Abstract
Purpose
The therapeutic benefits of cell transplantation may depend on the survival of sufficient numbers of grafted cells. We evaluate four potent immunosuppressive medications aimed at preventing acute donor cell death.
Procedures and Results
Embryonic rat H9c2 myoblasts were stably transduced to express firefly luciferase reporter gene (H9c2-Fluc). H9c2-Fluc cells (3 × 106) were injected into thigh muscles of Sprague–Dawley rats (N = 30) treated with cyclosporine, dexamethasone, mycophenolate mofetil, tacrolimus, or saline from day −3 to day +14. Longitudinal optical bioluminescence imaging was performed over two weeks. Fluc activity was 40.0 ± 12.1% (dexamethasone), 30.5 ± 12.5% (tacrolimus), and 21.5 ± 3.5% (mycophenolate) vs. 12.0 ± 5.0% (control) and 8.3 ± 5.0% (cyclosporine) at day 4 (P < 0.05). However, by day 14, cell signals had decreased drastically to <10% for all groups despite drug therapy.
Conclusion
This study demonstrates the ability of optical molecular imaging for tracking cell survival noninvasively and raises important questions with regard to the overall efficacy of immunosuppressives for prolonging transplanted cell survival.
Keywords: Molecular imaging, Cell transplant, Cyclosporine, Tacrolimus, Mycophenolate
Introduction
Stem cell therapies are experimental approaches based on the transplantation of normal or genetically modified cells for treating specific diseases. Several studies have already shown promising results in neurodegenerative [1], cardiovascular [2], and diabetic diseases [3]. However, current methods of studying stem cell survival rely on conventional histology, which preclude longitudinal monitoring because animals need to be killed at each time point. Thus, the development of “molecular markers” that could help determine the nature of engrafted cells and their progeny noninvasively would be extremely useful.
Novel techniques that allow noninvasive and repetitive imaging of cell transplants have recently been proposed and evaluated in a limited number of cell delivery models. One is imaging of radiolabeled stem cells by gamma camera or positron emission tomography (PET) [4]. However, the short physical half-lives of most available radioisotopes limit these studies to less than two to three days in duration. Another technique is magnetic resonance imaging (MRI) of iron-particle-labeled cells [5]. Although high spatial resolution images can be obtained, MRI is several log orders of magnitude less sensitive than radionuclide and bioluminescence imaging [6]. In addition, the engulfment of dead cells containing iron by neighboring macrophages limits its ability to distinguish viable from nonviable cells reliably, because iron may still remain in the region of interest [7]. The third technique involves the use of reporter gene technology for bioluminescence, fluorescence, MRI, single-photon emission computed tomography (SPECT), or PET imaging [8, 9]. With this approach, cell survival of genetically modified cells can be monitored more accurately because only viable cells will express the reporter genes. Moreover, because the reporter genes are passed on to daughter cells, cell proliferation can also be reliably quantified [10].
Undoubtedly, cell therapies depend on an appropriate knowledge of the donor cell biology and physiology after transplantation. One critical factor is a better understanding of the survival pattern of donor cells. For example, precise mechanisms of early donor cell death involving myoblast transplantation for Duchenne’s muscular dystrophy (DMD) remain unknown [11]. Although previous studies have addressed the dynamics of cell survival, their histology-based methods are less than ideal because the animals have to be killed at different time points for postmortem histology, enzyme assays, or DNA quantification [12–14]. In this respect, reporter gene technology can provide invaluable information on cell survival after transplantation in a noninvasive way. Thus, we conducted the present study with the aim of understanding the kinetics of cell survival within individual living subjects longitudinally and noninvasively. We also evaluated whether clinically used immunosuppressive drugs can help modulate this process.
Methods
Cell Culture
Rat H9c2 embryonic cardiomyoblast cells (derived from BDIX rats) were purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were grown in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 10 U/ml penicillin, and 10 μg/ml streptomycin. Although the H9c2 cell line was derived originally from embryonic rat myocardium, its morphologic, biochemical, and electrophysiologic properties more closely resemble those of a skeletal muscle cell line [15].
Cloning and Transfection
The firefly luciferase cDNA was inserted into plasmid pcDNA3.1 (Invitrogen) by using NheI and XhoI restriction enzyme sites. The final construct contains the human cytomegalovirus promoter driving firefly luciferase and SV40 promoter driving the aminoglycoside phosphotransferase (Neo) cDNA, followed by an SV40 poly-A fragment (pCMV-Fluc-SV40-neo). H9c2 cells were stably transfected by using the Superfect protocol (Invitrogen). Cells were plated at a density of 1 × 103 cells/cm2 with G418 sulfate (neomycin; 0.5 mg/ml). Colonies resistant to G418 were selected and the highest expressing clone (H9c2-Fluc) was used for the study.
Bright-Field and Immunofluorescence Microscopy
Cells were fixed in 95% alcohol, washed, and stored at 4°C. Nonspecific antibody was blocked with 5% horse serum for 15 minutes at 37°C. Cells were exposed to primary antibody: α-smooth muscle actin, followed by secondary antibody, mouse IgG conjugated to TRITC (ZyMed® Lab, 1:100). 4′,6-Diamidino-2-phenylindole (DAPI) staining was used to determine the number of nuclei and to assess gross cell morphology as described [16].
Cell Proliferation After Transduction
Control nontransduced H9c2 and H9c2-Fluc cells were distributed uniformly in 96-well plates at a density of 10,000 cells per well. Eight samples were assayed and the highest and lowest values were ignored. The CyQuant cell proliferation assay (Molecular Probes, Eugene, OR, USA) was used according to the manufacturer’s protocol. After 24, 48, and 72 hours of incubation, the density of surviving cells was measured with a microplate spectrofluorometer (Gemini EM, Sunnyvale, CA, USA).
Western Blot Analysis
H9c2 and H9c2-Fluc cells were washed once with ice-cold PBS and lysed (150 mM NaCl, 20 mM Tris, pH 7.5, 1 mM NaF, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM glycerol phosphate, and 1% Triton X-100) in the presence of Complete Mini Protease cocktail inhibitor (Roche) and 10 mM sodium orthovanadate (Sigma) on ice for 15 minutes. Cell lysates were cleared by centrifugation at 15,000 rpm for 15 minutes at 4°C. To detect firefly luciferase, protein was resolved using 4–12% NU-PAGE gradient gel (Invitrogen) and transferred onto a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in buffer containing 0.01% Tween 20 for one hour and probed overnight at 4°C with the appropriate primary antibodies. Detection was performed with horseradish-peroxidase-conjugated mouse polyclonal antibody against firefly luciferase (1:5,000; Promega) and mouse monoclonal antibody against human α-tubulin (1:5,000; Cell Signaling) and secondary goat antimouse IgG horseradish peroxidase conjugate (1:3,000; Cell Signaling).
Reverse Transcription–Polymerase Chain Reaction Analysis
Total RNA was prepared from H9c2 and H9c2-Fluc cells using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. Specific polymerase chain reaction (PCR) primers were designed for firefly luciferase and β-actin. Fluc primers (antisense, 5′-ACCGGACATAATCATAGGACCTCT-3′; sense, 5′-ATAG ATTTGAAGAAGAGCTGTTTC-3′) generated a 395-bp fragment. β-Actin primers (antisense, 5′-CCCCAGGCACCAGGGC GTGAT-3′; sense, 5′-GGTCATCTTCTCGCGGTTGGCCTTGG GGT-3′) generated a 250-bp fragment. Reverse transcription (RT) – PCR was performed using the SuperScript III one-step RT–PCR with Platinum Taq kit according to the manufacturer’s protocol (Invitrogen). Gel electrophoresis confirmed that each PCR product comprised a band of the correct size.
Immunosuppressive Regimen and Bioluminescence Imaging in Living Animals
H9c2-Fluc cells were implanted into the right thigh of allogeneic male Sprague–Dawley rats (n = 5 for each group, N = 30 total; weight 200–250 g). Animals were treated with cyclosporin A (5 mg kg−1 d−1) [17], dexamethasone (400 mg kg−1 d−1) [18], mycophenolate mofetil (Cellcept, 15 mg kg−1 d−1; Roche Laboratories Inc.) [19], tacrolimus (FK506, 1 mg kg−1 d−1; Fujisawa Healthcare) [20], or saline (1 ml kg−1) intraperitoneally on a daily basis from day −3 to day +14 of cell transplant. The drug dosages chosen were comparable to those of previously cited reports. After transplant, imaging was performed at 6, 12, and 24 hours after cell transplant. Because the Fluc activity at 24 hours typically yields the highest value, the Fluc activities at subsequent time points were expressed as percentage (%) of day 1. The animals were first placed supine in a light-tight chamber, and a gray scale reference image was obtained under low-level illumination. Bioluminescence imaging was performed after intraperitoneal injection of reporter substrate D-luciferin (375 mg kg−1 body weight) as previously described [8]. Photons emitted from transplanted H9c2-Fluc cells were quantified using the Igor analysis software (WaveMetrics, Lake Oswego, OR, USA). Activities were recorded as maximum photons per second per centimeter square per steradian (photons sec−1 cm−2 sr−1).
Postmortem Identification of Transplanted Cells by Enzyme Assay and Histology
After imaging, animals were killed and thigh skeletal muscles were removed and homogenized in Passive Lysis Buffer (Promega, Madison, WI, USA) at 4 ml g−1 tissue. After freezing and thawing three times at −80°C for 15 minutes each, the homogenate was centrifuged at 14,000 rpm for 15 minutes. Fluc activity was assessed using 20 μl of supernatant with 100 μl of Luciferase Assay Reagent (Promega). A luminometer (Lumat LB9504, Wildbad, Germany) was used to measure total light emission according to the manufacturer’s protocol. The results were normalized to RLU per milligram of protein as measured by Bio-Rad Protein Assay System (Bio-Rad, Hercules, CA). Results from these in vitro assays were compared to the maximum RLU/min per minute obtained from the cooled CCD camera for all rats.
Statistics
All results are expressed as mean ± standard deviation. The Student’s t test was used and P values of < 0.05 were considered to indicate significant differences between two groups.
Results
Morphology, Viability, and Proliferation of H9c2 and H9c2-Fluc Cells
To evaluate if plasmid transfection could adversely affect rat H9c2 myoblasts, we compared control nontransfected H9c2 cells vs. stably transfected H9c2-Fluc cells. Bright-field microscopy showed no difference in their gross morphology. Immunofluorescence microscopy showed similar patterns of cytoskeletal protein α-actin and nuclear DNA stain in control H9c2 and H9c2-Fluc cells (Fig. 1A). There was no significant difference in cell proliferation at 24, 48, and 72 hours between control H9c2 and H9c2-Fluc cells (P value 0.8, 0.15, and 0.23, respectively) (Fig. 1B).
Fig. 1.
Comparison of morphology and proliferation between control H9c2 and H9c2-Fluc cells. (A) Fusion of bright-field (grayscale) and immunofluorescent (color) images reveals that both control H9c2 and H9c2-Fluc cells have similar gross morphology and pattern of nuclear (purple) and cytoplasmic smooth muscle α-actin (red) staining. (B) Furthermore, both cell lines have comparable growth rate, as indicated by their optical density (OD) at 24, 48, and 72 hours post plating.
Confirmation of Firefly Luciferase Reporter Gene Expression in H9c2-Fluc Cells
After transfection with plasmids, 12 stable H9c2-Fluc clones were isolated by G418 antibiotic selection. The highest expressing clone was used for in vitro analysis and subsequent in vivo imaging. Fluc mRNA and protein expression were first confirmed by RT–PCR (Fig. 2A) and Western blots (Fig. 2B), respectively. As expected, stable H9c2-Fluc cells showed positive bands in Fluc mRNA transcript (0.4 kb) and protein (62 kDa) on the gels. In contrast, control H9c2 cells produced no Fluc transcript and protein expression. Moreover, firefly luciferase-mediated signals are detected in H9c2-Fluc cells only (Fig. 2C).
Fig. 2.

Confirmation of firefly luciferase expression in H9c2 cells. (A) RT–PCR of control H9c2 and H9c2-fluc cells reveals a 395-bp band (right lane), corresponding to the reversely transcribed firefly luciferase mRNA in H9c2-fluc cells, and no corresponding band for the H9c2 cells (left lane). (B) Western blotting reveals a 62 kDa band corresponding to the size of firefly luciferase in H9c2-Fluc (left lane) but not in H9c2 cells (right lane). (C) In vitro bioluminescence imaging reveals firefly luciferase-mediated signals only in H9c2-Fluc cells (bottom well) and not in control H9c2 cells (top well).
Effects of Cyclosporine, Dexamethasone, Mycophenolate, and Tacrolimus on Cell Survival
To determine if different immunosuppressive drugs can help prolong cell survival, H9c2-Fluc cells derived originally from BDIX rats were transplanted into the skeletal muscles of allogeneic Sprague–Dawley rats. Animals were treated with cyclosporine (5 mg kg−1 d−1), dexamethasone (0.4 mg kg−1 d−1), mycophenolate (Cellcept) (15 mg kg−1 d−1), tacrolimus (Prograf) (1 mg kg−1 d−1), or saline (1 ml kg−1 d−1) as control. For the control group, Fluc imaging signals were (2.29 ± 0.78) × 107 (day 1), (3.28 ± 1.27) × 106 (day 3), (4.62 ± 1.75) × 105 (day 6), (1.34 ± 0.20) × 104 (day 10), and (4.38 ± 2.28) × 103 photons sec−1 cm−2 sr−1 (day 14). A representative bioluminescence image is shown in Fig. 3A. Thus, the relative cell survival as reflected by Fluc activities were 19.9 ± 4.1% (day 3), 2.9 ± 0.9% (day 6), 0.4 ± 0.2% (day 10), and 0.2 ± 0.1% (day 14) compared to day 1 (Fig. 3B). Animals treated with tacrolimus, dexamethasone, and mycophenolate showed significantly stronger Fluc activities at days 2, 3, 4, 6, 10, and 14 compared to the control (P < 0.05). Interestingly, the cyclosporine group did not seem to improve cell survival compared to the control (P = NS). Despite these early positive results, the Fluc activity at day 14 was low in all groups: 6.8 ± 2.6% for tacrolimus, 8.0 ± 2.2% for dexamethasone, and 1.4 ± 0.2% for mycophenolate mofetil (Fig. 3B). A separate group of animals was treated with a combination regimen consisting of tacrolimus, dexamethasone, and mycophenolate (similar to the clinical transplant immunosuppressive protocol). Cell survival in the combination therapy group was significantly higher at later time points compared to the control group (P < 0.05). However, the percentage of cell survival at week 2 was still relatively low compared to day 1 (12.0 ± 3.5%). Finally, by week 3, both bioluminescence signals and histologic samplings could not detect the presence of transplanted cells (data not shown).
Fig. 3.
Differential effect of immunosuppressive medications on posttransplantation cell survival. (A) Representative bioluminescence images of animals treated with saline (control), mycophenolate, tacrolimus, and a combination of tacrolimus, dexamethasone, and mycophenolate. Notably, animals treated with the combination regimen have longer detectable signal (14 days) compared to other treatment groups. (B) The survival kinetics of the drug treatment groups show similar trends in that cell signal declines significantly (> 35%) on day 2 or 3 and falls to negligible levels by the end of second week. In contrast, the cell signal is no longer visible as early as the first week for the control group. Statistical significance compared to control is indicated at two ranges of P values: #P < 0.05 and *P < 0.01.
Correlation of Bioluminescence Imaging Signals with Traditional ex Vivo Assays
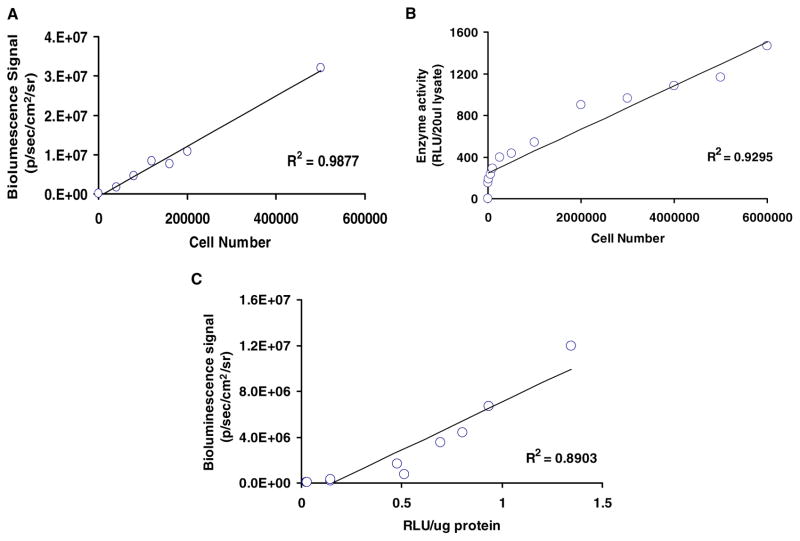
To confirm that enzyme assays and imaging signals correspond to cell number, we first plated different numbers of cells on six-well plates. The Fluc activity correlated with cell numbers as determined by bioluminescence imaging (Fig. 4A) and enzyme assay (Fig. 4B). The correlations were R2 = 0.98 and R2 = 0.93, respectively. Next, we sacrificed animals injected with H9c2-Fluc cells at different time points. The skeletal muscles were homogenized and Fluc activity was determined. Comparison of the in vivo bioluminescence signals to the ex vivo Fluc enzyme activity also showed a robust correlation (R2 = 0.89) (Fig. 4C). This suggests that bioluminescence imaging of cell survival can be used in parallel or in lieu of conventional postmortem enzyme assays.
Fig. 4.
Quantification of cell number using bioluminescence imaging. (A) H9c2-Fluc cells plated at different densities yield linearly proportional and highly correlated (r2 = 0.98) bioluminescence signals after D-luciferin substrate exposure. (B) The level of in vitro firefly luciferase enzyme activity correlates linearly with cell number. (C) A strong correlation exists between in vivo cell-mediated bioluminescence signal and in vitro firefly luciferase enzyme activity, attesting to the feasibility of using bioluminescence imaging to monitor cells noninvasively.
Discussion
To our knowledge, this is the first imaging study to systemically address cell survival and immunosuppressive modulators in an allogeneic cell transplant model. Our major findings are as follows: (1) Molecular imaging can be used to monitor cell survival in living subjects. (2) Dexamethasone, mycophenolate, and tacrolimus can improve cell survival during the first week, but the difference becomes minimal by the second week. (3) More importantly, the in vivo imaging results correlate with traditional ex vivo enzyme assays. Taken together, these imaging results represent a new paradigm for analyzing cell survival in living subjects non-invasively, repetitively, and longitudinally.
The process of donor cell death is not well understood and remains controversial. In clinical trials, myoblast transplant as a potential cell-based gene therapy to restore dystrophin expression in the muscles of patients with Duchenne muscular dystrophy (DMD) has been unsuccessful primarily due to the low levels of donor cell survival [21]. Thus, most of the published literature on donor cell death to date has been related to myoblast transfer therapy [22, 23]. The extent of donor cell survival can vary from 0.7% of donor cells remaining at two days [24] to 70% at three days [25], depending on the methods (e.g., histology, TUNEL apoptosis, or DNA quantification) used for assessing cell survival. However, all of these techniques require different subsets of animals to be sacrificed at various time points without being able to monitor cell survival within the same animal longitudinally. This can be a source of statistical discrepancy because of interindividual differences among animals. In this study, we showed that noninvasive imaging can be used to monitor early as well as late cell survival after transplantation.
Clinically, the acute rejection process can be controlled by immunosuppressing patients with drugs such as anti-inflammatory modulators (dexamethasone, prednisone), calcineurin inhibitors (cyclosporine, tacrolimus), and anti-proliferative agents (mycophenolate, azathioprine) [26]. Cyclosporine and tacrolimus act by blocking IL-2 promoter induction after T-cell activation, whereas mycophenolate inhibits mitogen-stimulated T- and B-lymphocyte proliferation [20]. The different phases of donor cell survival could be categorized into early survival (during the immediate hours or days after transplantation), acute rejection (mediated by inflammatory cells such as neutrophils, macrophages, and natural killer cells), and long-term survival (the period needed for clinical benefit) [11]. Previous reports have implicated neutrophils as being responsible for killing cells within the 24 hours and disappearing by day 2, whereas macrophages are present six hours after transplantation and increase further at day 7 during the period of acute rejection [14]. Cytolytic CD8+ T cells and natural killer cells also infiltrate the pockets of donor cells about the first week to initiate the acute rejection process. Although acquired immune response is an important factor in cell death, the findings of graft rejection after myoblast transplant performed in the muscles of histocompatible or severe combined immunodeficient (SCID) mice or immunosuppressed animals in other studies highlight the importance of other unknown innate factors that are involved in graft rejection [27, 28].
One limitation of our study is that we evaluated immunosuppressive drugs at single dosages. The dosages chosen were based on previous published reports [17, 19, 20]. Although a more detailed analysis involving toxicology profiles would be ideal, the main purpose of our study was to establish the proof of principle that molecular imaging can be used to study cell survival in vivo. Another limitation is that we did not evaluate viable cell number by traditional histologic sectioning. Because of sampling errors and inherent bias, several groups have noticed that it is difficult to accurately quantify the total number of surviving cells vs. dead or apoptotic cells based on tissue slides [29–31]. In contrast, recent studies have shown a robust correlation between bioluminescence signals and cell numbers, further attesting to the use of molecular imaging for following cell survival noninvasively, quantitatively, and longitudinally [32, 33]. Finally, we evaluated cell survival only within the context of a myoblast transplant model. How bone marrow stem cells, mesenchymal stem cells, endothelial progenitor cells, and other cell types survive in different disease models will need to be addressed in the future [1–3]. Indeed, donor cell death is not a problem unique to myoblast cell transplant strategies. Transplantations of isolated pancreatic islet cells for treatment of insulin-dependent diabetes [34], hepatocytes for inborn errors of metabolism [35], embryonic nervous tissues for experimental Parkinson’s disease [36], and fetal cardiomyocytes for ischemic myocardium all face the same challenge [30]. Thus, findings from similar imaging studies using these cell types will be important as the field of stem cell therapy advances.
In conclusion, we used a molecular imaging technique to analyze cell survival after transplant in living animals. Interestingly, clinically used immunosuppressive medications increased the survival of transplanted cells only modestly under the conditions tested in our study. Further studies will be needed to systemically evaluate factors important for the success of cell therapies, such as cell type, cell dosage, cell delivery route, and cell survival in different tissues or organs. We believe molecular imaging techniques will prove useful in expediting research on monitoring transplanted cell survival and therapeutic progress.
Acknowledgments
This work was supported in part by grants from the ASNC, GSK, AHA, and NHLBI (J.C.W.); NCI ICMIC-P50, NHLBI R01 HL078632, NCI SAIRP (S.S.G.); and Fund for Scientific Research Belgium—Flanders (O.G.). O. Gheysens and S. Lin contributed equally to this work.
References
- 1.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Karsenty G. Longevity and lineages: Toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120:533–544. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 5.Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 6.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 7.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 8.Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, Fishbein MC, Gambhir SS. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 10.Wu JC, Tseng JR, Gambhir SS. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11:491–505. doi: 10.1016/j.nuclcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Skuk D, Tremblay JP. Cell therapies for inherited myopathies. Curr Opin Rheumatol. 2003;15:723–729. doi: 10.1097/00002281-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller-Ehmsen J, Peterson KL, Kedes L, et al. Rebuilding a damaged heart: Long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation. 2002;105:1720–1726. doi: 10.1161/01.cir.0000013782.76324.92. [DOI] [PubMed] [Google Scholar]
- 14.Skuk D, Caron N, Goulet M, Roy B, Espinosa F, Tremblay JP. Dynamics of the early immune cellular reactions after myogenic cell transplantation. Cell Transplant. 2002;11:671–681. doi: 10.3727/000000002783985378. [DOI] [PubMed] [Google Scholar]
- 15.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 16.Tarnowski BI, Spinale FG, Nicholson JH. DAPI as a useful stain for nuclear quantitation. Biotech Histochem. 1991;66:297–302. [PubMed] [Google Scholar]
- 17.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94:II332–II336. [PubMed] [Google Scholar]
- 18.Wang C, Sun J, Sheil AG, McCaughan GW, Bishop GA. A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplantation. 2001;72:44–51. doi: 10.1097/00007890-200107150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Skuk D, Goulet M, Roy B, Tremblay JP. Efficacy of myoblast transplantation in nonhuman primates following simple intramuscular cell injections: Toward defining strategies applicable to humans. Exp Neurol. 2002;175:112–126. doi: 10.1006/exnr.2002.7899. [DOI] [PubMed] [Google Scholar]
- 20.Camirand G, Caron NJ, Asselin I, Tremblay JP. Combined immunosuppression of mycophenolate mofetil and FK506 for myoblast transplantation in mdx mice. Transplantation. 2001;72:38–44. doi: 10.1097/00007890-200107150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Smythe GM, Hodgetts SI, Grounds MD. Immunobiology and the future of myoblast transfer therapy. Mol Ther. 2000;1:304–313. doi: 10.1006/mthe.2000.0049. [DOI] [PubMed] [Google Scholar]
- 22.Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 23.Mendell JR, Kissel JT, Amato AA, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 24.Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 25.Rando TA, Pavlath GK, Blau HM. The fate of myoblasts following transplantation into mature muscle. Exp Cell Res. 1995;220:383–389. doi: 10.1006/excr.1995.1329. [DOI] [PubMed] [Google Scholar]
- 26.Hardinger KL, Koch MJ, Brennan DC. Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy. 2004;24:1159–1176. doi: 10.1592/phco.24.13.1159.38094. [DOI] [PubMed] [Google Scholar]
- 27.Huard J, Roy R, Guerette B, Verreault S, Tremblay G, Tremblay JP. Human myoblast transplantation in immunodeficient and immunosuppressed mice: Evidence of rejection. Muscle Nerve. 1994;17:224–234. doi: 10.1002/mus.880170214. [DOI] [PubMed] [Google Scholar]
- 28.Sammels LM, Bosio E, Fragall CT, Grounds MD, van Rooijen N, Beilharz MW. Innate inflammatory cells are not responsible for early death of donor myoblasts after myoblast transfer therapy. Transplantation. 2004;77:1790–1797. doi: 10.1097/01.tp.0000131150.76841.75. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 30.Reinecke H, Murry CE. Taking the death toll after cardiomyocyte grafting: A reminder of the importance of quantitative biology. J Mol Cell Cardiol. 2002;34:251–253. doi: 10.1006/jmcc.2001.1494. [DOI] [PubMed] [Google Scholar]
- 31.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Cao YA, Bachmann MH, Beilhack A, et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- 33.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 34.Swift SM, Clayton HA, London NJ, James RF. The potential contribution of rejection to survival of transplanted human islets. Cell Transplant. 1998;7:599–606. doi: 10.1177/096368979800700610. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowska A, Karrer FM, Bilir BM. Histological identification of purified and cryopreserved allogeneic hepatocytes following transplantation in a murine model without host immunosuppression. Transpl Int. 1999;12:188–194. doi: 10.1007/s001470050209. [DOI] [PubMed] [Google Scholar]
- 36.Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]