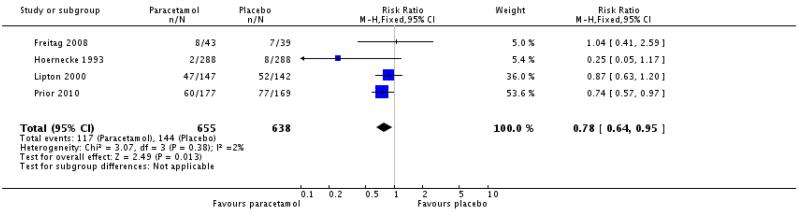
|
Analysis 2.1. Comparison 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg, Outcome 1 Headache relief at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 2 Paracetamol 1000 mg plu metoclopramide 10 mg versus sumatriptan 100 mg
Outcome: 1 Headache relief at 2 hours
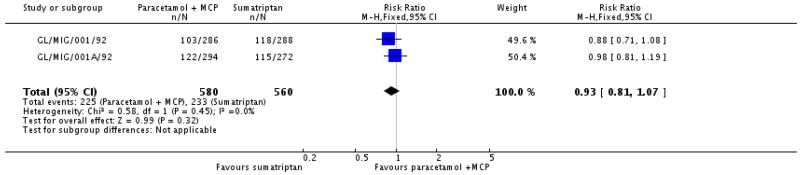
|
Analysis 2.2. Comparison 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg, Outcome 2 Relief of light/noise sensitivity at 2 hours.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg
Outcome: 2 Relief of light/noise sensitivity at 2 hours
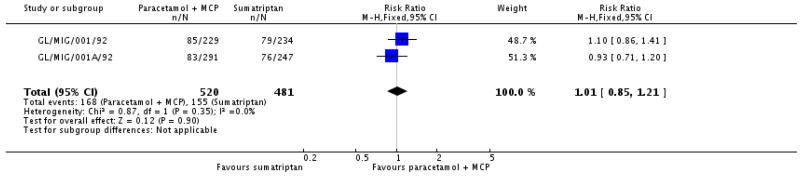
|
Analysis 2.3. Comparison 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg, Outcome 3 Use of rescue medication at 24 h.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg
Outcome: 3 Use of rescue medication at 24 h
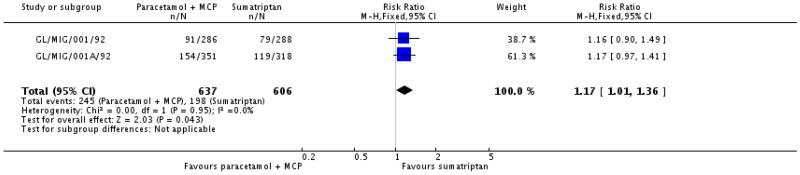
|
Analysis 2.4. Comparison 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg, Outcome 4 Any adverse event.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg
Outcome: 4 Any adverse event
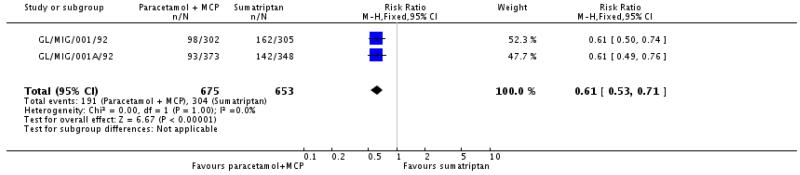
|
Analysis 2.5. Comparison 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg, Outcome 5 Major adverse event.
Review: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults
Comparison: 2 Paracetamol 1000 mg plus metoclopramide 10 mg versus sumatriptan 100 mg
Outcome: 5 Major adverse event
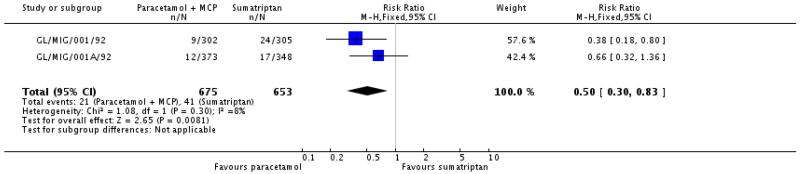
|
HISTORY
Protocol first published: Issue 4, 2009
Review first published: Issue 11, 2010
Appendix 1. Search strategy for MEDLINE (via OVID)
Acetaminophen/
(acetaminophen OR paracetamol OR Paramax OR Migraeflux OR Metomax).mp.
1 OR 2
Headache/OR exp Headache Disorders/
exp Migraine Disorders/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/8-15
3 AND 7 AND 16
Appendix 2. Search strategy for EMBASE (via OVID)
Paracetamol/
(acetaminophen OR paracetamol OR Paramax OR Migraeflux OR Metomax).mp.
1 OR 2
exp Headache and facial pain
exp migraine
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double-blind procedure.sh.
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/8-15
3 AND 7 AND 16
Appendix 3. Search strategy for CENTRAL
MeSH descriptor Acetaminophen
(acetaminophen OR paracetamol OR Paramax OR Migraeflux OR Metomax):ti,ab,kw.
1 OR 2
MeSH descriptor Headache/OR MeSH descriptor Headache Disorders explode all trees
MeSH descriptor Migraine Disorders explode all trees
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw.
4 OR 5 OR 6
Randomized controlled trial:pt
MESH descriptor Double-blind Method
random*:ti,ab,kw.
OR/8-10
3 AND 7 AND 11
Limit 12 to Clinical Trials (CENTRAL)
Appendix 4. Summary of efficacy outcomes: headache relief, pain-free, and use of rescue medication
Appendix 4. Summary of efficacy outcomes: headache relief, pain-free, and use of rescue medication.
| Study ID | Treatment | HR 1 h | HR 2 h | PF 2 h | 24 h SHR | 24 h SPF | Use of rescue medication |
|---|---|---|---|---|---|---|---|
| Dexter 1985 |
|
No data | No data | No data | No data | No data | Over 24 h:
Up to 3 doses of study medication taken |
| Dowson 2000 |
161 participants took medication, 120 completed both attacks and analysed for efficacy |
No data |
|
No data | No data | No data | No data |
| Freitag 2008 |
|
No data |
|
|
|
|
No usable data |
| GL/MIG/001/92 |
|
No data | Moderate/severe baseline pain Attack 1:
After 2nd dose:
Attack 2:
Attack 3:
|
No data | No 24 h data 48 h, any baseline pain: Attack 1:
Attack 3:
|
No data | Over 24 h, any baseline pain: Attack 1:
Attack 2:
Attack 3:
|
| GL/MIG/001A/92 |
|
No data | Moderate/severe baseline pain Attack 1:
Attack 2:
Attack 3:
|
No data | No 24 h data 48 h, any baseline pain: Attack 1:
Attack 2:
Attack 3:
|
No data | Over 24 h, any baseline pain: Attack 1:
Attack 2:
Attack 3:
|
| Hoernecke 1993 |
288 participants completed all four treatments |
No data |
|
Pain free or score of 1 (scale 0-9):
|
No data | No data | Over 2 h:
|
| Lipton 2000 |
|
|
|
|
No data | No data | Over 6 h:
|
| MacGregor 1993 |
46participants treated at least 1 attack, 44 treated all 3 attacks |
No data | No data | No data | No data | No data | Over 24 h:
Up to 4 doses of study medication taken |
| Norrelund 1989 |
116 participants treated both attacks |
No data | No data | No data | No data | No data | No significant difference but paracetamol numerically better than placebo |
| Prior 2010 |
|
|
|
|
No data at 6 h: (1) 98/17, (2)56/169 |
No data at 6 h: (1) 62/17, (2) 35/169 |
Over 6 h:
|
Appendix 5. Summary of efficacy outcomes: migraine-associated symptoms and functional disability
Appendix 5. Summary of efficacy outcomes: migraine-associated symptoms and functional disability.
| Study ID | Treatment | Nausea | Vomitting | Photophobia | Phonophobia | Functional disability | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any at 2h | Relief at 2h | Any at 2h | Relief at 2h | Any at 2h | Relief at 2h | Any at 2h | Relief at 2h | Severe at 2h | Relief at 2h | ||
| Dexter 1985 |
|
No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Dowson 2000 |
161 participants took medication, 120 completed both attacks and analysed for efficacy |
1. 44/120 2. 47/120 |
1. 41/85 2. 34/81 |
1. 6/120 2. 7/120 |
1. 5/11 2. 5/12 |
No data | No data | No data | No data | No data | No data |
| Freitag 2008 |
|
1. 6/43 2. 8/43 3. 4/48 4. 11/39 |
No data | No data | No data | 1. 20/43 2. 11/43 3. 15/48 4. 17/39 |
No data | 1. 17/43 2. 7/43 3. 7/48 4. 13/39 |
No data | 1. 22/43 2. 16/43 3. 17/48 4. 23/39 |
No data |
| GL/MIG/001/92 |
Data for attack 1 |
1. 48/286 2. 53/288 |
1. 44/92 2. 28/81 |
1. 8/286 2. 9/288 |
1. 6/14 2. 5/14 |
Light/noise sensitivity 1. 144/286 2. 155/288 |
Light/noise sensitivity 1. 85/22 2. 979/234 |
No data | No data | ||
| GL/MIG/001A/92 |
Data for attack 1 |
No data | No data | No data | No data | Light/noise sensitivity 1. 208/351 2. 171/318 |
Light/noise sensitivity 1. 83/291 2. 76/247 |
No data | No data | ||
| Hoernecke 1993 |
288 participants completed all four treatments |
1. 49/288 2. 55/288 3. 41/288 4. 76/288 |
1. 112/16 2. 112/18 3. 138/179 4. 101/177 |
1. 12/288 2. 12/288 3. 3/288 4. 13/288 |
1. 20/32 2. 29/41 3. 38/41 4. 30/43 |
1. 65/288 2. 87/288 3. 49/288 4. 94/288 |
1. 124/188 2. 104/191 3. 139/188 4. 94/188 |
1. 59/288 2. 75/288 3. 52/288 4. 87/288 |
1. 120/179 2. 112/187 3. 133/185 4. 90/177 |
No data | No data |
| Lipton 2000 |
|
No sig diff between groups | <2% at baseline | 1. 99/146 2. 115/142 |
1. 47/143 2. 27/132 |
1. 99/146 2. 112/142 |
1. 47/139 2. 30/130 |
1. 103/147 2. 121/142 |
1. 44/141 2. 21/137 |
||
| MacGregor 1993 |
46 participants treated at least 1 attack, 44 treated all 3 attacks |
No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Norrelund 1989 |
116 participants treated both attacks |
No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Prior 2010 |
|
1. 59/177 2. 78/169 |
1. 42/101 2. 19/97 |
No data | No data | 1. 141/177 2. 138/169 |
1. 34/175 2. 21/159 |
1. 125/177 2. 137/169 |
1. 37/162 2. 20/157 |
1. 138/177 2. 144/169 |
1. 30/168 2. 20/164 |
Appendix 6. Summary of outcomes: adverse events and withdrawals
Appendix 6. Summary of outcomes: adverse events and withdrawals.
| Study ID | Treatment | Any AE | Specific AEs | Serious AEs | AE withdrawal | Other withdrawals/exclusions |
|---|---|---|---|---|---|---|
| Dexter 1985 |
|
No “adverse reactions” reported | None | None |
|
Excluded:
|
| Dowson 2000 |
161 participants took medication, 120 completed both attacks and analysed for efficacy |
<15% had “side effects” with either treatment | Dizziness:
Nausea:
Denominator unknown |
None | No data | Excluded: 41 pts took at least one treatment, but did experience a second attack during the study. |
| Freitag 2008 |
|
Before rescue medication:
|
Dizziness: (1) 2/43, (2) 2/43, (3) 5/48, (4) 1/39 Nausea: (1) 0/43, (2) 1/43, (3) 3/48, (4) 2/39 Chest discomfort in 2 placebo pts |
None | None | Excluded: (1) 2 lost to follow-up, (2) 2 lost to follow-up, 1 withdrew consent, (3) 1 lost to follow-up, (4) 3 lost to follow-up, 1 withdrew consent) Discontinued:(1) 3/43, (2)3/43, (3) 6/48, (4) 6/39 No reasons given for discontinuation |
| GL/MIG/001/92 |
Data for all three attacks |
“Minor” + “major” over 48 hours:
|
Most common: Dizziness:
Drowsiness:
Tiredness:
Nausea:
Light headed:
|
“Major” - any serious AE or clinical or laboratory AE leading to withdrawal:
1 pt had chest pain (major AE) with sumatriptan |
|
Withdrawals:
|
| GL/MIG/001A/92 |
Data for all three attacks |
Over 48 h:
|
Most common: Dizziness
Drowsiness
Nausea:
Light headed:
|
“Major” - any serious AE or clinical or laboratory AE leading to withdrawal:
4 pts had chest pain (major AE) with sumatriptan |
|
Withdrawals:
“Other” probably includes pts who did not have 2nd or 3rd attacks - data for 1st attack used for efficacy |
| Hoernecke 1993 |
288 participants completed all four treatments |
Over 2 h:
All transient |
Most common: Tiredness:
Drowsiness:
Nausea:
|
None | None | Excluded: 186 due to missing data or protocol violations Withdrawal after treatment:
Denominator unknown, since number of participants taking each treatment not reported Overall 85 participants discontinued: 72 had non-medical reasons (attacks not frequent enough, study ended), 7 LoE (one withdrew after the combination, 2 each after paracetamol, DHE, and placebo), in 6 cases there were ‘no useful data’ |
| Lipton 2000 |
|
Over 6 h:
|
Digestive system:
Special senses:
Nervous system:
CV system:
|
None | None | Withdrawals:
|
| MacGregor 1993 |
46 participants treated at least 1 attack, 44 treated all 3 attacks |
Over 24 h:
|
|
None | None | Excluded after initial visit: 12/58 pts (10 lost to follow-up, 1 had no qualifying attack, 1 withdrew consent). Unclear whether they took any medication 2 excluded from analyses for (1)and (3) because treated attacks without adequate washout |
| Norrelund 1989 |
116 participants treated both attacks |
Over 3 h:
|
No data | No data | No data | Excluded: 7 had only 1 attack |
| Prior 2010 |
|
Over 6 h:
|
Dizziness:
Nausea:
Vomiting:
|
None |
(nausea, vomiting, sensitivity to to light and noise) |
Withdrawn: (1) 3/177 (1 vomited within 0.5 h, 2 fell asleep), (2) 5/169 (vomited within 0.5 h) |
Appendix 7. Associated symptoms: persistence 2 hours after treatment
Appendix 7. Associated symptoms: persistence 2 hours after treatment.
| Associated symptoms: symptom present 2 hours after taking study medication | ||||||
|---|---|---|---|---|---|---|
| Intervention | Studies | Attacks treated | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNTp (95% CI) |
| Nausea | ||||||
| Paracetamol 1000 mg versus placebo (Freitag 2008; Hoernecke 1993; Prior 2010) | 3 | 1004 | 22 | 33 | 0.67 (0.55 to 0.82) | 9.2 (6.1 to 19) |
| Paracetamol 1000 mg versus placebo (Freitag 2008; Prior 2010) for moderate to severe pain | 2 | 428 | 30 | 43 | 0.69 (0.54 to 0.89) | 7.6 (4.5 to 24) |
| Paracetamol 1000 mg versus dihydroergotamine 2 mg (Hoernecke 1993) | 1 | 576 | 17 | 19 | - | - |
| Paracetamol + domperidone 1000 + 20 mg (Dowson 2000) | 1 | 240 | 37 | 39 | - | - |
| Paracetamol + metoclopramide 1000 + 10 mg (GSK1) | 1 | 574 | 17 | 18 | - | - |
| Photophobia | ||||||
| Paracetamol 1000 mg versus placebo (Freitag 2008; Hoernecke 1993; Lipton 2000; Prior 2010) | 4 | 1292 | 50 | 57 | 0.86 (0.79 to 0.94) | 14 (7.8 to 52) |
| Paracetamol 1000 mg versus placebo (Freitag 2008; Lipton 2000; Prior 2010) for moderate to severe pain | 3 | 716 | 71 | 77 | 0.92 (0.85 to 1.0) | not calculated |
| Paracetamol 1000 mg versus dihydroergotamine 2 mg (Hoernecke 1993) | 1 | 576 | 23 | 30 | ||
| Phonophobia | ||||||
| Paracetamol 1000 mg versus placebo (Freitag 2008; Hoernecke 1993; Lipton 2000; Prior 2010) | 4 | 1292 | 46 | 55 | 0.83 (0.75 to 0.91) | 11 (7.0 to 29) |
| Paracetamol 1000 mg versus placebo (Freitag 2008; Lipton 2000; Prior 2010) for moderate to severe pain | 3 | 716 | 66 | 75 | 0.88 (0.80 to 0.97) | 11 (6.4 to 42) |
| Paracetamol 1000 mg versus dihydroergotamine 2 mg (Hoernecke 1993) | 1 | 576 | 20 | 26 | - | - |
| Combined “light/noise sensitivity” | ||||||
| Paracetamol + metoclopramide 1000 + 10 mg versus sumatriptan 100 mg (1st attack, GL/MIG/001/92; GL/MIG/001A/92) | 2 | 1243 | 55 | 54 | 1.0 (0.92 to 1.1) | not calculated |
| Paracetamol + metoclopramide 1000 + 10 mg versus sumatriptan 100 mg (all 3 attacks, GL/MIG/001/92; GL/MIG/001A/92) | 2 | 3274 | 57 | 52 | 1.1 (1.02 to 1.2) | 20 (12 to 65) |
Footnotes
DECLARATIONS OF INTEREST RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM, HJM and SD have received research support from charities, government and industry sources at various times. Support for this work was from Pain Research Funds, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme. None had any input into the review at any stage.
References to studies included in this review
- Dexter 1985 {published data only} .Dexter SL, Graham AN, Johnston ES, Ratcliffe DM, Wilkinson MI, Rose AJ. Double-blind controlled study of paramax in the acute treatment of common and classical migraine. British Journal of Clinical Practice. 1985;39(10):388–92. [PubMed] [Google Scholar]
- Dowson 2000 {published data only} .Dowson A, Ball K, Haworth D. Comparison of a fixed combination of domperidone and paracetamol (Domperamol) with sumatriptan 50 mg in moderate to severe migraine: a randomised UK primary care study. Current Medical Research and Opinion. 2000;16(3):190–7. [PubMed] [Google Scholar]
- Freitag 2008 {published data only} .Freitag F, Diamond M, Diamond S, Janssen I, Rodgers A, Skobieranda F. Efficacy and tolerability of coadministration of rizatriptan and acetaminophen vs rizatriptan or acetaminophen alone for acute migraine treatment. Headache. 2008;48(6):921–30. doi: 10.1111/j.1526-4610.2007.01053.x. DOI: 10.1111/j.1526-4610.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- GL/MIG/001/92 {unpublished data only} .A double-blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Original protocol) 1992 Available at: www.gsk-clinicalstudyregister.com/
- GL/MIG/001A/92 {unpublished data only} .A double-blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Amended protocol) 1992 Available at: www.gsk-clinicalstudyregister.com/
- Hoernecke 1993 {published data only} .Hoernecke R, Doenicke A. Treatment of migraine attacks: combination of dihydroergotamine tartrate and paracetamol in comparison with individual drugs and placebo [Behandlung des Migraneanfalls: die Kombination Dihydroergotamintartrat und Paracetamol im Vergleich zu den Einzelsubstanzen und Placebo] Medizinische Klinik (Munich) 1993;88(11):642–8. [PubMed] [Google Scholar]
- Lipton 2000 {published data only} .Lipton RB, Baggish JS, Stewart WF, Codispoti JR, Fu M. Efficacy and safety of acetaminophenin the treatment of migraine. Results of a randomized, double-blind, placebo-controlled, population-based study. Archives of Internal Medicine. 2000;160(22):3486–92. doi: 10.1001/archinte.160.22.3486. [DOI] [PubMed] [Google Scholar]
- MacGregor 1993 {published data only} .MacGregor EA, Wilkinson M, Bancroft K. Domperidone plus paracetamol in the treatment of migraine. Cephalalgia. 1993;13(2):124–7. doi: 10.1046/j.1468-2982.1993.1302124.x. [DOI] [PubMed] [Google Scholar]
- Norrelund 1989 {published data only} .Nørrelund N, Christiansen LV, Plantener S. Tolfenamic acid versus paracetamol in migraine attacks. A double-blind study in general practice [Tolfenamsyre versus paracetamol ved migraeneanfald. En dobbeltblind undersogelse i almen praksis] Ugeskrift for Laeger. 1989;151(38):2436–8. [PubMed] [Google Scholar]
- Prior 2010 {published data only} .Prior MJ, Codispoti JR, Fu M. A randomized, placebo-controlled trial of acetaminophen for treatment of migraine headache. Headache. 2010;50(5):819–33. doi: 10.1111/j.1526-4610.2010.01638.x. DOI: 10.1111/j.1526-4610.2010.01638.x. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Diamond 1976 {published data only} .Diamond S. Treatment of migraine with isometheptene, acetaminophen, and dichloralphenazone combination: a double-blind, crossover trial. Headache. 1976;15(4):282–7. doi: 10.1111/j.1526-4610.1976.hed1504282.x. [DOI] [PubMed] [Google Scholar]
- Diener 2005 {published data only} .Diener HC, Pfaffenrath V, Pageler L, Peil H, Aicher B. The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study. Cephalalgia. 2005;25(10):776–87. doi: 10.1111/j.1468-2982.2005.00948.x. DOI: 10.1111/j.1468-2982.2005.00948.x. [DOI] [PubMed] [Google Scholar]
- Larsen 1990 {published data only} .Larsen BH, Christiansen LV, Andersen B, Olesen J. Randomized double-blind comparison of tolfenamic acid and paracetamol in migraine. Acta Neurologica Scandinavica. 1990;81(5):464–7. doi: 10.1111/j.1600-0404.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
Additional references
- Ad Hoc 1962 .Ad Hoc Committee on the Classification of Headache National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA. 1962;179(9):717–8. [Google Scholar]
- Bigal 2008 .Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–66. doi: 10.1212/01.wnl.0000323925.29520.e7. DOI: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- Botting 2000 .Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clinical Infectious Diseases. 2000;31(5):S203–S210. doi: 10.1086/317520. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan 2002 .Chandrasekharan NV. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke 1996 .Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. The Quarterly Journal of Medicine. 1996;89(1):77–84. doi: 10.1093/oxfordjournals.qjmed.a030141. [DOI] [PubMed] [Google Scholar]
- Cook 1995 .Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSM 1997 .Committee on Safety of Medicines Medicines Control Agency. Paracetamol and aspirin. Current Problems in Pharmacovigilance. 1997;23:9. [Google Scholar]
- Dart 2000 .Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. American Journal of Medicine. 2000;7(2):123–34. doi: 10.1097/00045391-200007020-00009. [DOI] [PubMed] [Google Scholar]
- Diamond 2007 .Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47(3):355–63. doi: 10.1111/j.1526-4610.2006.00631.x. DOI: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- Diener 1998 .Diener HC, Kaube H, Limmroth V. A practical guide to the management and prevention of migraine. Drugs. 1998;56(5):811–24. doi: 10.2165/00003495-199856050-00006. [DOI] [PubMed] [Google Scholar]
- Edmeads 1993 .Edmeads J, Findlay H, Tugwell P, Pryse-Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension-type headache on life-style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences. 1993;20(2):131–7. doi: 10.1017/s0317167100047697. [DOI] [PubMed] [Google Scholar]
- Evers 2009 .Evers S, Afra J, Frese A, Goads by PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine - revised report of an EFNS task force. European Journal of Neurology. 2009;16(9):968–81. doi: 10.1111/j.1468-1331.2009.02748.x. DOI: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- Ferrari 1998 .Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics. 1998;13(6):667–76. doi: 10.2165/00019053-199813060-00003. [DOI] [PubMed] [Google Scholar]
- Flower 1972 .Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol) Nature. 1972;240(5381):410–1. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- Friedman 2005 .Friedman BW, Corbo J, Lipton RB, Bijur PE, Esses D, Solorzano C, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463–8. doi: 10.1212/01.WNL.0000150904.28131.DD. DOI: 10.1212/01.WNL.0000150904.28131.DD. [DOI] [PubMed] [Google Scholar]
- Graham 2005 .Graham GG, Scott KF. Mechanism of action of paracetamol. American Journal of Therapeutics. 2005;12(1):46–55. doi: 10.1097/00045391-200501000-00008. DOI: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Gunnell 1997 .Gunnell D, Hawton K, Garnier V, Bismuth C, Fagg J. Use of paracetamol for suicide and non-fatal poisoning in the UK and France: are restrictions on availability justified? Journal of Epidemiology and Community Health. 1997;51(2):175–9. doi: 10.1136/jech.51.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins 2007 .Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Safety. 2007;30(6):465–79. doi: 10.2165/00002018-200730060-00002. [DOI] [PubMed] [Google Scholar]
- Hawton 2001 .Hawton K, Townsend E, Deeks J, Appleby L, Gunnell D, Bennewith O, et al. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ. 2001;322(7296):1203–7. doi: 10.1136/bmj.322.7296.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazard 2009 .Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health. 2009;12(1):55–64. doi: 10.1111/j.1524-4733.2008.00404.x. DOI: 10.1111/j.1524-4733.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- Hinz 2008 .Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. Federation of American Societies for Experimental Biology Journal. 2008;22(2):383–90. doi: 10.1096/fj.07-8506com. DOI: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- Hu 1999 .Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States:disability and economic costs. Archives of Internal Medicine. 1999;159(8):813–8. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- IHS 1988 .Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- IHS 2000 .International Headache Society Clinical Trials Subcommittee Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20(9):765–86. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- IHS 2004 .Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Jadad 1996a .Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain. 1996;66(2-3):239–46. doi: 10.1016/0304-3959(96)03033-3. [DOI] [PubMed] [Google Scholar]
- Jadad 1996b .Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jhingran 1996 .Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health-related quality of life with sumatriptan treatment for migraine. Journal of Family Practice. 1996;42(1):36–42. [PubMed] [Google Scholar]
- L’Abbe 1987 .L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine. 1987;107(2):224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Lipton 1999 .Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache. 1999;39(Suppl 2):S20–S26. [Google Scholar]
- Lipton 2007 .Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Lofland 1999 .Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine. 1999;159(8):857–63. doi: 10.1001/archinte.159.8.857. [DOI] [PubMed] [Google Scholar]
- Lucas 2006 .Lucas C, Géraud G, Valade D, Chautard MH, Lantéri-Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population-based survey. Headache. 2006;46(5):715–25. doi: 10.1111/j.1526-4610.2006.00430.x. DOI: 10.1111/j.1526-4610.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- Moens 2007 .Moens G, Johannik K, Verbeek C, Bulterys S. The prevalence and characteristics of migraine among the Belgian working population. Acta Neurologica Belgica. 2007;107(3):84–90. [PubMed] [Google Scholar]
- Moore 1998 .Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78(3):209–16. doi: 10.1016/S0304-3959(98)00140-7. DOI: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Morris 1995 .Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors. Statistics with confidence - confidence intervals and statistical guidelines. British Medical Journal; London: 1995. pp. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris 2008 .Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Current Opinion in Gastroenterology. 2008;24(3):287–97. doi: 10.1097/MOG.0b013e3282f9764b. [DOI] [PubMed] [Google Scholar]
- Oldman 2002 .Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain. 2002;97(3):247–57. doi: 10.1016/S0304-3959(02)00024-6. DOI: 10.1016/S0304-3959(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Osterhaus 1994 .Osterhaus JT, Townsend RJ, Gandek B, Ware JE., Jr Measuring the functional status and well-being of patients with migraine headache. Headache. 1994;34(6):337–43. doi: 10.1111/j.1526-4610.1994.hed3406337.x. [DOI] [PubMed] [Google Scholar]
- PIC 2009 .PharmWeb . Paracetamol Information Centre: [accessed 30 July 2009]. www.pharmweb.net/pwmirror/pwy/paracetamol/pharmwebpic.html [Google Scholar]
- Prescott 2000 .Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction? American Journal of Therapeutics. 2000;7(2):99–114. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]
- Radtke 2009 .Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache. 2009;49(1):79–89. doi: 10.1111/j.1526-4610.2008.01263.x. DOI: 10.1111/j.1526-4610.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- Ross-Lee 1983 .Ross-Lee LM, Eadie MJ, Heazlewood V, Bochner F, Tyrer JH. Aspirin pharmacokinetics in migraine. The effect of metoclopramide. European Journal of Clinical Pharmacology. 1983;24(6):777–85. doi: 10.1007/BF00607087. [DOI] [PubMed] [Google Scholar]
- Salazar-Tortolero 2008 .Salazar-Tortolero G, Huertas-Campistol A, Vergez-Pinto L, Ramos-Brunet A, Lluch-López J. Metoclopramide as a painkiller for intense migraine headache in emergency departments [Metoclopramida como analgésicoen la cefalea migrañosa intensa en urgencias] Revista de Neurologia. 2008;47(10):506–8. [PubMed] [Google Scholar]
- Schwab 2003 .Schwab JM, Schluesener HJ, Laufer S. COX-3: just another COX or the solitary elusive target of paracetamol? Lancet. 2003;361(9362):981–2. doi: 10.1016/S0140-6736(03)12841-3. [DOI] [PubMed] [Google Scholar]
- Solomon 1997 .Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics. 1997;11(Suppl 1):1–10. doi: 10.2165/00019053-199700111-00003. [DOI] [PubMed] [Google Scholar]
- Soyka 1988 .Soyka D. Diagnosis of migraine [Diagnostik der Migrane] Deutsche Medizinische Wochenschrift. 1988;113(18):735–6. doi: 10.1055/s-2008-1067713. [DOI] [PubMed] [Google Scholar]
- Tramer 1997 .Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta-analysis: a case study. BMJ. 1997;315(7109):635–9. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volans 1974 .Volans GN. Absorption of effervescent aspirin during migraine. British Medical Journal. 1974;4(5939):265–8. doi: 10.1136/bmj.4.5939.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volans 1975 .Volans GN. The effect of metoclopramide on the absorption of effervescent aspirin in migraine. British Journal of Clinical Pharmacology. 1975;2(1):57–63. doi: 10.1111/j.1365-2125.1975.tb00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel 2003 .Wenzel RG, Sarvis CA, Krause ML. Over-the-counter drugs for acute migraine attacks: literature review and recommendations. Pharmacotherapy. 2003;23(4):494–505. doi: 10.1592/phco.23.4.494.32124. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study