Abstract
We report the use of fluorescein-polyethylene glycol (Fluor-PEG) to non-covalently functionalize single-walled carbon nanotubes (SWNTs) for obtaining aqueous-soluble nanotube conjugates (Fluor-PEG/SWNT) and simultaneously affording fluorescence labels to nanotubes. We find serendipitously that fluorescein, a widely used fluorophore, can strongly adsorb onto the sidewall of the SWNTs likely via π-stacking, and the hydrophilic PEG chain imparts high aqueous solubility. Interaction between fluorescein and SWNT is pH dependent; it weakens as the pH is increased, causing the Fluor-PEG/SWNT conjugate to be less stable at high pHs. Fluorescein molecules bound to SWNTs exhibit interesting pH dependent optical absorbance and fluorescence properties that are distinct from free molecules, as a result of pH dependent interactions with SWNT sidewalls. Fluorescence emission from fluorescein adsorbed on SWNT is quenched by ~67%, but remains sufficient and useful as a fluorescent label. The utility of Fluor-PEG/SWNT as a simultaneous fluorescent marker and an intracellular transporter is demonstrated by uptake of Fluor-PEG/SWNT by mammalian cells and detection of fluorescence inside the cells. Raman detection of SWNTs in the cells is also carried out and used to prove the co-localization of fluorescein and SWNT.
Carbon nanotubes are interesting 1D nanomaterials,1 and to explore nanotubes as macromolecules, various functionalization schemes, both covalent and non-covalent, have been developed to impart water solubility and chemical functionalities.2 Non-covalent modifications of nanotubes include the use of surfactants and aromatic molecules (e.g., pyrene).3 Here, we report non-covalent functionalization of single-walled carbon nanotubes (SWNTs) by fluorescein-polyethylene glycol (Fluor-PEG) (1) based on a serendipitous observation of strong binding of the molecule on SWNTs. The simple functionalization approach imparts aqueous solubility and simultaneously affords fluorescent labels to nanotubes. Interestingly, the optical absorbance and fluorescence of fluorescein bound to SWNTs exhibit distinct pH dependence from those of free fluorescein, displaying pH dependent non-covalent binding interactions between molecules and nanotubes. The results are important to the supramolecular chemistry of nanomaterials and potential applications such as pH sensing.
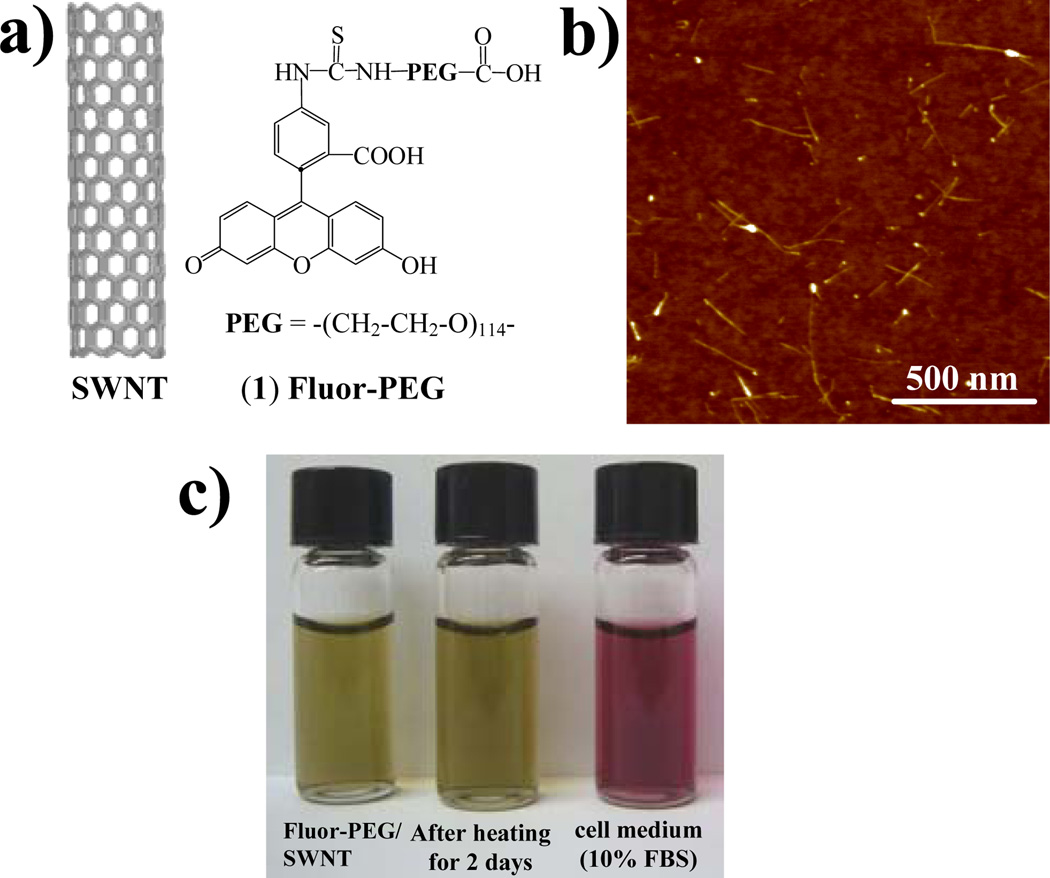
By simple sonication of as-grown SWNTs in an aqueous solution of (1) followed by centrifugation to remove large impurities and dialysis of the supernatant to remove free molecules (see Supp. Info.), we obtained SWNTs (average length~150nm, Fig.1b) stably suspended in water by physisorbed Fluor-PEG (Fig.1c). The hydrophobic aromatic fluorescein group binds to the sidewall of SWNTs (likely via π-stacking) while the PEG group extends into water. The nanotube suspension was stable in water without aggregation even after heating to 70 °C for 2 days (Fig.1c). High stability was also observed in cell culture medium containing 10% fetal bovine serum and ~150mM salt (Fig.1c), suggesting strong binding of Fluor-PEG on SWNTs.
Figure 1.
Fluor-PEG functionalized SWNTs. a) Schematic showing SWNT and Fluor-PEG (1). b) Atomic force microscopy image of Fluor-PEG/SWNTs deposited on substrate. c) Photo of Fluor-PEG/SWNT in water (left, yellow-green color due to SWNT bound Fluor), after heating at 70 °C for 2 days (center), and in cell culture medium supplemented with 10% serum (right, red color due to cell medium). Unbound Fluor-PEG was dialyzed in the starting solution.
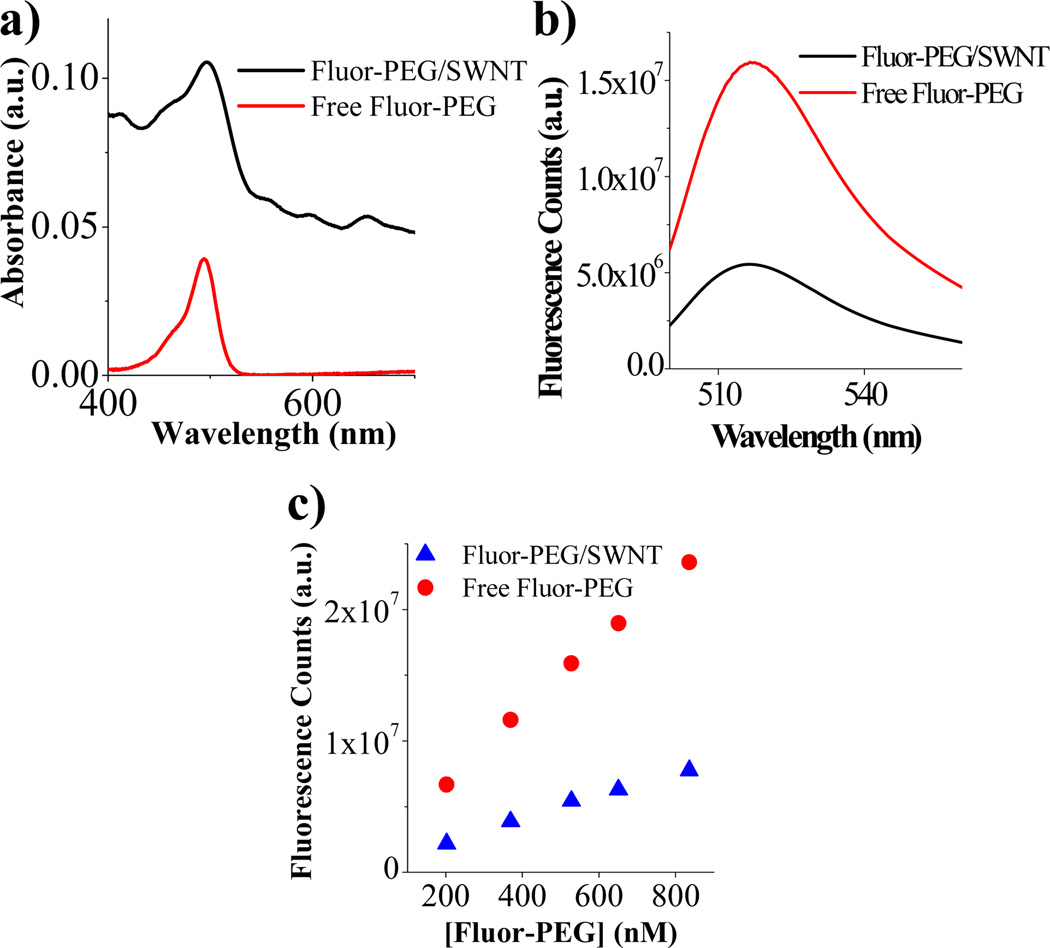
We investigated the optical absorbance and fluorescence characteristics of fluorescein bound to SWNTs in phosphate buffered saline (PBS) at pH 7.4 (note: free unbound fluorescein in all of our SWNT suspensions were removed by dialysis). UV-vis-NIR spectrum clearly revealed an absorbance peak (at ~497 nm) of fluorescein bound to SWNTs (Fig. 2a in which the background spectrum with small peaks was due to SWNTs), and the peak was red-shifted (by ~3 nm) relative to free fluorescein. The number of Fluor-PEG per tube (average length ~150nm) was estimated to be ~90 with a ~12% coverage of the SWNT sidewall area (see supplementary Fig.S1). We observed ~67% quenching of the fluorescence of SWNT-bound fluorescein relative to free fluorescein at the same concentration (495 nm excitation) (Fig.2b) due to interactions between fluorescein and SWNT. Similar fluorescence quenching was reported for SWNT bound pyrene due to energy transfer.4,5 The ~67% quenching effect was observed for various SWNT concentrations up to 10nM with fluorescein concentrations up to ~900 nM (Fig. 2c).
Figure 2.
Optical properties of SWNT bound Fluor-PEG. a) Absorbance of Fluor-PEG/SWNT and free Fluor-PEG. b) Corresponding fluorescence emission spectra. c) Emission peak intensity of SWNT bound Fluor-PEG and free Fluor-PEG in a fluorescein concentration range of 200–900 nM.
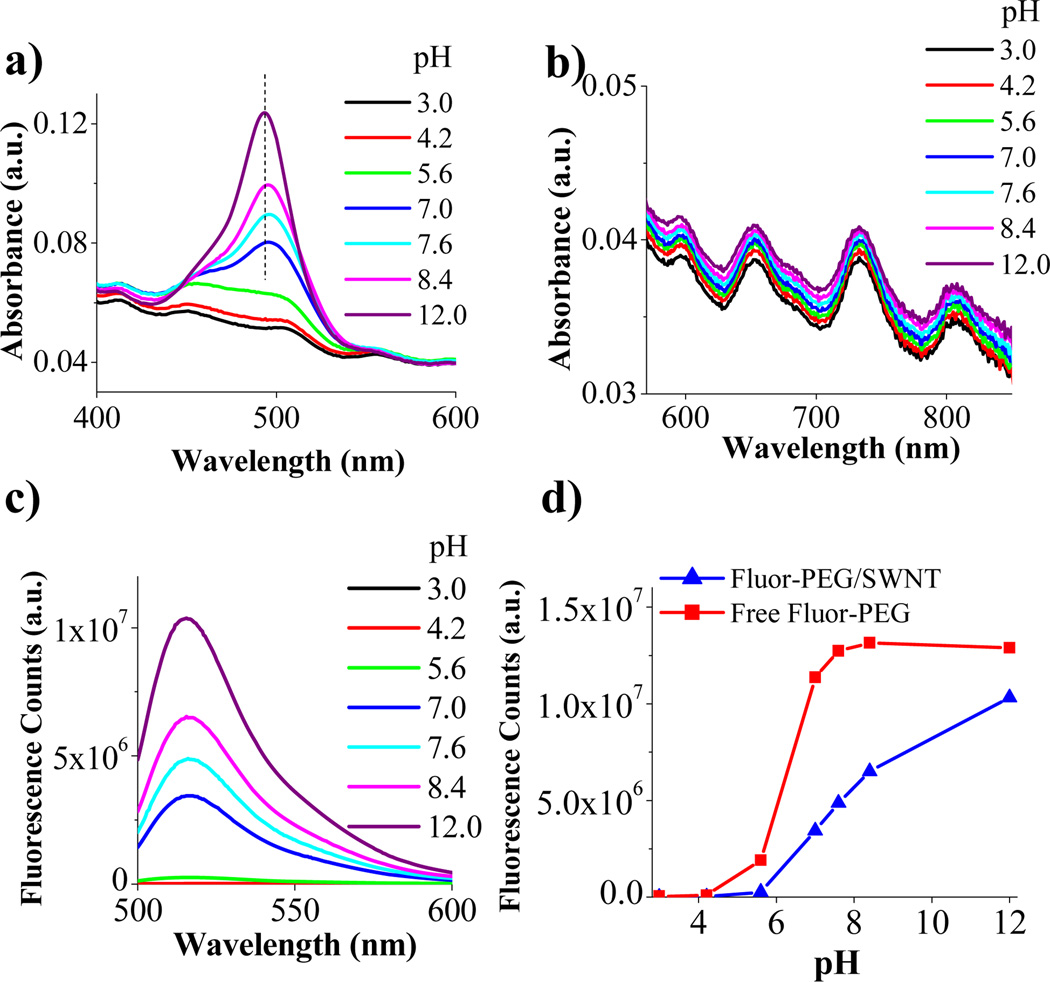
The absorbance (at 490 nm) and fluorescence of fluorescein are known to increase at higher pHs under higher degree of deprotonation and saturate around pH~8.6–8 Though similar trend was observed for fluorescein bound to SWNTs under increasing pH, no saturation was seen even up to pH~12 (Fig.3a,c&3d). No significant pH dependence was observed in the absorbance (Fig.3b) and photoluminescence (Fig.S3) of our SWNTs, in contrast with SWNTs solubilized by surfactants with charged groups and without PEGylation.9,10 This difference is currently not understood but could be due to the different functionalization schemes used. The absorbance peaks of SWNT bound fluorescein exhibited monotonic blue-shift (Fig.3a) at higher pHs, approaching the characteristics of free fluorescein (i.e., reversing the effects of fluorescein-SWNT binding). This suggested that increase in pH led to weakening of the interaction between fluorescein and SWNT, reducing fluorescence quenching and shifting the spectral peaks back to near that of free fluorescein. At pH 12, we observed slow but noticeable precipitation of Fluor-PEG/SWNTs from the solution after standing for ~48 h while no precipitates were seen in lower pH solutions (see Supp. Info. Fig.S2). This confirmed weakened interaction between fluorescein and SWNT at higher pHs, causing Fluor-PEG desorption over time. The mechanism of reduced interaction at higher pHs is not yet fully understood, but we speculate that higher pH and deprotonation impart higher hydrophilicity to fluorescein and thus higher affinity for water solvation. The monotonic increase of Fluor-PEG/SWNT fluorescence without saturation at pH~8 expected for free fluorescein could also be due to delayed deprotonation of fluorescein when bound to SWNT, or competition of hydroxyl ion with (1) for SWNT binding, though further work is needed to fully understand the various possible mechanisms.
Figure 3.
Fluor-PEG/SWNT fluorescence and absorbance dependence on pH. Absorption curves of Fluor-PEG/SWNT at various pHs in the a) fluorescein region (dashed line marks the peak at pH~12) and b) SWNT region (curves are displaced for clarity). c) Corresponding fluorescence emission spectra (λexcitation = 495 nm). c) Comparison of emission peak intensity dependence on pH of free Fluor-PEG and Fluor-PEG/SWNT with the same Fluor-PEG concentration.
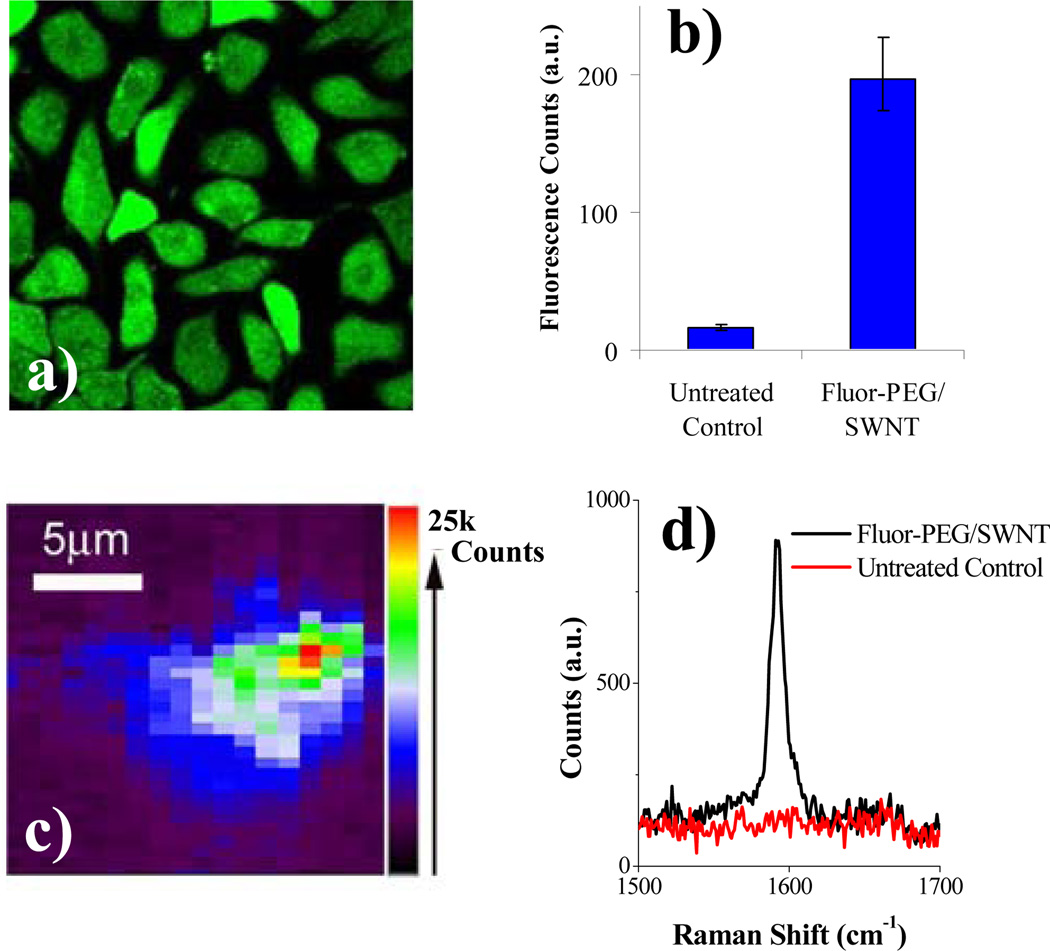
Our simple functionalization imparts solubility to nanotubes in p hysiological buffers and simultaneously affords fluorescent labels. The fluorescence intensity of fluorescein on SWNT is ~33% of free fluorescein, which can still be utilized in chemical and biological settings. To this end, we investigated the cellular uptake of Fluor-PEG/SWNT and used fluorescence detections for characterization. After incubation of BT474 breast cancer cells in a solution of Fluor-PEG/SWNT, we observed fluorescein signals within cells by confocal fluorescence microscopy (Fig. 4a) and flow cytometry (or fluorescence activated cell sorting, FACS, Fig. 4b). Further, owing to the strong resonance raman signatures of SWNTs, we used the G-band raman peak at ~1600cm−1, characteristic of graphitic stretching mode, to directly probe SWNTs in live cells. Micro-Raman image formed by spatially mapping out the G-band integrated intensity showed the existence of SWNTs at the cells (Fig.4c&4d), and the co-localization with fluorescein fluorescence suggested the cellular uptake of Fluor/SWNT conjugates, similar to internalization of other molecular complexes with nanotubes.11,12 Control cells without exposure to Fluor-PEG/SWNT showed minimal fluorescence and SWNT G-band raman signal (Fig.4b&4d).
Figure 4.
Cellular uptake of Fluor-PEG/SWNT. a) Confocal fluorescence image of BT474 cells incubated with Fluor-PEG/SWNT. b) Fluorescence intensities of large population of cells treated with Fluor-PEG/SWNT vs. untreated control cells by flow cytometry. c) Mirco-raman image of the cells incubated with Fluor-PEG/SWNT. d) Representative raman spectra showing the presence and absence of the SWNT G-band from Fluor-PEG/SWNT incubated cells and control untreated cells.
We have used PEGylated fluorescein to non-covalently tionalize SWNTs for aqueous stably conjugates with pH dependent optical properties. We showed that the finite fluorescence intensity of fluorescein-PEG/SWNT can be utilized for detection, imaging and cell sorting in biological applications. Lastly, since the terminal group of Fluor-PEG on the SWNT conjugate is a carboxylic acid (Fig.1a), one can envision conjugation of various molecules to the conjugates to impart further chemical or biological functionalities.
Supplementary Material
Acknowledgement
This work was partly supported by NIH-NCI CCNE-TR and a Stanford Translational Research Grant.
Footnotes
Supporting Information Available: Experimental details are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Dresselhaus MS, Dresselhaus G, Eklund PC. Science of Fullerenes and Carbon Nanotubes. San Diego: Academic Press; 1996. pp. 1–985. [Google Scholar]
- 2.Britz DA, Khlobystov AN. Chem. Soc. Rev. 2006;35:637. doi: 10.1039/b507451g. [DOI] [PubMed] [Google Scholar]
- 3.Chen RJ, Zhan YG, Wang DW, Dai HJ. J.Am. Chem. Soc. 2001;123:3838. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 4.Tomonari Y, Murakami H, Nakashima N. Chem. Eur. J. 2006;12:4027. doi: 10.1002/chem.200501176. [DOI] [PubMed] [Google Scholar]
- 5.Paloniemi H, Aaritalo T, Laiho T, Liuke H, Kocharova N, Haapakka K, Terzi F, Seeber R, Lukkari J. J. Phys. Chem. B. 2005;109:8634. doi: 10.1021/jp0443097. [DOI] [PubMed] [Google Scholar]
- 6.Invitrogen Molecular Probes. The Handbook — A Guide to Fluorescent Probes and Labeling Technologies. Web Edition. accessed 11/2006; Sec. 20. [Google Scholar]
- 7.Diehl H. Talanta. 1989;36:413. doi: 10.1016/0039-9140(89)80212-7. [DOI] [PubMed] [Google Scholar]
- 8.Diehl H, Markuszewski R. Talanta. 1989;36:416. doi: 10.1016/0039-9140(89)80213-9. [DOI] [PubMed] [Google Scholar]
- 9.Strano MS, Huffman CB, Moore VC, O'Connell MJ, Haroz EH, Hubbard J, Miller M, Rialon K, Kittrell C, Ramesh S, Hauge RH, Smalley RE. J. Phys. Chem. B. 2003;107:6979. [Google Scholar]
- 10.Dukovic G, White B, Zhou Z, Wang F, Jockusch S, Steigerwald M, Heinz T, Friesner R, Turro NJ, Brus LE. J.Am.Chem.Soc. 2004;126:15269. doi: 10.1021/ja046526r. [DOI] [PubMed] [Google Scholar]
- 11.Kam NWS, Liu Z, Dai HJ. J. Am. Chem. Soc. 2005;127:12492. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- 12.Kam NWS, Jessop TC, Wender PA, Dai HJ. J. Am. Chem. Soc. 2004;126:6850. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.