Abstract
Working memory (WM) impairment is a core feature of schizophrenia, but the contributions of different WM components are not yet specified. Here, we investigated the potential role of inefficient encoding in reduced WM performance in patients with schizophrenia (PSZ). Twenty-eight PSZ, 16 patients with bipolar disorder (PBP), 16 unaffected and unmedicated relatives of PSZ (REL), and 29 demographically matched healthy controls (HC) performed a spatial delayed response task with either low or high WM demands. The demands on attentional selection were also manipulated by presenting distractor stimuli during encoding in some of the trials. After each trial, participants rated their level of response confidence. This allowed us to analyze different types of WM responses. WM was severely impaired in PSZ compared to HC; this reduction was mainly due to an increase in the amount of false memory responses (incorrect responses that were given with high confidence) rather than an increase in the amount of incorrect and not-confident responses. Although PBP showed WM impairments, they did not have increased false memory errors. In contrast, reduced WM in REL was also accompanied by an increase in false memory errors. The presentation of distractors led to a decline in WM performance, which was comparable across groups indicating that attentional selection was intact in PSZ. These findings suggest that inefficient WM encoding is responsible for impaired WM in schizophrenia and point to differential mechanisms underlying WM impairments in PSZ and PBP.
Keywords: schizophrenia, bipolar disorder, spatial working memory encoding, false memory, attention, relatives, high-risk subjects
Impairments in working memory (WM) are considered a core cognitive deficit in schizophrenia (Goldman-Rakic, 1994; Lee & Park, 2005). WM refers to the short-term storage of information in the service of the active guidance of behavior (Baddeley, 1986). Thus, the ability to store information in WM is crucial for a broad range of cognitive operations, and WM impairments have significant consequences on social and occupational functioning (Cervellione, Burdick, Cottone, Rhinewine, & Kumra, 2007). Spatial WM deficits in particular are present in high-risk populations (Simon et al., 2007), in spectrum disorders (Mitropoulou et al., 2005), and in unaffected relatives (Myles-Worsley & Park, 2002; Park, Holzman, & Goldman-Rakic, 1995), and therefore have been discussed as a potential endophenotypic marker of the disorder (Glahn et al., 2003). In addition, the neurophysiological underpinnings of spatial WM as measured with the spatial delayed response task (DRT) and its deficits in schizophrenia are well documented (Goldman-Rakic, 1990, 1994). Despite the evidence for WM deficits in schizophrenia, only recently researchers have started to disentangle the component processes of the complex WM system that are most impaired.
Successful performance in a spatial DRT depends on the efficient operation of several processes at different stages of the task. First, during the encoding phase, subjects need to perceive the stimulus and transform the perceptual representation into a stable WM representation. Second, the internal representation needs to be accurately maintained across the retention interval. Finally, the memory representation must be compared with the presented probe to prepare and initiate the response.
Following Goldman-Rakic’s model on the importance of the dorsolateral prefrontal cortex for WM storage, many studies of spatial WM impairments in patients with schizophrenia (PSZ) have focused on the maintenance phase (e.g., Keefe et al., 1995; Park & Holzman, 1992; Park, Püschel, Sauter, Rentsch, & Hell, 1999; Piskulic, Olver, Noran, & Maruff, 2007). However, performance deficits occur even with very short delays and do not necessarily increase with longer delays (Gold et al., 2010; Javitt, Strous, Grochowski, Ritter, & Cowan, 1997; Keefe et al., 1995; Lee & Park, 2005; Park & Holzman, 1992). These findings point to a major locus of impairment at the stage of encoding (Lee & Park, 2005).
One issue that complicates the investigation of encoding deficits in behavioral paradigms is the difficulty in isolating the encoding process because performance measures in terms of accuracy and reaction time (RT) are compound measurements that potentially reflect processes associated with the encoding, maintenance, and/or retrieval phase. In this study, we therefore used a different approach to investigate the role of inefficient encoding in WM impairments in PSZ by analyzing different types of responses, in addition to analyzing overall WM performance.
Participants performed a spatial DRT that required them to memorize either the locations of two (low demand on WM) or four (high demand on WM) geometric shapes (squares and circles). In each trial, we asked participants to rate their level of response confidence. This approach allowed us to examine four types of accurate and erroneous responses. Our classifications were as follows: if a subject gave the correct response with high confidence, this was classified as true memory, in contrast to a correct response that was given without confidence (= correct/not-confident response). If an incorrect response was giving with confidence, this was classified as false memory, in contrast to an incorrect response that was given without confidence (= incorrect/not-confident response). We were particularly interested in the rate of false memory responses in comparison to the amount of incorrect/not-confident responses among patients and healthy controls (HC). We reasoned, that false memory errors most likely reflect a problem at the encoding stage. Thus, patients may transfer erroneously and/or imprecisely encoded information into WM, but successfully maintain and retrieve this information from WM, resulting in judgments of high confidence. In contrast, if patients had difficulties in maintaining a stable memory representation, one could also expect an increase in the amount of incorrect responses that are given without confidence.
In line with the encoding hypothesis, we have previously shown in healthy participants that the percentage of false memories in a spatial DRT decreases when the processes that support WM encoding are facilitated (Mayer, Kim, & Park, 2011). Moreover, Lee, Folley, Gore, and Park (2008) reported similar activation patterns in the prefrontal cortex for correct responses and false memory responses in a spatial DRT in PSZ, suggesting that the maintenance of the internal representation was intact whether that representation was correctly or incorrectly encoded. Therefore, if reduced WM performance in PSZ was due to a failure in the encoding rather than the maintenance of the items, we expected a higher percentage of false memory errors rather than incorrect/not-confident responses in PSZ compared to HC. This effect should be most pronounced when the demands on WM are increased.
To test the specificity of this potential encoding deficit, we included a group of patients with bipolar disorder (PBP) who were comparable in terms of symptom severity to the schizophrenia sample. It is not clear whether and to what degree visual WM is impaired in bipolar disorder (e.g., Glahn, Bearden, et al., 2006; McGrath, Chapple, & Wright, 2001; Park & Holzman, 1992, 1993; Sweeney, Kmiec, & Kupfer, 2000). However, even though PBP suffered from WM deficits as well, the comparison of the response types might allow us to differentiate the underlying mechanisms.
We also included a group of unaffected and unmedicated first-degree relatives (REL) of PSZ. Given the evidence for common deficits in WM in PSZ and their unaffected relatives (Conklin, Conklin, Curtis, Calkins, & Iacono, 2005; Myles-Worsley & Park, 2002; Park et al., 1995; Snitz, MacDonald, & Carter, 2006), we expected an increase in the amount of false memory errors in REL as well.
A second aim of this study was to specify the processes that contribute to the potential WM encoding deficit in PSZ. Increasing evidence suggests that deficits in early-stage visual processing (Badcock, Badcock, Read, & Jablensky, 2008; Hartman, Steketee, Silva, Lanning, & McCann, 2003; Javitt, Liederman, Cienfuegos, & Shelley, 1999; Javitt et al., 1997; Tek et al., 2002) and/or higher-level cognitive processes associated with the consolidation process itself (Fuller, Luck, McMahon, & Gold, 2005; Fuller et al., 2009) can lead to abnormal encoding in schizophrenia (for a review see Haenschel & Linden, 2011). Moreover, attentional processes can modulate the encoding process (Fine & Minnery, 2009; Mayer et al., 2011; Vogel, McCollough, & Machizawa, 2005) and might be impaired in PSZ (Gold, Fuller, Robinson, Braun, & Luck, 2007; Hahn et al., 2010; Luck & Gold, 2008; Tanaka et al., 2007). Thus, difficulties in selecting relevant information or deploying attention to the relevant feature efficiently (Nestor et al., 1992; Posner, Early, Reiman, Pardo, & Dhawan, 1988; Sereno & Holzman, 1996) may result in imprecise encoding or in encoding wrong stimuli which then may lead to increased false memory responses.
To test the hypothesis of impaired selection as a cause of WM encoding deficits in schizophrenia, we manipulated the demands on the selection process during the encoding phase in the task described above. In the no-distraction condition, two squares were presented and participants were instructed to memorize their locations. In the distraction condition, two squares and two circles were presented and participants were instructed to memorize the locations of only the squares. We expected that WM performance would be reduced in the condition of distraction versus no distraction in all groups due to increased demands on attentional selection (Vogel et al., 2005). However, if a failure in the selection of the items to be encoded into WM was responsible for reduced WM performance in PSZ, the distraction-induced decline in WM performance should be stronger for this group compared to HC.
Method
Participants
Twenty-eight patients with schizophrenia or schizoaffective disorder, 16 PBP, and 29 HC participated in this study. Diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) criteria (American Psychiatric Association, 1994) using structured clinical interviews. Demographic information is summarized in Table 1. The three groups were matched for age, F(2, 72) = 0.32, p = .73, premorbid IQ, F(2, 72) = 0.21, p = .81, as measured with the National Adult Reading Test (Nelson, 1982), and handedness, F(2, 72) = 2.59, p = .08, as measured with the Edinburgh Handedness Inventory (Oldfield, 1971). Years of education were lower in both patient groups compared to controls (all t values > 2.1, all p values < .05), but did not differ between PSZ and PBP, t(42) = 0.68, p = .50.
Table 1.
Demographic Information
| PSZ (n = 28) |
PBP (n = 16) |
HC (n = 29) |
REL (n = 16) |
|
|---|---|---|---|---|
| Age | 38.32 (9.29) | 36.06 (10.04) | 37.28 (8.41) | 34.63 (11.13) |
| Range | 24–57 | 20–51 | 25–55 | 20–57 |
| Female/male | 9/19 | 7/9 | 12/17 | 9/7 |
| AA: A: C: O | 13: 1: 14: 0 | 2: 0: 14: 0 | 7: 1: 20: 1 | 12: 0: 4: 0 |
| Handedness | 39.79 (64.05) | 64.38 (25.02) | 70.69 (52.40) | 67.19 (30.60) |
| Education | 14.25 (2.29) | 13.75 (2.41) | 15.41 (1.80) | 14.56 (3.61) |
| IQ | 105.57 (8.68) | 107.31 (11.4) | 106.31 (6.69) | 99.50 (11.72) |
| CPE, mg/day | 361.12a (381.78) | 140.50b (174.42) | n/a | n/a |
Note. AA = African American; A = Asian; C = Caucasian; O = Other; CPE = Chlorpromazine equivalent; Mean values are shown. SD is given in parenthesis.
CPEs for three PSZ who were treated with Iloperidone and Paliperidone are not included.
CPEs for one PBP who was treated with Iloperidone is not included.
PSZ were clinically stable outpatients (mean duration of illness: 16.85, SD = 9.10). At the time of testing, PSZ obtained a mean total score of 12.74 (SD = 5.74) on the Brief Psychiatric Rating Scale (BPRS) (Overall & Gorman, 1962), 23.67 (SD = 13.81) on the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983), and 13.41 (SD = 10.23) on the Scale for the Assessment of Positive Symptoms (Andreasen, 1984). Twenty-four PSZ were medicated, three with a first-generation antipsychotic and 21 with a second-generation antipsychotic. In addition, 10 PSZ also received antidepressants, two PSZ benzodiazepines, and one PSZ was treated with lithium. The mean chlorpromazine equivalent dose (CPE) was 361.12 (SD = 381.78). PSZ were taking stable medications for a minimum of 4 weeks prior to testing.
In the bipolar sample, 10 patients had a history of psychotic symptoms when acutely manic or depressed. PBP were clinically stable outpatients (mean duration of illness: 14.07, SD = 8.63). At the time of testing, patients obtained a mean total score of 12.94 (SD = 9.18) on the BPRS, 6.63 (SD = 5.97) on the Young Mania Rating Scale (YMRS) (Young, Biggs, Ziegler, & Meyer, 1978), and 11.81 (SD = 7.82) on the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). Based upon symptom ratings, five of the PBP were considered mildly to moderate depressed (HRSD > 16), two were in a manic (YMRS > 12) or mixed episode (HRSD > 16 and YMRS > 12) and seven were euthymic (HRSD < 8 and YMRS < 6). Fifteen PBP were medicated; seven were taking mood-stabilizing medications (lithium: n = 1, anticonvulsants: n = 6); 10 patients were taking antidepressants, two patients were taking benzodiazepines, and two were taking a psychostimulant. In addition, eight PBP were currently receiving a second-generation antipsychotic. The mean CPE dose was 140.50 (SD = 174.42). PSZ and PBP were comparable in terms of symptom severity as measured with the BPRS, t(41) = −.09, p = .93, and duration of illness, t(39) = 1.15, p = .26.
HC were recruited from the community. They had no history of DSM–IV Axis I disorders and no family history of psychosis. They were medication-free and screened to rule out schizotypal personality using the Schizotypal Personality Questionnaire (SPQ) (Raine, 1991). No control participant scored high on the SPQ (M = 10.90, SD = 7.71, range: 0–26).
In addition, 16 unaffected first-degree relatives of PSZ were tested. REL were recruited through our patients and local advertisement. They had no history of DSM–IV Axis I and were medication-free. The mean score on the SPQ was 22.94 (SD = 13.89, range: 3–57) for this group. REL were matched with PSZ and HC for age (all t values < 1.18, all p values > .24), years of education (all t values < 1.09, all p values > .28), and handedness (all t values < 1.60, all p values > .12). However, premorbid IQ was lower in REL than PSZ, t(42) = 1.96, p = .06, and HC, t(43) = 2.49, p < .05.
All subjects had normal or corrected-to-normal vision. Exclusion criteria were a history of head injury, neurological disorder, or substance abuse in the 6 months preceding the study. All subjects gave written informed consent approved by the Vanderbilt University Institutional Review Board and were paid.
Stimuli, Task, and Procedure
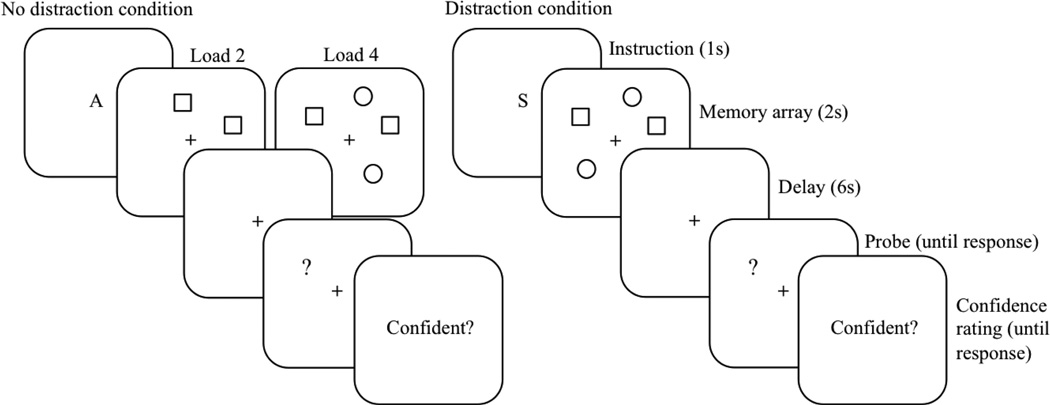
Stimuli were geometric shapes (circles and squares) of approximately 0.71° visual angle, displayed on a white background (see Figure 1). Stimuli appeared along an imaginary circle (4.7° radius) including 16 positions around a centrally presented fixation cross (0.71°).
Figure 1.
Schematic diagram of the procedure and stimuli in the spatial delayed response task. In the no-distraction condition, participants were asked to memorize either the locations of two (Load 2) or four (Load 4) geometric shapes (squares and circles). In the distraction condition, they were instructed to memorize the locations of only the squares. At the end of each trial, participants rated their level of response confidence.
Participants performed a spatial DRT. Each trial began with presenting a fixation cross at the center position for 1 s, then the task instruction was given in the form of the letter A or S which was followed by the presentation of several geometric shapes (circles and squares) for 2 s. The letter A instructed participants to memorize the locations of all shapes (no-distraction condition), whereas participants were asked to memorize the locations of only the squares when the letter S appeared before (distraction condition). In the distraction condition, subjects needed to remember the locations of two squares (targets) and ignore two circles (distractors). In the no-distraction condition, the number of targets (WM load) was either two (only squares) or four (two squares and two circles).1 Within each trial, the target positions were determined pseudorandomly with the constraint that in WM load 4/no-distraction trials, the targets appeared in at least three different quadrants of the screen and that a maximum of two target items could be presented in adjacent locations. After a 6 s delay interval, a question mark (0.71°) was presented as a probe until a response was given. Participants indicated whether the position of the question mark matched one of the target positions by a left or right key press for match and nonmatch, respectively. Half of the trials were matches. In the nonmatch trials, the question mark appeared at one of the remaining positions that had not been occupied by either a target or a distractor during the stimulus presentation phase. Participants made the response with the index finger and the middle finger of their dominant hand, and they were instructed to respond as fast and accurately as possible. Immediately after the decision, participants indicated the confidence level for their response by pressing the A button for “confident” and the S button for “not confident.” The participants were given as much time as they wanted to make an accurate response. An intertrial interval (ITI) of 2 s followed the confidence rating before a new trial began. See Figure 1 for an illustration of the sequence of events at each trial. Each of the three experimental conditions (WM load 2/distraction, WM load 2/no distraction, WM load 4/no distraction) was presented equally often (32 trials per condition). Participants performed one practice block (10 trials) followed by two experimental blocks of 48 trials each. The trials were presented fully randomized across blocks.
Analysis
WM performance was analyzed in terms of response accuracy, RT, and the amount of type of response (true memory responses, correct but not-confident responses, false memory responses, incorrect and not-confident responses). A subsequent analysis tested the relationship between error rates (false memory responses and incorrect and not-confident responses) and the distance from the probe to the nearest target (near vs. far). This analysis was restricted to the nonmatch trials in the WM load 4/no-distraction condition as the probability that the probe appeared at locations adjacent to a target location differed between task conditions and was highest in this condition. A “near probe-to-target location” was defined as adjacent to the target location (one location apart from the target). A “far probe-to-target location” included locations that were two or more locations apart from the target.
Results
We first report the findings on response accuracy and RTs in the two WM load conditions for PSZ compared to HC and PBP in order to assess WM deficits. To test whether WM deficits reflect difficulties of WM encoding rather than maintenance, we then present the findings on different response types based on the confidence rating in these groups. This is followed by the presentation of the findings on response accuracy and RTs in the distractor condition. This condition was included to test whether the potential WM encoding deficit can be explained by impaired attentional selection. To rule out medication effects, we also included a group of unaffected and unmedicated first-degree REL of PSZ. The results on REL are reported in the second part of the result section.
Schizophrenia, Bipolar, and Healthy Control Participants
WM load conditions
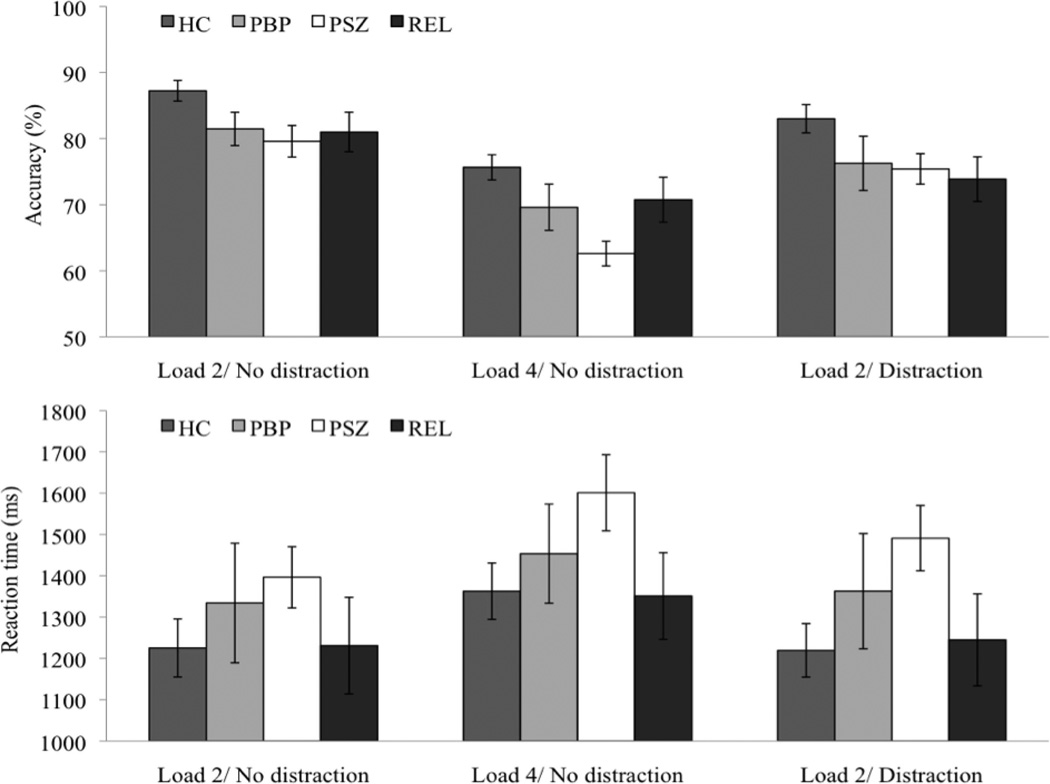
Two separate repeated-measures ANOVAs were performed on response accuracy and RTs as a function of WM load (two vs. four targets) and group (PSZ, PBP, HC). For response accuracy, the analysis revealed a significant main effect of group, F(2, 70) = 7.92, p < .01, η2 = .18. Planned comparisons using independent t test indicated that for WM load 2, accuracy was lower in both patient groups compared to HC [PSZ versus HC, t(55) = −2.69, p < .05; PBP versus HC, t(43) = −2.05, p < .05] but did not differ between PSZ and PBP, t(42) = −.51, p = .62 (see Figure 2). For WM load 4, accuracy was lowest in PSZ, [PSZ versus HC, t(55) = −4.89, p < .001] followed by PBP [PSZ versus PBP, t(42) = −1.95, p = .06] and HC [PBP versus HC, t(43) = −1.66, p = .10]. This pattern was also reflected in a significant interaction between group and WM load, F(2, 70) = 3.05, p = .05, η2 = .08. Overall, accuracy was lower for WM load 4 versus 2, F(1, 70) = 154.27, p < .001, η2 = .69.
Figure 2.
WM accuracy and RTs in the three experimental conditions in healthy controls (HC), patients with bipolar disorder (PBP), patients with schizophrenia (PSZ), and unaffected first-degree relatives of PSZ (REL). Vertical bars represent the standard error of the mean.
Consistent with the accuracy data, RTs increased from WM load 2 to WM load 4, F(1, 70) = 42.76, p < .001, η2 = .38. This load-dependent increase was found to be similar across all groups [nonsignificant interaction between the factors group and WM load, F(2, 70) = 1.29, p = .28]. RTs did not significantly differ between groups, F(2, 70) = 1.64, p = .20.
Neither the severity of positive nor negative symptoms correlated with accuracy or RTs in PSZ in any of the WM load conditions (all p values > .12). In PBP, there was no relationship between WM performance and ratings of mania or depression (all p values > .08). In PSZ, accuracy correlated negatively with the daily CPE dose in the WM load 4 condition, r −.45, p < .05, but not the WM load 2 condition, p = .56. RTs did not correlate with the CPE dose in any of the load conditions (all p values > .86). In PBP, neither response accuracy nor RTs correlated with the daily CPE dose in any of the load conditions (all p values > .12). In PSZ, there was no relationship between WM performance (accuracy and RTs) and duration of the illness in any of the load conditions (all p values > .28). In PBP, we found in both load conditions a significant positive correlation between duration of illness and RTs (r values > .65, p values < .01) but not accuracy (all p values > .40).
Confidence rating
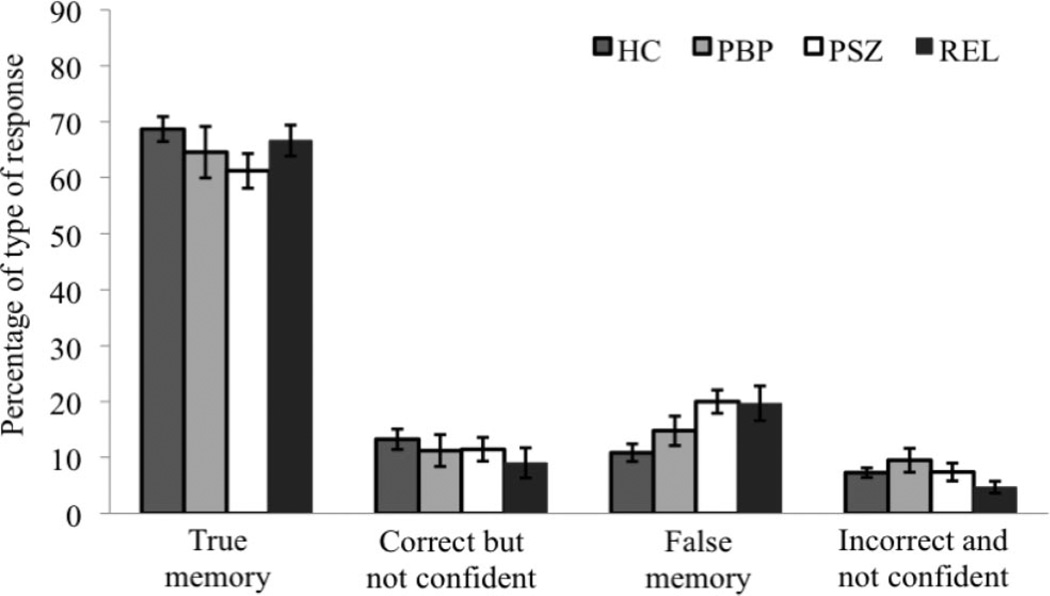
A 3 × 4 × 3 repeated-measures ANOVAs was conducted to test whether the percentage of type of response (true memories, correct but not-confident responses, false memories, incorrect and not-confident responses) differed between groups (PSZ, PBP, HC) in the three experimental conditions (WM load 2/no distraction, WM load 2/distraction, WM load 4/no distraction). (See Figure 3 and Supplementary Figure 1 for the results for each experimental condition). Specifically, we were interested whether PSZ showed an increase in the rate of false memory rather than incorrect/not-confident responses compared to HC reflecting problems specifically at the stage of encoding. As indicated by a significant interaction between the factors group and response type, F(6, 210) = 2.18, p < .05, η2 = .06, the percentage of type of response differed between groups. Because there was no effect of experimental condition on the percentage of type of responses, F(2, 140) = 0.17, p = .84, data was collapsed across conditions for post hoc analyses using separate one-way ANOVAS (see Supplementary Figure 1 for the results for each experimental condition). A significant group difference emerged only in the percentage of false memories, F(2, 70) = 6.09, p > .05, η2 = .15, corrected for multiple comparisons using Bonferroni correction. Post hoc tests corrected for multiple comparisons indicated that PSZ made significantly more false memory errors than HC, p < .003. PSZ made also more false memories than PBP, however the difference of 5.2% did not reach significance, p = .29. There was no difference in the percentage of false memories between PBP and HC, p = .62. The percentage of true memory responses, correct and incorrect responses given without being confident did not significantly differ between groups (all F values < 1.70, all p values > .19).
Figure 3.
Results from the confidence rating across the three experimental conditions. The percentage of four types of responses is shown: True memory (correct and confident response), correct but not-confident responses, false memory (incorrect and confident response) and incorrect and not-confident responses. Vertical bars represent the standard error of the mean. HC = healthy controls; PBP = patients with bipolar disorder; PSZ = patients with schizophrenia; REL = unaffected first-degree relatives of PSZ.
The percentage of false memories calculated across experimental conditions did not correlate with symptom ratings in PSZ (all p values > .19). In PBP, the percentage of false memories correlated positively with the severity of mania symptoms, r = .49, p < .05, but not depressive symptoms, p = .36. In addition, the percentage of false memories did not correlate with the daily CPE dose, neither when calculated across both groups, p = .47, nor separately for PSZ, p = .59, and PBP, p = .33. There was also no relationship between the percentage of false memories and duration of the illness, when calculated across both groups, p = .79, and separately for PSZ, p = .38, and PBP, p = .22.
Was the increase in the amount of false memory errors specifically related to WM deficits in our task?
To answer this question we correlated the percentage of false memory responses and the percentage of incorrect/not-confident responses with response accuracy in our task and WM capacity derived from an independent task.2 This analysis was restricted to the WM load 4/No distraction condition that was associated with the highest rate of false memory errors (see Supplementary Figure 1). When collapsing across all participants, there was a strong negative correlation between the percentage of false memory responses and accuracy, r= −.65, p < .001, whereas the correlation between the amount of incorrect and not-confident responses and accuracy was considerably weaker, r = −.28, p < .05. In addition, the percentage of false memories correlated negatively with WM capacity, r= −.67, p < .001, but there was no correlation between capacity estimates and the amount of incorrect and not-confident responses, r = −.20, p = .22.
Did the increased rate of false memory errors reflect a failure of WM retrieval?
RTs usually increase as a function of the demands on memory search in order to retrieve information from WM (Sternberg, 1966). If the increased rate of false memory errors in PSZ reflected a failure of WM retrieval we therefore expected that increases in RTs would be accompanied by higher rates of false memory responses. To test this hypothesis we correlated RTs in the WM load 4/no-distraction condition with the amount of false memory and the amount of incorrect and not-confident responses in PSZ and HC. For PSZ, RTs were positively correlated with the amount of incorrect and not-confident responses, r = .70, p < .001, however there was a trend toward a negative relationship between RT increases and the amount of false memory responses, r = −.33, p = .08. For HC, RTs correlated positively with the amount of false memory responses, r = .40, p < .05, whereas there was no relationship with the amount of incorrect and not-confident responses, r = .13, p = .52.
Distractor condition
Response accuracy was significantly lower when distractors were presented in the stimulus display [ANOVA, main effect of distractor, F(1, 70) = 15.74, p < .001, η2 = .18] (see Figure 2). This decrease was found to be similar across all groups [nonsignificant interaction between the factors distraction and group, F(2, 70) = .07, p = .93]. The analysis also revealed a significant group effect on accuracy, F(2, 70) = 3.82, p < .05, η2 = .10. Accuracy was higher in HC compared to PSZ, t(55) = −2.41, p < .05, but did not significantly differ between HC and PBP, t(43)= −1,61, p = .12 and between the two patient groups, t(42) = −0.19, p = .85.
Consistent with the accuracy data, RTs significantly increased in the distractor condition, F(1, 70) = 4.48, p < .05, η2 = .06, however this increase differed between groups as indicated by a significant interaction between the factors distractor and group, F(2, 70) = 3.17, p < .05, η2 = .08. RTs increased in the distractor versus no distractor condition in PSZ, t(27)= −2.80, p < .01, but did not differ between these conditions in HC, t(28) = 0.23, p = .82, and PBP, t(15) = −0.96, p = .35. There was no significant group effect on RTs, F(2, 70) = 1.95, p = .15.
Furthermore, RTs were faster in the WM load 2/distraction condition than the WM load 4/no-distraction condition in HC, PSZ, and PBP [ANOVA, main effect of task condition, F(1, 70) = 30.91, p < .001, η2 = .31; nonsignificant interaction between group and task condition, F(2, 70) = 0.55, p = .58].
There were no significant correlations between symptom ratings and neither response accuracy nor RTs in the distraction condition in both PSZ and PBP (PSZ, all p values > .36; PBP, all p values > .25). In PSZ, accuracy, r = −40, p = .05, but not RTs, p = .66, correlated negatively with the daily CPE dose. In PBP, there was no significant correlation between neither accuracy nor RTs and daily CPE dose (all p values > .25). WM performance did not correlate with duration of the illness in PSZ (all p values > .44). In PBP, there was a significant correlation between duration of illness and RTs, r = .69, p < .01, but not accuracy, p > .86.
Did the increased rate of false memory errors reflect less precise WM encoding?
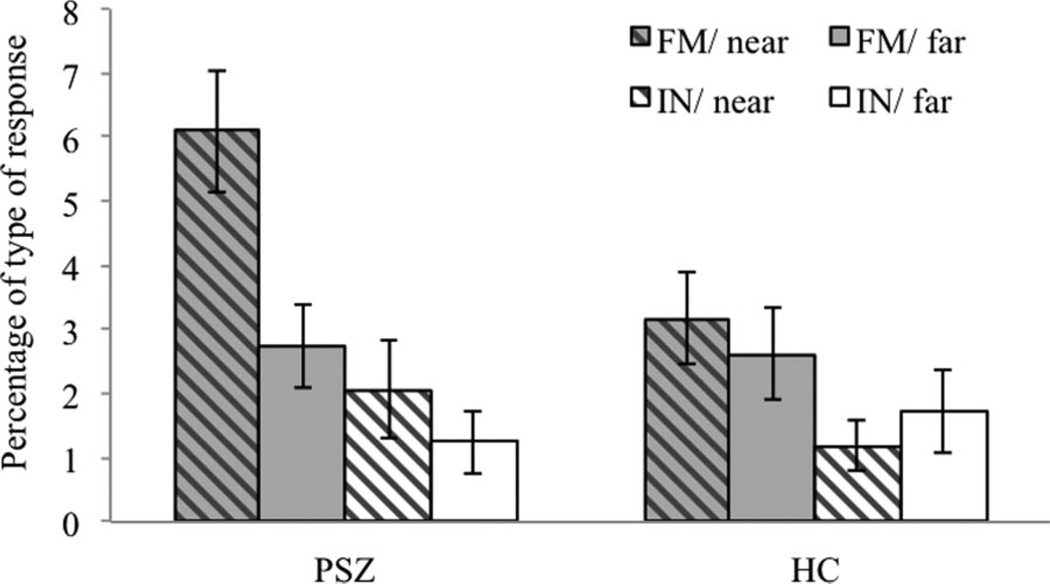
To test whether spatial WM encoding was less precise in PSZ compared to HC, we analyzed the relationship between error rates (false memory responses and incorrect and not-confident responses) and the distance from the probe to the nearest target (see Methods). If PSZ encoded the locations spatially less precisely than HC, we expected a higher rate of false memories rather than incorrect and not-confident responses in PSZ than HC when the probe was located at locations adjacent to a target location versus locations that were farther apart from targets. A 2 × 2 × 2 repeated-measures ANOVA with the factors group (PSZ vs. HC), error type (false memory vs. incorrect and not-confident responses), and probe location (near vs. far) revealed a significant main effect of probe location, F(1, 55) = 8.80, p < .01, η2 = .14, with increased error rates for near versus far probe locations. Most important, this increase in errors for near versus far probe locations differed between PSZ and HC, as reflected in the significant group by probe location interaction, F(1, 55) = 8.74, p < .01, η2 = .14. The interaction between all three factors did not reach significance, F(1, 55) =0.69, p = .41, η2 = .012. This might be due to insufficient statistical power because planned comparisons using paired t tests showed the appropriate pattern for the three-way interaction (see Figure 4). PSZ made more false memory errors when the probe was located near versus far from a target position, t(27) = 3.87, p < .01. In contrast, there was no difference between near and far probe-to-target locations for the amount of incorrect and not-confident responses, t(27) = 1.13, p = .27. In HC, both the amount of false memories, t(28) = 0.64, p = .53, and the amount of incorrect and not-confident responses, t(28)= −0.77, p = .45, did not differ for probe locations that were adjacent to targets versus locations that were farther from targets.
Figure 4.
The percentage of false memory (FM) and incorrect and not-confident (IN) responses for probes that were presented near vs. far from the target location. Vertical bars represent the standard error of the mean. HC = healthy controls; PSZ = patients with schizophrenia.
Patients With Schizophrenia, First-Degree Relatives and Healthy Control Participants
WM load conditions
In the WM load 2 condition, accuracy was lower in REL than HC, t(43) = 2.04, p < .05, but did not differ from PSZ, t(42) = −0.36, p = .72. For WM load 4, accuracy was significant higher in REL compared to PSZ, t(42) = −2.30, p < .05, and did not differ from HC, t(43) = 1.36, p = .18 (see Figure 2). The latter result was mainly driven by two outliers in the REL group showing better performance for WM load 4 versus WM load 2. When the data from the outliers were disregarded, accuracy was significant lower in REL compared to HC, t(41) = 2.20, p < .05 and did not differ from PSZ, t(40) = −1.54, p = .13. In both load conditions, RTs did not differ significantly between REL and HC (all p values > .92) but there was a trend for faster RTs compared to PSZ in the WM load 4/no-distraction condition, t(42) = 1.72, p = .09, but not the WM load 2/no-distraction condition, p = .22.
There was no relationship between WM performance (accuracy and RTs) and SPQ ratings in REL in both WM load conditions (all p values > .50).
Confidence rating
Separate one-way ANOVAs revealed a significant group difference in the percentage of false memories calculated across experimental conditions, F(2, 70) = 6.65, p > .01, η2 = .16, corrected for multiple comparisons. As indicated by post hoc tests, REL made more false memory errors than HC, p < .05, corrected for multiple comparisons, but did not differ from PSZ in the amount of false memories, p = 1.0. The percentage of true memory responses, correct and incorrect responses given without being confident, did not significantly differ between groups (all F values < 2.20, all p values > .12).
The amount of false memories did not correlate with SPQ ratings in REL, p > .86.
The percentage of false memory responses in the WM load 4/no-distraction condition correlated negatively with accuracy, r = −.92, p < .001. In contrast, there was no relationship between the amount of incorrect and not-confident responses and accuracy, r = .02, p = .95. RTs were not correlated with neither the amount of false memories nor the amount of incorrect and not-confident responses in REL (all p values > .29).
Distractor condition
The distractor effect, that is, lower accuracy when the distractors were present versus not present, was also found in REL, t(15) = 2.89, p < .05. In addition, accuracy was lower in REL than HC, t(43) = 2.40, p < .05, but did not differ from performance observed in PSZ, t(42) = 0.39, p = .70.
RTs did not differ between the two distraction conditions in REL, t(15) = −0.48, p = .64. In addition, RTs did not significantly differ from HC, t(43)= −0.21, p = .83, whereas there was a trend for faster RTs compared to PSZ, t(42) = 1.83, p = .07.
For REL, there was no relationship between WM performance (accuracy and RTs) and SPQ ratings in the distraction condition (p > .59).
Discussion
The present findings provide new insight into the mechanisms underlying WM impairments in schizophrenia by analyzing different response types. Consistent with previous studies (Gold et al., 2010; Goldman-Rakic, 1994; Haenschel & Linden, 2011; Lee & Park, 2005), WM performance was markedly reduced in PSZ, especially when the demands on WM were high. Here, we demonstrate that this reduction in WM performance in schizophrenia is mainly due to an increase in the amount of false memory responses rather than the amount of incorrect/not-confident responses. The percentage of false memories was almost doubled in PSZ compared to HC, whereas the amount of incorrect responses that were given without being confident did not differ between groups. Moreover, the percentage of false memory responses rather than the percentage of incorrect/not-confident responses correlated strongly negatively with WM accuracy in the condition of high WM load as well as with WM capacity estimates derived from an independent task. This suggests that the degree to which participants made false memory errors was related to their degree of WM impairment.
The finding of an increased rate of false memory but not incorrect/not-confident responses in PSZ suggests that their WM deficit is most likely due to difficulties in processes required during the early phase of WM encoding rather than due to a failure of WM storage. In line with an encoding deficit (Lee & Park, 2005), we have previously shown in healthy participants that the percentage of false memories in a spatial DRT decreases when the processes that support WM encoding are facilitated (Mayer et al., 2011). Furthermore, neuroimaging studies that compared true memory and false memory responses in PSZ reported similar delay-related activation patterns in the prefrontal cortex for both types (Lee et al., 2008) suggesting that the mechanisms that support the maintenance of the internal representation were intact whether that representation was correctly or incorrectly encoded.
In addition, consistent with previous studies (Park et al., 1995) the RT data did not indicate that the increased rate of false memory responses reflected a failure of WM retrieval. Although the increase in RTs was related to higher rates of incorrect and not-confident responses in PSZ, we did not find a positive correlation with the rate of false memories. Instead our data suggests a trend toward a negative correlation between RT and false memory errors, which indicates that patients with higher rates of false memory responses might be finding retrieval less demanding, resulting in faster RTs.
It might also be argued that the increased rate of false memories in PSZ was a consequence of deficits in metamemory (Flavell, 1979). Patients might be less aware of their own memory capacity, which would lead to a problem at the stage of the confidence judgments rather than WM encoding. For instance, in tasks on episodic long-term memory (LTM), PSZ show overconfidence in errors, as observed by higher retrospective judgment ratings for error trials (Moritz & Woodward, 2006; Moritz, Woodward, Whitman, & Cuttler, 2005). Similarly, PSZ may have given more false memory responses in the present WM task due to an overall response bias for confidence responses. However, if patients were less successful in subjectively assessing the correctness of their WM representation, we expected a similar distribution of confident and not-confident responses among correct and incorrect responses in PSZ. The results in the condition WM load 4/no distraction were not consistent with this hypothesis. Seventy-nine percent of the correct responses were true memories and 21% of the correct responses were given without being confident. In contrast, among the errors about 74% were false memory errors whereas 26% were incorrect and not-confident responses. A t test for paired samples indicated that the difference in the percentage of confident and not-confident responses was significant for correct and incorrect responses, t(27) = 2.01, p < .05. In addition, the distribution of confident and not-confident correct responses was similar in PSZ and HC [77% versus 23% for HC, no significant group difference in the difference between percent correct/confident and correct/not confident, t(55) = .32, p = .75]. This is in contrast to LTM tasks where PSZ also show less confidence in correct responses (Moritz & Woodward, 2006). Together, these findings do not suggest a failure of metamemory monitoring in our patients, which is also consistent with recent reports on intact response monitoring in schizophrenia (Thakkar, Schall, Boucher, Logan, & Park, 2011). Even in the context of episodic LTM tasks, which demand monitoring processes to a higher degree than WM, PSZ show some preservation of their monitoring and control abilities (Bacon & Izaute, 2009; Bacon, Izaute, & Danion, 2007).
Taken together, by demonstrating an increased rate of false memory responses in PSZ compared to HC in a spatial DRT, we provide evidence that the severe impairments in spatial WM observed in PSZ can be attributed, at least to some degree, to deficits in processes associated with WM encoding. This finding supports and extends recent studies that examined different encoding conditions rather than response types and found impaired encoding as well (Badcock et al., 2008; Fuller et al., 2009; Hahn et al., 2010; Hartman et al., 2003; Tek et al., 2002; for a review see Haenschel & Linden, 2011).
Similar to PSZ, PBP showed lower visual WM performance compared to HC. However, this deficit was less pronounced compared to PSZ when the demands on WM were high. These findings are consistent with neuropsychological studies indicating that PBP show a cognitive profile similar to that of PSZ, but that the impairments are less severe (Schretlen et al., 2007; Seidman et al., 2002). However, results from studies focusing on visual WM in bipolar disorder have been inconsistent. Several studies reported similar performance in PBP and HC (Kéri, Kelemen, Benedek, & Janka, 2001; Park & Holzman, 1992, 1993; Pirkola et al., 2005). Other studies provide evidence for impaired visual WM storage in PBP, particularly in the manic phase of the illness (Badcock, Michiel, & Rock, 2005; McGrath et al., 2001; Sweeney et al., 2000) and in patients with a history of psychotic symptoms (Bora, Yücel, & Pantelis, 2010; Glahn, Bearden, et al., 2006). About half the participants in our sample reported psychotic symptoms, which might explain our finding of moderate impairments on the group level in the PBP compared to more severe impairments in the PSZ in the condition of high WM demand. Most strikingly, even though PBP showed WM deficits, the percentage of false memory errors did not differ from HC. These findings support and extend previous reports on WM dysfunctions in bipolar disorder and schizophrenia by indicating that WM impairments in these patients might differ not only in severity but also quality (Badcock et al., 2005; Glahn, Barrett, et al., 2006). The demonstration of increased false memory rates in PSZ but not PBP compared to HC suggests that inefficient WM encoding contributes to WM deficits in PSZ but not PBP. These findings point to differential mechanisms underlying WM impairments in PSZ and PBP and thus have important implications for the development of differential cognitive remediation strategies in schizophrenia and bipolar disorder.
In PSZ, neither the severity of current positive nor negative symptoms was related to any of the WM performance measures. This is consistent with the literature suggesting that WM deficits in schizophrenia represent deficits that are stable and exist independently of clinical state (Glahn et al., 2003; Park et al., 1999). In PBP, the stability of WM deficits across different states of the disease is less clear. Some studies indicate that WM deficits are in particular pronounced during the manic phases of the illness (McGrath et al., 2001; Sweeney et al., 2000). It is interesting that in our sample of PBP we found that the rate of false memories increased with the severity of manic symptoms. As psychotic symptoms such as delusion, hallucinations, and positive thought disorder have a high prevalence in mania (e.g., Canuso, Bossie, Zhu, Youssef, & Dunner, 2008; Rosen, Grossman, Harrow, Bonner-Jackson, & Faull, 2011; Schürhoff et al., 2003), our finding suggests that the PBP who are more similar to PSZ in terms of their symptomatology also show a more severe encoding deficit.
A second aim of this study was to elucidate the processes that contribute to the WM encoding deficit in PSZ. We tested the hypothesis of impaired attentional selection as a cause of WM encoding deficits in schizophrenia, by manipulating the demands on the selection process during the encoding phase. For this purpose, we presented distractors in some of the trials. We expected that the distraction effect, that is, lower WM accuracy in the condition with distraction versus no distraction, would be stronger in PSZ than HC reflecting reduced selection capabilities. Our findings did not support this hypothesis. The decline in accuracy when distractors were presented in the stimulus display was similar in PSZ, HC, and PBP. Moreover, RTs were significantly faster in the WM load 2/distraction condition compared to the WM load 4/no-distraction condition both in HC and patients, indicating that all participants were effectively ignoring the distractors. However, PSZ showed a stronger increase in RTs in the condition with distraction versus no distraction compared to HC and PBP. Thus, PSZ were able to ignore the distractors as effectively as HC and PBP only at the cost of additional processing time. Although this finding may suggest that processes related to WM retrieval are altered in PSZ when distractors were presented, they do not provide evidence that the selection process itself was impaired during WM encoding. This was surprising in the light of previous reports on reduced selectivity during WM encoding in healthy individuals with low WM capacity (Vogel et al., 2005). However, the lack of a selection deficit in our group of chronic PSZ replicates previous findings indicating that attentional selection is not necessarily impaired in PSZ, in particular, when the selection is based on simple and salient cues and the distractors do not strongly compete for attentional resources (Gold et al., 2006, 2007; Tanaka et al., 2007). Consistent with theses reports, target selection and/or distractor inhibition was intact in our patients as well, and thus cannot explain their WM encoding deficit that was reflected in the increased false memory rate. However, recent evidence suggests that when the selection process requires a high degree of top-down control, such as in the presence of highly distracting inputs, WM encoding is indeed markedly reduced in schizophrenia (Hahn et al., 2010). Moreover, in our previous study we have demonstrated that false memory errors are linked to top-down attentional selection necessary for WM encoding (Mayer et al., 2011). Following these findings, one can speculate that increased rates of false memory errors in PSZ may be specifically associated with a failure of top-down attentional control necessary for WM encoding, a hypothesis that remains to be tested in future studies.
If reduced attentional selection cannot account for the encoding deficit observed in PSZ in our task, which other processes might contribute? Given that participants were granted long encoding time (Tek et al., 2002), perceptual deficits might not have played a major role. Another plausible and prominent hypothesis is that WM encoding is less precise in PSZ (Javitt et al., 1997, 1999; Lencz et al., 2003; see also Badcock et al., 2008). Consistent with this hypothesis, false memory errors were elevated in PSZ but not incorrect and not-confident responses when the probe was presented nearer rather than farther from a target location. In contrast, the amount of false memories and the amount of incorrect and not-confident responses did not differ in HC depending on the distance between probe and targets. These findings provide preliminary evidence that imprecise encoding of stimuli is one factor that can lead to false memory errors and contribute to WM deficits observed in patients with schizophrenia. Future studies are required to determine whether other factors also play a role.
While both patient groups were comparable with regard to the severity of symptoms as measured with the BPRS and duration of illness, they were taking different classes of psychotropic drugs. Therefore, one limitation of this study concerns potential medication effects. Indeed, correlational analyses indicated that the current antipsychotic medication dosage was related to a reduction in WM performance in PSZ. We therefore tested a group of unaffected and unmedicated first-degree relatives of PSZ. REL showed WM deficits that were quantitatively and qualitatively similar to those observed in PSZ. Thus, similar to PSZ, WM accuracy was lower and the percentage of false memories rather than the percentage of incorrect/not-confident responses was higher in REL compared to HC. These findings suggest that the WM deficit observed in PSZ cannot be explained solely in terms of medication but rather reflect a neurocognitive marker of the disease. In addition, many PBP were taking atypical antipsychotic drugs but not all. It was not possible to match the two psychosis groups on medication. Thus, the differences between the two groups may be partly driven by differential medication. We compared WM performance between PBP who did receive (n = 9) and did not receive atypical antipsychotics (n = 7) and found no group differences in neither WM accuracy, F(1, 14) = 0.02, p = .90, RTs, F(1, 14) = 0.01, p = .91, nor the percentage of false memories across task conditions, F(1, 14) = 0.94, p = .35. Thus, differential medication did not affect WM performance in PBP. Therefore it seems also unlikely that performance differences between PBP and PSZ were solely driven by effects of antipsychotic medication.
Furthermore, one could argue that the comparison between REL, PSZ, and HC was confounded by differences in premorbid IQ, which was significantly lower in REL. If poor general intellectual functioning was the main factor explaining WM deficits, we would have expected the lowest performance in REL. However, WM accuracy was similar in REL and PSZ in all three experimental conditions. This finding indicates that the WM deficits observed in PSZ cannot be explained solely in terms of poor general intellectual abilities.
Finally, there is a caveat. The bipolar sample was heterogeneous and included patients with bipolar I and bipolar II disorders. Due to the small sample size we were not able to address differences in performance among bipolar patients with different characteristics. Future studies will be needed to investigate the various patterns of cognitive impairment and their underlying mechanisms which may well exist in this heterogeneous disorder.
Taken together, in this study we demonstrated spatial WM deficits that are accompanied by an increased rate of false memories in PSZ and their unaffected first-degree relatives but not PBP. These findings indicate that reduced WM in schizophrenia can be explained, at least partially, by deficits of processes associated with the encoding phase. We suggest that future studies will benefit from combining the analysis of responses types with conventional strategies of modulating encoding processes in order to determine the various mechanisms of impaired WM in schizophrenia and other psychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by NIH grant (R01 MH073028) to Sohee Park and NICHD Grant P30 HD15052 to the Vanderbilt Kennedy Center for Research on Human Development. We thank Jejoong Kim for his advice on programming the experiment; Heath Nichols, Katy Thakkar, and Joel Peterman for their assistance with clinical interviews and subject recruitment; and Professor Stephan Heckers with his help in recruitment.
Footnotes
The design was not balanced with regard to the target shapes, as circles were presented as targets only in the WM load 4/no-distraction condition. We cannot exclude that if circles and squares differed in perceptual salience, this could have confounded the effect of WM load. However, given that perceptual salience is encoded very fast (in the range of tenths of ms) (e.g., Nothdurft, 2002; Mayer et al., 2011; Wolfe, 1998) and the targets were presented rather long (2 s), a potential salience effect might be disregarded.
This analysis included data from 19 PSZ and 21 HC who also participated in a visual change detection task. In this task, participants were presented with arrays of two, four, six, or eight colored squares for 150 ms in each trial. After a retention interval of 900 ms, one colored square was presented at the location of one of the items from the memory array. Participants made an unspeeded button press to indicate whether the color of the test probe matched or did not match the color of the original memory item in that location. Each individual’s accuracy for each array size was transformed into a K estimate using a standard formula: K = (hit rate + correct rejection rate − 1) × N (Cowan, 2001). This approach allows quantifying the number of items held in memory, K, from an array size of N items, taking guessing into account. For each subject, the values for array sizes 2, 4, 6, and 8 were averaged into a single WM capacity estimate.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Bacon E, Izaute M. Metacognition in schizophrenia: Processes underlying patients’ reflections on their own episodic memory. Biological Psychiatry. 2009;66:1031–1037. doi: 10.1016/j.biopsych.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Bacon E, Izaute M, Danion JM. Preserved memory monitoring but impaired memory control during episodic encoding in patients with schizophrenia. Journal of the International Neuropsychological Society. 2007;13:219–227. doi: 10.1017/S1355617707070245. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Badcock DR, Read C, Jablensky A. Examining encoding imprecision in spatial working memory in schizophrenia. Schizophrenia Research. 2008;100:144–152. doi: 10.1016/j.schres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Michiel PT, Rock D. Spatial working memory and planning ability: Contrasts between schizophrenia and bipolar I disorder. Cortex. 2005;41:753–763. doi: 10.1016/s0010-9452(08)70294-6. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, UK: Oxford University Press; 1986. [Google Scholar]
- Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: A meta-analytic study. Journal of Affective Disorders. 2010;127:1–9. doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Bossie CA, Zhu Y, Youssef E, Dunner DL. Psychotic symptoms in patients with bipolar mania. Journal of Affective Disorders. 2008;111:164–169. doi: 10.1016/j.jad.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: Longitudinal stability and predictive utility for short-term functional outcome. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:867–878. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Conklin HM, Curtis CE, Calkins ME, Iacono WG. Working memory functioning in schizophrenia patients and their first-degree relatives: Cognitive functioning shedding light on etiology. Neuropsychologia. 2005;43:930–942. doi: 10.1016/j.neuropsychologia.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Fine MS, Minnery BS. Visual salience affects performance in a working memory task. Journal of Neuroscience. 2009;29:8016–8021. doi: 10.1523/JNEUROSCI.5503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental enquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired visual working memory consolidation in schizophrenia. Neuropsychology. 2009;23:71–80. doi: 10.1037/a0013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. Journal of Abnormal Psychology. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Barrett J, Bearden CE, Mintz J, Green MF, Serap Monkul E, Velligan DI. Dissociable mechanisms for memory impairment in bipolar disorder and schizophrenia. Psychological Medicine. 2006;36:1085–1095. doi: 10.1017/S0033291706007902. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: Effects of lifetime history of psychosis. Bipolar Disorders. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Archives of General Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Progress in Brain Research. 1990;85:325–336. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neuroscience. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Linden D. Exploring intermediate phenotypes with EEG: Working memory dysfunction in schizophrenia. Behavioural Brain Research. 2011;216:481–495. doi: 10.1016/j.bbr.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biological Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M, Steketee M, Silva S, Lanning K, McCann H. Working memory and schizophrenia: Evidence for slowed encoding. Schizophrenia Research. 2003;59:99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophrenia Bulletin. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davis KL. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: Spatial working memory in patients with schizophrenia. Schizophrenia Research. 1995;17:25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: A neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Lee J, Folley BS, Gore J, Park S. Origins of spatial working memory deficits in schizophrenia: An event-related FMRI and nearinfrared spectroscopy study. PLoS One. 2008;12:e1760. doi: 10.1371/journal.pone.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Archives of General Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Kim J, Park S. Enhancing visual working memory encoding: The role of target novelty. Visual Cognition. 2011;19:863–885. doi: 10.1080/13506285.2011.594459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Chapple B, Wright M. Working memory in schizophrenia and mania: Correlation with symptoms during the acute and subacute phases. Acta Psychiatrica Scandinavica. 2001;103:181–188. doi: 10.1034/j.1600-0447.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: Importance of working memory. The American Journal of Psychiatry. 2005;162:1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS. The contribution of metamemory deficits in schizophrenia. Journal of Abnormal Psychology. 2006;115:15–25. doi: 10.1037/0021-843X.15.1.15. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Whitman JC, Cuttler C. Confidence in errors as a possible basis for delusions in schizophrenia. The Journal of Nervous and Mental Disease. 2005;193:9–16. doi: 10.1097/01.nmd.0000149213.10692.00. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. American Journal of Medical Genetics. 2002;114:609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National adult reading test. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Nestor PG, Faux S, McCarley R, Penhune V, Shenton M, Pollak S. Attentional cues in chronic schizophrenia: Abnormal disengagement of attention. Journal of Abnormal Psychology. 1992;101:682–689. doi: 10.1037//0021-843x.101.4.682. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC. Attention shifts to salient targets. Vision Research. 2002;42:1287–1306. doi: 10.1016/s0042-6989(02)00016-0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophrenia Research. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Park S, Püschel J, Sauter B, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: A 4-month follow-up study. Biological Psychiatry. 1999;46:392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppä T, Haukka J, Kaprio J, Cannon TD. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biological Psychiatry. 2005;58:930–936. doi: 10.1016/j.biopsych.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: A quantitative literature review. Psychiatry Research. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Archives of General Psychiatry. 1988;45:814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSMIII-R criteria. Schizophrenia Bulletin. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Rosen C, Grossman LS, Harrow M, Bonner-Jackson A, Faull R. Diagnostic and prognostic significance of Schneiderian first-rank symptoms: A 20-year longitudinal study of schizophrenia and bipolar disorder. Comprehensive Psychiatry. 2011;52:126–131. doi: 10.1016/j.comppsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biological Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürhoff F, Szoke A, Meary A, Bellivier F, Rouillon F, Pauls D, Leboyer M. Familial aggregation of delusional proneness in schizophrenia and bipolar pedigrees. The American Journal of Psychiatry. 2003;160:1313–1319. doi: 10.1176/appi.ajp.160.7.1313. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MTA. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophrenia Research. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Spatial selective attention in schizophrenic, affective disorder, and normal subjects. Schizophrenia Research. 1996;20:33–50. doi: 10.1016/0920-9964(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, Umbricht D. Cognitive functioning in the schizophrenia prodrome. Schizophrenia Bulletin. 2007;33:761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Tanaka G, Mori S, Inadomi H, Hamada Y, Ohta Y, Ozawa H. Clear distinction between preattentive and attentive process in schizophrenia by visual search performance. Psychiatry Research. 2007;149:25–31. doi: 10.1016/j.psychres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan GD, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biological Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. What can 1,000,000 trials tell us about visual search? Psychological Science. 1998;9:33–39. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.