Abstract
Background
Topical creams with capsaicin are used to treat pain from a wide range of chronic conditions including neuropathic pain. Following application to the skin capsaicin causes enhanced sensitivity to noxious stimuli, followed by a period with reduced sensitivity and, after repeated applications, persistent desensitisation. There is uncertainty about the efficacy and tolerability of capsaicin for treating painful chronic neuropathies.
Objectives
To review the evidence from controlled trials on the efficacy and tolerability of topically applied capsaicin in chronic neuropathic pain in adults.
Search methods
Cochrane CENTRAL, MEDLINE, EMBASE and Oxford Pain Relief Database, searched in May 2009.
Selection criteria
Randomised, double blind, placebo controlled studies of at least six weeks’ duration, using topical capsaicin to treat neuropathic pain.
Data collection and analysis
Two review authors independently assessed trial quality and validity, and extracted data. Information was extracted on numbers of participants with pain relief (clinical improvement) after at least six weeks, and with local skin reactions, and used to calculate relative risk and numbers needed to treat to benefit (NNT) and harm (NNH). Details of definition of pain relief and specific adverse events were sought.
Main results
Six studies (389 participants in total) compared regular application of low dose (0.075%) capsaicin cream with placebo cream; the NNT for any pain relief over six to eight weeks was 6.6 (4.1 to 17). Two studies (709 participants in total) compared a single application of high dose (8%) capsaicin patch with placebo patch; the NNT for ≥ 30% pain relief over twelve weeks was 12 (6.4 to 70). Local skin reactions were more common with capsaicin, usually tolerable, and attenuated with time; the NNH for repeated low dose application was 2.5 (2.1 to 3.1). There were insufficient data to analyse either data set by condition or outcome definition. All studies satisfied minimum criteria for quality and validity, but maintenance of blinding remains a potential problem.
Authors’ conclusions
Capsaicin, either as repeated application of a low dose (0.075%) cream, or a single application of a high dose (8%) patch may provide a degree of pain relief to some patients with painful neuropathic conditions. Local skin irritation, which is often mild and transient but may lead to withdrawal, is common. Systemic adverse effects are rare. Estimates of benefit and harm are not robust due to limited amounts of data for different neuropathic conditions and inconsistent outcome definition.
Medical Subject Headings (MeSH): Administration, Topical; Analgesics [*administration & dosage; adverse effects]; Capsaicin [*administration & dosage; adverse effects]; Chronic Disease; Diabetic Neuropathies [drug therapy]; HIV Infections [complications]; Neuralgia [*drug therapy]; Neuralgia, Postherpetic [drug therapy]; Ointments; Pain, Postoperative [drug therapy]
MeSH check words: Adult, Humans
BACKGROUND
Description of the condition
Neuropathic pain occurs as a consequence of damage to the central nervous system (CNS) (e.g. cerebrovascular accident, multiple sclerosis or spinal cord injury) or peripheral nervous system (PNS) (e.g. painful diabetic neuropathy (PDN), postherpetic neuralgia (PHN), surgery). Topical agents are most likely to be used for localised, peripheral neuropathies. This review includes studies in which participants had experienced neuropathic pain for at least three months.
Description of the intervention
Topical medications are applied externally and are taken up through the skin. They exert their effects close to the site of application, and there is no substantial systemic uptake or distribution. This compares with transdermal application, where the medication is applied externally and is taken up through the skin, but relies on systemic distribution for its effect.
Topical creams with capsaicin are used to treat pain from a wide range of chronic conditions including postherpetic neuralgia and diabetic neuropathy, osteoarthritis, and rheumatoid arthritis, in addition to pruritus, psoriasis, mastectomy pain, and cluster headaches (Reynolds 1999). Capsaicin is available in the United Kingdom on prescription only but, according to the British National Formulary, may be present in small quantities in topical rubefacients (a substance for external application producing redness of the skin, or erythema) sold through pharmacies. It is also available over the counter in some other countries. In England in 2007 there were almost 149,000 prescriptions for topical capsaicin products (PCA 2007).
How the intervention might work
Capsaicin is the active compound present in chilli peppers, responsible for making them hot when eaten. It binds to nociceptors (sensory receptors responsible for sending signals that cause the perception of pain) in the skin, releasing proinflammatory neuropeptides, such as substance P, which cause neurogenic inflammation. There is an initial excitation of the neurons and a period of enhanced sensitivity to noxious stimuli, usually perceived as itching, pricking or burning sensations. Enhanced sensitivity is followed by a refractory (unresponsive) period with reduced sensitivity and, after repeated applications, persistent desensitisation. The mechanisms of desensitisation remain unclear. It is thought that degenerative changes in the primary sensory neuron result in loss of peripheral or central nociceptor-specific macromolecules and receptors, and that these degenerative changes can affect distant parts of the primary sensory neuron (Jancso 2008). The resulting reduction of sensation is associated with degeneration of epidermal nerve fibres, which is at least partially reversible (Nagy 2004; Nolano 1999; Simone 1998). It is the theoretical ability of capsaicin to desensitise nociceptors that is exploited for therapeutic pain relief (Holzer 2008).
Adverse events from capsaicin are mainly at the application site (burning, stinging, erythema), and systemic events are rare. Achieving double blind conditions in placebo controlled trials using capsaicin can therefore be difficult. Respiratory irritation has also been reported from inhalation of the dried cream (Rains 1995).
Why it is important to do this review
A review in 2004 (Mason 2004) found only a limited amount of information for limited efficacy in pain relief, but suggested that capsaicin might be a useful adjunct or used as a sole therapy for some patients unresponsive to, or intolerant of, other treatments. Advances in the formulation of preparations of capsaicin for skin application together with a better understanding of trial methodology may mean that more information is now available. A review of the current evidence for the efficacy of topical capsaicin for chronic neuropathic pain in adults is needed for purchasers of healthcare, prescribers and consumers to make informed choices about their use.
OBJECTIVES
To review the evidence from controlled trials on the efficacy and tolerability of topically applied capsaicin in neuropathic pain in adults.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled double blind trials comparing topical capsaicin with placebo or other active treatment for neuropathic pain, with at least 10 participants per treatment arm. Studies published only as abstracts or studying experimentally induced pain were excluded.
Types of participants
Adult participants (16 years or more) with neuropathic pain of at least moderate intensity (Collins 1997) resulting from any cause, with a duration of at least three months and as defined in the study using accepted diagnostic criteria.
Types of interventions
Included studies had at least one treatment arm using topical capsaicin, and a comparator arm using placebo or other active treatment. Treatment had to be applied three to four times daily for a minimum of six weeks in studies using low dose (< 0.1%) formulations. In studies using high dose formulations (8%) a single application was accepted.
Types of outcome measures
Information was sought on participant characteristics: age, sex, and condition treated.
Primary outcomes
The primary outcome was “clinical improvement”, defined as at least a 50% reduction in pain, or equivalent measure such as a “very good” or “excellent” global assessment of treatment, or “none” or “slight” pain on rest or movement, measured on a categorical scale (Moore 1998a). The following hierarchy of outcomes, in order of preference, was used to extract data for the primary outcome:
patient reported reduction in pain of at least 50%;
patient reported global assessment of treatment;
pain on movement;
pain on rest or spontaneous pain;
undefined “improvement”.
Physician or investigator reported outcomes of efficacy was not used.
Secondary outcomes
Secondary outcomes sought were:
numbers of participants with adverse events: local and systemic, and cough;
numbers of withdrawals: all cause, lack of efficacy and adverse events.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported as close to eight weeks as possible, but not less than six weeks. Where longer duration outcomes were available these were also extracted. We also anticipated that reporting of adverse events would vary between trials with regard to the terminology used, method of ascertainment, and categories that are reported (e.g. occurring in at least 5% of patients or where there is a statistically significant difference between treatment groups). Care was be taken to identify these details.
Search methods for identification of studies
Electronic searches
The following databases were searched:
MEDLINE via Ovid (May 2009);
EMBASE via Ovid (May 2009);
Cochrane CENTRAL (issue 2, 2009);
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, and Appendix 3 for the CENTRAL search strategy.
There was no language restriction.
Searching other resources
Reference lists of review articles and included studies were also searched. Manufacturers have previously (2004) been asked for details of unpublished studies. The manufacturers of a new high dose capsaicin patch have been asked for details of unpublished data, but it is unlikely that any will be released until pending approvals from the US Food and Drug Administration and European Medicines Agency are resolved.
Data collection and analysis
Two review authors independently selected the studies for inclusion, assessed methodological quality and study validity, and extracted data. Disagreements were resolved through discussion.
Selection of studies
Titles and abstracts of studies identified by the searches were reviewed on screen to eliminate those that clearly did not satisfy inclusion criteria. Full reports of the remaining studies were obtained to determine inclusion in the review. Crossover trials were considered only if data from the first treatment period was reported separately. Studies in oral, ocular or buccal diseases, and in musculoskeletal conditions were excluded.
Assessment of methodological quality
Included studies were assessed for quality using the five-point Oxford Quality Scale (Jadad 1996b) that considers randomisation, blinding, and study withdrawals and dropouts. Study validity was assessed using a 16-point scale (Smith 2000).
Data extraction
Information on participants, interventions, and outcomes from the original reports was abstracted into a standard data extraction form. Authors were not contacted for further information.
Data analysis
For efficacy we planned to use data from all randomised participants who fulfilled inclusion criteria and received at least one dose of study medication. Participants contributing no efficacy data were assumed to have had no benefit. For safety, we planned to use data from all randomised participants who received at least one dose of study medication.
Where appropriate, relative benefit (RB) and risk (RR) estimates were calculated with 95% confidence intervals (CI) using a fixed-effect model (Morris 1995). NNT and number needed to treat to harm (NNH) and 95% CIs were calculated using the pooled number of events, using the method devised by Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the RR or RB did not include the number one. Homogeneity was examined visually using L’Abbé plots (L’Abbe 1987).
Sensitivity analyses for the primary outcome were planned for:
diagnosis of neuropathic pain;
high versus low quality (two versus three or more) and validity (eight or less versus nine or more) scores;
study size (39 or less versus 40 or more participants per treatment arm);
outcome (undefined ‘improvement’ versus others);
dose.
At least 200 participants had to be available in any of these different contexts before information was pooled (Moore 1998b).
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Nine studies, with 1600 participants in total, fulfilled the entry criteria (Backonja 2008; Bernstein 1989; Biesbroeck 1995; CSG 1992; Ellison 1997; Low 1995; Simpson 2008; Watson 1992; Watson 1993). Details of included studies are in the ‘Characteristics of included studies’ table. Four studies identified (Basha 1991; Donofrio 1991; Scheffler 1991; Tandan 1992) were reports of parts of a multicentre study (CSG 1992), only one of which (Tandan 1992, 22 participants) provided any additional data. Eleven other studies were excluded after reading the full report (Chad 1990; Fusco 1992; McCarty 1994; McCleane 2000; Paice 2000; Peikert 1991; Pfeifer 1993; Robbins 1998; Vickers 1998; Watson 1988; Watson 1989). Reasons for exclusion are in the ‘Characteristics of excluded studies’ table.
Neuropathic pain conditions studied were postherpetic neuralgia, diabetic neuropathy, HIV neuropathy, postmastectomy pain, and postsurgical cancer pain. In all studies pain was of at least moderate severity and was frequently unresponsive to, or poorly controlled by, conventional therapy.
Capsaicin 0.075% in a cream base was used by 449 participants in seven studies, and was applied four times daily to sites of neuropathic pain for six (Bernstein 1989; Watson 1992; Watson 1993), eight (CSG 1992; Ellison 1997) or 12 weeks (Low 1995). A total of 325 participants received placebo cream, and 117 received the active comparator oral amitriptyline, in these studies.
Two studies (Backonja 2008; Simpson 2008) used a single dose of 8% capsaicin applied with a patch covering the painful site that was left in place for 30 to 90 minutes. This high concentration of capsaicin was expected to cause intense burning sensations, so the area was pretreated with topical local anaesthetic cream for 60 minutes before application of the patch. A total of 431 participants were treated with capsaicin patch and 278 with control patch. Participants were followed up for 12 weeks.
Because application of capsaicin to the skin initially causes redness and a burning or stinging sensation in many individuals, maintaining the double blind status of studies is problematic. Two studies using low dose (0.075%) capsaicin (Biesbroeck 1995; Low 1995) included methyl nicotinate (a rubefacient, without established analgesic effects) in the first tube of placebo cream to mimic these side effects. The two studies using high dose (8%) capsaicin patch (Backonja 2008; Simpson 2008) used a low dose (0.04%) of capsaicin in the control patch to produce some degree of skin irritation without effective analgesia. The study using amitriptyline as an active control (Biesbroeck 1995) included in the placebo capsules benztropine to mimic dry mouth, and for the first two weeks diazepam to mimic sedation; these are expected side effects of amitriptyline.
Eight studies allowed concomitant oral or transdermal drugs for neuropathic pain without change in dose or frequency (Backonja 2008; Bernstein 1989; Biesbroeck 1995; CSG 1992; Ellison 1997; Simpson 2008; Watson 1992; Watson 1993) but all topical medications were discontinued at least seven days before the study. One study did not mention concomitant medication (Low 1995). One study (Low 1995) included only participants with bilateral symmetric chronic painful peripheral neuropathy and participants were randomised by side, left versus right leg.
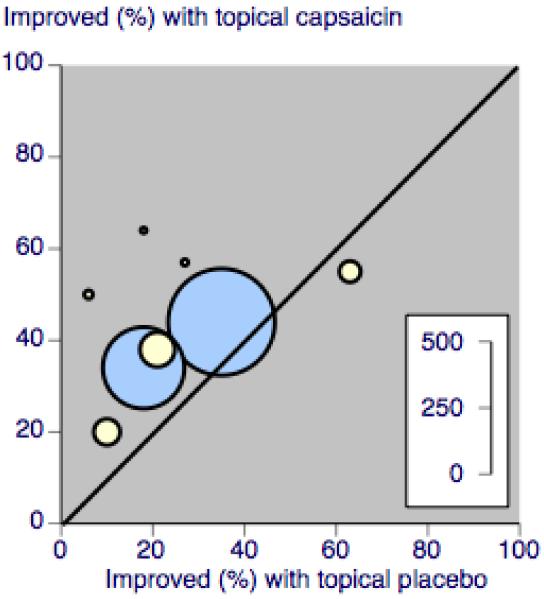
A L’Abbé plot (Figure 1) shows considerable scatter for the studies using multiple applications of low dose capsaicin, which may be accounted for by random chance due to the small numbers of participants in these studies, but may also reflect clinical heterogeneity in the neuropathic pain conditions studied.
Figure 1. L’Abbé plot showing response rates in studies using multiple application low dose capsaicin (yellow) and single application high dose capsaicin (blue). Size of circle is proportional to size of study (inset scale).
Risk of bias in included studies
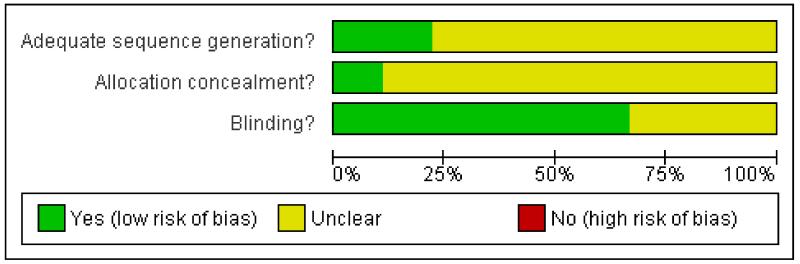
Each study was scored for methodological quality. One study was given a quality score of five (Backonja 2008), two a score of four (Bernstein 1989; Biesbroeck 1995), and six a score of three (CSG 1992; Ellison 1997; Low 1995; Simpson 2008; Watson 1992; Watson 1993). Points were lost for failure to provide adequate details of the methods of randomisation and blinding, and of withdrawals.
Each study was also scored for validity. All scored greater than 9/16 (range 11 to 16), indicating adequate study validity.
The risk of bias table has been completed for all studies for sequence generation, allocation concealment and blinding.
Full details can be found in the ‘Characteristics of included studies’ table, and Figure 2.
Figure 2. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Details of study efficacy outcomes are in Table 1, and details of adverse events and withdrawals are in Table 2.
Number of participants achieving clinical improvement
Six studies compared regular application of low dose (0.075%) capsaicin cream with placebo cream. Only one of the studies (Watson 1992) used the achievement of at least 50% pain relief as a primary outcome measure. One study (Bernstein 1989) reported the proportion of participants achieving at least 40% pain relief, and another (Ellison 1997) reported the proportion of participants with a decreased pain score of at least 75%, while Tandan 1992 (part of CSG 1992) reported pain reduction by at least one category, and Low 1995 reported the proportion of participants with unspecified improvement. These studies were pooled to determine clinical efficacy. Watson 1993 reported the percentage of participants with unspecified improvement, but did not provide the number in each treatment arm after post-randomisation exclusions for failure to meet entry criteria and protocol violations. For the purposes of this review it was assumed that the 12 participants not included were equally distributed between the two groups. A large multicentre study (CSG 1992) reported only mean results for pain relief, but one single centre report from that study did provide dichotomous data (Tandan 1992).
Two studies compared a single application of high dose (8%) capsaicin patch with control patch containing very low dose (0.04%) capsaicin patch (Backonja 2008; Simpson 2008). These studies reported the number of participants with at least 30% pain relief during weeks 2 to 12, based on average pain for each 24 hour period of the study, and were pooled for analysis.
For the purposes of efficacy analysis, control treatments containing small quantities of ingredients to mimic side effects of capsaicin were treated as placebos since quantities of active ingredients were not expected to have any analgesic effects.
Only one active controlled trial with 237 participants (Biesbroeck 1995) was included in this review and it reported only mean data for efficacy. There were insufficient data to draw any conclusions concerning relative efficacy for alternative therapy.
Topical capsaicin 0.075% versus placebo, multiple dose
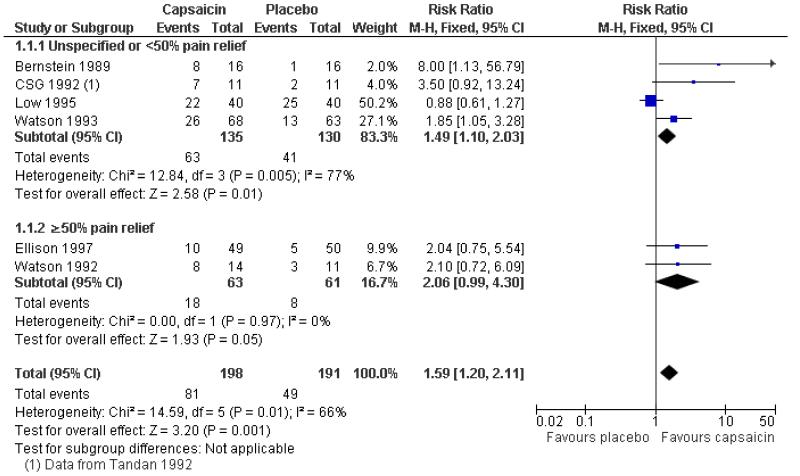
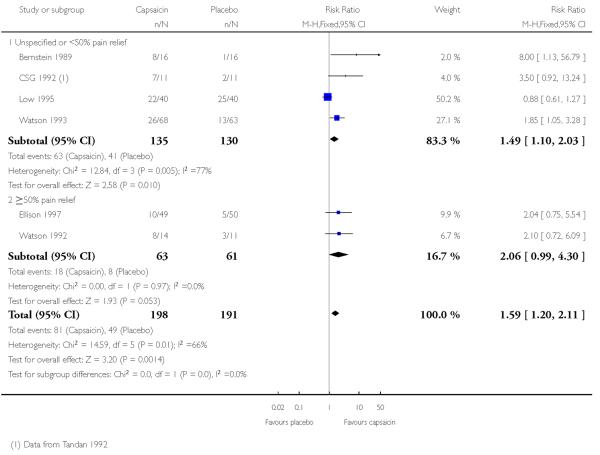
Six studies provided dichotomous outcome data (Bernstein 1989; Ellison 1997; Low 1995; Tandan 1992 (CSG 1992); Watson 1992; Watson 1993): 198 participants were treated with topical capsaicin and 191 with placebo (Figure 3 - see total).
The proportion of participants experiencing successful treatment with capsaicin (0.075%) was 41% (81/198, range 20% to 64%);
The proportion of participants experiencing successful treatment with placebo was 26% (49/191, range 6% to 63%);
The RB of treatment compared with placebo was 1.6 (1.2 to 2.1);
The NNT for successful treatment over 6 to 8 weeks was 6.6 (4.1 to 17). For every seven participants treated with topical capsaicin, one would experience successful treatment who would not have done so with placebo.
Figure 3. Forest plot of comparison: 1 0.075% capsaicin versus placebo, outcome: 1.1 Clinical success.
One multicentre study (CSG 1992), with 277 participants, reported the mean percentage decrease in pain intensity over 8 weeks. This was significantly greater for participants using capsaicin (40%) than for those using placebo (28%). For 22 of these participants, dichotomous data was provided in Tandan 1992, and is included in the analysis above.
Topical capsaicin 8% versus placebo, single dose
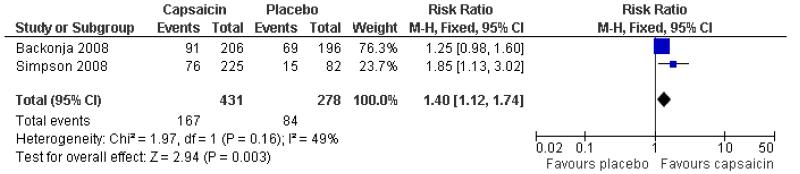
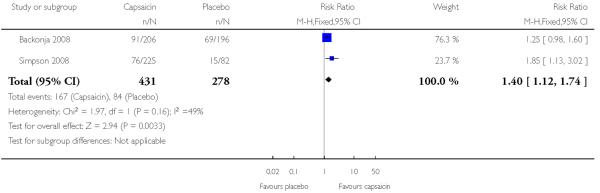
Two studies provided dichotomous outcome data (Backonja 2008; Simpson 2008): 431 participants were treated with capsaicin and 278 with placebo. Simpson 2008 used periods of exposure from 30 min to 90 min, but found no dose response, so data for all exposures are combined for analysis (Figure 4).
The proportion of participants experiencing successful treatment with capsaicin (8%) was 39% (167/431, range 34% to 44%);
The proportion of participants experiencing successful treatment with placebo was 30% (84/278, range 18% to 35%);
The RB of treatment compared with placebo was 1.4 (1.1 to 1.7);
The NNT for successful treatment over 12 weeks was 12 (6.4 to 69). For every 12 participants treated with topical capsaicin, one would experience successful treatment who would not have done so with placebo.
Figure 4. Forest plot of comparison: 2 8% capsaicin versus placebo (single dose), outcome: 2.1 ≥30% pain relief.
There were high levels of statistical heterogeneity in these comparisons, which is likely to be due to the different neuropathic conditions treated, the different outcome measurement and the random play of chance in small studies. Post hoc analysis using a random-effects model did not change the results.
Sensitivity analysis
Outcome definition
For capsaicin 0.075% multiple dose placebo controlled studies, there were insufficient data for participants with ≥ 50% improvement (124 participants) to allow separate analysis and compare with less strict improvements. The four studies (Bernstein 1989; Low 1995; Tandan 1992 (CSG 1992); Watson 1993) with outcomes including less than 50% improvement included 265 participants and gave a RR of 1.5 (1.1 to 2.0) and NNT of 6.6 (3.7 to 28) (Figure 3). This does not differ from the estimate for all studies regardless of outcome definition. With such limited data, particularly for the higher level of pain relief, it is difficult to draw conclusions about the effect of outcome definition.
One of the studies using the high dose patch reported no significant difference between groups for the outcome of at least 50% pain relief for weeks 2 to 12 (Backonja 2008).
There were insufficient data to allow sensitivity analyses for neuropathic pain condition, study quality and validity, or study size. The effect of dose of capsaicin could not be investigated because although two different doses were used, studies for each dose used very different designs that prevented direct comparison.
Adverse events
Reporting of adverse events was inconsistent and incomplete (Table 2). One study (CSG 1992) specified that side effects of concurrent systemic medication were not reported, and Biesbroeck 1995, in which capsaicin was compared to amitriptyline, specified that adverse events were excluded if they were judged likely to be caused by the mimicking agents or unrelated to either drug. Other studies said that they recorded only “adverse drug experiences”, or “side effects” or “adverse reactions to study medication”. The two high dose studies (Backonja 2008; Simpson 2008) reported only those adverse events occurring in at least 3% and at least 2% respectively of participants in either treatment arm. Most studies did not report the methods used to collect adverse event data.
Local skin reactions
All the included studies reported on local skin reactions to capsaicin. Generally they were more common with capsaicin than placebo or amitriptyline, appeared early in treatment, and either disappeared or were reduced in frequency and severity after one to two weeks of treatment for studies using multiple doses. For the single dose application the local anaesthetic appears to have helped to prevent, or mask, the worst of the early reactions, with local skin reactions reported as mostly mild to moderate in intensity and transient or self-limiting.
Local skin reactions were variously reported as numbers of participants with individual symptoms, such as “burning”, “stinging”, “erythema”, “redness”, “rash”, “pruritus”, and “itch”, or with combinations of these symptoms, or with “total skin adverse effects”. It was not usually possible to determine the number of participants with any kind of local skin reaction since more than one symptom may appear in an individual participant. We chose to analyse “burning, stinging, erythema and pruritus”, using the number of participants reporting the most common of these symptoms in individual studies. This almost certainly underestimates the number of participants affected by these symptoms.
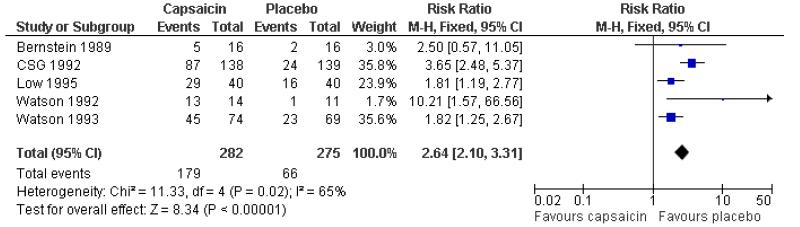
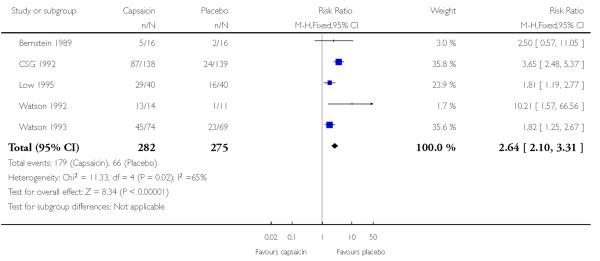
Five studies using multiple low doses of capsaicin contributed data for comparison with placebo (Bernstein 1989; CSG 1992; Low 1995; Watson 1992; Watson 1993) (Figure 5).
The proportion of participants experiencing a local skin reaction with capsaicin (0.075%) was 63% (179/282, range 31% to 93%);
The proportion of participants experiencing a local skin reaction with placebo was 24% (66/275, range 9% to 40%);
The RR of treatment compared with placebo was 2.6 (2.1 to 3.3);
The NNH for experiencing a local skin reaction over 6 to 8 weeks of treatment was 2.5 (2.1 to 3.1).
Figure 5. Forest plot of comparison: 1 0.075% capsaicin versus placebo, outcome: 1.2 Adverse event: burning, stinging, erythema.
Removing the study that included a mimicking agent in the first tube of placebo cream (Low 1995) did not change the result. One study (Ellison 1997) did not provide dichotomous data, but reported that there was more burning, redness and coughing with capsaicin than placebo (P < 0.0001).
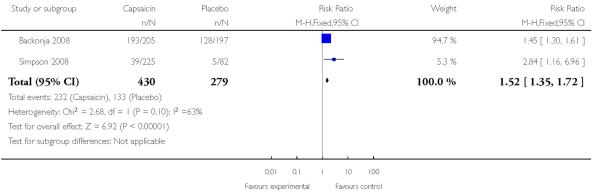
Both studies using single high dose capsaicin (Backonja 2008; Simpson 2008) provided data. Despite being almost identical trials conducted in slightly different populations, the most common event in Backonja 2008 was erythema at 94%, while this was not reported in Simpson 2008, where application site pain, at 21%, was the most common event. It was felt inappropriate to combine these two studies. Both studies reported higher local adverse event rates with capsaicin than with placebo.
Coughing and sneezing
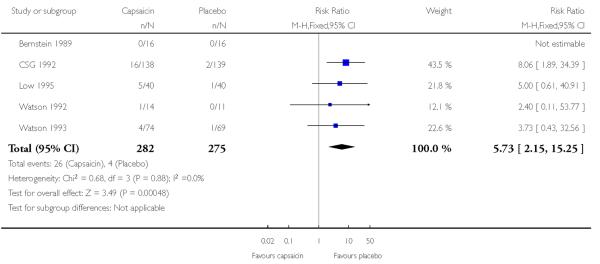
Coughing and sneezing are thought to result from inhalation of particles of dried capsaicin cream. Five studies using multiple low doses of capsaicin contributed data for comparison with placebo (Bernstein 1989; CSG 1992; Low 1995; Watson 1992; Watson 1993; Table 2; Analysis 1.3).
The proportion of participants experiencing coughing or sneezing with capsaicin (0.075%) was 9.2% (26/282, range 0% to 13%);
The proportion of participants experiencing coughing or sneezing with placebo was 1.4% (4/275, range 0% to 3%);
The RR of treatment compared with placebo was 5.7 (2.1 to 15);
The NNH for experiencing coughing or sneezing over 6 to 8 weeks of treatment was 13 (8.7 to 25).
In single dose studies, Backonja 2008 did not report coughing or sneezing, indicating that the incidence was below 3%, and Simpson 2008 reported cough at 2.2% and 1.2% in capsaicin and placebo groups respectively (Table 2). A lower incidence might be expected in these studies due to the single application and careful cleaning of the application site afterwards.
Systemic adverse events
A number of studies did not report any information for systemic adverse events, and in those that did, they were uncommon and did not differ from placebo. No analysis was possible.
Serious adverse events
Serious adverse events were uncommon, and only one was judged possibly related to study medication. This participant was treated with a single dose of 8% capsaicin and experienced increased blood pressure on the day of treatment, which was associated with changes in pain.
Withdrawals
Withdrawals due to adverse events were nearly all due to skin reactions. A total of 37 withdrawals were reported in 242 participants (15%) with 0.075% capsaicin and seven in 235 participants (3%) with placebo in four studies using multiple doses, giving a relative risk of 5.0 (2.3 to 11) and a NNT of 8.1 (5.7 to 14) (Analysis 1.4). Three studies (Biesbroeck 1995; Ellison 1997; Low 1995) did not provide data for comparison. A total of three withdrawals in 430 participants (0.7%) with 8% capsaicin and one in 278 participants (0.4%) with placebo were reported in the two single dose studies. There were insufficient events for further analysis. Withdrawals were more common with repeated low dose application than the single high dose application.
Withdrawals for other reasons were generally poorly reported. Some studies reported withdrawals or exclusions due to protocol violation, non-compliance, treatment failure and other health problems (Table 2). No further statistical analysis of withdrawals was carried out.
See Table 1 for details of efficacy outcomes and Table 2 for details of adverse events and withdrawals.
DISCUSSION
Summary of main results
Capsaicin appears to provide some degree of improvement in a range of neuropathic pain conditions over periods of 6 to 12 weeks, with either repeated application of a low dose (0.075%) cream or a single dose of a high dose (8%) patch. For every seven people treated with low dose application for 6 to 8 weeks, and for every 12 individuals treated with a single dose of high dose capsaicin, one will experience improvement in pain over 6 to 8 weeks and 12 weeks respectively, who would not have done with placebo.
These results might be compared with an NNT of 5.1 (3.9 to 7.3) for the outcome of at least 50% pain relief with 60 or 120 mg duloxetine daily in trials over 12 weeks (Sultan 2008), or an NNT of 5.2 (4.5, 6.4) over 10 to 18 weeks for pregabalin in neuropathic pain across 150 to 600 mg daily, though in that case there was a clear dose response with lower NNTs at higher doses (Straube 2008). Some of the participants treated with capsaicin in this review had neuropathies resistant to conventional treatments, which may lead to a higher NNT than for more treatment-responsive participants. Reviews that include shorter duration studies or lesser outcomes than at least 30% pain relief are likely to overestimate treatment efficacy compared with the strategy used for topical capsaicin.
Use of capsaicin is associated with increased local skin reactions, primarily burning, stinging and erythema. For every five individuals treated with low dose capsaicin for 6 to 8 weeks, two will experience local skin reactions who would not have done so with placebo. These effects tend to be mild to moderate in intensity, but lead to withdrawal in some individuals. In those who can tolerate them, they generally disappear or become less intense after one to two weeks of use.
Overall completeness and applicability of evidence
There were very limited amounts of evidence available for analysis, so that confidence intervals for estimates of efficacy are wide. A single new large study could significantly change the estimates. Importantly there were insufficient data to allow analysis by condition, but there is now evidence that different response rates to treatment are seen in different neuropathic conditions (Moore 2009). The L’Abbé plot shows considerable scatter for the studies using multiple applications of low dose capsaicin, which reflects the small numbers of participants in these studies, but may also be due, in part, to the different neuropathic pain conditions studied (Figure 1).
The decisions a priori to exclude studies of less than six weeks’ duration, and not to use physician reported outcomes, both reduced the amount of evidence available to us. We feel this is justified because other studies in neuropathic pain have shown that studies of shorter duration tend to overestimate treatment effect, sometimes considerably (Moore (in press)), and do not accurately reflect treatment of chronic conditions. Similarly, investigator or physician reported outcomes do not reliably correspond to patient outcomes. In particular, four studies of 4 weeks’ duration were excluded (Chad 1990; McCarty 1994; McCleane 2000; Paice 2000). These studies enrolled 329 participants, 90 of whom were treated with 0.075% capsaicin, and 21 with 0.025% capsaicin. Only one study (59 participants) provided dichotomous data for the efficacy outcome, so the amount of data excluded was small.
All studies used standard validated scales to measure pain intensity and pain relief, but outcome definitions for improvement in pain differed between studies. Only two studies reported at least substantial levels of pain relief (≥ 75% in Ellison 1997, and ≥ 50% in Watson 1992) according to the recent IMMPACT consensus criteria (Dworkin 2008). Three studies reported at least moderate levels of pain relief (30% Backonja 2008 and Simpson 2008; 40% Bernstein 1989), while one (Tandan 1992 (CSG 1992)) used pain decreased by at least one category, and two did not define improvement (Low 1995; Watson 1993). The remaining two studies reported only mean data or physician assessments for pain. Such heterogeneity in outcomes makes combining studies for analysis difficult and reduces the validity of the result. There were insufficient data for the planned sensitivity analysis for outcome definition.
We found only two published studies using a high dose capsaicin patch, but are aware that there are a number of unpublished ongoing or completed studies listed on a clinical trials website (www.clinicaltrials.gov), and one additional completed study listed on the manufacturer’s website (www.neurogesx.com/ngx.4010). In addition, the manufacturer’s website states that a new drug application for management of PHN, with data from over 2300 patients, was submitted to the US Food and Drug Administration in October 2008. The manufacturer is also awaiting final marketing authorisation from the European Medicines Agency for use of the patch in peripheral neuropathic pain in non-diabetic adults. The manufacturer is unable to provide us with further information at this time.
Quality of the evidence
Overall the methodological quality was adequate, with all included studies indicating that they were both randomised and double blind, and scoring a minimum of 3/5 on the Oxford Quality Score. Points were lost for failure to report details of the methods of randomisation and blinding, and also for failure to adequately report withdrawals in some of the earlier studies using low dose capsaicin. Additionally all included studies scored more than the minimum of 9/16 on the Oxford Pain Validity Score.
While all the studies claimed to be double blind, the propensity for capsaicin to cause skin irritation makes maintenance of blinding of treatment groups difficult. This was recognised in all studies, and two took steps to include a mimicking agent (a rubefacient) while another two included a sub-therapeutic dose of capsaicin in the placebo treatment. In all control groups, some individuals experienced skin irritation, reporting events similar to those in the capsaicin groups, but the frequency of such reactions was substantially different, and doubt must remain about the adequacy of the blinding.
The two studies using the high dose patch used a low dose (0.04%) of capsaicin in the “placebo” patch. While there is unlikely to be a significant analgesic effect with a single dose of 0.04% over 12 weeks, there remains a theoretical possibility that the efficacy of the high dose patch is underestimated due to some efficacy of the “placebo” patch.
Reporting of adverse events was inconsistent, making analysis difficult. Estimates for skin reactions presented here may underestimate their frequency. Problems with reporting of adverse events have been highlighted before (Edwards 1999; Ioannidis 2001; Loke 2001).
The relatively small number of participants and the relatively poor (high) NNT makes the result susceptible to non publication of negative results. For example, zero effect data from only about 300 participants would be needed to increase the best NNT for efficacy from 6.6 to 12 (Moore 2008), a point at which the clinical utility of the intervention would be questioned. This is a smaller number of participants than actually included in the review, meaning that the results obtained cannot be regarded as robust.
Agreements and disagreements with other studies or reviews
This review is in broad agreement with an earlier review (Mason 2004), which looked at both neuropathic and musculoskeletal conditions. It differs in adopting more rigorous criteria for study inclusion and sensitivity analyses, and more rigorous outcome measures. That review, which included only studies using repeated applications of 0.075% capsaicin for neuropathic pain, reported an NNT of 5.7 (4.0 to 10) for improvement in pain at eight weeks, which is better (lower) than the estimate here, but not significantly so.
AUTHORS’ CONCLUSIONS
Implications for practice
Capsaicin, either as repeated application of a low dose (0.075%) cream, or a single application of a high dose (8%) patch may provide a degree of pain relief to some patients with painful neuropathic conditions. Local skin irritation, which is often mild and transient but may lead to withdrawal, is common. Systemic adverse effects are rare. The limited amount of data and inconsistent definition of outcome means that estimates for the number of participants achieving clinically useful levels of pain relief are not robust. As an add-on therapy for patients with inadequate response or intolerance to other treatment, even a small degree of pain relief may be considered worthwhile, if the adverse events are tolerable.
Implications for research
Earlier studies used repeated applications of low dose capsaicin, and have not convincingly demonstrated good efficacy. After a gap of almost 10 years, a new strategy has emerged, using a single application of high dose capsaicin, facilitated by pretreatment with local anaesthetic. Further developments in methods of application and formulation may lead to improved clinical efficacy. New trials need to be large and have consistent reporting of both dichotomous patient-reported outcomes with clinical relevance, and adverse events. Trials combining capsaicin with another therapy may also be helpful.
PLAIN LANGUAGE SUMMARY.
Capsaicin applied to the skin for chronic neuropathic pain in adults
Topical (rubbed on the skin) capsaicin may provide some pain relief in various neuropathic pain conditions, but at the expense of local skin irritation such as burning and stinging. The amount of pain relief experienced remains uncertain because of different definitions used in these studies, and confidence in our estimates of the number of individuals likely to benefit is not strong because of the small numbers of participants in the studies and probable differences in response for different neuropathic conditions. Capsaicin, either alone or in combination with other treatment, may provide useful pain relief in individuals who fail to respond to, or cannot tolerate, other available therapies.
Acknowledgments
SOURCES OF SUPPORT
Internal sources
Oxford Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
NIHR Biomedical Research Centre Programme, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT, DB, multicentre, parallel groups, single application, 12 week duration Oral pain medication continued without change. Transdermal opioids (≤ 60 mg morphine/day equivalent) permitted, but not topical analgesics Pain assessed daily (average pain for last 24 hours). Clinic visits at 4, 8, 12 weeks |
|
| Participants | Post herpetic neuropathy with at least moderate pain N = 402 M = 190, F = 212 Mean age 71 years Baseline pain 30-90 mm (mean 60 mm) |
|
| Interventions |
Topical local anaesthetic applied for 60 min, then patch applied for 60 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11 point numeric pain rating scale Patient global: PGIC - 7 point scale Adverse events Withdrawals |
|
| Notes | R2, DB2, W1 OPVS 16/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Yes | Remote treatment assignment, using unique number on printed labels affixed to outside of patch envelope |
| Blinding? All outcomes |
Yes | Low concentration of capsaicin in “identically formulated” control patch to mimic local skin reaction of active treatment |
| Methods | RCT, DB, parallel study, 6 week duration 7 day washout for topical medications, current oral medication continued with no changes to dose or frequency Pain assessment at 0, 2, 4 and 6 weeks |
|
| Participants | Chronic severe postherpetic neuralgia (at least 12 months duration). Pain poorly or incompletely controlled with oral analgesics, antidepressants and neuroleptic agents Mean age 72 years (range 54 to 90) N = 32 M = 12, F = 20 Mean baseline pain >70 mm |
|
| Interventions | Capsaicin 0.075% cream, n = 16 Vehicle cream, n = 16 Cream applied to painful areas × 3 to × 4 daily |
|
| Outcomes | PI: standard 5 point scale and 100 mm VAS PR: 100 mm VAS Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W0 OPVS 14/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | computer-generated randomisation schedule |
| Allocation concealment? | Unclear | not described |
| Blinding? All outcomes |
Yes | “identical appearing” |
| Methods | RCT, DB, parallel study, 8 week duration 7 day washout for all topical medication and tricyclic antidepressants. Other long-term oral therapy permitted with no changes to dose or frequency Pain assessment at 0, 2, 4, 6 and 8 weeks |
|
| Participants | Diabetic neuropathy involving feet. At least moderate daily pain interfering with activities or sleep Mean age 60 years (range 21 to 85) N = 235 M = 132, F = 103 Mean baseline pain > 60 mm |
|
| Interventions | Capsaicin 0.075% cream + placebo capsule(s), n = 118 Oral amitriptyline capsule (titrated from ×1 to ×5 25 mg/day) + placebo cream, n = 117 Capsaicin 0.075% cream + oral amitriptyline capsule(s) - not analysed Cream applied to painful area ×4 daily For first 2 weeks, placebo cream contained methyl nicotinate, a rubefacient that can produce a stinging/burning sensation and erythema (to mimic capsaicin). Placebo capsules contained 0.25 mg benztropine to mimic dry mouth of amitriptyline, and also for first 2 weeks 2 mg diazepam to mimic CNS effects such as sedation |
|
| Outcomes | PI: 100 mm VAS PR: 100 mm VAS Interference with activities of life: 4 point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W0 OPVS 14/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | computer generated randomisation schedule |
| Allocation concealment? | Unclear | not described |
| Blinding? All outcomes |
Yes | Agents to mimic adverse effects of active treatments (but without effect on efficacy) added to placebo treatments |
| Methods | RCT, DB, multicentre, parallel groups, 8 week duration 7 day washout for topical medication. Oral medication continued without change. No new medication Pain assessment at 2, 4, 6 and 8 weeks. |
|
| Participants | Peripheral polyneuropathy and/or radiculopathy, with at least moderate pain that was unresponsive or intolerant to conventional therapy and interfering with functional activities and/or sleep N = 277 M = 139, F = 138 Mean age 60 years (range 27 to 92) Baseline pain 78% severe or very severe |
|
| Interventions | Capsaicin 0.075% cream, n = 138 Vehicle cream, n = 139 Cream applied ×4 daily |
|
| Outcomes | PI: 100 mm VAS Interference with activities of life: 4 point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 OPVS 12/16 Additional data for pain relief for 22 participants in Tandan 1992 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | not described |
| Allocation concealment? | Unclear | not described |
| Blinding? All outcomes |
Unclear | not described |
| Methods | RCT, DB, two-arm crossover study. Only first 8 week period used in this review 7 day washout for topical medication. Stable (≥ 10 days) oral analgesic medication continued without change Pain assessment weekly through questionnaires and at 2-week intervals by study nurses for 8 week treatment phase |
|
| Participants | Post-surgical neuropathic pain of ≥ moderate severity and ≥ 3 months duration N = 99 M = 27, F = 72 Mean age 64 years Median baseline pain 60 mm |
|
| Interventions | Capsaicin 0.075% cream n = 49 Placebo cream, n = 50 |
|
| Outcomes | PI: 100 mm VAS PR: 100 mm VAS Interference with activities of life: 4 point scale Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0 OPVS 14/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “identical-appearing” |
| Methods | RCT, DB, parallel groups, 12 week duration (8 week DB then 4 week SB placebo treatment) Randomisation by side (left vs right). Each participant treated one leg with capsaicin and the other with placebo No details of washout or concomitant medication Pain assessment at 4, 8 and 12 weeks |
|
| Participants | Bilateral symmetric painful polyneuropathy involving distal lower extremities, of ≥6 months’ duration and refractory to at least one other form of treatment N = 40 M = 24, F = 16 Mean age 59 years (30 to 78) Median baseline pain > 80 mm |
|
| Interventions | Capsaicin 0.075% cream, n = 40 Placebo (contained methyl nicotinate), n = 40 Cream applied × 4 daily First tube of placebo contained methyl nicotinate, a rubefacient that can produce a stinging/burning sensation and erythema (to mimic capsaicin) |
|
| Outcomes | PI: 10 cm VAS PR: 10 cm VAS and 7 point scale Interference with activities of life: 4 point scale Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0 OPVS 14/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “identical appearance” |
| Methods | RCT, DB, multicentre, parallel groups, single application, 12 week duration Oral pain medication continued without change. No topical analgesics Pain assessment at weekly intervals |
|
| Participants | HIV neuropathy with ≥2 months’ moderate to severe pain in both feet N = 307 M = 268, F = 39 Mean age 48 years (29 to 74) Mean baseline pain ~60 mm (25 to 96 mm) |
|
| Interventions | Capsaicin patch 8% 30 min, n = 72 Capsaicin patch 8% 60 min, n = 78 Capsaicin patch 8% 90 min, n = 75 Control patch, n = 82 Topical local anaesthetic applied for 60 min before patch was applied for specified time Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11 point numeric pain rating scale Patient global: PGIC - 7 point scale Adverse events Withdrawals |
|
| Notes | R1, DB1, W1 OPVS 13/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Unclear | control patch contained low concentration of capsaicin to mimic local skin reaction of active treatment, but does not say “identical” or use similar wording |
| Methods | RCT, DB, parallel group, 6 week duration 7 day washout for topical agents, and no new oral agents permitted during the trial Pain assessment at baseline and weekly intervals, and with daily pain diaries |
|
| Participants | Postmastectomy pain syndrome, persisting for more than 3 months and with at least moderate pain for at least 12 hours per day N = 25 All F Median age 58 years (range 36-78) Baseline pain moderate in 15 cases and severe in 10 cases |
|
| Interventions | Capsaicin 0.075% cream, n = 14 Vehicle cream, n = 11 Cream applied ×4 daily |
|
| Outcomes | PI: 10 cm VAS and 4 point scale PR: 10 cm VAS and percentage improvement Patient global evaluation: 4 point scale Interference with activities of life: 4 point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 OPVS 11/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Unclear | Not described |
| Methods | RCT, DB, parallel group, 6 week duration 7 day washout for topical medication. Oral medication continued without change. No new medication Pain assessment at 0, 2, 4 and 6 weeks |
|
| Participants | Chronic post-herpetic neuralgia of at least 6 months duration, with pain poorly or incompletely controlled with oral analgesic, antidepressants or neuroleptic agents in most participants N = 143 M = 53, F = 90 Mean age 71 years Baseline pain 90% moderate or severe, 10% very severe |
|
| Interventions | Capsaicin 0.075% cream, n = 74 Vehicle cream, n = 69 Cream applied ×4 daily |
|
| Outcomes | PI: 100 mm VAS and 5 point scale PR: 100 mm VAS Functional capacity: 5 point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W0 OPVS 13/16 |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “identical appearing” |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Chad 1990 | Study only 4 weeks’ duration |
| Fusco 1992 | Too few patients (12 total), open label |
| McCarty 1994 | Study only 4 weeks’ duration |
| McCleane 2000 | Study only 4 weeks’ duration |
| Paice 2000 | Study only 4 weeks’ duration |
| Peikert 1991 | Not randomised controlled trial |
| Pfeifer 1993 | Not randomised controlled trial |
| Robbins 1998 | Not randomised controlled trial, too few participants (10 total) |
| Vickers 1998 | Not randomised controlled trial |
| Watson 1988 | Not randomised controlled trial |
| Watson 1989 | Not randomised controlled trial |
DATA AND ANALYSES
Comparison 1. 0.075% capsaicin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical improvement | 6 | 389 | Risk Ratio (M-H, Fixed, 95% CI) | 1.59 [1.20, 2.11] |
| 1.1 Unspecified or <50% pain relief | 4 | 265 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [1.10, 2.03] |
| 1.2 ≥50% pain relief | 2 | 124 | Risk Ratio (M-H, Fixed, 95% CI) | 2.06 [0.99, 4.30] |
| 2 Adverse event: burning, stinging, erythema | 5 | 557 | Risk Ratio (M-H, Fixed, 95% CI) | 2.64 [2.10, 3.31] |
| 3 Adverse event: coughing, sneezing | 5 | 557 | Risk Ratio (M-H, Fixed, 95% CI) | 5.73 [2.15, 15.25] |
| 4 Adverse event withdrawal | 4 | 477 | Risk Ratio (M-H, Fixed, 95% CI) | 5.02 [2.28, 11.08] |
Comparison 2. 8% capsaicin versus placebo (single dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥30% pain relief | 2 | 709 | Risk Ratio (M-H, Fixed, 95% CI) | 1.40 [1.12, 1.74] |
| 2 Adverse event: erythema, pruritus | 2 | 709 | Risk Ratio (M-H, Fixed, 95% CI) | 1.52 [1.35, 1.72] |
Analysis 1.1. Comparison 1 0.075% capsaicin versus placebo, Outcome 1 Clinical improvement.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 1 0.075% capsaicin versus placebo
Outcome: 1 Clinical improvement
 |
Analysis 1.2. Comparison 1 0.075% capsaicin versus placebo, Outcome 2 Adverse event: burning, stinging, erythema.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 1 0.075% capsaicin versus placebo
Outcome: 2 Adverse event: burning, stinging, erythema
 |
Analysis 1.3. Comparison 1 0.075% capsaicin versus placebo, Outcome 3 Adverse event: coughing, sneezing.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 1 0.075% capsaicin versus placebo
Outcome: 3 Adverse event: coughing, sneezing
 |
Analysis 1.4. Comparison 1 0.075% capsaicin versus placebo, Outcome 4 Adverse event withdrawal.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 1 0.075% capsaicin versus placebo
Outcome: 4 Adverse event withdrawal
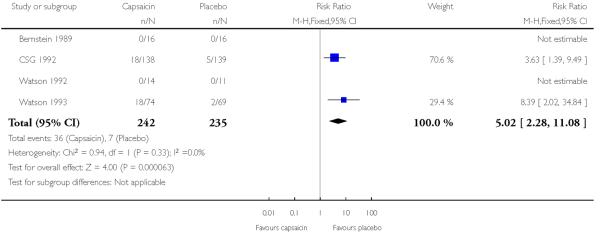 |
Analysis 2.1. Comparison 2 8% capsaicin versus placebo (single dose), Outcome 1 ≥30% pain relief.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 2 8% capsaicin versus placebo (single dose)
Outcome: 1 ≥0% pain relief
 |
Analysis 2.2. Comparison 2 8% capsaicin versus placebo (single dose), Outcome 2 Adverse event: erythema, pruritus.
Review: Topical capsaicin for chronic neuropathic pain in adults
Comparison: 2 8% capsaicin versus placebo (single dose)
Outcome: 2 Adverse event: erythema, pruritus
 |
ADDITIONAL TABLES
Table 1. Summary of outcomes: successful treatment.
| Study ID | Treatment | Clinical improvement |
|---|---|---|
| Backonja 2008 |
|
≥ 30% pain reduction from baseline to weeks 2 to 12:
|
| Bernstein 1989 |
|
≥ 40% pain relief at 6 weeks:
|
| Biesbroeck 1995 |
|
Mean decrease in pain intensity at 8 weeks:
|
| CSG 1992 |
|
Mean decrease in pain intensity at 8 weeks:
|
| Ellison 1997 |
|
≥75% decrease in pain score:
|
| Low 1995 |
|
Undefined improvement at 8 weeks:
|
| Simpson 2008 |
|
≥30% pain reduction from baseline to weeks 2 to 12:
Capsaicin 1 to 3 combined 76/225 |
| Tandan 1992 |
|
Reduction in pain by at least one category at 8 weeks:
|
| Watson 1992 |
|
≥ 50% pain relief at 6 weeks:
|
| Watson 1993 |
|
Improvement in pain severity at 6 weeks: Assuming 12 participants not included in analysis are evenly distributed
|
Table 2. Summary of outcomes: adverse events and withdrawals.
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals |
|---|---|---|---|---|---|
| Backonja 2008 |
|
Erythema:
Cough not reported, so <3% |
Various, all ≤ 5% in both groups |
(1 in capsaicin group judged related to medication) |
AE:
Other:
|
| Bernstein 1989 |
|
Burning, stinging, erythema
|
None | None | AE: none Other: 3 lost to follow up - no details of group or time |
| Biesbroeck 1995 |
|
Total skin AEs:
Cough/sneeze:
|
None with capsaicin Limited therapeutic dose for majority of amitriptyline participants |
|
AE: none related to capsaicin Other:
|
| Capsaicin study group 1992 |
|
Burning:
Cough/sneeze:
Burning, cough, sneeze, rash, erythema:
|
Other AE:
Increased pain:
|
No data | AE:
Other:
|
| Ellison 1997 |
|
Significantly more burning, redness and coughing with capsaicin than placebo (P <0.0001) | No data | No data | AE: no data from first phase Other: no data |
| Low 1995 |
|
Burning:
Sneezing:
Itching:
Rash:
|
No data | No data | 1 did not complete entire study - no further details |
| Simpson 2008 |
|
Pruritus:
Burning:
Cough:
|
Various, ≤ 5% in both groups, except URTI (8%, 6%) |
(all deaths, all judged unrelated to study medication) |
AE:
Other:
|
| Watson 1992 |
|
Burning:
Cough:
|
No data | No data | AE:
Other:
|
| Watson 1993 |
|
Burning,stinging, erythema:
Cough:
|
No more than 3/group with any systemic AE. No significant difference between groups | None | AE:
Other:
Exclusions: 12 participants treated did not meet entrance criteria, further 6 had protocol violations |
Appendix 1. MEDLINE search strategy (via Ovid)
Capsaicin.sh
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin-P OR dolorac OR gelcen OR katrum OR “No pain-HP” OR priltam OR “R-gel” OR zacin OR zostrix OR capsicum).ti,ab,kw
1 OR 2
exp Administration, topical.sh
(topical$ OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).ti,ab,kw
4 OR 5
Diabetic neuropathies.sh OR Peripheral neuropathies.sh OR Polyneuropathies.sh OR Neuralgia.sh
(neuropath$ OR diabet$ post-herpetic OR neuralgia).ti,ab,kw
7 OR 8
(pain OR painful OR analgesi$).ti,ab,kw
randomized controlled trial.pt
controlled clinical trial.pt
randomized.ab
placebo.ab
drug therapy.fs
randomly.ab
trial.ab
groups.ab
OR/11-18
3 AND 6 AND 9 AND 19
Appendix 2. EMBASE search strategy (via Ovid)
Capsaicin.sh
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin-P OR dolorac OR gelcen OR katrum OR “No pain-HP” OR priltam OR “R-gel” OR zacin OR zostrix OR capsicum).ti,ab,kw
1 OR 2
exp Topical Drug Administration.sh
(topical$ OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).ti,ab,kw
4 OR 5
Diabetic Neuropathy.sh OR Peripheral neuropathy.sh OR Polyneuropathy.sh OR Neuralgia.sh
(neuropath$ OR diabet$ post-herpetic OR neuralgia).ti,ab,kw
7 OR 8
(pain OR painful OR analgesi$).ti,ab,kw
clinical trials.sh
controlled clinical trials.sh
randomized controlled trial.sh
double-blind procedure.sh
(clin$ adj25 trial$).ab
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ab
placebo$.ab
random$.ab
OR/11-18
3 AND 6 AND 9 AND 10 AND 19
Appendix 3. CENTRAL search strategy
MESH descriptor Capsaicin
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin-P OR dolorac OR gelcen OR katrum OR “No pain-HP” OR priltam OR “R-gel” OR zacin OR zostrix OR capsicum):ti,ab,kw
1 OR 2
exp MESH descriptor Administration, topical
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster):ti,ab,kw
4 OR 5
MESH descriptor (Diabetic neuropathies OR Peripheral neuropathies OR Polyneuropathies OR Neuralgia)
(neuropath* OR diabet* post-herpetic OR neuralgia):ti,ab,kw
7 OR 8
(pain OR painful OR analgesi*):ti,ab,kw
Randomized Controlled Trial:pt.
MESH descriptor Double-Blind Method
(double or treble or triple) NEXT (blind* or mask*)):ti,ab,kw.
random*:ti,ab,kw.
OR/11-14
3 AND 6 AND 9 AND 10 AND 15
Limit 16 to Clinical Trials (CENTRAL)
HISTORY
Protocol first published: Issue 4, 2008
Review first published: Issue 4, 2009
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The protocol specified that treatment should be applied at least three times daily, based on published studies known to the authors. Since then, new studies using a single high dose capsaicin patch that are of high quality and include large numbers of participants, have been published. It was felt that these studies should be included and analysed separately because of their different study design, rather than be excluded because they did not satisfy the original inclusion criteria.
Footnotes
DECLARATIONS OF INTEREST
RAM, HJM, SD have received research support from charities, government and industry sources at various times. RAM and HJM have consulted for various pharmaceutical companies and have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions.
References to studies included in this review
* Indicates the major publication for the study
- Backonja 2008 {published data only} .Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Jr, Rauck R, et al. NGX-4010 C107 Study Group NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurology. 2008;7(12):1106–12. doi: 10.1016/S1474-4422(08)70228-X. DOI: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- Bernstein 1989 {published data only} .Bernstein JE, Korman NJ, Bickers DR, Dahl MV, Millikan LE. Topical capsaicin treatment of chronic postherpetic neuralgia. Journal of the American Acadamy of Dermatology. 1989;21(2):265–70. doi: 10.1016/s0190-9622(89)70171-7. [DOI] [PubMed] [Google Scholar]
- Biesbroeck 1995 {published data only} .Biesbroeck R, Bril V, Hollander P, Kabadi U, Schwartz S, Singh SP, et al. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Advances in Therapy. 1995;12(2):111–20. [PubMed] [Google Scholar]
- CSG 1992 {published data only} .Basha KM, Whitehouse FW. Capsaicin: a therapeutic option for painful diabetic neuropathy. Henry Ford Hospital Medical Journal. 1991;39:138–40. [PubMed] [Google Scholar]
- *; Capsaicin Study Group Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Diabetes Care. 1992;15(2):159–65. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- Donofrio P, Walker F, Hunt V, Tandan R, Fries T, Lewis G, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Archives of Internal Medicine. 1991;151:2225–9. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- Scheffler NM, Sheitel PL, Lipton MN. Treatment of painful diabetic neuropathy with capsaicin 0.075% Journal of the American Podiatric Medical Association. 1991;81:288–93. doi: 10.7547/87507315-81-6-288. [DOI] [PubMed] [Google Scholar]
- Tandan R, Lewis GA, Krusinski PB, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care. 1992;15:8–14. doi: 10.2337/diacare.15.1.8. [DOI] [PubMed] [Google Scholar]
- Ellison 1997 {published data only} .Ellison N, Loprinzi CL, Kugler J, Hatfield AK, Miser A, Sloan JA, et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. Journal of Clinical Oncology. 1997;15(8):2974–80. doi: 10.1200/JCO.1997.15.8.2974. [DOI] [PubMed] [Google Scholar]
- Low 1995 {published data only} .Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy WJ, O’Brien PC. Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain. 1995;62(2):163–8. doi: 10.1016/0304-3959(94)00261-C. [DOI] [PubMed] [Google Scholar]
- Simpson 2008 {published data only} .Simpson DM, Brown S, Tobias J, NGX-4010 C107 Study Group Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–13. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- Watson 1992 {published data only} .Watson CP, Evans RJ. The postmastectomy pain syndrome and topical capsaicin: a randomized trial. Pain. 1992;51(3):375–9. doi: 10.1016/0304-3959(92)90223-X. [DOI] [PubMed] [Google Scholar]
- Watson 1993 {published data only} .Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clinical Therapeutics. 1993;15(3):510–26. [PubMed] [Google Scholar]
References to studies excluded from this review
- Chad 1990 {published data only} .Chad DA, Aronin N, Lundstrom R, McKeon P, Ross D, Molitch M, et al. Does capsaicin relieve the pain of diabetic neuropathy? Pain. 1990;42(3):387–8. doi: 10.1016/0304-3959(90)91153-A. [DOI] [PubMed] [Google Scholar]
- Fusco 1992 {published data only} .Fusco BM, Alessandri M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesthesia and Analgesia. 1992;74:375–7. doi: 10.1213/00000539-199203000-00011. [DOI] [PubMed] [Google Scholar]
- McCarty 1994 {published data only} .McCarty DJ, Csuka M, McCarthy G, Trotter D. Treatment of pain due to fibromyalgia with topical capsaicin: A pilot study. Seminars in Arthritis and Rheumatism. 1994;23(Suppl 3):41–7. [Google Scholar]
- McCleane 2000 {published data only} .McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double-blind, placebo-controlled study. British Journal of Clinical Pharmacology. 2000;49(6):574–9. doi: 10.1046/j.1365-2125.2000.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paice 2000 {published data only} .Paice JA, Ferrans CE, Lashley FR, Shott S, Vizgirda V, Pitrak D. Topical capsaicin in the management of HIV-associated peripheral neuropathy. Journal of Pain and Symptom Management. 2000;19(1):45–52. doi: 10.1016/s0885-3924(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Peikert 1991 {published data only} .Peikert A, Hentrich M, Ochs G. Topical 0.025% capsaicin in chronic post-herpetic neuralgia: efficacy, predictors of response and long-term course. Journal of Neurology. 1991;238:452–6. doi: 10.1007/BF00314653. [DOI] [PubMed] [Google Scholar]
- Pfeifer 1993 {published data only} .Pfeifer MA, Ross DR, Schrage JP, Gelber DA, Schumer MP, Crain GM, et al. A highly successful and novel model for treatment of chronic painful diabetic peripheral neuropathy. Diabetes Care. 1993;16:1103–15. doi: 10.2337/diacare.16.8.1103. [DOI] [PubMed] [Google Scholar]
- Robbins 1998 {published data only} .Robbins WR, Staats PS, Levine J, Fields HL, Allen RW, Campbell JN, et al. Treatment of intractable pain with topical large-dose capsaicin: preliminary report. Anesthesia and Analgesia. 1998;86:579–83. doi: 10.1097/00000539-199803000-00027. [DOI] [PubMed] [Google Scholar]
- Vickers 1998 {published data only} .Vickers ER, Cousins MJ, Walker S, Chisholm K. Analysis of 50 patients with atypical odontalgia. A preliminary report on pharmacological procedures for diagnosis and treatment. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 1998;85:24–32. doi: 10.1016/s1079-2104(98)90393-6. [DOI] [PubMed] [Google Scholar]
- Watson 1988 {published data only} .Watson CP, Evans RJ, Watt VR. Post-herpetic neuralgia and topical capsaicin. Pain. 1988;33:333–40. doi: 10.1016/0304-3959(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Watson 1989 {published data only} .Watson CP, Evans RJ, Watt VR. The post-mastectomy pain syndrome and the effect of topical capsaicin. Pain. 1989;38:177–86. doi: 10.1016/0304-3959(89)90236-4. [DOI] [PubMed] [Google Scholar]
Additional references
- Collins 1997 .Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1-2):95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- Cook 1995 .Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ (Clinical Research Ed) 1995;310:452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin 2008 .Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. DOI: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Edwards 1999 .Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management. 1999;18(6):427–37. doi: 10.1016/s0885-3924(99)00093-7. DOI: 10.1016/S0885-3924(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Holzer 2008 .Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. British Journal of Pharmacology. 2008;155(8):1145–62. doi: 10.1038/bjp.2008.351. DOI: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis 2001 .Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437–43. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- Jadad 1996a .Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain. 1996;66:239–46. doi: 10.1016/0304-3959(96)03033-3. [DOI] [PubMed] [Google Scholar]
- Jadad 1996b .Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jancso 2008 .Jancsó G, Dux M, Oszlács O, Sántha P. Activation of the transient receptor potential vanilloid-1 (TRPV1) channel opens the gate for pain relief. British Journal of Pharmacology. 2008;155(8):1139–41. doi: 10.1038/bjp.2008.375. DOI: 10.1038/bjp.2008.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Abbe 1987 .L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine. 1987;107:224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Loke 2001 .Loke YK, Derry S. Reporting of adverse drug reactions in randomised controlled trials - a systematic survey. BMC Clinical Pharmacology. 2001;1:3. doi: 10.1186/1472-6904-1-3. DOI: 10.1186/1472-6904-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason 2004 .Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ (Clinical Research Ed) 2004;328(7446):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore (in press) .Moore RA, Straube S, Derry S, McQuay HJ. Pregabalin in fibromyalgia - responder analysis from individual patient data. Annals of the Rheumatic Diseases. doi: 10.1186/1471-2474-11-150. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 1998a .Moore RA, Tramèr MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316(7128):333–8. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 1998b .Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78(3):209–16. doi: 10.1016/S0304-3959(98)00140-7. DOI: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Moore 2008 .Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors. Systematic reviews in pain research: methodology refined. IASP Press; Seattle: 2008. pp. 15–23. ISBN: 978–0–931092–69–5. [Google Scholar]
- Moore 2009 .Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD007076.pub2. DOI: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris 1995 .Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors. Statistics with confidence - confidence intervals and statistical guidelines. British Medical Journal; London: 1995. pp. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy 2004 .Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. European Journal of Pharmacology. 2004;500(1-3):351–69. doi: 10.1016/j.ejphar.2004.07.037. DOI: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Nolano 1999 .Nolano M, Simone DA, Wendelschafer-Crabb G, et al. Topical capsaicin in humans: parallel loss of epidermal nerve fibres and pain sensation. Pain. 1999;81(1-2):135–45. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- PCA 2007 .Prescription Cost Analysis, England. NHS The Information Centre; 2008. ISBN: 978–1–84636–210–1. [Google Scholar]
- Rains 1995 .Rains C, Bryson HM. Topical Capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7(4):317–28. doi: 10.2165/00002512-199507040-00007. [DOI] [PubMed] [Google Scholar]
- Reynolds 1999 .Anonymous . In: Martindale: the extra pharmacopoeia. 2nd Edition Reynolds JEF, editor. Royal Pharmaceutical Society; London: 1999. [Google Scholar]
- Simone 1998 .Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. Journal of Neuroscience. 1998;18(21):8947–59. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith 2000 .Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain. 2000;86:119–32. doi: 10.1016/s0304-3959(00)00234-7. [DOI] [PubMed] [Google Scholar]
- Straube 2008 .Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology. 2008;66(2):266–75. doi: 10.1111/j.1365-2125.2008.03200.x. DOI: 10.1111/j.1365-2125.2008.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan 2008 .Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology. 2008;8:29. doi: 10.1186/1471-2377-8-29. DOI: 10.1186/1471-2377-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]