Abstract
Objective
To determine whether previously reported socioeconomic status (SES)-related disparities in cystic fibrosis (CF) health outcomes vary by the indicator used (median household income by zip code [MIZ], maternal educational attainment [MEA], and state insurance coverage [MA]), and whether these disparities can be explained by differences in medical treatment.
Study design
A cross-sectional analysis of data on patients age <18 years from the Epidemiologic Study of Cystic Fibrosis (ESCF).
Results
Disease severity showed a similar inverse correlation with all 3 SES measures. The number of stable clinic visits was unrelated to SES. Patients with MA had more sick outpatient visits and more courses of intravenous (IV) antibiotics for pulmonary exacerbations, and were more likely to be prescribed all chronic therapies. Low-MIZ patients had slightly fewer sick visits and more courses of IV antibiotics, and were more likely to receive oral nutrition supplements but less likely to receive macrolide prescriptions. Low-MEA patients were less likely to receive IV antibiotics at home, more likely to receive oral nutrition supplements, but less likely to receive macrolide prescriptions.
Conclusions
CF health outcomes are correlated with the SES spectrum, but these disparities are not explained by differential use of health services or prescription of chronic therapy. Future investigations should focus on the possible impact of environmental exposures and differences in disease self-management.
Socioeconomic status (SES) is a strong predictor of outcomes in patients with cystic fibrosis (CF).1,2 Barriers to accessing quality care are an important cause of SES-related disparities in many patient populations,3 but previous analyses of CF Foundation Registry data have failed to show any SES-related difference in care at accredited CF care centers.2 Until recently, however, the CF Registry collected only limited data on the use of specific therapies and practice patterns. Reports from the Epidemiologic Study of Cystic Fibrosis (ESCF) show large variations in the use of chronic medications of proven efficacy4 and in outpatient monitoring,5 and also demonstrate an association between these variations in care and outcomes.6 It is plausible that some of this variability in treatment and monitoring might be related to some aspect of SES and serve as a possible explanation for the disparities in outcomes.
There is no ideal measure of SES; different aspects impact health in different ways. Family income affects overall financial resources, which can affect the ability to access care and medications; measures of neighborhood income partly reflect individual income but have an independent effect related to features of the physical, social, or service environments, such as public transportation or distance from a CF center.7 Maternal education level affects disease self-management abilities. State insurance eligibility reflects financial resources, but this differs by state and is determined in part by healthcare expenditures, leading to the possible misinterpretation of the direction of causality in terms of conclusions regarding the link between SES and health.8-13 Whether any specific indicator of SES most accurately predicts disease outcomes in CF is not clear; to date, no comparison of different variables associated with SES in the CF population has been reported.
We analyzed data from the ESCF to ascertain whether SES is associated with different patterns of routine outpatient monitoring or the likelihood of prescription of various chronic CF therapies. Given that a small minority of children with CF are uninsured,2 we hypothesized that due to potential access barriers, such as cost and transportation,7 the most likely effect of SES would be in the number of outpatient visits, and any disparity in prescribed medications would not be seen for Medicaid recipients. In addition, we investigated whether any of 3 different indicators of SES for which ESCF tracked data (median household income by zip code, maternal education, or insurance status) might have a stronger association with disease severity or practice patterns compared with the others. We further hypothesized that state insurance status might have the strongest association with disease severity because of the higher likelihood that sicker patients are eligible for Medicaid, and that neighborhood income might have a weaker association because of the ecological nature of the variable.14
Methods
The design and implementation of the ESCF have been described previously.15 The ESCF is a large, multicenter, longitudinal, prospective observational study of the clinical course of patients with CF in the United States and Canada from 1994 through 2005. Data collected on each patient included demographics, use of chronic therapies, results of pulmonary function tests, results of respiratory tract cultures, information on growth and nutrition, and details of antibiotic, anti-inflammatory, and other treatments. ESCF II, the second phase of the ESCF, was conducted from 2003 to 2005 and included several additional variables, including those relevant to SES. Institutional review board approval was obtained at each individual study site.
The data collection form was completed by the study site personnel from medical records and identified certain prescribed therapies as chronic; there was no ascertainment of patient adherence. Data also were collected on treatment of pulmonary exacerbations, including site (home vs hospital) where intravenous (IV) antibiotics were administered.
Cohort Definition and SES Measures
This analysis included patients who were age <18 years at the time of enrollment in ESCF II, had 2 or more medical encounters at least 30 days apart recorded in the ESCF II database during 2004, and had no missing data on the outcome variables of interest and at least 1 SES variable. SES was evaluated using 3 indicators, defined as follows:
Median income of zip code of residence (MIZ). ESCF data on zip code of residence were linked to United States Census reports from the year 2000 on median annual income for each zip code in the United States. These data were categorized into 4 groups: < $40 000, $40 000 to < $50 000, $50 000 to < $60 000, and $ $60 000.
Maternal educational attainment (MEA), reported by participating centers in 3 categories: less than high school, high school diploma, or college diploma
Eligibility for Medicaid or state health insurance cover age (MA), reported as a dichotomous yes/no variable, independent of whether or not the patient had any other form of health insurance.
Outcome Measures and Covariates
We evaluated the relationships between SES and disease severity, use of prescription or chronic medications, and healthcare utilization. Disease severity for patients age < 6 years was categorized using the first stable weight-for-age percentile (ie, < 5, 5 to < 10, 10 to < 25, 25 to < 50, $ 50) in 2003 or 2004. For older patients, the first stable percent predicted prebronchodilator forced expiratory volume in 1 second (FEV1) value (ie, < 40, 40 to < 70, 70 to < 100, $ 100) in 2003 or 2004 was used to categorize disease severity. Values for percent predicted FEV1 were calculated using the formula of Wang et al16 for males up to age 17 years and females up to age 15 years. The equations of Hankinson et al17 were used for all older patients. In terms of chronic medications, the reported use of oral and enteral nutritional supplements, vitamins, oral macrolide antibiotics, inhaled antibiotics, and dornase alfa in 2004 was evaluated. Health-care utilization was evaluated as the number of outpatient visits (distinguished by report as “stable” or “sick”) and number of pulmonary exacerbations requiring IV antibiotics (as diagnosed by treating physician and distinguished by a report as treated in the hospital or at home) in 2004. Age at enrollment and disease severity were considered potential confounders and included as covariates in the analysis of chronic medication use and healthcare utilization.
Statistical Analysis
Differences among patients in each SES category were evaluated by ×2, Mantel-Haenszel ×2, or t tests, as appropriate. Separate multivariate models were used to evaluate each SES indicator as a predictor of each outcome measure, controlling for age at enrollment in the ESCF II and for disease severity. Additional models containing either all 3 SES categories or 2 categories at a time provided no novel outcomes and thus are not reported here. Because disease severity was classified differently for children age < 6 and those age 6 to < 18, the model was established with a different set of severity effects for these 2 age groups. Logistic regression was used to model the likelihood of receiving chronic therapies and medications, and linear regression was used to model the number of healthcare visits.
Results
Of the 32 585 patients enrolled in the ESCF between 1994 and 2005, 17 722 were enrolled in the ESCF II. Of these patients, 11 447 were age < 18 years at the time of enrollment in the ESCF II. Approximately 83% of these patients (n = 9534) had at least 2 medical encounters in 2004 spanning a minimum of 30 days (mean number of encounters, 4.93 ± 2.65), and no missing SES or disease severity data. MIZ was known for 9134 patients, MEA was reported for 3731 patients, and MA status was known for 9168 patients. Figure 1 shows the overlap of subjects with SES data.
Figure 1.
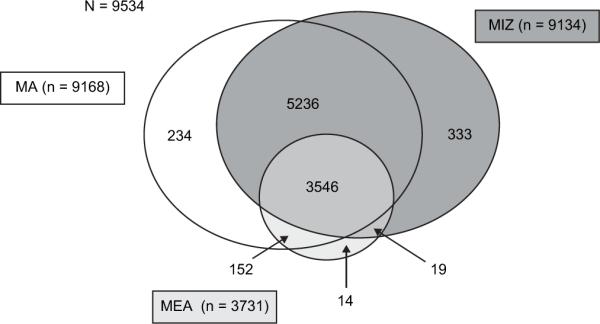
Distribution of subjects for each SES cohort.
The cohorts were similar with respect to mean age, sex, race/ethnicity, percent predicted FEV1, height-for-age and weight-for-age percentiles, body mass index (BMI), and disease severity (data not shown). The distributions of mother's education, median household income, and type of healthcare insurance also were similar among the cohorts. For the MIZ and MEA cohorts, there were no differences between the study population analyzed and those patients excluded from the analysis because of missing SES data (data not shown). However, compared with the group included in the MA cohort, those with missing insurance data tended to be older (mean age, 9.1 ± 5.0 vs 8.3 5.2 years; P = .007), to be non-Hispanic white (94.3% vs 85.3%; P < .001), to have higher BMI (mean BMI, 18.7 ± 3.4 vs 18.3 ± 3.3; P = .013), and to have higher percent predicted FEV1 (mean, 94.9 ± 19.7 vs 91.6 ± 21.6; P = .014).
As assessed by Spearman correlation coefficients, the correlation among SES indicators was small to moderate18: 0.193 between MIZ and MEA, 0.260 between MA and MEA, and 0.343 between MA and MIZ (P < .001 for all).
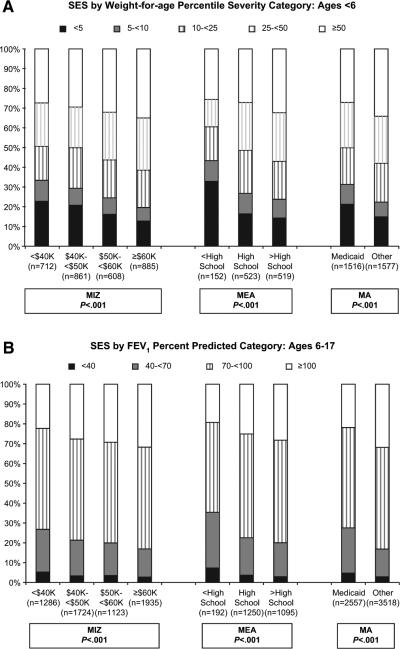
Comparisons of disease severity, as measured by both weight-for-age percentile and percent predicted FEV1, and all measures of SES exhibited a linear inverse correlation (ie, low SES associated with greater disease severity; all P < .0001) (Figure 2). There was no difference in the prevalence of positive airway cultures for Pseudomonas aeruginosa by any SES measure.
Figure 2.
Distribution of SES by disease severity, defined by weight-for-age percentile in children age < 6 years A, and percent predicted FEV1 in children age 6 to 17 years B.
Likelihood of Chronic Medication Use
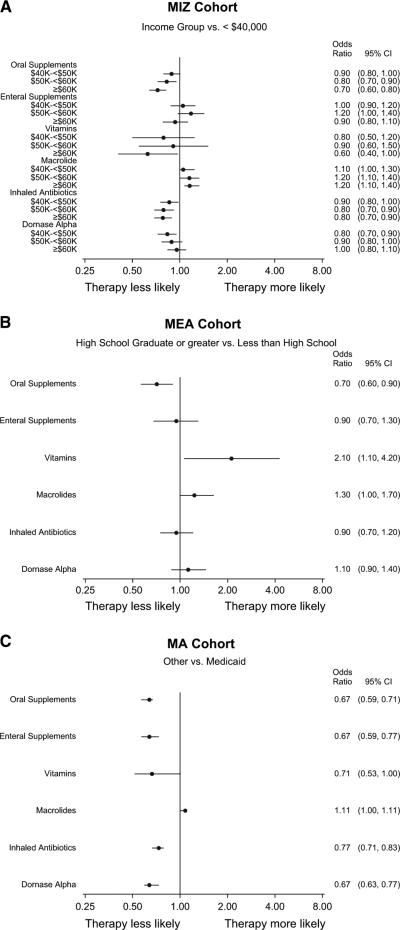
In models adjusting for age and disease severity, patients living in zip codes with the lowest median income were more likely to receive oral nutritional supplements and inhaled antibiotics but less likely to receive macrolides; there were no discernible trends in the prescription of other chronic therapies (Figure 3, A). Similarly, patients whose mothers had less than a high school education were more likely to be prescribed oral nutritional supplements but less likely to be prescribed vitamins and macrolides compared with patients whose mothers were high school graduates (Figure 3, B). There was no significant difference between high school and college graduates in the use of any chronic therapies, so the results from a model using a dichotomous MEA variable are shown. Patients with MA were about 30% to 50% more likely than patients without MA to be prescribed all chronic therapies and medications except macrolides (Figure 3, C).
Figure 3.
Adjusted odds ratios for the likelihood of being prescribed chronic therapy according to SES status, as defined by MIZ A, MEA B, and MA C. Logistic regression models include age at enrollment and severity as covariates.
Healthcare Utilization
In models adjusting for age and disease severity, no SES-related differences in the number of stable outpatient visits were found. Patients living in zip codes with the lowest median income had the fewest sick outpatient visits (Table). There was a trend across the category for lower-income patients to receive more IV antibiotics for pulmonary exacerbations. MEA was not associated with any difference in outpatient visits or treatment for IV exacerbations, but the children of mothers who did not graduate high school were less likely to be treated for IV exacerbations at home. There was no significant difference between high school and college graduates in terms of healthcare utilization, so the results from a model using a dichotomous MEA variable are shown. Patients with MA had more sick visits than those without MA, and they also had more pulmonary exacerbations treated with IV antibiotics.
Table.
Adjusted* averages for healthcare utilization by SES cohort
| Healthcare utilization (mean ± SE) | MIZ |
MEA |
MA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <$40 000 (n = 1998) | $40 000-<$50 000 (n = 2585) | $50 000-<$60 000 (n = 1731) | ≥$60 000 (n = 2820) | P † | < High school (n = 344) | ≥ High school (n = 3387) | P ‡ | Medicaid (n = 4073) | Other (n = 5095) | P ‡ | |
| Stable visits | 3.56 ± 0.04 | 3.61 ± 0.04 | 3.67 ± 0.04 | 3.65 ± 0.03 | .18 | 3.48 ± 0.10 | 3.66 ± 0.03 | .078 | 3.59 ± 0.03 | 3.61 ± 0.03 | .72 |
| Sick visits | 0.94 ± 0.04 | 1.05 ± 0.03 | 1.16 ± 0.04 | 1.07 ± 0.03 | <.001 | 0.99 ± 0.10 | 1.14 ± 0.03 | .15 | 1.22 ± 0.03 | 0.93 ± 0.02 | <.001 |
| Total IV exacerbations | 0.47 ± 0.02 | 0.48 ± 0.02 | 0.45 ± 0.02 | 0.41 ± 0.02 | .006 | 0.51 ± 0.05 | 0.46 ± 0.02 | .43 | 0.56 ± 0.01 | 0.37 ± 0.01 | <.001 |
| Hospital IV exacerbations | 0.44 ± 0.02 | .44 ± 0.02 | 0.40 ± 0.02 | 0.35 ± 0.02 | <.001 | 0.47 ± 0.05 | 0.41 ± 0.01 | .17 | 0.51 ± 0.01 | 0.32 ± 0.01 | <.001 |
| Home IV exacerbations | 0.19 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.22 ± 0.01 | .091 | 0.14 ± 0.03 | 0.24 ± 0.01 | .003 | 0.23 ± 0.01 | 0.21 ± 0.01 | .062 |
Linear regression models include age at enrollment and severity as covariates.
Calculated from F-test statistics.
Calculated from t-test statistics.
Discussion
SES is a strong predictor of disease severity in CF; patients of low SES have increased mortality, worse lung function, and worse nutrition.1,2 This is confirmed by our ESCF data, which also show a similar association whether SES is defined by median household income by zip code, by maternal education, or by Medicaid status. Our findings suggest the existence of some previously unappreciated SES-related differences in healthcare utilization patterns in patients attending CF care centers in the United States that vary, to some degree on the marker of SES used. Patients with low SES as indicated by MIZ) have fewer outpatient sick visits, but patients with MA have more visits, and MEA does not seem to influence the number of visits. The relationship between MIZ and number of sick visits might reflect reduced access for patients with this marker, particularly if they live at a distance from the CF center.19 Pulmonary exacerbations requiring IV antibiotics are diagnosed and treated more frequently in patients with lower SES, as indicated by MIZ or MA status but not by MEA. The reason for this is unclear; perhaps the CF providers sense that patients with fewer resources are likely to deteriorate faster and thus need more aggressive care. Among the SES measures, MEA primarily seems to influence whether a patient's pulmonary exacerbations are treated with IV medications at home or in the hospital, suggesting that providers are less likely to believe that low-MEA mothers can perform all of the necessary treatments on their own or that the mothers perceive barriers to home care.
Most chronic medications are used at similar or slightly greater rates in patients with lower SES. Patients with MA in particular are more likely to receive all medications evaluated except macrolides, perhaps reflecting the absence of financial barriers faced by patients who have private insurance and need to make copayments for medications. Oral nutritional supplements are more likely to be prescribed to low-MIZ and low-MEA patients. Low-MIZ patients receive more inhaled antibiotics, even though they are no more likely than others to have Pseudomonas airway infection when rates are adjusted for disease severity and age. The greater prescription of chronic therapies in this group might indicate that healthcare providers recognize that patients with low SES have worse disease outcomes and attempt to treat these patients more aggressively.
Children of more highly educated mothers and those living in wealthier zip codes are more likely to be prescribed macro-lides. This analysis evaluated practice in 2004, the year after publication of a major study documenting the efficacy of azithromycin in CF.20 It took several years before the use of chronic macrolide therapy was consistently adopted at CF centers, and even now it is used nationally in fewer than 60% of patients for whom it would be appropriate.21 One possible explanation for this is that CF healthcare providers are prompted by wealthier or better-educated mothers to prescribe macrolides sooner than they might have otherwise. This is consistent with the theory that wealth or parental education influences disease outcomes in children through their impact on disease self-management.22 Similarly, the greater use of vitamins in children with more educated mothers also might be related to parental prompting.
Overall, we uncovered no compelling differences in health-care utilization or the prescription of chronic therapies that would explain the significant and striking disparities in health outcomes in low-SES groups. This contrasts with findings in other chronic diseases, such as asthma,3 for which access to quality care is an important contributor to SES-related disparities. Other mechanism are probably of greater importance in the CF population.23 For example, disease self-management is likely an important determinant of long-term outcomes for chronic illnesses such as CF and appears to be associated with SES.22,24 Health behaviors, such as cigarette smoking, also are clearly linked to SES;25 secondhand smoke exposure is a likely contributing factor to SES disparities in CF.26 Other potential risk factors include exposure to psychological stress and environmental pollutants.27
No previous comparison of SES measures has been undertaken in patients with CF; in fact, very few comparison studies are available in the general pediatric literature.28 SES is a broad sociological concept with different attributes and no single ideal measure.8,29 Income is an intuitively obvious measure of SES and is a good measure of material resources, but it is potentially confounded by age, education, family size, regional cost of living, and whether the income is generated by one or more family members.8 We do not have direct information on family income in the ESCF database, but we used US Census data to link to median household income by zip code. This approach has the usual weaknesses and imprecision of an ecological measure,14 but any resulting misclassification would typically weaken statistical associations rather than cause spurious ones.30 Furthermore, some specific neighborhood effects are probably relevant to an analysis of the impact of SES on health.31 In particular, in our population of primarily Caucasian patients with CF, low median household income is more likely to be associated with rural areas than inner-city urban areas, and rural populations are more likely to encounter barriers due to distance from a CF center.19 Educational attainment may best predict disease self-management skills. Furthermore, because the resources required to care for a sick child may drain a family of wealth and time for work, low education is more likely than low income to be the cause, rather than the result, of greater disease severity.22 Unfortunately, we have information on MEA for only one-third of our patients, making it difficult to ensure that our sample is not biased. State insurance (Medicaid) eligibility is closely tied to family income and is readily ascertained, making it a simple measure to use. But eligibility is determined differently by individual states, undermining its usefulness when comparing outcomes across centers. Of medical expenses may be considered when evaluating eligibility;32 thus, sicker patients are more likely to be eligible for MA at any given income level. The finding that MA was associated with greater likelihood of receiving many therapeutic interventions suggests that finances might play a role in prescribing patterns for low-SES patients, but this also may be because higher medical expenses increase the likelihood of being eligible for MA. This makes the finding that MA was no stronger a predictor of disease severity than the other 2 SES measures even more interesting.
The low correlation among SES measures is intuitively surprising (although it has been reported previously7) and reflects the fact that SES is a heterogeneous category with different components. This has presented significant methodological challenges in attempting to differentiate the relative contribution of SES to racial disparities33 in CF along with other conditions; the contradictory reports on the relative impact of race on outcomes in CF stem from the use of different SES measures to control for that factor.1,2 Attempts to combine components of SES into a single composite measure have met with limited acceptance;8 in our own analysis, models combining all measures provided essentially the same results as those containing only 1 or 2 measures.
Because this was a cross-sectional study, the direction and causality of the relationship between SES and disease severity could not be determined definitively. Nevertheless, our findings are consistent with those from previous longitudinal analyses. In addition, without performing a prospective cohort study, we cannot technically conclude that SES status preceded the differences (or similarities) in treatment, or that those treatments preceded the SES-related outcome disparities, but it is likely that both contribute to these findings.
In conclusion, we have confirmed previous observations regarding SES and disease severity in CF and have found that this relationship exists in association with all 3 of the SES markers that we studied. Although our data show mild differences in the use of outpatient and inpatient services, these are unlikely to have an important relationship with SES-associated disparities. The differences in the use of chronic therapies suggest that low-SES patients are treated equally or more intensively than higher-SES patients, so again this aspect of care is unlikely to explain the SES disparities. MA eligibility is clearly associated with an increased likelihood of receiving various prescribed treatments and appears to increase the use of the outpatient clinic for sick visits. □
Acknowledgments
We thank David J. Pasta, MS (ICON Clinical Research, Inc, funded by Genentech) for providing biostatical and analytical services. We gratefully acknowledge the participation of the more than 400 ESCF site investigators and coordinators in collecting this comprehensive database, and the helpful discussions with members of the North American Scientific Advisory Group for ESCF (Appendix 1).
Glossary
- BMI
Body mass index
- CF
Cystic fibrosis
- ESCF
Epidemiologic Study of Cystic Fibrosis
- FEV1
Forced expiratory volume in 1 second
- IV
Intravenous
- MA
Medicaid or state insurance
- MEA
Maternal educational attainment
- MIZ
Median household income by zip code
- SES
Socioeconomic status
Appendix 1
Appendix 1.
ESCF Investigators and NASAG Members
| ESCF Investigators | |
|---|---|
| Dana Brasfield | Alabama |
| Raymond Lyrene | Alabama |
| Lawrence Sindel | Alabama |
| Dion Roberts | Alaska |
| John Carroll | Arkansas |
| Robert Warren | Arkansas |
| Louay Nassri | Arkansas |
| Paula Anderson | Arkansas |
| Mark Brown | Arizona |
| Amy Silverthorn | Arizona |
| Peggy Radford | Arizona |
| Gerald Gong | Arizona |
| Gregory Legris | Arizona |
| Gerald Greene | California |
| Reddivalam Sudhakar | California |
| Arnold Platzker | California |
| Bruce Nickerson | California |
| Karen Hardy | California |
| Ivan Harwood | California |
| Gregory Shay | California |
| Bryon Quick | California |
| Allan Lieberthal | California |
| Richard Moss | California |
| Chris Landon | California |
| Yvonne Fanous | California |
| Jay Lieberman | California |
| Eugene Spiritus | California |
| Bradley Chipps | California |
| Ruth McDonald | California |
| Mark Pian | California |
| Gerd Cropp | California |
| Nancy Lewis | California |
| Dennis Nielson | California |
| Bertrand Shapiro | California |
| Jeff Wagener | Colorado |
| Frank Accurso | Colorado |
| Milene Saavedra | Colorado |
| Karen Daigle | Connecticut |
| Jacob Hen | Connecticut |
| Regina Palazzo | Connecticut |
| Kathryn Dodds | Delaware |
| Raj Padman | Delaware |
| John Goodill | Delaware |
| Glenna Winnie | District of Columbia |
| Lea Davies | District of Columbia |
| Tony Kriseman | Florida |
| Jorge Sallent | Florida |
| Joseph Chiaro | Florida |
| Martin Kubiet | Florida |
| Sue Goldfinger | Florida |
| Morton Schwartzman | Florida |
| Carlosenrique Diaz | Florida |
| Kevin Maupin | Florida |
| Eduardo Riff | Florida |
| David Geller | Florida |
| Floyd Livingston | Florida |
| Kunjana Mavunda | Florida |
| Jose Birriel Jr. | Florida |
| Luis Faverio | Florida |
| David Rosenberg | Florida |
| David Schaeffer | Florida |
| James Sherman | Florida |
| Mary Wagner | Florida |
| Michael Light | Florida |
| Bruce Schnapf | Florida |
| Gary Montgomery | Georgia |
| Kevin Kirchner | Georgia |
| Mark Weatherly | Georgia |
| Daniel Caplan | Georgia |
| Margaret Guill | Georgia |
| Valera Hudson | Georgia |
| Javeed Akhter | Illinois |
| Donald Davison | Illinois |
| Steven Boas | Illinois |
| Susanna McColley | Illinois |
| Youngran Chung | Illinois |
| Rennee Latner | Illinois |
| Gabriel Aljadeff | Illinois |
| Youngran Chan | Illinois |
| Jerome Kraut | Illinois |
| Arvey Stone | Illinois |
| John Lloyd Still | Illinois |
| Girish Sharma | Illinois |
| Lanie Eagleton | Illinois |
| Patricia Hopkins | Illinois |
| Umesh Chatrath | Illinois |
| Lucille Lester | Illinois |
| Young-Jee Kim | Illinois |
| Veena Anthony | Indiana |
| Howard Eigan | Indiana |
| Michelle Howenstine | Indiana |
| Pushpom James | Indiana |
| Edward Gergesha | Indiana |
| James Harris | Indiana |
| Robert Plant | Indiana |
| Veljko Zivkovich | Iowa |
| Angela Collins | Iowa |
| Edward Nassif | Iowa |
| Richard Ahrens | Iowa |
| Daniel Doornbos | Kansas |
| Joseph Kanarek | Kansas |
| Richard Leff | Kansas |
| Pamela Shaw | Kansas |
| Elanor Demoss | Kansas |
| Maria Riva | Kansas |
| Leonard Sullivan | Kansas |
| Michael Anstead | Kentucky |
| Jamshed Kanga | Kentucky |
| Nemr Eid | Kentucky |
| Ron Morton | Kentucky |
| Bettina Hilman | Louisana |
| Kim Jones | Louisana |
| Scott Davis | Louisana |
| Ralph Harder | Maine |
| Tom Lever | Maine |
| Anne Marie Cairns | Maine |
| Edgar Caldwell | Maine |
| Jonathan Zuckerman | Maine |
| Peter Mogayzel | Maryland |
| Beryl Rosenstein | Maryland |
| John McQuestion | Maryland |
| Donna Perry | Maryland |
| Samuel Rosenberg | Maryland |
| Robert Gerstle | Massachusetts |
| Andrew Colin | Massachusetts |
| Mary Ellen Wohl | Massachusetts |
| Allan Lapey | Massachusetts |
| William Yee | Massachusetts |
| Brian O'Sullivan | Massachusetts |
| Robert Zwerdling | Massachusetts |
| Ibrahim Abdulhamid | Michigan |
| Adrian O'Hagan | Michigan |
| John Schuen | Michigan |
| Lawrence Kurlandsky | Michigan |
| Richard Honicky | Michigan |
| Douglas Homnick | Michigan |
| John Marks | Michigan |
| Bohdan Pichurko | Michigan |
| Norma Maxvold | Michigan |
| Samya Nasr | Michigan |
| Richard Simon | Michigan |
| Wan Tsai | Michigan |
| Dana Kissner | Michigan |
| John Mc Namara | Minnesota |
| Nancy Henry | Minnesota |
| Stephen Marker | Minnesota |
| Michael Pryor | Minnesota |
| Warren Regelmann | Minnesota |
| Lynn Walker | Minnesota |
| Jim Woodward | Mississippi |
| Louis Mizell | Mississippi |
| Suzanne Miller | Mississippi |
| Daniel Rosenbluth | Missouri |
| Philip Black | Missouri |
| Michael McCubbin | Missouri |
| Alan Cohen | Missouri |
| Thomas Ferkol | Missouri |
| George Mallory | Missouri |
| Anthony Rejent | Missouri |
| Bruce Rubin | Missouri |
| Gavin Graff | Missouri |
| Peter Konig | Missouri |
| John Colombo | Nebraska |
| Peter Murphy | Nebraska |
| William Boyle H | New Hampshire |
| Worth Parker | New Hampshire |
| Chandler Patton | New Jersey |
| Robert Zanni | New Jersey |
| Arthur Atlas | New Jersey |
| Nelson Turcios | New Jersey |
| Lourdes Laraya-Cuasay | New Jersey |
| Dorothy Bisberg | New Jersey |
| Helen Aguila | New Jersey |
| Sarah Allen | New Mexico |
| David James | New Mexico |
| Elizabeth Perkett | New Mexico |
| Marsha Thompson | New Mexico |
| Sonia Budhecha | Nevada |
| Ruben Diaz | Nevada |
| Jonathan Rosen | New York |
| Robert Kaslovsky | New York |
| Ronald Percciacante | New York |
| Drucy Borowitz | New York |
| Joseph Cronin | New York |
| Colin McMahon | New York |
| Lynne Quittell | New York |
| Robert Giusti | New York |
| Rubin Cohen | New York |
| Joan DeCelie-Germana | New York |
| Jack Gorvoy | New York |
| Kalpan Patel | New York |
| Meyer Kattan | New York |
| Allen Dozor | New York |
| Emily DiMango | New York |
| Maria Berdella | New York |
| Ran Anbar | New York |
| Debra Ianuzzi | New York |
| James Sexton | New York |
| Catherine Tayag-Kier | New York |
| John McBride | New York |
| Clement Ren | New York |
| Karen Voter | New York |
| Mary Dimaio | New York |
| Gerald Georgitis | North Carolina |
| Joseph Marc Majure | North Carolina |
| Maria Martinez J | North Carolina |
| Clarke McIntosh | North Carolina |
| Margaret Leigh | North Carolina |
| Michael Schechter | North Carolina |
| Hugh Black | North Carolina |
| James Hughes | North Dakota |
| Anand Kantak | North Dakota |
| Robert Wilmott | Ohio |
| Gregory Omlor | Ohio |
| Robert Stone | Ohio |
| Karen McCoy | Ohio |
| James Acton | Ohio |
| Carl Doershuk | Ohio |
| Michael Konstan | Ohio |
| Robert Fink | Ohio |
| Michael Steffan | Ohio |
| Pierre Vauthy | Ohio |
| Patricia Joseph | Ohio |
| Santiago Reyes | Oklahoma |
| John Kramer | Oklahoma |
| James Royall | Oklahoma |
| Jay Eisenberg | Oregon |
| Michael Wall | Oregon |
| Stanley Fiel | Pennsylvania |
| Thomas Scanlin | Pennsylvania |
| Shroti Phadke | Pennsylvania |
| Glenna Winnie | Pennsylvania |
| Joel Weinberg | Pennsylvania |
| William Sexauer | Pennsylvania |
| Stephen Wolf | Pennsylvania |
| Douglas Holsclaw | Pennsylvania |
| Debra Klein W. | Pennsylvania |
| Stuart Warren | Pennsylvania |
| Robert Kinsey | Pennsylvania |
| Carlos Perez | Pennsylvania |
| Muttiah Ganeshanathan | Pennsylvania |
| James Shinnick | Pennsylvania |
| Howard Panitch | Pennsylvania |
| Laurie Varlotta | Pennsylvania |
| Cynthia Robinson | Pennsylvania |
| Jose Rodriguez Santana | Puerto Rico |
| Mary Ann Passero | Rhode Island |
| Jane Gwinn | South Carolina |
| Robert Baker C. | South Carolina |
| Michael Bowman | South Carolina |
| Patrick Flume | South Carolina |
| Daniel Brown | South Carolina |
| Roxanne Marville | South Carolina |
| James Wallace | South Dakota |
| Rodney Parry | South Dakota |
| Don Ellenburg | Tennessee |
| John Rogers | Tennessee |
| Ricky Mohon | Tennessee |
| Joel Ledbetter | Tennessee |
| Aram Hanissian | Tennessee |
| Robert Schoumacher | Tennessee |
| Preston Campbell | Tennessee |
| Christopher Harris | Tennessee |
| Bonnie Slovis | Tennessee |
| Dennis Stokes | Tennessee |
| Kathryn Hale | Texas |
| Marcia Katz | Texas |
| Dan Seilheimer | Texas |
| Marianne Sockrider | Texas |
| Allan Frank | Texas |
| James Daniel | Texas |
| James Cunningham | Texas |
| Iley Browning | Texas |
| John Bray | Texas |
| Amanda Dove J. | Texas |
| Fernando Mandujano | Texas |
| Larry Tremper | Texas |
| Martha Morse | Texas |
| Donna Willey-Courand | Texas |
| Steven Copenhaver | Texas |
| John Pohl | Texas |
| Bennie McWilliams | Texas |
| Marie Martine-Logvinoff | Texas |
| Marsh Wallace | Texas |
| Robert Klein | Texas |
| Rodolfo Amaro | Texas |
| Leslie Couch | Texas |
| Michael Brown | Texas |
| Claude Prestidge | Texas |
| Stephen Inscore | Texas |
| Andrew Lipton | Texas |
| Barbara Chatfield | Utah |
| Theodore Liou | Utah |
| Bruce Marshall | Utah |
| Karl Karlson | Virginia |
| Ignacio Ropoll | Virginia |
| Thomas Rubio | Virginia |
| Joel Schmidt | Virginia |
| David Thomas | Virginia |
| John Osborn | Virginia |
| Deborah Froh | Virginia |
| Benjamin Gaston | Virginia |
| Greg Elliott | Virginia |
| Thomas Lahiri | Vermont |
| Donald Swartz | Vermont |
| Laurie Whittaker | Vermont |
| Ronald Gibson | Washington |
| Bonnie Ramsey | Washington |
| Michael McCarthy | Washington |
| Lawrence Larson | Washington |
| David Ricker | Washington |
| Mark Robbins | Washington |
| Moira Aitken | Washington |
| Julia Emerson | Washington |
| Julie Biller | Wisconsin |
| Mark Splaingard | Wisconsin |
| Bradley Sullivan | Wisconsin |
| Paul Pritchard | Wisconsin |
| Stu Adair | Wisconsin |
| Peter Holzwarth | Wisconsin |
| Guillermo Dopico | Wisconsin |
| Keith Meyer | Wisconsin |
| Christopher Green | Wisconsin |
| Michael Rock | Wisconsin |
| Stephen Aronoff | West Virginia |
| Kathryn Moffett | West Virginia |
| NASAG Members | |
| Wayne Morgan (chair) | |
| Michael Konstan (co-chair) | |
| Ted Liou | |
| Susanna McColley | |
| Ann McMullen | |
| Alexandra Quittner | |
| Warren Regelmann | |
| Clement Ren | |
| Michael Schechter | |
| Jeff Wagener | |
| Marlyn Woo |
Appendix 2
Funding and Conflict of Interest Disclosures
M.S.S., S.A.M., M.W.K., and J.S.W. have received honoraria during the last 3 years to attend meetings as members of the North American Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF), and their respective institutions previously received grant support from Genentech, Inc, for participating in the study. S.A.M. is a member of a speakers’ bureau for Genentech and a consultant to Axcan Pharma and Novartis; T.H. is a consultant to Genentech; M.W.K. is a member of a speakers’ bureau for Genentech and has received grant support from and is a consultant to Axcan Pharma, Digestive Care Inc, Genentech, and Novartis; and J.S.W. is a member of a speakers bureau for Genentech and is a consultant to Genentech and Novartis, and previously was an employee of Genentech. S.S. is an employee of ICON Clinical Research, which was paid by Genentech to provide biostatistical and analytical services for ESCF. No honoraria, grants, or other form of payment were given to any of these authors to produce this manuscript. The authors were responsible for the study design, interpretation of data, and drafting and completion of the manuscript. The initial draft and all subsequent drafts of the manuscript were written by Dr Schechter in consultation with the coauthors. The decision to submit the manuscript was made by the authors and was approved by Genentech.
Footnotes
Funding and conflict of interest disclosure information is available at www.jpeds.com (Appendix 2).
References
- 1.O'Connor GT, Quinton HB, Kahn R, Robichaud P, Maddock J, Lever T, et al. Case-mix adjustment for evaluation of mortality in cystic fibrosis. Pediatr Pulmonol. 2002;33:99–105. doi: 10.1002/ppul.10042. [DOI] [PubMed] [Google Scholar]
- 2.Schechter MS, Shelton BJ, Margolis PA, FitzSimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163:1331–7. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 3.Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics. 1993;91:56–61. [PubMed] [Google Scholar]
- 4.Konstan MW, Butler SM, Schidlow DV, Morgan WJ, Julius JR, Johnson CA. Patterns of medical practice in cystic fibrosis, part II: use of therapies. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol. 1999;28:248–54. doi: 10.1002/(sici)1099-0496(199910)28:4<248::aid-ppul3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Butler SM, Schidlow DV, Morgan WJ, Julius JR, Johnson CA. Patterns of medical practice in cystic fibrosis, part I: evaluation and monitoring of health status of patients. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol. 1999;28:242–7. doi: 10.1002/(sici)1099-0496(199910)28:4<242::aid-ppul2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123:20–7. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 8.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi I, Kennedy BP. Income inequality and health: pathways and mechanisms. Health Serv Res. 1999;34(1 Pt 2):215–27. [PMC free article] [PubMed] [Google Scholar]
- 10.Gazmararian JA, Adams MM, Pamuk ER. Associations between measures of socioeconomic status and maternal health behavior. Am J Prev Med. 1996;12:108–15. [PubMed] [Google Scholar]
- 11.Davey Smith G, Hart C, Hole D, MacKinnon P, Gillis C, Watt G, et al. Education and occupational social class: which is the more important indicator of mortality risk? J Epidemiol Community Health. 1998;52:153–60. doi: 10.1136/jech.52.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fein O. The influence of social class on health status: American and British research on health inequalities. J Gen Intern Med. 1995;10:577–86. doi: 10.1007/BF02640369. [DOI] [PubMed] [Google Scholar]
- 13.Zill N. Parental schooling and children's health. Public Health Rep. 1996;111:34–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]
- 15.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, et al. Epidemiologic Study of Cystic Fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the US and Canada. Pediatr Pulmonol. 1999;28:231–41. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 19.Blumenthal SJ, Kagen J. The effects of socioeconomic status on health in rural and urban America. JAMA. 2002;287:109. [PubMed] [Google Scholar]
- 20.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 21.Cystic Fibrosis Foundation Patient Registry, 2005 Annual Data Report. Cystic Fibrosis Foundation; Bethesda, MD: 2006. [Google Scholar]
- 22.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998;129:406–11. doi: 10.7326/0003-4819-129-5-199809010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Schechter MS. Non-genetic influences on cystic fibrosis lung disease: the role of sociodemographic characteristics, environmental exposures, and healthcare interventions. Semin Resp Crit Care Med. 2003;24:639–52. doi: 10.1055/s-2004-815660. [DOI] [PubMed] [Google Scholar]
- 24.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proc Natl Acad Sci USA. 2002;99:10929–34. doi: 10.1073/pnas.162086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156:1094–100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 26.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299:417–24. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169:816–21. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 28.Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol. 1999;28:394–401. doi: 10.1002/(sici)1099-0496(199912)28:6<394::aid-ppul2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–120. doi: 10.1093/oxfordjournals.epirev.a036030. [DOI] [PubMed] [Google Scholar]
- 30.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 31.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–9. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kronebusch K, Elbel B. Simplifying children's Medicaid and SCHIP. Health Aff (Millwood) 2004;23:233–46. doi: 10.1377/hlthaff.23.3.233. [DOI] [PubMed] [Google Scholar]
- 33.LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health. 2005;(2 Suppl. 3):82, iii26–34. doi: 10.1093/jurban/jti061. [DOI] [PMC free article] [PubMed] [Google Scholar]