Abstract
Background
Gestational diabetes mellitus (GDM) is a form of diabetes that occurs in pregnancy. Although GDM usually resolves following birth, it is associated with significant morbidities for mother and baby both perinatally and in the long term. There is strong evidence to support treatment for GDM. However, there is little consensus on whether or not screening for GDM will improve maternal and infant health and if so, the most appropriate protocol to follow.
Objectives
To assess the effects of different methods of screening for gestational diabetes mellitus and maternal and infant outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (April 2010).
Selection criteria
Randomised and quasi-randomised trials evaluating the effects of different methods of screening for gestational diabetes mellitus.
Data collection and analysis
Two review authors independently conducted data extraction and quality assessment. We resolved disagreements through discussion or through a third author.
Main results
We included four trials involving 3972 women were included in the review. One quasi-randomised trial compared risk factor screening with universal or routine screening by 50 g oral glucose challenge testing. Women in the universal screening group were more likely to be diagnosed with GDM (one trial, 3152 women, risk ratio (RR) 0.44 95% confidence interval (CI) 0.26 to 0.75). Infants of mothers in the risk factor screening group were born marginally earlier than infants of mothers in the routine screening group (one trial, 3152 women, mean difference −0.15 weeks, 95% CI −0.27 to −0.53).
The remaining three trials evaluated different methods of administering a 50 g glucose load. Two small trials compared glucose monomer with glucose polymer testing, with one of these trials including a candy bar group. One trial compared a glucose solution with food. No differences in diagnosis of GDM were found between each comparison. Overall, women drinking the glucose monomer experienced fewer side effects from testing than women drinking the glucose polymer (two trials, 151 women, RR 2.80, 95% CI 1.10 to 7.13). However, we observed high heterogeneity between the trials for this result (I2 = 61%).
Authors’ conclusions
There was insufficient evidence to determine if screening for gestational diabetes, or what types of screening, can improve maternal and infant health outcomes.
Medical Subject Headings (MeSH): Diabetes, Gestational [*diagnosis; therapy]; Glucose Tolerance Test [adverse effects; *methods]; Infant Welfare; Infant, Newborn; Mass Screening [*methods]; Maternal Welfare; Pregnancy Outcome; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) is defined as ‘carbohydrate intolerance of varying degrees of severity with onset or first recognition during pregnancy’ (Metzger 1998). GDM therefore includes type I or type II diabetes previously undetected or with first presentation during pregnancy. GDM typically resolves following birth. However, these women are at risk for type II diabetes in the future (Kim 2002).
Epidemiology
Gestational diabetes affects up to 14% of pregnant women every year and accounts for 90% of pregnancies affected by diabetes mellitus (Coustan 1995; Setji 2005). There is growing concern over the increasing prevalence of gestational diabetes and its effects for individual mothers and infants and its impact on public health (Ferrara 2007; Hunt 2007; Metzger 2007). GDM is associated with numerous risk factors. Maternal age and body mass index (BMI) are among the most common risk factors (Di Cianni 2003; O’Sullivan 1973). Specific ethnicities are also at higher risk of developing gestational diabetes, namely Hispanic, black, Native American, South or East Asian, Pacific Islander and Indigenous Australian (Kjos 2005). These ethnicities are similar to those at high risk of type II diabetes mellitus, with suggestions that parallels may be drawn between these two forms of diabetes (Ben-Haroush 2004; Kuhl 1998). Other risk factors include previous birth of a large baby, a family history of diabetes mellitus, weight gain and cigarette smoking (Davey 2001; Di Cianni 2003; O’Sullivan 1973; Solomon 1997).
Aetiology/pathophysiology
Normally, insulin is released by pancreatic beta cells in response to increasing blood glucose levels to achieve euglycaemia (normal blood glucose levels). This system can be disrupted in two ways. A problem with the release of insulin from beta cells can occur, such as in type I or insulin dependent diabetes mellitus. Alternatively, insulin may not act as effectively in promoting glucose uptake. This is known as insulin resistance, and is seen in the development of type II or non insulin dependent diabetes mellitus and GDM. Placental hormones such as progesterone, cortisol, prolactin and human placental lactogen released mid-pregnancy contribute to decreased insulin action in pregnancy (Kuhl 1998). Physiologically, this ensures sufficient nutrient transport to the fetus as it develops, and promotes growth (Setji 2005). In a normal pregnancy, the action of these placental hormones is adequately compensated by increasing insulin release, creating an equilibrium between insulin supply and insulin demand.
In pregnant women with abnormal glucose intolerance, the insulin resistance of pregnancy is not adequately compensated for, resulting in carbohydrate or glucose intolerance. It is suggested that women who develop gestational diabetes may also have an underlying insulin resistance, such as high maternal adiposity, or beta cell dysfunction that potentiates the insulin resistance of pregnancy (Buchanan 2005; Kuhl 1998; Richardson 2007). Recent theories relating to the pathogenesis of gestational diabetes include inflammation (Richardson 2007).
These effects culminate in a disruption of the action of insulin in maintaining glucose levels, resulting in maternal hyperglycaemia (high blood glucose). Glucose is transferred, via the placenta, to the fetus. Maternal hyperglycaemia therefore stimulates a fetal hyperinsulinaemia to counter the excess placental glucose transfer. There is strong evidence confirming the continuum of risk associated with increasing carbohydrate intolerance (Dodd 2007; HAPO 2008; Sermer 1995). The point at which this increasing carbohydrate intolerance becomes pathological remains uncertain.
Clinical features
Infant
Excess insulin due to maternal hyperglycaemia acts in two ways on the fetus. Firstly, insulin promotes fat deposition due to the state of nutrient excess (Pedersen 1954; Whitelaw 1977). Secondly, insulin acts as a growth factor, stimulating further growth of the infant in utero (Hunt 2007). Thus, fetal hyperinsulinaemia results in excessive growth of the fetus, leading to one of the major perinatal concerns in GDM, macrosomia (birthweight greater than 4000 g). Macrosomia may lead to birth trauma including shoulder dystocia, nerve palsies and fractures (Dodd 2007; Metzger 1998). GDM is associated with respiratory distress syndrome, neonatal hypoglycaemia (low blood glucose), hyperbilirubinaemia (high bilirubin levels), polycythaemia (excess red blood cells), and hypocalcaemia (low calcium) (ADA 2003; Metzger 1998). In utero exposure to hyperglycaemia has long lasting effects on the infant, increasing their risk of future obesity and type II diabetes mellitus (Pettitt 1985; Silverman 1998).
Maternal
With the implementation of screening protocols, GDM is usually diagnosed before it becomes symptomatic during pregnancy. However, where GDM is undetected, the pregnant woman may experience polyuria (increased urinary frequency), polydipsia (excessive thirst) or fatigue. Macrosomia in utero or polyhydramnios (excess amniotic fluid volume) may also indicate GDM.
In the mother, evidence supports an association between GDM and increased rates of caesarean delivery and pre-eclampsia (ACOG 2001). As with their infants, the consequences of GDM for the mother extend beyond the perinatal period. There are strong links between GDM and future development of type II diabetes mellitus. Within 10 years of developing gestational diabetes, half the women develop type II diabetes mellitus (Kim 2002).
Diagnosis of GDM
Although diagnostic criteria vary (ACOG 2001; ADA 2003; Berger 2002; IADPSG 2010; NICE 2008; Oats 2004; RANZCOG 2008; WHO 1999), the oral glucose tolerance test (OGTT) is considered the ‘gold standard’ for diagnosis of GDM (Scott 2002). Minor degrees of abnormal carbohydrate tolerance, such as one abnormal value on OGTT or positive oral glucose challenge test (OGCT) with normal OGTT are also associated with similar outcomes to GDM. This is in line with the increasing awareness of the continuum of risk associated with increasing carbohydrate intolerance (Dodd 2007; HAPO 2008; Sermer 1995).
Management of GDM
The importance of management for women with gestational diabetes has been widely accepted and is evaluated by several Cochrane reviews (Alwan 2009; Boulvain 2001; Ceysens 2006) and the treatment of GDM is widely supported (ADA 2003; Crowther 2005; Hoffman 1998; Metzger 1998; O’Sullivan 1966).
Treatment focuses on reducing the hyperglycaemia driving the complications of GDM (Metzger 1998). In general, management includes any or all of: nutritional therapy, exercise, blood glucose monitoring and insulin therapy. The results from two large, multi-centred randomised controlled trials provide strong support for the treatment of women with mild gestational diabetes (Crowther 2005; Landon 2009). Crowther and colleagues reported reduced infant morbidity in those treated for GDM in addition to suggesting that maternal quality of life was improved by treatment. Landon and colleagues showed treatment for GDM reduced the risks of fetal overgrowth, shoulder dystocia and pre-eclampsia.
Medical nutrition therapy
The American Diabetes Association and Australasian Diabetes in Pregnancy Society, in line with other governing bodies, recommend nutrition therapy in the treatment of GDM (ADA 2003; ADA 2007; Hoffman 1998). Both guidelines focus on managing carbohydrate intake for blood glucose maintenance.
Exercise
Exercise is often used in conjunction with dietary therapy to maintain normal glucose levels. The Cochrane review ‘Exercise in diabetic pregnancy’ found that there was insufficient evidence to make a recommendation (Ceysens 2006). However, there is growing consensus on the safety of moderate exercise in pregnancy and its benefits in the treatment of gestational diabetes.
Blood glucose monitoring
Blood glucose monitoring is often recommended (ACOG 2001; Hoffman 1998). Postprandial hyperglycaemia monitoring demonstrates a closer association with rates of fetal macrosomia and obviously correlates with peaks of blood glucose (Hoffman 1998). Blood glucose monitoring provides the health professional with a representation of glycaemic control while providing the woman with feedback on her management progress.
Insulin/oral hypoglycaemic agents
Where the maternal hyperglycaemia cannot be managed by dietary or exercise advice and blood glucose levels remain elevated, insulin is added for greater control (Metzger 1998). The methods for administering insulin are discussed in the Cochrane review ‘Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes’ (Farrar 2007). Oral hypoglycaemics such as glyburide (Langer 2000) and metformin (Rowan 2008) have been suggested as alternatives to insulin therapy.
Birth
The Cochrane review ‘Elective delivery in diabetic pregnant women’ suggested that induction of labour at 38 to 39 weeks may be suitable for diabetic women treated with insulin (Boulvain 2001).
Following pregnancy
It is recommended that women whose pregnancies were affected by GDM receive an OGTT between six and 12 weeks postpartum to detect diabetes (ACOG 2009; Berger 2002; Hoffman 1998; Metzger 2007; Oats 2004; RANZCOG 2008). Because of the high risk of future diabetes, these women are often advised to be retested on a regular basis (Hoffman 1998; Metzger 2007; Oats 2004; RANZCOG 2008).
Description of the intervention
Screening
A screening tool establishes the risk of disease in an otherwise well person (NSC 2007). Ordinarily, the presentation of symptoms prompts testing for disease. However, screening aims to identify the illness earlier, before symptoms arise. Identification of an illness by screening allows for earlier management, which may result in better health outcomes. While screening can be beneficial, it can also cause unnecessary anxiety due to the testing process itself. This is further complicated by the occurrence of false positives, where screening has suggested an increased risk for the disease but the diagnostic test does not show evidence of the disease.
An accepted screening process must first meet certain criteria (NSC 2003). In addition to the illness being an important health problem, the screening process must benefit the individual. This includes the acceptability of the screening process clinically, socially and ethically and the availability of an effective treatment. These benefits must outweigh any possible harms such as discomfort from any testing and costs of administering the screening process. From an economic perspective, the screening process must also be cost effective.
Screening does not always involve a clinical test, and may include, for example, a series of history questions. Furthermore, it is important to distinguish screening from diagnostic procedures which provide a diagnosis in an already symptomatic or high-risk individual. It is important to distinguish between a screening and diagnostic test. While a screening test will identify those at risk for a disease, diagnostic tests are generally designed to give a definitive yes or no. Diagnostic tests are also often more complex and expensive than screening tests.
While screening tools will identify those at risk of an illness, it is the subsequent management of the result that ultimately affects health outcomes. ‘Screening’ can be used to refer to an individual screening tool or to a screening program, protocol or guideline, which includes the screening tool and subsequent management such as diagnostic testing and treatment of any illness identified. By identifying individuals at high and low risk of a particular illness, a screening tool therefore identifies those who require diagnostic testing and those who do not. It therefore follows that the implementation of a screening program, which includes a combination of screening tool, subsequent diagnostic testing and management, is able to affect health outcome.
Screening for GDM
Whether to screen for gestational diabetes, and which methods to use, remain controversial. This is compounded by the lack of clearly defined, universally accepted screening criteria, and the uncertainty as to the severity of glucose intolerance at which treatment is beneficial. Even with screening protocols in place, GDM is diagnosed at the end of the second trimester or early third trimester based on physiology. This leaves little time for management of GDM. Without screening, the diagnosis of GDM, and therefore treatment, is potentially delayed.
Screening for GDM is often implemented despite the uncertainty of its utility. A wide variety of strategies have been employed in screening for gestational diabetes that provide varying degrees of sensitivity and specificity. Universal or routine screening, usually where all women are offered a 50 g OGCT, and risk factor screening (by women’s history) are the most commonly used methods and combinations of these and other methods have been used to form various screening protocols (ACOG 2001; Gabbe 2004; Metzger 2007; Mires 1999; Rumbold 2001).
The OGCT was originally proposed by O’Sullivan and colleagues to provide a more sensitive screening process than risk factor screening (O’Sullivan 1973). An OGCT involves a 50 g glucose drink and a blood glucose measurement after one hour. While the predefined risk factors used vary between centres and countries, they commonly include maternal age, BMI, ethnicity, previous GDM and family history of diabetes mellitus (ADA 2003; Berger 2002; Hoffman 1998; Metzger 2007; USPSTF 2008). Other methods used to screen for GDM include urine testing for glucosuria, fructosamine testing, random plasma glucose measurements, fasting plasma glucose measurements and HbA1c (a measure of how well blood glucose has been controlled over the previous two to three months) (Scott 2002).
The variation in screening protocols is reflected in surveys conducted around the world. In a UK survey of obstetric units in 1996, it was found that 89% screened for GDM, with 81% of those units using risk factor based screening (Mires 1999). There was a lack of consensus on the appropriate screening method (Mires 1999). In a similar Australian survey of obstetric practice conducted in 1999, it was found that 87% of the obstetric population was being screened for GDM. Again there was no strong consensus on how to screen (Rumbold 2001). An American survey in 2004 found that 95.2% of obstetricians screening for GDM adopted a universal one hour 50 g OGCT (Gabbe 2004). This diversity in preferred screening protocols may reflect a number of factors, such as the cost of screening, the expected prevalence of GDM and test accuracy, in addition to the lack of definitive evidence in favour of a particular screening protocol.
A largely accepted time for screening is the end of the second trimester, ranging between 24 to 28 weeks’ gestation (ACOG 2001; Hoffman 1998; Metzger 2007; Oats 2004; RANZCOG 2008). This value reflects a balance between having adequate time to manage GDM and the ability to detect the development of carbohydrate intolerance (ACOG 2001; Brody 2003). There is little evidence on the benefits and detriments of screening prior to 24 weeks’ gestation (USPSTF 2008).
The negative impact of screening for GDM also needs to be considered. The importance of identification of GDM should be weighed against any discomfort experienced by the woman and anxiety from testing. While the 50 g OGCT is considered to be a quick and simple test, it is unpleasant to drink and is associated with side effects such as dizziness, headaches, nausea and vomiting, and requires a blood test. For many women, the inconvenience of testing can be significant. Screening by any method can create anxiety for the mother, including women identified as having risk factors for GDM, those identified through routine OGCT and those with elevated random blood glucose levels. In particular, a false positive result has been associated with a decline in women’s perception of health (Kerbel 1997; Rumbold 2002). It also follows that with the introduction of screening, that more women are offered diagnostic testing, usually an OGTT, which requires them to fast overnight, drink a higher glucose load, requires more blood tests and can take up to three hours to complete.
An evaluation of cost is imperative with any screening procedure. While screening processes may affect detection, management and therefore improve maternal and infant health, this must also be weighed against the cost of screening all pregnant women, any subsequent diagnostic tests and treatment for additional women diagnosed with GDM.
Why it is important to do this review
Many suggest that the incidence of GDM, the adverse outcomes arising from GDM and the benefit of treatment suggest a need for some screening process. However, high-quality evidence demonstrating the effectiveness of screening on detection of GDM and subsequent maternal and infant health is required for screening to be implemented (NSC 2003). Whether a screening protocol adequately identifies those at risk of GDM, and whether this knowledge improves the health outcomes for women with GDM and their babies through subsequent management of a screening result, are important factors to consider when recommending a screening process. It is also equally important that a recommended screening protocol does not harm women without GDM. Moreover, given the lack of consensus on a method for screening, an evaluation of the different screening protocols on the detection of GDM and subsequent maternal and infant health is required.
This review aims to evaluate the effects of screening for gestational diabetes mellitus as an intervention on maternal and infant health. The evaluation of test performance of individual screening methods is not included in the scope of this review. The strategies for diagnosis of GDM will be evaluated in a separate Cochrane review ‘Alternative strategies for glucose tolerance testing to diagnose gestational diabetes and impaired glucose tolerance during pregnancy’ currently in progress (Farrar 2008). The Cochrane review ‘Treatments for gestational diabetes’ (Alwan 2009) assesses management after diagnosis of GDM or impaired glucose tolerance and therefore is looking at a later stage in the pathways of care and management than this review which addresses the process from screening onwards
OBJECTIVES
To assess the effects of different methods of screening for gestational diabetes mellitus on maternal and infant outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, quasi-randomised controlled trials and cluster-randomised trials.
Types of participants
Pregnant women, excluding women who have already been diagnosed with gestational diabetes in this pregnancy or who have pre-existing diabetes mellitus.
Types of interventions
Any individual screening tool or screening program, protocol or guideline for gestational diabetes compared with the absence of screening; or any individual screening tool or screening program, protocol or guideline for gestational diabetes with another.
Types of outcome measures
Primary outcomes
Maternal
Perinatal
Diagnosis of gestational diabetes*;
positive screen for gestational diabetes*;
mode of birth (normal vaginal birth, operative vaginal birth, caesarean section).
Offspring
Neonatal
Large-for-gestational age (birthweight greater than or equal to 90th percentile);
macrosomia (greater than 4000 g or greater than 4500 g). * as defined by author(s)
Secondary outcomes
Maternal
Perinatal
Pre-eclampsia;
induction of labour;
perineal trauma;
weight gain in pregnancy;
augmentation of labour;
insulin or oral hypoglycaemic agent required to treat gestational diabetes;
women who screen positive and are not subsequently diagnosed with gestational diabetes mellitus;
placental abruption;
postpartum haemorrhage*;
postpartum infection*;
women’s sense of wellbeing and quality of life*.
Long-term
Development of type II diabetes mellitus;
gestational diabetes in subsequent pregnancy;
development of type I diabetes mellitus;
impaired glucose tolerance*;
insulin sensitivity*;
body mass index (BMI);
BMI greater than 25;
BMI greater than 30;
women’s sense of wellbeing and quality of life*.
Offspring
Neonatal
Stillbirths;
death of liveborn infants prior to hospital discharge;
infant death (up to one year of life);
shoulder dystocia;
bone fractures;
nerve palsy;
birthweight;
birth centile;
ponderal index;
gestational age at birth;
preterm birth (less than 37 weeks’ gestation);
respiratory distress syndrome;
hypoglycaemia requiring treatment;
hyperbilirubinaemia requiring treatment;
five minute Apgar score less than seven;
five minute Apgar score less than four.
Childhood
BMI;
BMI greater than 25;
BMI greater than 30;
weight;
height;
fat mass/fat-free mass;
skinfold thickness measurements;
blood pressure;
impaired glucose tolerance*;
type I diabetes;
type II diabetes;
insulin sensitivity*;
dyslipidaemia.
Adulthood
BMI;
BMI greater than 25;
BMI greater than 30;
weight;
height;
fat mass/fat-free mass;
skinfold thickness measurements;
blood pressure;
impaired glucose tolerance*;
type I diabetes;
type II diabetes;
insulin sensitivity*;
dyslipidaemia;
educational achievement.
Acceptability of testing
Side effects of testing (e.g. nausea, vomiting);
women’s acceptance of screening protocol*.
Costs
Cost of screening each woman;
number of hospital visits/antenatal visits for mother;
dietitian visits;
medical physician visits;
length of postnatal stay (mother);
length of postnatal stay (baby);
cost of maternal care;
cost of offspring care.
* as defined by author(s)
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (April 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two authors individually assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreement through discussion, or consulted a third review author.
Data extraction and management
We designed a form to extract data. Two review authors extracted data using the agreed form. We resolved discrepancies through discussion. We used Review Manager software (RevMan 2008) to enter all data.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
adequate (e.g., random number table; computer random number generator; tossing a coin, minimisation);
inadequate (odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will also provide any information relating to whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
In this assessment, we defined ‘adequate’ as when there was blinding or where we assess that the outcome or the outcome measurement is not likely to have been influenced by lack of blinding.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported; the numbers (compared with the total randomised participants); reasons for attrition or exclusion where reported; and any re-inclusions in analyses which we undertook.
We assessed the methods as:
adequate (e.g. where there was no missing data or where reasons for missing data are balanced across groups);
inadequate (e.g. where missing data are likely to be related to outcomes or are not balanced across groups);
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to me made).
(5) Selective reporting bias
We described for each included study how we examined the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data-dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses - see ‘Sensitivity analysis’.
Measures of treatment effect
We planned an assessment of trials comparing any screening protocol with none, with data analysed separately for different methods of screening. We analysed trials comparing one method of screening with another, with data from different comparisons analysed separately. We carried out statistical analysis using the Review Manager software (RevMan 2008). We used random-effects meta-analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If we had found heterogeneity, we planned to explore this by sensitivity analysis followed by random-effects if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods. If there is evidence of skewness, we would have reported this.
Unit of analysis issues
Cluster-randomised trials
We planned to include cluster-randomised trials in the analyses along with individually randomised trials. Their sample sizes were to be adjusted using the methods described in Gates 2005 using an estimate of the intracluster correlation co-efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources were used, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster-randomised trials and individually randomised trials, we planned to synthesise the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We would also acknowledge heterogeneity in the randomisation unit and perform a separate meta-analysis. Therefore, we would perform the meta-analysis in two parts as well.
Cross-over trials
We excluded cross-over trials due to the nature of the primary outcomes we considered; and because of the difficulties in extracting the relevant data.
Dealing with missing data
We aimed to analyse data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there is sufficient information in the trial report, we planned to attempt to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I2 statistic. Where we identified high levels of heterogeneity among the trials (exceeding 50%), we planned to explore it by prespecified subgroup analysis and perform sensitivity analysis.
Subgroup analyses
We planned to conduct subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001.
We planned to carry out the following subgroup analyses for primary outcomes:
high risk for gestational diabetes mellitus (variously defined) (we will explore risk factors individually if sufficient data become available);
gestational age at screening (less than 24 weeks, 24 to 30 weeks, 30 weeks or more);
number of stages in the screening protocol;
type of management protocol.
There were insufficient data to conduct subgroup analyses.
Sensitivity analyses
We planned to carry out sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment (rated inadequate). If cluster-randomised trials or quasi-randomised trials had been included, we planned to perform sensitivity analysis of these trials.
We would then carry out sensitivity analysis to explore the effect of trial quality on primary outcomes. This would have involved analysis based on an assessment of selection bias and attrition bias. We planned to exclude studies of poor quality in the analysis (those rating unclear or inadequate) in order to assess for any substantive difference in the overall result.
There were insufficient data to conduct sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The Cochrane search identified 31 trials to be considered for inclusion into the review. Following application of eligibility criteria, we included four of these trials in this review (Bergus 1992; Griffin 2000; Martinez Collado 2003; Murphy 1994); we excluded 25 and two are awaiting assessment.
Included studies
One quasi-randomised study compared the effect of screening by risk factors and universal (routine) screening by a 50 g oral glucose challenge test (OGCT) on health outcomes (Griffin 2000). Three studies compared screening by different glucose loading methods (Bergus 1992; Martinez Collado 2003; Murphy 1994). Of the four studies, Griffin 2000 was the largest study with 3742 women enrolled in the study. Murphy 1994 recruited 124 women, Bergus enrolled 76 women into the study and Martinez Collado 2003 included 30 women.
Participants
All studies included pregnant women in Western societies. Bergus 1992 and Murphy 1994 were conducted in the United States, while Griffin 2000 took place in Ireland and Martinez Collado 2003 recruited women in Mexico. Women in all studies were recruited from obstetric clinics. Gestational age at entry was specified in Bergus 1992 and Martinez Collado 2003, where women were between 24 and 28 weeks’ gestation. Murphy 1994 screened women routinely at 24 to 28 weeks’ gestation and also screened women at their first antenatal visit if they were found to have one of the following risk factors for gestational diabetes: past history of glucose intolerance, first-degree relative with diabetes mellitus, age greater than 35 years, past macrosomia, habitual abortion, unexplained stillbirth, congenital anomalies, current pregnancy with glycosuria, hypertension, suspected large-for-gestational-age fetus, polyhydramnios or obesity. Martinez Collado 2003 included women with a ‘high-risk’ pregnancy, although a description of ‘high risk’ was not reported. This trial excluded women with diabetes, previously diagnosed gestational diabetes and those treated with steroid or tocolytics. No other exclusion criteria were listed for the studies.
Baseline characteristics of the participants by treatment group were compared in two studies (Griffin 2000; Murphy 1994). There was an imbalance in age and parity at screening with participants in the candy bar group being younger and having a lower parity than those in the d-Glucose group. No baseline imbalances were reported between women in the candy bar group and the polymer group. Participants in the risk factor group and universal group in Griffin 2000 were similar with respect to age, weight at 36 weeks, body mass index, gestational age at delivery, parity and prevalence of risk factors for gestational diabetes.
Interventions
Risk factor versus universal (routine) screening
Griffin 2000 compared risk factor screening with universal (routine) screening. Participants in the risk factor group of Griffin 2000 were screened on the basis of historical and current risk factors, including having a first-degree relative with diabetes mellitus, weighing more than 100 kg in the current pregnancy, having a previous baby greater than 4.5 kg, previous unexplained still-birth or intrauterine death, previous major malformation, previous gestational diabetes, glycosuria in second fasting urine sample, macrosomia in the current pregnancy and polyhydramnios in the current pregnancy. Women received glucose testing by a 100 g oral glucose tolerance test (OGTT) at 32 weeks’ gestation where they were found to have any of the risk factors listed.
The universal screening group used a 50 g OGCT at 26 to 28 weeks’ gestation. A one-hour plasma glucose of greater than or equal to 7.8 mmol/L was considered positive. A positive screening test was an indication for a full 100 g OGTT using the National Diabetes Data Group criteria for diagnosis. The 50 g OGCT was repeated in those with a negative OGCT and with risk factors for gestational diabetes four to six weeks after the initial OGCT. Women who were diagnosed with gestational diabetes were treated by standard diabetes management, maintaining otherwise similar antenatal care for both groups. Griffin 2000 referred women for obstetric and endocrinology review fortnightly and weekly after 36 weeks’ gestation, with treatment including diabetic diet and insulin as required.
Glucose method
Bergus 1992, Martinez Collado 2003 and Murphy 1994 compared different methods of glucose loading as screening tests for gestational diabetes. Both Bergus 1992 and Murphy 1994 assessed solutions of glucose monomer (d-glucose) and glucose polymer. Murphy 1994 included an additional group where participants ate 50 g chocolate bars in place of a glucose drink. In both studies, all women underwent a glucose tolerance test within three to seven days of their 50 g challenge test. Both used a 100 g OGTT by O’Sullivan criteria to diagnose gestational diabetes. Martinez Collado 2003 compared a 50 g glucose solution with a food mix, which included 50 g of glucose, and did not report on further testing to diagnose gestational diabetes. It is not reported whether the glucose solution is a glucose monomer or polymer.
Outcomes
Griffin 2000 reported clinical measures of maternal health outcome and infant health outcome and size. Bergus 1992, Martinez Collado 2003 and Murphy 1994 focused primarily on the efficiency of the methods by which glucose was administered, reporting on diagnosis of gestational diabetes, glucose levels following testing and the adverse effects of the different methods. Murphy 1994 reported side effects only where they were rated moderate to severe by women on a five-point scale.
Excluded studies
Fifteen studies identified by the literature search assessed strategies for diagnosis of gestational diabetes rather than screening (Berkus 1995; Brustman 1995; Buhling 2004; Cheng 1992; Court 1984; Court 1985; Fung 1993; Harlass 1991; Jones 1993; Olarinoye 2004; Sammarco 1993; Soonthornpun 2003; Stavrianos 2004; Weiss 1998; Zhang 1995). One study was not randomised (Dornhorst 2000). Seven of the trials identified were crossover studies (Eslamian 2007; Eslamian 2008; Helton 1989; Hidar 2001; Lamar 1999a; Lamar 1999b; Soonthornpun 2008) and two studies included women who had already undergone diagnostic testing for gestational diabetes (Kjos 2001; Lewis 1993).
Risk of bias in included studies
Allocation
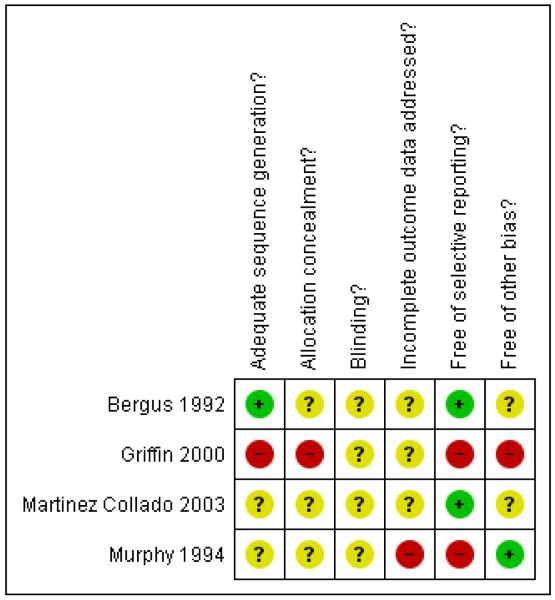
The generation of randomisation sequence was specified only by Bergus 1992 and Griffin 2000. Bergus 1992 used consecutive numbers from a random number table to allocate participants to study group while Griffin 2000 was quasi-randomised, allocating women to study group by the day of their clinic visit. While reported as randomised controlled trials, Martinez Collado 2003 and Murphy 1994 did not specify the method of allocation of participants.
None of the studies reported allocation concealment.
Blinding
Bergus 1992 describes a ‘double-blind design’, but did not specifically state who was blinded. Griffin 2000, Martinez Collado 2003 and Murphy 1994 did not report on blinding of participants, clinicians or outcome assessors.
Incomplete outcome data
Ten out of 76 women (13%) did not complete the symptom questionnaire in Bergus 1992 and it is reported that their baseline characteristics were comparable to those who were followed up. Griffin 2000 reported that 590, or 31%, of the women in the universal screening group did not consent to glucose challenge testing and were excluded from analysis. No participants in the risk factor screening group were lost to follow up. Routine care in this centre was risk factor screening, which contributes to the differential loss to follow-up rate. There were no significant differences between those who consented to glucose challenge testing and those who did not in Griffin 2000. Sixteen women in Murphy 1994 were lost to follow up. However, no comparison of baseline characteristics of those lost to follow up and those who remained in the study was made and it is unclear which groups these women were from. It is also uncertain as to how many women in Murphy 1994 were being screened in their first trimester or at 24 to 28 weeks’ gestation.
Selective reporting
Outcome data in Griffin 2000 was analysed primarily by gestational diabetes diagnosis rather than by original group allocation by day of visit, which affects the ability to interpret results of pre-specified outcomes. Although a comparison of baseline characteristics between women who were able or unable to complete the symptom questionnaire was made, Bergus 1992 did not report the baseline characteristics of participants by intervention group. Murphy 1994 only reported side effects of testing were women had rated their symptoms as moderate or severe, equivalent to a three to five out of a possible five. Mild symptoms were unreported.
Other potential sources of bias
Analysis of Griffin 2000 was based on diagnosis of gestational diabetes rather than original group allocation by day of visit. Because no baseline comparison of the intervention groups was made in Bergus 1992 and Martinez Collado 2003, it is uncertain whether or not baseline imbalances are present.
Overall risk of bias
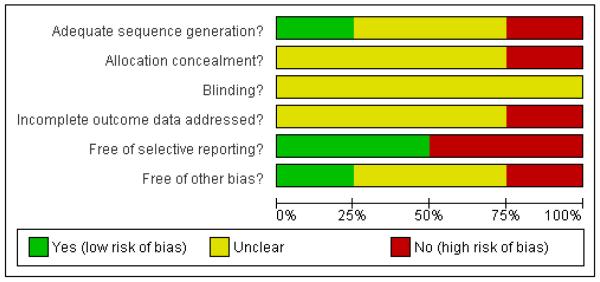
In general, assessment of the included studies for methodological quality revealed a moderate to high risk of bias, which is likely to have affected the results of the review (Figure 1; Figure 2) by making the results of trials less certain. Most studies were unclear or did not adequately report on sequence generation, allocation concealment and blinding. Missing data affected the assessment of incomplete outcome data, and selective reporting and other biases were also likely.
Figure 1 . Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 2 . Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Effects of interventions
We included four studies (Bergus 1992; Griffin 2000; Martinez Collado 2003; Murphy 1994), with data available from 3382 of the 3972 women randomised. One study compared risk factor screening with routine screening and three trials compared the method of glucose administration at screening.
Risk factor versus routine screening
Primary outcomes
More women were diagnosed with gestational diabetes in the universal screening group than the risk factor screening group (Griffin 2000). Thirty-five women were diagnosed with GDM in the routine screening group compared with 22 in the risk factor group (one trial, 3152 women, risk ratio (RR) 0.44, 95% confidence interval (CI) 0.26 to 0.75) (Analysis 1.1).
Secondary outcomes
Infants of mothers in the risk factor screening group were born earlier than infants of mothers in the routine screening group (one trial, 3152 women, mean difference (MD) −0.15 weeks, 95% CI −0.27 to −0.53) (Analysis 1.2).
The remaining data from this trial were reported according to diagnosis of GDM and we are in correspondence with the authors of this paper for additional data. In particular, data were collected for the primary outcomes of mode of birth, large-for-gestational age and macrosomia.
Glucose monomer versus glucose polymer
Primary outcomes
Two studies, Bergus 1992 and Murphy 1994, compared a glucose monomer (d-glucose) with a glucose polymer drink for screening for gestational diabetes. No women were diagnosed with gestational diabetes in either group in Bergus 1992. Three women in the glucose monomer group and two women in the glucose polymer group were diagnosed with GDM in Murphy 1994 (two trials, 161 women, RR 1.61, 95% CI 0.28 to 9.15) (Analysis 2.1). Numbers of women screening positive also showed no significant differences between groups (RR 2.36, 95% CI 0.90 to 6.21) (Analysis 2.2).
Secondary outcomes
Both trials reported a number of side effects from the method of glucose administration. Although not pre-specified, any symptom, sick, tired, taste and bloating were included as measures of acceptability of testing. Women in the glucose monomer group were more likely to experience ‘any symptom’ than those in the glucose polymer group (two trials, 151 women, RR 2.80, 95% CI 1.10 to 7.13). There was significant heterogeneity in this result, with an I2 value of 61%)(Analysis 2.3). Nausea was experienced more often by women in the glucose monomer group than glucose polymer (two trials, 151 women, RR 2.62, 95% CI 1.01 to 6.79). There was no heterogeneity in this result (I2 = 0%). No statistically significant difference was found for all other measures of acceptability of testing, including headache, dizziness, abdominal discomfort, vomiting, feeling sick, tired, bloating; or for taste in one trial (Analysis 2.4).
Neither study reported on additional maternal and infant health outcomes.
Glucose monomer versus candy bar
Primary outcomes
Murphy 1994 included a third group, who consumed a chocolate bar as an alternative to the two types of glucose drinks. There was no significant difference in diagnosis of gestational diabetes between the candy bar and glucose monomer groups, with three women diagnosed with GDM in the glucose monomer group, compared with none in the candy bar group (one trial, 80 women, RR 6.67, 95% CI 0.36 to 125.02) (Analysis 3.1). However, significantly more women in the monomer group screened positive for GDM (RR 3.49, 95% CI 1.05 to 11.57) (Analysis 3.2).
Secondary outcomes
The candy bar was given the highest rating for taste more often than glucose monomer (one trial, 80 women, RR 0.35, 95% CI 0.17 to 0.74) (Analysis 3.4). No significant differences were seen overall (RR 1.90, 95% CI 0.97 to 3.72) or for each individual symptom of dizziness, nausea, abdominal discomfort and bloating (Analysis 3.3).
No maternal and infant health outcomes were reported for this comparison.
Glucose polymer versus candy bar
Primary outcomes
No significant difference was found in diagnosis of gestational diabetes between the two groups (one trial, 83 women, RR 4.44, 95% CI 0.22 to 89.84) (Analysis 4.1) or for screening positive (RR 1.48, 95% CI 0.38 to 5.78) (Analysis 4.2).
Secondary outcomes
Again, the candy bar was preferred for taste more often than the glucose polymer drink (one trial, 83 women, RR 0.42, 95% CI 0.22 to 0.82) (Analysis 4.4). Other measures of acceptability of testing were not significantly different between the two groups (RR 0.39, 95% CI 0.13 to 1.18) (Analysis 4.3).
No maternal and infant health outcomes were reported for this comparison.
Glucose solution versus food
Primary outcomes
In one trial (Martinez Collado 2003), there was no significant difference in having a positive screening test for gestational diabetes between the two glucose solution and food mix groups (30 women, RR 7.00, 95% CI 0.39 to 124.83) (Analysis 5.1).
Secondary outcomes
Women receiving the glucose solution were more likely to experience a side effect of the screening, including nausea, vomiting, migraine, diarrhoea and feeling sick, than those receiving the food mix (80% versus 7%, one trial, 30 women, RR 12.00, 95% CI 1.78 to 81.06) (Analysis 5.2).
No maternal and infant health outcomes were reported for this comparison.
There were insufficient data to perform subgroup analyses for all comparisons of the review.
DISCUSSION
Gestational diabetes mellitus (GDM) is widely accepted as a serious health issue, associated with serious maternal and infant morbidity. Recent, high-quality evidence exists to suggest that treatment of GDM is beneficial (Crowther 2005; Landon 2009). The variation in screening practices and guidelines, both in national surveys and worldwide, further demonstrates the need for large, high-quality trials evaluating the effect of screening for gestational diabetes (ACOG 2001; ADA 2003; CDA 2008; Gabbe 2004; Hanna 2008; Mires 1999; NICE 2008; Oats 2004; RANZCOG 2008; Rumbold 2001). Despite its use, this review found little evidence on the effect of screening on maternal and infant health outcomes. This is consistent with other reviews on screening for gestational diabetes (Hillier 2008; Hollander 2007; NICE 2008; Scott 2002).
We included three trials evaluating various methods of screening for GDM, which can be considered in two categories. Griffin 2000 evaluated different screening protocols. Bergus 1992, Martinez Collado 2003 and Murphy 1994 compared different types of glucose for a 50 g OGCT.
Griffin 2000 was a large quasi-randomised trial comparing universal screening by 50 g OGCT with risk factor screening and reported on diagnosis of gestational diabetes, positive screening for gestational diabetes and gestational age at birth. Of the 1299 women in the universal screening group who completed an OGCT, 366 were referred for an oral glucose tolerance test (OGTT). By comparison, fewer women (249 of the 1853 women in the risk factor group) were referred for an OGTT. Unsurprisingly, with more women offered diagnostic testing for GDM in the universal screening group, more women were diagnosed with GDM. It is, however, difficult to interpret how these increases in diagnoses and subsequent management translate to clinical maternal and infant health outcomes in the review. The marginal reduction seen in gestational age at birth is likely a result of the large sample size rather than a clinically relevant difference. The trial reported on the remaining health outcome data with women reorganised into three groups, those who were not diagnosed with GDM, women who were diagnosed with GDM from the universal screening group and those diagnosed with GDM in the risk factor screening group.
While more women received glucose testing in the universal screening group, compared to those in the risk factor group, Griffin 2000 did not report on women’s views on the two forms of screening. In addition to side effects of receiving a glucose load, practical issues for testing and the anxiety from screening and false positive results, the anxiety created by receiving an abnormal result on screening compared with receiving information on background risk of GDM is also important in selecting an appropriate screening protocol. Women’s views on their health status and the screening protocol would be further affected by whether or not they are subsequently diagnosed and managed for GDM. Given that some screening protocols will result in more women being diagnosed with GDM than others, this may influence women’s views on their health status.
Furthermore, although management of diagnosed gestational diabetes improves maternal and infant health outcomes, the diagnosis of GDM may also be associated with increased intervention or monitoring such as induction of labour and neonatal intensive care unit admission (Alwan 2009). Therefore, with various protocols for screening, the subsequent management of women with a positive or negative screening result as part of these protocols may impact on maternal and infant health. Large studies are required to address these issues and given that only a proportion of women are subsequently diagnosed with GDM, these trials require sufficient power for subgroup analyses by diagnosis of GDM are meaningful.
Interestingly, women in the risk factor group with risk factors for GDM received an oral glucose tolerance test at 32 weeks. Women in the universal screening group underwent a glucose challenge test at 26 to 28 weeks’ gestation and were referred for subsequent OGTT if this was positive. Further to this, if glucose testing was negative, repeat screening was performed where the woman had risk factors for GDM. The risk factor group is therefore more likely to be diagnosed at a later stage in pregnancy than those in the universal screening group. It is unclear, however, if the timing of glucose testing has affected health outcomes.
The trials (Bergus 1992; Martinez Collado 2003; Murphy 1994) evaluating different types of glucose for the challenge test were primarily interested in the side effects and number of women diagnosed by different forms of glucose. Therefore, unlike Griffin 2000, the women in these trials were all offered the same diagnostic testing pathway regardless of the screening result. Although no health outcome data were included in the review, data on health outcome would be unlikely to be affected by the screening test because the women received the same subsequent management.
Bergus 1992 and Murphy 1994 included a comparison of glucose monomer (d-glucose) and glucose polymer. Overall, fewer side effects were reported by women receiving a glucose polymer drink than glucose monomer. There was, however, significant heterogeneity in this result, with a stronger effect seen in Murphy 1994 than Bergus 1992. This may be explained by the different approaches taken by the trials, with Murphy 1994 only reporting side effects where they were rated moderate to severe. Bergus 1992 however used a binary approach. The differences in data presentation therefore limit interpretation of these outcomes. Maternal and infant health outcomes were not recorded by the trials. Although not statistically significant, the glucose polymer group generally reported fewer side effects in the moderate to severe range than the candy bar group.
Interpretation of the results of this arm of the review are, however, limited by the number of participants in each trial. Although women were screened at 24 to 28 weeks’ gestation in the trials, Murphy 1994 also included women in their first trimester with risk factors for GDM and Martinez Collado 2003 included women with a ‘high-risk’ pregnancy. Both trials used a 100 g OGTT for diagnosis of GDM.
The results of this review were limited by the number of participants and methodological quality of the trials, which, overall, was assessed to be moderate to low. Therefore, the results of this review are to be interpreted with caution. Bergus 1992, Martinez Collado 2003 and Murphy 1994 included a total of 230 women and were both primarily interested in the side effects and numbers of women diagnosed by different forms of glucose. As a consequence, most of the maternal and infant health outcomes included in this review were not reported by these trials. While Griffin 2000 was a large quasi-randomised trial enrolling 3792 women, the format of outcome reporting precluded the outcome data of interest for this review from being included.
The trials included in this review were conducted in the United States, Ireland and Mexico. Geographical location, socio-economic circumstances and ethnicity are important factors that can alter the most appropriate method of screening with regard to feasibility and practicality. Compliance is probably not only related to side effects, as investigated by Bergus 1992, Martinez Collado 2003 and Murphy 1994, but also the requirements for the screening protocol. For example, the inconvenience posed by a one-hour 50 g OGCT should be considered against random capillary blood glucose testing or risk factor screening. This emphasises the need for future research to report not only on subsequent management and maternal and infant health outcomes, but also on the acceptability of and adherence to particular screening protocols.
One trial (Bebbington 1999) published as an abstract, and awaiting assessment, also compared universal and selected screening in 2401 women with no reported risk factors for gestational diabetes. In abstract form, there were insufficient data to include results in the review. The abstract reports no significant difference in birth-weight and the incidence of macrosomia between the two groups. Although no trial evaluating screening with no screening for GDM was included in the review, there is one trial awaiting assessment reported as an abstract that has not yet been published (Meltzer 2005). This trial compared screening by 50 g OGCT with 100 g OGTT if required, 50 g OGCT with a 75 g OGTT if required or 75 g OGTT only. This trial has been planned to report on the cost-effectiveness of the different protocols and maternal and infant health outcomes.
AUTHORS’ CONCLUSIONS
Implications for practice
There was insufficient evidence from this review to determine the effects of screening for gestational diabetes and its subsequent management, or the comparative effects of different protocols for screening. Although women who were routinely screened by 50 g glucose challenge testing were more likely to be diagnosed with gestational diabetes than those screened by their risk factors, effects of subsequent management on health outcome are unclear.
Implications for research
Large, high-quality trials are required to evaluate the effects of screening and subsequent management of gestational diabetes. As only a proportion of women will be subsequently diagnosed with gestational diabetes in these trials, a large number of participants are required for sufficient power to detect statistically significant differences and subgroup analyses by diagnosis. Future studies should assess the value of screening compared with no screening in addition to comparing different types of screening tools. The 50 g oral glucose challenge test and screening tools that are more easily implemented such as risk factor screening, glucosuria and the use of capillary blood glucose testing need to be evaluated as part of screening protocols. Furthermore, assessment of the optimal gestational age for screening is required. Trials should include data on health outcomes for mother and baby, acceptability of the screening protocol and cost effectiveness.
PLAIN LANGUAGE SUMMARY.
Screening for gestational diabetes and subsequent management for improving maternal and infant health
Gestational diabetes mellitus (GDM) is a form of diabetes that can develop during pregnancy, usually toward the end of the second trimester. Having GDM increases the risk of complications during the rest of the pregnancy. Women with GDM are more likely to develop pre-eclampsia (a combination of high blood pressure and protein in the urine) and require a caesarean section. For the baby, potential problems include the baby growing larger than it normally would, causing difficulties with birth. The baby can also have low blood sugar levels after birth. Although GDM usually resolves following birth, both mother and child are at risk of developing type 2 diabetes in the future. There is strong evidence that treatment of GDM is beneficial and improves health outcomes.
It may therefore help if pregnant women are screened to identify as many as possible of those who do have GDM before they have symptoms, such as excessive thirst, frequent urination or fatigue. The two main approaches to screening approaches are ‘universal’ where all women undergo a screening test for GDM; and a selective approach where only those women at high risk are screened. The main risk factors are maternal age, high body mass index, family history and cigarette smoking. The different screening strategies used around the world to identify women with GDM include identifying women based on their risk factors, a blood sugar test one hour after a 50 g glucose drink, and random blood sugar measurements. It is however unclear whether screening for GDM leads to better health outcomes and if so, which screening strategy is the most appropriate.
This review of four trials involving 3972 women found that there is little high-quality evidence on the effects of screening for GDM on health outcomes for mothers and their babies. Further research is required to see which recommendations for screening practices for gestational diabetes are most appropriate.
ACKNOWLEDGEMENTS
We thank Dr George Bergus for contributing extra information and clarification on Bergus 1992. We also acknowledge the efforts of Dr Richard Firth and Dr Michael Bebbington in locating extra data.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. Funding: unspecified. |
|
| Participants | Location: Ketchikan Native Health Clinic, Ketchikan, Alaska and Mt. Edgecumbe Hospital, Sitka, Alaska Inclusion criteria: pregnant native Alaskan women at 24-28 weeks’ gestation, with no history of diabetes mellitus, between January 1988 and May 1990 Exclusion criteria: none specified. 76 women were enrolled into the study. |
|
| Interventions | Participants received either a 50 g glucose monomer or 50 g glucose polymer according to their randomly allocated group, regardless of time of last meal Both groups: venous and capillary samples were collected after 1 hour. A result of greater than or equal to 7.8 mmol/L was considered positive. All participants underwent a 100 g 3 hour oral glucose tolerance test within 3 days of their glucose challenge test |
|
| Outcomes | Maternal: venous plasma glucose following glucose challenge test, capillary blood glucose following glucose challenge test, oral glucose tolerance test and symptom questionnaire (’felt sick’, ’felt nauseated’, ’headache’, ’felt dizzy’, ’felt bloated’, ’felt tired’, ’vomited’ and ’felt abdominal discomfort’) Infant: none. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ’Randomisation was achieved by using consecutive numbers from a random number table.’ |
| Allocation concealment? | Unclear risk | Not specified. |
| Blinding? All outcomes |
Unclear risk | ’Double-blind’ implies that participants and personnel were blinded to randomised group, although this is not stated. Blinding of outcome assessors was not reported |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | ’10 women did not complete the symptom questionnaire, but their baseline characteristics did not differ significantly from those with complete data collection.’ These women (13%) were excluded from the analysis of symptoms |
| Free of selective reporting? | Low risk | All pre-specified outcomes were reported. |
| Free of other bias? | Unclear risk | Baseline characteristics were not reported. It is therefore, unclear whether baseline imbalances exist |
| Methods | Quasi-randomised controlled trial. Funding: grant from Bayer Diagnostics and research grant from National Maternity Hospital, Dublin |
|
| Participants | Location: outpatient obstetric clinics at the National Maternity Hospital, Dublin Inclusion criteria: first visit to outpatient clinic of National Maternity Hospital, over a 24-month period Exclusion criteria: none specified. 3742 women were enrolled into the study, with 1853 randomised to the risk factor screening group and 1889 randomised to the universal screening group |
|
| Interventions | Risk factor group: 100 g 3-hour oral glucose tolerance test performed at 32 weeks’ gestation if any risk factors are present (historical - first-degree relative with diabetes mellitus, > 100 kg in current pregnancy, previous baby >4.5 kg, previous unexplained stillbirth/intrauterine death, previous major malformation, previous gestational diabetes mellitus, current - glycosuria in 2nd fasting urine sample, macrosomia in current pregnancy or polyhydramnios in current pregnancy) Universal group: 50 g 1 -hour oral glucose challenge test at 26-28 weeks’ gestation without regard to time of last meal. This was considered positive if 1-hour plasma glucose was greater than or equal to 7.8 mmol/L. In those with a positive glucose challenge test, a 100 g oral glucose tolerance test was performed (using National Diabetes Data Group criteria). Women with risk factors for GDM (i.e. those listed for the risk factor group) , had a repeat OGCT if the first OGCT was negative or if the OGTT was negative (following a positive OGCT) Both groups: there was uniform diabetic and obstetric management for all participants, regardless of randomly allocated screening group. Women diagnosed with GDM were referred to both an obstetrician and endocrinologist, reviewed every 2 weeks until 36 weeks and awaited until 42 weeks unless medically contraindicated. All participants diagnosed with GDM were instructed in appropriate diabetic diet and intensive insulin treatment was instituted if fasting and postprandial (1.5 hour) blood glucose following a standard breakfast were not maintained (< 5.9 mmol/L or < 7.9 mmol/L respectively) |
|
| Outcomes | Maternal: diagnosis of gestational diabetes, spontaneous vaginal birth at term, emergency caesarean section, pre-eclampsia and insulin treatment required Infant: LGA, macrosomia (> 4500 g), hypoglycaemia, hyperbilirubinaemia, gestational age at birth, ponderal index, admission to neonatal intensive care unit and preterm birth |
|
| Notes | We are in correspondence with authors for additional data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | High risk | This trial was quasi-randomised. ’Randomisation to group was performed on the basis of which day the clinic was held as patients were randomly assigned clinics at booking.’ |
| Allocation concealment? | High risk | Not feasible since randomisation was based on the day women came to the clinic |
| Blinding? All outcomes |
Unclear risk | Not specified. It was probably unfeasible for the study to blind participants and personnel. Blinding of outcome assessors was not reported |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | ’There were no significant differences between those women who consented to glucose challenge test and those who refused with respect to weight, BMI, age, socioeconomic group or presence of risk factors for GDM.’ 590 (31%) women in the universal group were lost with no losses in the routine care group seen since routine care in this centre is the care received in the risk factor group |
| Free of selective reporting? | High risk | Outcome data for participants were not available by original group allocation by day of visit, and were analysed by GDM diagnosis rather than randomly allocated group, affecting interpretation of outcome data |
| Free of other bias? | High risk | As mentioned above, outcome data were analysed by GDM diagnosis rather than by original group allocation by day of visit |
| Methods | Randomised controlled trial. Funding: not specified. |
|
| Participants | Location: Hospital Regional 1° de Octubre. Inclusion criteria: pregnant women at 24 to 28 weeks’ gestation with a high-risk pregnancy Exclusion criteria: women with diabetes mellitus, previously diagnosed with gestational diabetes or on steroid or tocolytic therapy 30 women were enrolled into the study, with 15 allocated to a 50 g glucose challenge test and 15 women allocated to receive a food mix |
|
| Interventions | Glucose challenge test group: received a 50 g glucose solution orally Food group: received food mix containing carbohydrate, protein, fats and 50 g of glucose A venous blood sample was taken 1 hour after consuming the glucose solution or food mix. A glucose level greater than 140 mg/dL was considered abnormal |
|
| Outcomes | Maternal: positive screen for gestational diabetes mellitus and tolerance to food or solution Infant: none specified. |
|
| Notes | Study was reported in Spanish. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | The allocation of participants is described as being ’randomly assigned’, no further information is provided on the sequence generation |
| Allocation concealment? | Unclear risk | Not specified. |
| Blinding? All outcomes |
Unclear risk | Not specified. It is likely that blinding of participants was unfeasible. Blinding of study personnel and outcome assessors is not reported |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Losses to follow up were not reported. |
| Free of selective reporting? | Low risk | The prespecified outcomes of outcome of venous blood sample and tolerance to food or solution were reported |
| Free of other bias? | Unclear risk | There was no baseline comparison of participants in the 2 groups |
| Methods | Randomised controlled trial. Funding: not specified. |
|
| Participants | Location: Saint Luke’s Hospital, Kansas City, Missouri. Inclusion criteria: pregnant women at the Medical Education Clinics, Saint Luke’s Hospital, Kansas City, Missouri Exclusion criteria: none specified. Of the 124 women were enrolled into the study, 44 were allocated to group 1 (glucose polymer), 41 were allocated to group 2 (d-glucose) and 39 were allocated to group 3 (candy bar). 16 of the 124 women were unable to complete the glucose tolerance test within 1 week of screening, as required by all participants. Of these 16 women, 5 vomited or were too symptomatic and 11 did not complete the glucose tolerance test for logistical reasons or incomplete data |
|
| Interventions | Those recruited, following hospital protocol, were screened at 24-28 weeks. Where the woman possessed one of the following risk factors, they also receive GDM screening at their initial visit (past history of glucose intolerance, first-degree relative with diabetes mellitus, age > 35 years, past macrosomia, habitual abortion, unexplained stillbirth, congenital anomalies or current pregnancy with glycosuria, hypertension, suspected large-for-gestational-age fetus, polyhydramnios or obesity). For each of the screening methods, the carbohydrate source was ingested without regard to time of last meal. Within 1 week of the screening test, all women were required to undergo a 100 g 3 hour glucose tolerance test (in 300 ml of carbonated water). GDM was diagnosed using O’Sullivan criteria Group 1 (n = 44): participants received 50 g of glucose polymer. This was made from 100 ml of 43% polymer solution with 1.5 g unsweetened flavouring and 50 ml of unsweetened club soda Group 2 (n = 41): participants received the standard 50 g d-glucose solution in 300 ml of carbonated water Group 3 (n = 39): participants received a total of 50 g of candy bar (containing milk chocolate, sucrose, corn syrup, partially hydrogenated soya bean oil, cocoa, salt, egg whites, malt extract, soybean protein and artificial flavour) |
|
| Outcomes | Maternal: diagnosis of GDM, serum glucose values following screening and symptom questionnaire (taste, abdominal pain, bloating, dizziness and nausea) Infant: none reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not specified. Women were ’prospectively enrolled and randomly assigned to receive 1 of 3 different carbohydrate sources for their GDM screening’ |
| Allocation concealment? | Unclear risk | Not specified. |
| Blinding? All outcomes |
Unclear risk | Not specified for participants, clinicians and outcome assessors |
| Incomplete outcome data addressed? All outcomes |
High risk | Data from 16 of the 124 women (13%) were not included in the final analysis as they were unable to complete the glucose tolerance test within 1 week. Of these women, 5 were unable to complete because they became symptomatic during the test and the remaining 11 were unable to complete the test for logistical reasons or because there was incomplete laboratory data. The number of women lost to follow up from each treatment group is not reported. A comparison of baseline characteristics was not made on those lost to follow up and those not Furthermore, it is not reported how many of the women included in the study were screened in their first trimester or at the standard 24 to 28 weeks’ gestation |
| Free of selective reporting? | High risk | Side effects were only reported where the women rated it in the moderate to severe range (3 to 5 out of a possible 5). Therefore, mild symptoms were not reported |
| Free of other bias? | Low risk | No obvious source of other bias. |
BMI: body mass index
LGA: large-for-gestational age
GDM: gestational diabetes mellitus
OGCT: oral glucose challenge test
OGTT: oral glucose tolerance test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Berkus 1995 | Study assessed strategies for diagnosis of GDM, not screening |
| Brustman 1995 | Study assessed strategies for diagnosis of GDM, not screening |
| Buhling 2004 | Study assessed strategies for diagnosis of GDM, not screening |
| Cheng 1992 | Study assessed strategies for diagnosis of GDM, not screening |
| Court 1984 | Study assessed strategies for diagnosis of GDM, not screening |
| Court 1985 | Study assessed strategies for diagnosis of GDM, not screening |
| Dornhorst 2000 | Not a randomised controlled trial. |
| Eslamian 2007 | Crossover study. |
| Eslamian 2008 | Crossover study. |
| Fung 1993 | Study assessed strategies for diagnosis of GDM, not screening |
| Harlass 1991 | Study assessed strategies for diagnosis of GDM, not screening |
| Helton 1989 | Crossover study. |
| Hidar 2001 | Crossover study. |
| Jones 1993 | Study assessed strategies for diagnosis of GDM, not screening |
| Kjos 2001 | Women in this study had already been diagnosed with GDM. |
| Lamar 1999a | Crossover study. |
| Lamar 1999b | Crossover study. |
| Lewis 1993 | Women in this study had already been diagnosed with GDM or had undergone a process for diagnosis of GDM |
| Olarinoye 2004 | Study assessed strategies for diagnosis of GDM, not screening |
| Sammarco 1993 | Study assessed strategies for diagnosis of GDM, not screening |
| Soonthornpun 2003 | Study assessed strategies for diagnosis of GDM, not screening |
| Soonthornpun 2008 | Crossover study. |
| Stavrianos 2004 | Study assessed strategies for diagnosis of GDM, not screening |
| Weiss 1998 | Study assessed strategies for diagnosis of GDM, not screening |
| Zhang 1995 | Study assessed strategies for diagnosis of GDM, not screening |
GDM: gestational diabetes mellitus
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Type of study: randomised controlled trial. Method of randomisation: unspecified. Loss of participants to follow up: 18 of 2401 women were lost to follow up Intention-to-treat analysis: unspecified. Blinding: unfeasible to blind participants or healthcare providers. ’Blinded assessors collected outcomes postpartum. Funding: unspecified. |
| Participants | Location: British Columbia Women’s Hospital, British Columbia, Canada Inclusion criteria: low-risk pregnancy at British Columbia Women’s Hospital Exclusion criteria: risk factors for gestational diabetes (specific risk factors not specified) 2401 women were enrolled into the study. 1197 women were allocated to the routine screening arm and 1204 women were allocated to the selected screening arm |
| Interventions | Routine testing group: received a 50 g glucose screen and a full 100 g glucose tolerance test was performed in women whose 1 hour glucose was > 7.8 mmol/L on 50 g screening Selected screening group: received glucose testing only for selected indications that arose during pregnancy |
| Outcomes | Maternal: none. Infant: birthweight and macrosomia. |
| Notes | The only publication of this trial is a conference abstract. We are in correspondence with the authors for additional data |
| Methods | Type of study: randomised controlled trial. Method of randomisation: not specified. Intention-to-treat analysis: ’If the screen or GTT needs to be repeated at some time during the pregnancy, patients will remain in the same study arm as originally assigned.’ Blinding: not specified. Funding: Canadian Diabetes Association. |
| Participants | Location: Royal Victoria Hospital, McGill University Health Centre, Montreal, Quebec, Canada Inclusion criteria: 18 to 50 year old women ’presenting for glucose screening in pregnancy between January 2001 and June 2004 at a tertiary care university hospital setting’ Exclusion criteria: ’insufficient knowledge of French or English; known diabetes; refusal to participate; error in protocol; not fasting when presenting for study test’ Estimated enrolment: 5800 women. |
| Interventions | Women were randomised to 1 of 3 groups:
|
| Outcomes | Incidence and cost-effectiveness of screening and diagnosis of GDM; gestational age at screening, diagnosis and initiation of treatment and maternal and neonatal outcomes |
| Notes | NCT00295659 |
GDM: gestational diabetes mellitus
GTT: glucose tolerance test
DATA AND ANALYSES
Comparison 1. Risk factor versus universal screening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diagnosis of gestational diabetes | 1 | 3152 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.26, 0.75] |
| 2 Gestational age at birth | 1 | 3152 | Mean Difference (IV, Random, 95% CI) | −0.15 [−0.27, −0.03] |
Comparison 2. Glucose monomer versus glucose polymer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diagnosis of gestational diabetes | 2 | 161 | Risk Ratio (M-H, Random, 95% CI) | 1.61 [0.28, 9.15] |
| 2 Positive screen for gestational diabetes | 1 | 85 | Risk Ratio (M-H, Random, 95% CI) | 2.36 [0.90, 6.21] |
| 3 Symptoms | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Any symptom | 2 | 151 | Risk Ratio (M-H, Random, 95% CI) | 2.80 [1.10, 7.13] |
| 3.2 Headache | 1 | 66 | Risk Ratio (M-H, Random, 95% CI) | 5.0 [0.62, 40.51] |
| 3.3 Dizziness | 2 | 151 | Risk Ratio (M-H, Random, 95% CI) | 2.50 [0.93, 6.76] |
| 3.4 Nausea | 2 | 151 | Risk Ratio (M-H, Random, 95% CI) | 2.62 [1.01, 6.79] |
| 3.5 Abdominal discomfort | 2 | 151 | Risk Ratio (M-H, Random, 95% CI) | 6.15 [0.76, 50.08] |
| 3.6 Vomiting | 1 | 66 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 3.7 Sick | 1 | 66 | Risk Ratio (M-H, Random, 95% CI) | 9.00 [0.50, 160.78] |
| 3.8 Tired | 1 | 66 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.18, 3.09] |
| 3.9 Bloating | 1 | 85 | Risk Ratio (M-H, Random, 95% CI) | 11.79 [0.67, 206.69] |
| 4 Taste | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.34, 2.04] |
Comparison 3. Glucose monomer versus candy bar.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diagnosis of gestational diabetes | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 6.67 [0.36, 125.02] |
| 2 Positive screen for gestational diabetes | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 3.49 [1.05, 11.57] |
| 3 Symptoms | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Any symptom | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 1.90 [0.97, 3.72] |
| 3.2 Dizziness | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 1.66 [0.53, 5.24] |
| 3.3 Nausea | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.17, 2.99] |
| 3.4 Abdominal discomfort | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 6.67 [0.36, 125.02] |
| 3.5 Bloating | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.34, 4.11] |
| 4 Taste | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.17, 0.74] |
Comparison 4. Glucose polymer versus candy bar.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diagnosis of gestational diabetes | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 4.44 [0.22, 89.84] |
| 2 Positive screen for gestational diabetes | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 1.48 [0.38, 5.78] |
| 3 Symptoms | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Any symptom | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.13, 1.18] |
| 3.2 Dizziness | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.16, 2.79] |
| 3.3 Nausea | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.06, 13.70] |
| 3.4 Abdominal discomfort | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 3.5 Bloating | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 0.10 [0.01, 1.78] |
| 4 Taste | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.22, 0.82] |
Comparison 5. Glucose versus food.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Screened positive for gestational diabetes | 1 | 30 | Risk Ratio (M-H, Random, 95% CI) | 7.0 [0.39, 124.83] |
| 2 Any symptom | 1 | 30 | Risk Ratio (M-H, Random, 95% CI) | 12.0 [1.78, 81.06] |
Analysis 1.1. Comparison 1 Risk factor versus universal screening, Outcome 1 Diagnosis of gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 1 Risk factor versus universal screening
Outcome: 1 Diagnosis of gestational diabetes
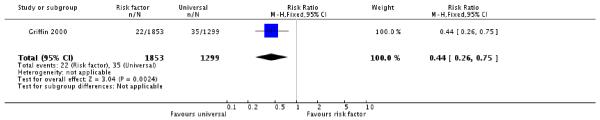 |
Analysis 1.2. Comparison 1 Risk factor versus universal screening, Outcome 2 Gestational age at birth.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 1 Risk factor versus universal screening
Outcome: 2 Gestational age at birth
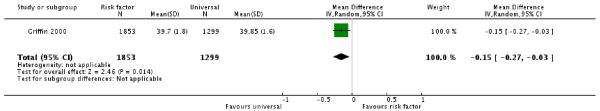 |
Analysis 2.1. Comparison 2 Glucose monomer versus glucose polymer, Outcome 1 Diagnosis of gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 2 Glucose monomer versus glucose polymer
Outcome: 1 Diagnosis of gestational diabetes
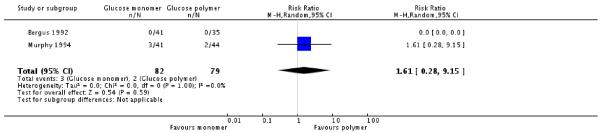 |
Analysis 2.2. Comparison 2 Glucose monomer versus glucose polymer, Outcome 2 Positive screen for gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 2 Glucose monomer versus glucose polymer
Outcome: 2 Positive screen for gestational diabetes
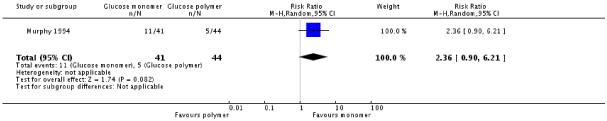 |
Analysis 2.3. Comparison 2 Glucose monomer versus glucose polymer, Outcome 3 Symptoms.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 2 Glucose monomer versus glucose polymer
Outcome: 3 Symptoms
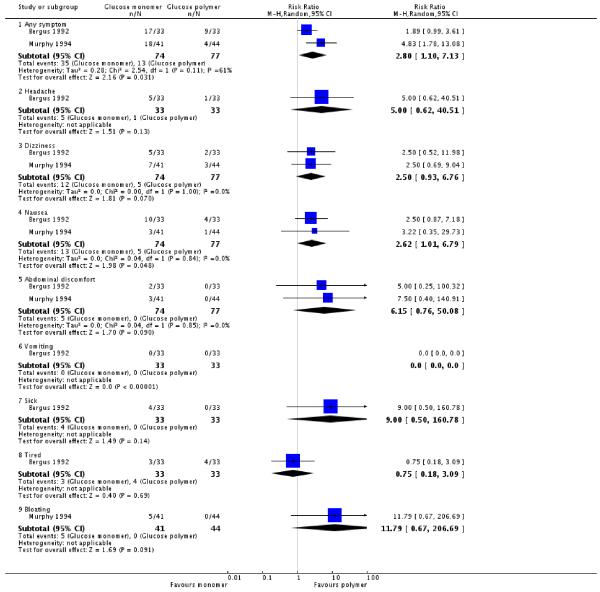 |
Analysis 2.4. Comparison 2 Glucose monomer versus glucose polymer, Outcome 4 Taste.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 2 Glucose monomer versus glucose polymer
Outcome: 4 Taste
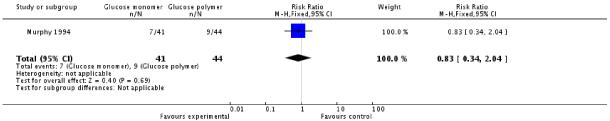 |
Analysis 3.1. Comparison 3 Glucose monomer versus candy bar, Outcome 1 Diagnosis of gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 3 Glucose monomer versus candy bar
Outcome: 1 Diagnosis of gestational diabetes
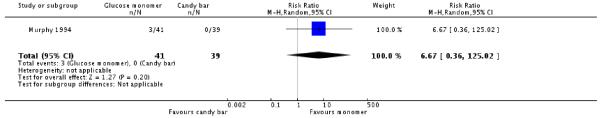 |
Analysis 3.2. Comparison 3 Glucose monomer versus candy bar, Outcome 2 Positive screen for gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 3 Glucose monomer versus candy bar
Outcome: 2 Positive screen for gestational diabetes
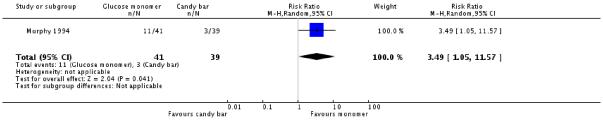 |
Analysis 3.3. Comparison 3 Glucose monomer versus candy bar, Outcome 3 Symptoms.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 3 Glucose monomer versus candy bar
Outcome: 3 Symptoms
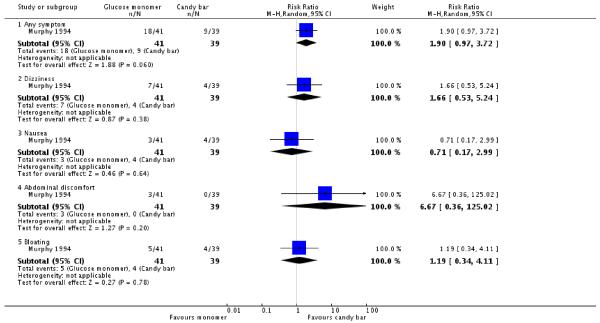 |
Analysis 3.4. Comparison 3 Glucose monomer versus candy bar, Outcome 4 Taste.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 3 Glucose monomer versus candy bar
Outcome: 4 Taste
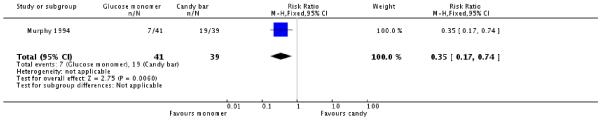 |
Analysis 4.1. Comparison 4 Glucose polymer versus candy bar, Outcome 1 Diagnosis of gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 4 Glucose polymer versus candy bar
Outcome: 1 Diagnosis of gestational diabetes
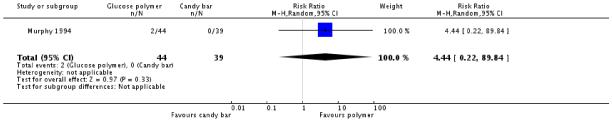 |
Analysis 4.2. Comparison 4 Glucose polymer versus candy bar, Outcome 2 Positive screen for gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 4 Glucose polymer versus candy bar
Outcome: 2 Positive screen for gestational diabetes
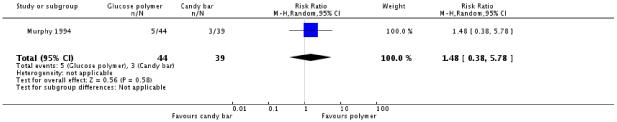 |
Analysis 4.3. Comparison 4 Glucose polymer versus candy bar, Outcome 3 Symptoms.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 4 Glucose polymer versus candy bar
Outcome: 3 symptoms
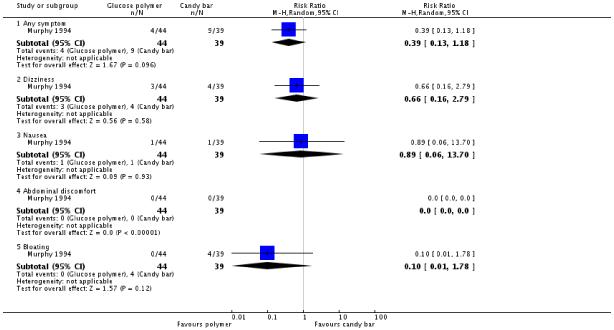 |
Analysis 4.4. Comparison 4 Glucose polymer versus candy bar, Outcome 4 Taste.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 4 Glucose polymer versus candy bar
Outcome: 4 Taste
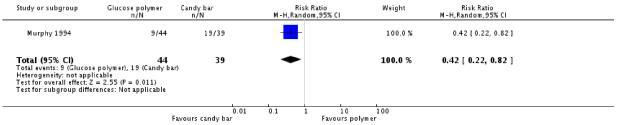 |
Analysis 5.1. Comparison 5 Glucose versus food, Outcome 1 Screened positive for gestational diabetes.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 5 Glucose versus food
Outcome: 1 Screened positive for gestational diabetes
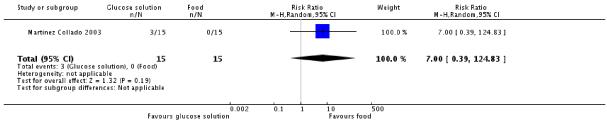 |
Analysis 5.2. Comparison 5 Glucose versus food, Outcome 2 Any symptom.
Review: Screening and subsequent management for gestational diabetes for improving maternal and infant health
Comparison: 5 Glucose versus food
Outcome: 2Any symptom
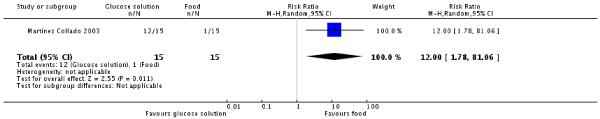 |
WHAT’S NEW
Last assessed as up-to-date: 7 June 2010.
| Date | Event | Description |
|---|---|---|
| 10 January 2011 | Amended | Contact details updated. |
HISTORY
Protocol first published: Issue 3, 2008
Review first published: Issue 7, 2010
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Bergus 1992 {published data only} .Bergus GR, Murphy NJ. Screening for gestational diabetes mellitus: comparison of a glucose polymer and a glucose monomer test beverage. Journal of the American Board of Family Practice. 1992;5(3):241–7. [PubMed] [Google Scholar]
- Griffin 2000 {published data only} .Griffin ME, Coffey M, Johnson H, Scanlon P, Foley M, Stronge J, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabetic Medicine. 2000;17:26–32. doi: 10.1046/j.1464-5491.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Martinez Collado 2003 {published data only} .Martinez Collado JH, Alvarado Gay FJ, Danel Beltran JA, Gonzalez Martinez E. Glucose screening test in pregnant women. A comparison between the traditional glucose load and diet [Tamiz de glucosa en emabarazadas. Comparacion de la carga tradicional contra la dieta] Medicina Interna de Mexico. 2003;19(5):286–8. [Google Scholar]
- Murphy 1994 {published data only} .Murphy NJ, Meyer BA, Hogard ME. Carbohydrate sources for gestational diabetes screening. A comparison. Journal of Reproductive Medicine. 1994;39:977–81. [PubMed] [Google Scholar]
References to studies excluded from this review
- Berkus 1995 {published data only} .Berkus MD, Langer O. Glucose tolerance test periodicity: the effect of glucose loading. Obstetrics & Gynecology. 1995;85:423–7. doi: 10.1016/0029-7844(94)00410-F. [DOI] [PubMed] [Google Scholar]
- Brustman 1995 {published data only} .Brustman LE, Gela BD, Moore M, Reilly KD, Langer O. Variations in oral glucose tolerance tests: the 100- versus 75-g controversy. Journal of the Association for Academic Minority Physicians. 1995;6(2):70–2. [PubMed] [Google Scholar]
- Buhling 2004 {published data only} .Buhling KJ, Elsner E, Wolf C, Harder T, Engel B, Wascher C, et al. No influence of high- and low-carbohydrate diet on the oral glucose tolerance test in pregnancy. Clinical Biochemistry. 2004;37(4):323–7. doi: 10.1016/j.clinbiochem.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Cheng 1992 {published data only} .Cheng LC, Salmon YM, Chen C. A double-blind, randomised, cross-over study comparing the 50g OGTT and the 75g OGTT for pregnant women in the third trimester. Annals of the Academy of Medicine, Singapore. 1992;21(6):769–72. [PubMed] [Google Scholar]
- Court 1984 {published data only} .Court DJ, Stone PR, Killip M. Comparison of glucose and a glucose polymer for testing oral carbohydrate tolerance in pregnancy. Obstetrics & Gynecology. 1984;64(2):251–5. [PubMed] [Google Scholar]
- Court 1985 {published data only} .Court DJ, Mann SL, Stone PR, Goldsbury SM, Dixon-McIvor D, Baker JR. Comparison of glucose polymer and glucose for screening and tolerance tests in pregnancy. Obstetrics & Gynecology. 1985;66(4):491–9. [PubMed] [Google Scholar]
- Dornhorst 2000 {published data only} .Dornhorst A, Frost G. Jelly-beans, only a colourful distraction from gestational glucose-challenge tests. Lancet. 2000;355(9205):674. doi: 10.1016/S0140-6736(00)90025-4. [DOI] [PubMed] [Google Scholar]
- Eslamian 2007 {published data only} .Eslamian L, Ramezani S. Breakfast as a screening test for gestational diabetes. International Journal of Gynecology & Obstetrics. 2007;96(1):34–5. doi: 10.1016/j.ijgo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Eslamian 2008 {published data only} .Eslamian L, Ramezani Z. Evaluation of a breakfast screening test for the detection of gestational diabetes. Acta Medica Iranica. 2008;46(1):43–6. [Google Scholar]
- Fung 1993 {published data only} .Fung H, Baldwin S, Rogers M. The influence of a glucose load on subsequent carbohydrate metabolism in pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1993;33(2):118–21. doi: 10.1111/j.1479-828x.1993.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Harlass 1991 {published data only} .Harlass FE, McClure GB, Read JA, Brady K. Use of a standard preparatory diet for the oral glucose tolerance test. Is it necessary? Journal of Reproductive Medicine. 1991;36:147–50. [PubMed] [Google Scholar]
- Helton 1989 {published data only} .Helton DG, Marin RW, Martin JN, Meeks GR, Morrison JC. Detection of glucose intolerance in pregnancy. Journal of Perinatology. 1989;9:259–61. [PubMed] [Google Scholar]
- Hidar 2001 {published data only} .Hidar S, Chaieb A, Baccouche S, Laradi S, Fkih M, Milled A, et al. Post-prandial plasma glucose test as a screening tool for gestational diabetes. A prospective randomized trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 2001;30:344–7. [PubMed] [Google Scholar]
- Jones 1993 {published data only} .Jones JS, Horger E. A comparative study of the standard oral and intravenous glucose tolerance tests in pregnancy. American Journal of Obstetrics and Gynecology. 1993;168:407. [Google Scholar]
- Kjos 2001 {published data only} .Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care. 2001;24:1904–10. doi: 10.2337/diacare.24.11.1904. [DOI] [PubMed] [Google Scholar]
- Lamar 1999a {published data only} .Lamar ME, Allen SR, Cooney AT, Gayle LJ, Holleman S, Kuehl TJ. Jelly beans as an alternative to the glucola for gestational diabetes screening. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 2):S36. doi: 10.1016/s0002-9378(99)70099-2. [DOI] [PubMed] [Google Scholar]
- Lamar 1999b {published data only} .Lamar ME, Kuehl TH, Cooney LJ, Holleman S, Allen SR. Jelly beans as an alternative to a fifty-gram glucose beverage for gestational diabetes screening. American Journal of Obstetrics and Gynecology. 1999;181:1154–7. doi: 10.1016/s0002-9378(99)70099-2. [DOI] [PubMed] [Google Scholar]
- Lewis 1993 {published data only} .Lewis GF, McNally C, Blackman JD, Polonsky KS, Barron WM. Prior feeding alters the response to the 50-g glucose challenge test in pregnancy. The Staub-Traugott Effect revisited. Diabetes Care. 1993;16(12):1551–6. doi: 10.2337/diacare.16.12.1551. [DOI] [PubMed] [Google Scholar]
- Olarinoye 2004 {published data only} .Olarinoye JK, Ohwovoriole AE, Ajayi GO. Diagnosis of gestational diabetes in Nigerian pregnant women-comparison between 75g and 100g oral glucose tolerance tests. West African Journal of Medicine. 2004;23(3):198–201. doi: 10.4314/wajm.v23i3.28120. [DOI] [PubMed] [Google Scholar]
- Sammarco 1993 {published data only} .Sammarco MJ, Mundy DC, Riojas JE. Glucose tolerance in pregnancy; 41st Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; Washington D.C., USA. 1993; May 1-6, pp. 10–1. 1993. [Google Scholar]
- Soonthornpun 2003 {published data only} .Soonthornpun S, Soonthornpun K, Aksonteiny J, Thamprasit A. A comparison between a 75-g and 100-g oral glucose tolerance test in pregnant women. International Journal of Gynecology & Obstetrics. 2003;81(2):169–73. doi: 10.1016/s0020-7292(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Soonthornpun 2008 {published data only} .Soonthornpun K, Soonthornpun S, Thamprasit A, Aksonteing J. Differences in postload plasma glucose levels between 100-g and 75-g oral glucose tolerance tests in normal pregnant women: a potential role of early insulin secretion. Journal of the Medical Association of Thailand. 2008;91(3):277–81. [PubMed] [Google Scholar]
- Stavrianos 2004 {published data only} .Stavrianos C, Anastasiou E. Oral glucose tolerance test evaluation with forearm and fingertip glucose measurements in pregnant women. Diabetes Care. 2004;27(2):627–8. doi: 10.2337/diacare.27.2.627. [DOI] [PubMed] [Google Scholar]
- Weiss 1998 {published data only} .Weiss PA, Haeusler M, Kainer F, Purstner P, Haas J. Toward universal criteria for gestational diabetes: relationships between seventy-five and one hundred gram glucose loads and between capillary and venous glucose concentrations. American Journal of Obstetrics and Gynecology. 1998;178:830–5. doi: 10.1016/s0002-9378(98)70500-9. [DOI] [PubMed] [Google Scholar]
- Zhang 1995 {published data only} .Zhang Y, Xu H. Screening for gestational diabetes with capillary blood glucose. Chinese Journal of Obstetrics and Gynecology (Zhonghua Fu Chan Ke Za Zhi) 1995;30(5):287–9. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Bebbington 1999 {published data only} .Bebbington MW, Milner R, Wilson RD, Harris S. A randomized controlled trial comparing routine screening vs selected screening for gestational diabetes in low risk population. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 2):S36. [Google Scholar]
- Meltzer 2005 {published data only} .Meltzer SJ. [accessed 2009];An RCT to evaluate incidence, cost and clinical outcomes using 75 vs 100g. screening methods for gestational diabetes. 2006 ClinicalTrials.gov http://clinicaltrials.gov/
- Meltzer SJ, Snyder J, Morin L, Nudi M, Karalis A. Prevalence of gestational diabetes mellitus (GDM) among 5489 multi-ethnic pregnant women in Montreal using a randomized trial of a 75 vs 100g glucose load. Diabetologia. 2005;48(Suppl 1):A23. [Google Scholar]
Additional references
- ACOG 2001 .American College of Obstetricians and Gynecologists ACOG practice bulletin. Clinical management guidelines of gestational diabetes for obstetrician-gynecologists. Obstetrics & Gynecology. 2001;98(3):525–38. [PubMed] [Google Scholar]
- ACOG 2009 .American College of Obstetricians and Gynecologists Postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstetrics & Gynecology. 2009;113:1419–21. doi: 10.1097/AOG.0b013e3181ac06b6. ACOG committee opinion No. 235. [DOI] [PubMed] [Google Scholar]
- ADA 2003 .American Diabetes Association Gestational diabetes. Diabetes Care. 2003;26:S103–S105. doi: 10.2337/diacare.26.2007.s103. [DOI] [PubMed] [Google Scholar]
- ADA 2007 .American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30:S48–S65. doi: 10.2337/dc07-S048. [DOI] [PubMed] [Google Scholar]
- Alwan 2009 .Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD003395.pub2. DOI: 10.1002/14651858.CD003395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haroush 2004 .Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabetic Medicine. 2004;21(2):103–13. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- Berger 2002 .Berger H, Crane J, Farine D. SOGC clinical practice guidelines: screening for gestational diabetes mellitus. Journal of Obstetrics and Gynecology Canada: JOGC. 2002;121:1–10. [Google Scholar]
- Boulvain 2001 .Boulvain M, Stan C, Irion O. Elective delivery in diabetic pregnant women. Cochrane Database of Systematic Reviews. 2001;(2) doi: 10.1002/14651858.CD001997. DOI: 10.1002/14651858.CD001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody 2003 .Brody SC, Harris R, Lohr K. Screening for gestational diabetes: a summary of evidence for the US Preventative Services Task Force. Obstetrics & Gynecology. 2003;101(2):380–92. doi: 10.1016/s0029-7844(02)03057-0. [DOI] [PubMed] [Google Scholar]
- Buchanan 2005 .Buchanan TA, Xiang AH. Gestational diabetes mellitus. Journal of Clinical Investigation. 2005;115(3):485–91. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDA 2008 .Thompson D, Capes S, Feig DS, Kader T, Keely E, Kozak S, et al. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Diabetes Association; Toronto: 2008. Diabetes and pregnancy; pp. S168–S180. [Google Scholar]
- Ceysens 2006 .Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD004225.pub2. DOI: 10.1002/14651858.CD004225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan 1995 .Coustan DR. Gestational diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. National Institute of Health; 1995. pp. 703–17. [Google Scholar]
- Crowther 2005 .Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes on pregnancy outcomes. New England Journal of Medicine. 2005;352(24):2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- Davey 2001 .Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: an evaluation of predictive risk factors. Medical Journal of Australia. 2001;174:118–21. doi: 10.5694/j.1326-5377.2001.tb143181.x. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Di Cianni 2003 .Di Cianni G, Volpe L, Lencioni C, Miccoli R, Cuccuru I, Ghio A, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Research and Clinical Practice. 2003;62:131–7. doi: 10.1016/j.diabres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dodd 2007 .Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2007;47(4):307–12. doi: 10.1111/j.1479-828X.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- Farrar 2007 .Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005542.pub2. DOI: 10.1002/14651858.CD005542.pub2. [DOI] [PubMed] [Google Scholar]
- Farrar 2008 .Farrar D, Duley L, Lawlor DA. Alternative strategies for diagnosing gestational diabetes mellitus to improve maternal and infant health. Cochrane Database of Systematic Reviews. 2008;(2) doi: 10.1002/14651858.CD007122.pub2. DOI: 10.1002/14651858.CD007122. [DOI] [PubMed] [Google Scholar]
- Ferrara 2007 .Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- Gabbe 2004 .Gabbe SG, Gregory RP, Power ML, Williams SB, Schulkin J. Management of diabetes mellitus by obstetrician-gynecologists. Obstetrics & Gynecology. 2004;103(6):1229–34. doi: 10.1097/01.AOG.0000128045.50439.89. [DOI] [PubMed] [Google Scholar]
- Gates 2005 .Gates S. Methodological Guidelines. In: The Editorial Team; Pregnancy and Childbirth Group, editor. About the Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2 2005. [Google Scholar]
- Hanna 2008 .Hanna FWF, Peters JR, Harlow J, Jones PW. Gestational diabetes screening and glycaemic management. National survey on behalf of the Association of British Clinical Diabetologists. Quarterly Journal of Medicine. 2008;101:777–84. doi: 10.1093/qjmed/hcn069. [DOI] [PubMed] [Google Scholar]
- HAPO 2008 .HAPO study cooperative research group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. updated September 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Hillier 2008 .Hillier TA, Vesco KK, Pedula KL, Beil TL, Whitlock EP, Pettitt DJ. Screening for gestational diabetes mellitus: a systematic review for the U.S. Preventative Services Task Force. Annals of Internal Medicine. 2008;148(10):766–75. doi: 10.7326/0003-4819-148-10-200805200-00009. [DOI] [PubMed] [Google Scholar]
- Hoffman 1998 .Hoffman L, Nolan C, Wilson JD, Oats JJN, Simmons D. Gestational diabetes mellitus--management guidelines. The Australasian Diabetes in Pregnancy Society. Medical Journal of Australia. 1998;169(2):93–7. doi: 10.5694/j.1326-5377.1998.tb140192.x. [DOI] [PubMed] [Google Scholar]
- Hollander 2007 .Hollander MH, Paarlberg KM, Huisjes AJM. Gestational diabetes: a review of the current literature and guidelines. Obstetrical and Gynecological Survey. 2007;62(2):125–36. doi: 10.1097/01.ogx.0000253303.92229.59. [DOI] [PubMed] [Google Scholar]
- Hunt 2007 .Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstetrics and Gynecology Clinics of North America. 2007;34:173–99. doi: 10.1016/j.ogc.2007.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IADPSG 2010 .International Association of Diabetes and Pregnancy Study Groups consensus panel International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel 1997 .Kerbel D, Glazier R, Holzapfel S, Yeung M, Lofsky S. Adverse effects of screening for gestational diabetes: a prospective cohort study in Toronto, Canada. Journal of Medical Screening. 1997;4(3):128–32. doi: 10.1177/096914139700400303. [DOI] [PubMed] [Google Scholar]
- Kim 2002 .Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–8. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- Kjos 2005 .Kjos S, Buchanan T, Langer O, Yariv Y, Most O, Xenakis EMJ. Gestational diabetes: the consequence of not treating. American Journal of Obstetrics and Gynecology. 2005;192:989–97. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Kuhl 1998 .Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21(Suppl 2):B19–B26. [PubMed] [Google Scholar]
- Landon 2009 .Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine. 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer 2000 .Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. New England Journal of Medicine. 2000;343(16):1134–8. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- Metzger 1998 .Metzger BE, Coustan DR. Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 1998;21(Suppl 2):B161–B167. [PubMed] [Google Scholar]
- Metzger 2007 .Metzger BE, Buchanan TA, Coustan DR, De Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- Mires 1999 .Mires GJ, Williams FL, Harper V. Screening practices for gestational diabetes mellitus in UK obstetric units. Diabetic Medicine. 1999;16:138–41. doi: 10.1046/j.1464-5491.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- NICE 2008 .National Collaborating Centre for Women’s and Children’s Health. National Institute for Health and Clinical Excellence . Diabetes in pregnancy: management of diabetes and its complications from pre-conception to the postnatal period. National Institute for Health and Clinical Excellence; [accessed 2009]. 2008. http://www.nice.org.uk/CG063 Royal College of Obstetricians and Gynaecologists. [Google Scholar]
- NSC 2003 .National Screening Committee . Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. National Screening Commitee; UK: 2003. [Google Scholar]
- NSC 2007 .UK National Screening Committee [accessed 14 October 2007];What is screening? UK Screening Portal. 2007 http://www.nsc.nhs.uk/whatscreening/whatscreen.ind.htm
- O’Sullivan 1966 .O’Sullivan JB, Gellis SS, Dandrow RV, Tenney BO. The potential diabetic and her treatment in pregnancy. Obstetrics & Gynecology. 1966;27:683–9. [PubMed] [Google Scholar]
- O’Sullivan 1973 .O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. American Journal of Obstetrics and Gynecology. 1973;116:895–900. doi: 10.1016/s0002-9378(16)33833-9. [DOI] [PubMed] [Google Scholar]
- Oats 2004 .Oats JJN, McIntyre HD. Revision of guidelines for the management of gestational diabetes mellitus. Medical Journal of Australia. 2004;181(6):342. doi: 10.5694/j.1326-5377.2004.tb06310.x. [DOI] [PubMed] [Google Scholar]
- Pedersen 1954 .Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinologica. 1954;16:330–42. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- Pettitt 1985 .Pettitt DJ, Bennett PH, Knowler WC, Baird HR, Aleck KA. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy: long-term effects on obesity and glucose tolerance in the offspring. Diabetes. 1985;34(Suppl 2):119–22. doi: 10.2337/diab.34.2.s119. [DOI] [PubMed] [Google Scholar]
- RANZCOG 2008 .College statement. Diagnosis of gestational diabetes mellitus. RANZCOG; Melbourne: 2008. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. [Google Scholar]
- RevMan 2008 .The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Richardson 2007 .Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstetrics and Gynecology Clinics of North America. 2007;34:213–24. doi: 10.1016/j.ogc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Rowan 2008 .Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine. 2008;358(19):2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- Rumbold 2001 .Rumbold AR, Crowther CA. Guideline use for gestational diabetes mellitus and current screening, diagnostic and management practices in Australian hospitals. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2001;41(1):86–90. doi: 10.1111/j.1479-828x.2001.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Rumbold 2002 .Rumbold AR, Crowther CA. Women’s experiences of being screened for gestational diabetes mellitus. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2002;42(2):131–7. doi: 10.1111/j.0004-8666.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- Scott 2002 .Scott DA, Loveman E, McIntyre L, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technology Assessment. 2002;6(11):1–161. doi: 10.3310/hta6110. [DOI] [PubMed] [Google Scholar]
- Sermer 1995 .Sermer M, Naylor CD, Gare DJ, Kenshole AB, Ritchie JWK, Farine D, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. American Journal of Obstetrics and Gynecology. 1995;173:146–56. doi: 10.1016/0002-9378(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Setji 2005 .Setji T, Brown A, Geinglos M. Gestational diabetes. Clinical Diabetes. 2005;23:17–24. [Google Scholar]
- Silverman 1998 .Silverman BL, Rizzo TA, Nam H, Metzger BE. Long term effects of the intrauterine environment: the Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl 2):B142–B149. [PubMed] [Google Scholar]
- Solomon 1997 .Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–83. [PubMed] [Google Scholar]
- USPSTF 2008 .U.S. Preventive Services Task Force Screening for gestational diabetes mellitus: U.S. preventive services task force recommendation statement. Annals of Internal Medicine. 2008;148:759–65. doi: 10.7326/0003-4819-148-10-200805200-00008. [DOI] [PubMed] [Google Scholar]
- Whitelaw 1977 .Whitelaw A. Subcutaneous fat in newborn infants of diabetic mothers: an indication of quality of diabetic control. Lancet. 1977;1(8001):15–8. doi: 10.1016/s0140-6736(77)91654-3. [DOI] [PubMed] [Google Scholar]
- WHO 1999 .World Health Organization . Report of a WHO consultation: definition, diagnosis and classification of diabetes mellitus. WHO; Geneva: 1999. [Google Scholar]
- *Indicates the major publication for the study