Abstract
To identify factors that affect manifestations of sickle cell anemia we compared patients 11-30 years of age from University of Ibadan, Nigeria (n = 214) and University of Illinois at Chicago, USA (n = 209). Paralleling findings in the general populations of the two countries, the Chicago patients were more often overweight or obese as defined by the Centers for Disease Control (Atlanta, GA, USA) guidelines, and more often had elevated blood pressure (BP) as defined by the National Heart, Lung, and Blood Institute (Bethesda, MD, USA) guidelines. The Ibadan sickle cell anemia patients less frequently received the pneumococcal vaccine or hydroxyurea (HU) therapy. Consistent with lower rates of elevated BP and increased body mass index (BMI), stroke history was less frequent in the Ibadan patients ≥18 years old. Furthermore, in combined analyses, systolic and diastolic BP directly correlated with BMI, and elevated weight status independently associated with history of stroke [odds ratio (OR) 2.7, p = 0.019]. Our findings are consistent with the possibility that higher values for BMI and BP in Chicago sickle cell anemia patients may contribute to an increased risk of stroke and highlights the need for measures to reduce these risk factors. On the other hand, lower pneumococcal vaccination and HU therapy rates in Ibadan patients highlights the need for more improved vaccination coverage and for studies to define the role of HU therapy in Africa.
Keywords: Hydroxyurea (HU), hypertension, pneumococcal vaccination, sickle cell anemia, weight
Introduction
Sickle cell anemia is one of the most common monogenetic diseases worldwide. It is caused by a single base pair mutation resulting in an amino acid substitution in the β-globin chain and results in a structurally abnormal hemoglobin (Hb) molecule, Hb S (HBB: c.20A>T). Heterozygous carriers of Hb S have protection from malaria mortality and carrier rates for Hb S range from 5 to 40% in malaria endemic regions (1). Sickle cell anemia is a consequence of homozygosity for the Hb S gene and results in polymerization of deoxygenated Hb in red blood cells. Approximately 20-25 million people have sickle cell anemia worldwide with 12-15 million living in sub-Saharan Africa (http://www.who.int/genomics/public/Maphaemoglobin.pdf). Migration patterns have led to the distribution of the Hb S gene to non endemic regions for malaria and the estimated prevalence of sickle cell anemia in the United States of America (USA) is approximately 100,000 (2).
Polymerization of Hb in the red blood cells (RBCs) of patients with sickle cell anemia leads to vaso-occlusion and hemolysis, and causes both acute and chronic complications. The disease severity varies markedly among patients with sickle cell anemia. Genetic factors, notably α-thalassemia (α-thal) and loci that regulate Hb F (α2γ2) expression contribute to this variation, and the pharmacological agent, hydroxyurea (HU), can have a substantial effect through increasing Hb F levels but much of the variation is still unexplained (3-7). Elevated blood pressure (BP) is another modifier of clinical severity in sickle cell anemia, being associated with stroke, renal dysfunction, pulmonary hypertension and early mortality (8-10). Increased body mass index (BMI), which may be a consequence of differences in life-style factors such as dietary habit or activity level as well as other genetic modifiers, is associated with higher BP in patients with sickle cell anemia (8,11-13).
General population comparisons between Nigerians and African Americans have shown that Nigerians have shorter height, lower BMI, lower systolic BP, and lower prevalence of hypertension (14). Furthermore, the rate of full childhood vaccination coverage is lower in the general population of Nigerians vs. African Americans (15,16). It is likely that these trends also occur among patients with sickle cell anemia. In this study, we compared differences in anthropometric, clinical, and hematological variables between two cohorts of patients with sickle cell anemia from the University of Ibadan, Nigeria and University of Illinois at Chicago (UIC), USA in light of background population differences. The objective of this study was to identify adverse outcome-influencing variables that may be amenable to public health interventions such as improving vaccination coverage, increasing appropriate HU therapy, promoting normal BMI, and preventing elevated BP.
Materials and methods
We analyzed 214 individuals with a diagnosis of sickle cell anemia (Hb SS) between the ages of 11 and 30 years old, receiving routine medical care at the University of Ibadan, Ibadan, Oyo, Nigeria and 209 individuals with sickle cell anemia between the ages of 11 and 30 years old, receiving routine medical care at UIC, Chicago, IL, USA. In both cohorts, all patients with a diagnosis of sickle cell anemia and between the ages of 11 and 30 years old at the time of the analysis were included. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The protocol was approved by the Institutional Review Board of the respective institutions prior to initiating the study.
Laboratory and clinical data were obtained from a steady-state visit, which we defined as a visit without mention of the patient being in an acute vaso-occlusive pain episode and at least 4 weeks after a blood transfusion or acute vaso-occlusive pain episode requiring medical attention. Laboratory variables that were collected included the white blood cell count (WBC) and differential, Hb concentration, mean corpuscular volume (MCV), platelet count, and Hb F%. Complete blood counts and WBC differentials were performed using the Sysmex KX-21 (Sysmex America, Lincolnshire, IL, USA) and Mindray 3000 plus (Mindray Medical International Ltd, Shenzhen, Guangdong, People's Republic of China) machines in the Ibadan cohort and the Siemens ADVIA 2120 (Siemens AG, Erlangen, Germany) machine in the Chicago cohort. The Hb F% was measured using the Kleihauer-Betke method in the Ibadan cohort and high performance liquid chromatography in the Chicago cohort. Clinical data was obtained by the medical health professionals and chart review from each institution. Clinical variables included age, sex, height, weight, systolic BP, diastolic BP, medication history (HU, pneumococcal vaccination), and a history of sickle cell anemia related complications [vaso-occlusive crisis episode frequency, acute chest syndrome (ACS), stroke, and RBC transfusion requirements]. In both cohorts, BP measurements were made using automated BP machines while the patients were in a seated position.
Weight status was defined as per the Centers for Disease Control and Prevention (Atlanta, GA, USA) guidelines (17,18). For individuals less than 18 years old, underweight was defined as a BMI <5th percentile, normal as a BMI in the 5-84th percentile, overweight as a BMI in the 85-94th percentile, and obesity for a BMI ≥95th percentile for age and gender-specific values. For individuals 18 years or older, underweight was defined as a BMI <18.5 kg/m2, normal as a BMI between 18.5-24.9 kg/m2, overweight as a BMI between 25-29.9 kg/m2, and obesity as a BMI ≥30 kg/m2.
Hypertension categories were defined as per the Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high BP guidelines (19). Pre hypertension was defined in individuals less than 18 years old as a systolic or diastolic BP in the 90-94th percentile and hypertension as a systolic or diastolic BP ≥95th percentile based on age, height, and gender-specific values. For those individuals 18 years or older, pre hypertension was defined as a systolic BP 120-139 mmHg or a diastolic BP 80-89 mmHg, and hypertension as a systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg. An elevated BP was defined as any individual with pre hypertension or hypertension.
Continuous variables were compared according to site or clinical outcomes with the Kruskall-Wallis test and categorical variables with Pearson's χ2 test. Comparisons between the two cohorts were stratified by age group (<18 years old and ≥18 years old). The Bonferroni correction was applied to adjust for multiple comparisons. Multivariate analysis was performed using logistic regression and linear regression analysis and adjustments for age, gender, HU use, and cohort were applied to all final models. Variables with a p≤0.1 in univariate analysis were entered into the initial model and a stepwise approach was applied to select the final regression models adjusted for age, gender, HU use and site. Systat 11 (Systat Software Corporation, Chicago, IL, USA) was used for statistical analyses.
Results
Patient characteristics
Gender distribution was similar between the Ibadan and Chicago cohorts in both age groups (Table 1). Hydroxyurea therapy, pneumococcal vaccination, and influenza vaccination use were observed in lower proportions of sickle cell anemia patients from the Ibadan cohort vs. the Chicago cohort in both sickle cell anemia patients <18 years and ≥18 years of age, while a difference in a lifetime history of >5 units of RBC transfusions was significantly different in sickle cell anemia patients ≥18 years of age.
Table 1.
Comparison of clinical and laboratory factors between all patients from the University of Ibadan, Ibadan, Oyo, Nigeria and the University of Illinois at Chicago, Chicago, IL, USA.
| <18 Years Old | ≥18 Years Old | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | n | UIC | n | Nigeria | p Value | n | UIC | n | Nigeria | p Value |
| Age | 32 | 14 (11-17) | 59 | 14 (11-17) | 0.6 | 177 | 24 (18-30) | 155 | 23 (18-30) | 0.04 |
| Gender (M; F) (%) | 32 | 47.0; 53.0 | 59 | 34.0; 66.0 | 0.02 | 177 | 45.0; 55.0 | 155 | 49.0; 51.0 | 0.02 |
| HU therapy (%) | 32 | 14 (44.0) | 58 | 4 (7.0) | <0.0001a | 177 | 81 (46.0) | 154 | 4 (3.0) | <0.0001a |
| Pneumovax (%) | 26 | 23 (88.0) | 59 | 0 (0.0) | <0.0001a | 131 | 119 (91.0) | 151 | 1 (1.0) | <0.0001a |
| Influenza vaccination (%) | 29 | 26 (90.0) | 57 | 0 (0.0) | <0.0001a | 132 | 121 (92.0) | 124 | 2 (2.0) | <0.0001a |
| Transfusion (>5 U/lifetime) (%) | 28 | 12 (43.0) | 59 | 17 (29.0) | 0.2 | 82 | 36 (44.0) | 155 | 29 (19.0) | <0.0001a |
| Stroke history (%) | 32 | 2 (6.0) | 59 | 4 (7.0) | 0.9 | 170 | 41 (24.0) | 155 | 3 (2.0) | <0.0001a |
| History of three or more VOC/year (%) | 31 | 6 (19.0) | 55 | 27 (49.0) | 0.0065 | 165 | 80 (48.0) | 153 | 65 (42.0) | 0.3 |
| History of ACS (%) | 32 | 17 (53.0) | 59 | 17 (29.0) | 0.022 | 164 | 100 (61.0) | 154 | 50 (32.0) | <0.0001a |
| Weight (kg) | 32 | 42 (26-66) | 59 | 33 (20-63) | 0.018 | 177 | 64 (42-113) | 155 | 52 (28-76) | <0.0001a |
| Height (cm) | 32 | 151 (122-171) | 59 | 147 (115-189) | 0.2 | 177 | 170 (140-191) | 155 | 165 (142-184) | <0.0001a |
| BMI (kg/m2) | 32 | 17.8 (12.6-26.9) | 59 | 15.7 (13.2-24.7) | 0.0093 | 177 | 22.1 (15.2-47.0) | 155 | 19.5 (14.1-28.0) | <0.0001a |
| Systolic BP (mmHg) | 32 | 112 (95-129) | 59 | 98 (78-124) | <0.0001a | 174 | 119 (89-160) | 155 | 108 (77-146) | <0.0001a |
| Diastolic BP (mmHg) | 32 | 61 (42-74) | 59 | 60 (44-83) | 0.8 | 174 | 69 (41-97) | 155 | 64 (47-117) | 0.0006a |
UIC: University of Illinois at Chicago; HU: hydroxyurea; VOC: vaso-occlusive crises; ACS: acute chest syndrome; BMI: body mass index; BP: blood pressure; continuous variables are presented as median values (range).
p Values significant after Bonferroni correction.
Anthropometric measures
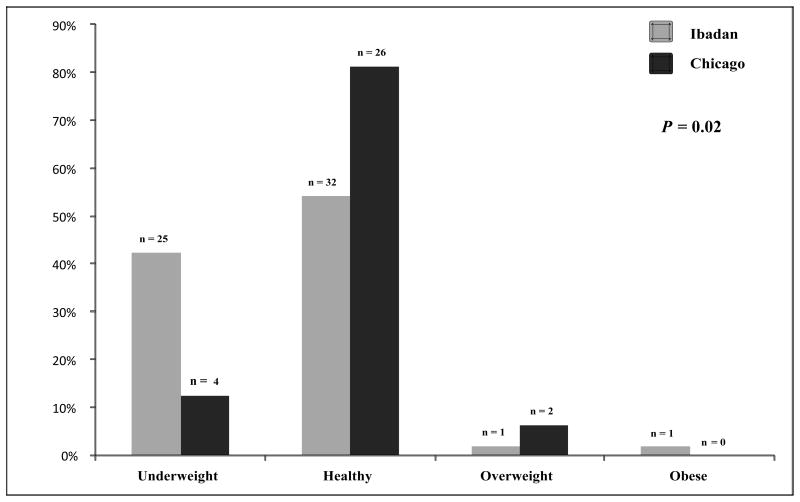
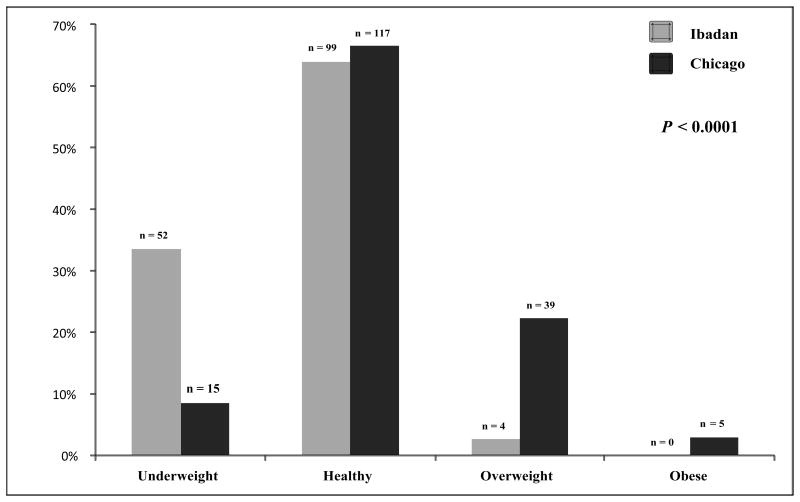
In sickle cell anemia patients ≥18 years old, weight, height, and BMI were lower in the Ibadan cohort vs. the Chicago cohort (Table 1). In sickle cell anemia patients <18 years old, these differences persisted for weight (33 vs. 42 kg) and BMI (15.7 vs. 17.8 kg/m2) between the Ibadan and Chicago cohorts, respectively, although the differences were not significant after the Bonferonni correction. A higher proportion of patients from the Ibadan cohort were underweight (Figure 1A and 1B), while a higher proportion of patients from the Chicago cohort were overweight or obese in both age groups of sickle cell anemia patients (Figure 1A and 1B).
Figure 1.
(A) Weight status in sickle cell anemia patients <18 years old from the Ibadan and Chicago cohorts. A higher proportion of sickle cell anemia patients from Ibadan were underweight (42.0 vs. 13.0%), while higher proportions of sickle cell anemia patients from Chicago were overweight or obese (6.0 vs. 3.0%), respectively. (B) Weight status in sickle cell anemia patients ≥18 years old from the Ibadan and Chicago cohorts. A higher proportion of sickle cell anemia patients from Ibadan were underweight (34.0 vs. 9.0%), while higher proportions of sickle cell anemia patients from Chicago were overweight or obese (25.0 vs. 3.0%), respectively.
Blood pressure
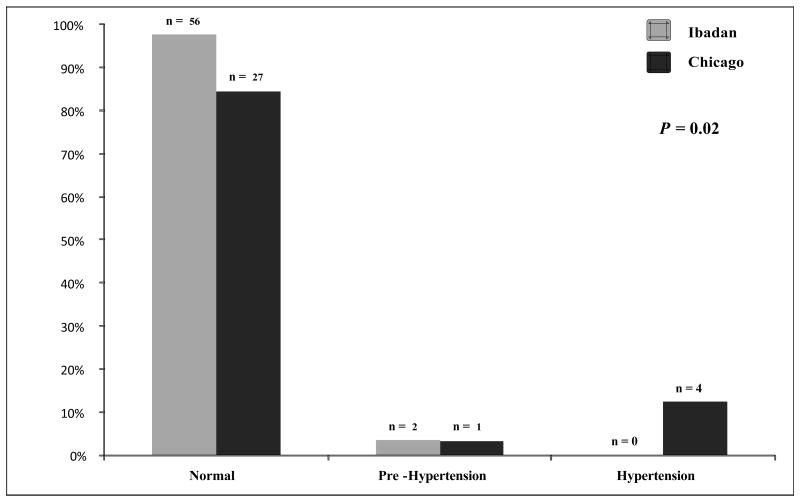
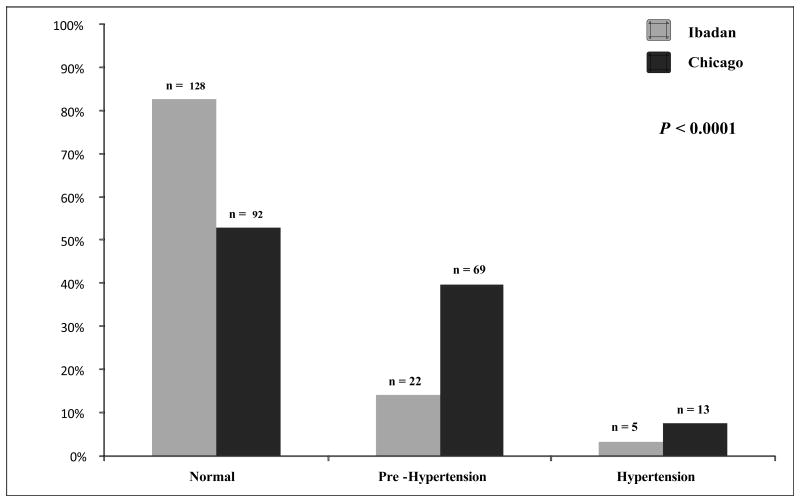
The systolic BP was higher in both age groups and the diastolic BP was higher in the adult sickle cell anemia patients from the Chicago cohort compared to the Ibadan cohort (Table 1). Correspondingly, the proportion of patients with an elevated BP (defined as being pre hypertensive or hypertensive) was higher in both age groups of sickle cell anemia patients from the Chicago cohort than the Ibadan cohort (Figure 2A and 2B).
Figure 2.
(A) Hypertension stage in sickle cell anemia patients <18 years old from the Ibadan and Chicago cohorts. Lower proportions of sickle cell anemia patients from Chicago were normotensive (84.0 vs. 98.0%), and higher proportions were pre hypertensive or hypertensive (16.0 vs. 3.0%) compared to sickle cell anemia patients from Ibadan, respectively. (B) Hypertension stage in sickle cell anemia patients ≥18 years old from the Ibadan and Chicago cohorts. Lower proportions of sickle cell anemia patients from Chicago were normotensive (53.0 vs. 83.0%) and higher proportions were pre hypertensive or hypertensive (47.0 vs. 17.0%) compared to sickle cell anemia patients from Ibadan, respectively.
Clinical complications
Lower proportions of adult sickle cell anemia patients from the Ibadan cohort had histories of stroke or ACS compared to the Chicago cohort, while similar proportions of patients had ≥3 vaso-occlusive crises per year requiring medical attention (Table 1). In the sickle cell anemia patients <18 years old, a history of stroke was present in similar proportions between the Ibadan and Chicago cohorts. Rates of ACS were lower and the proportion of sickle cell anemia patients with ≥3 vaso-occlusive crises per year requiring medical attention were higher in the Ibadan cohort of sickle cell anemia patients <18 years old, although the differences were not significant after Bonferroni correction.
Combined analyses
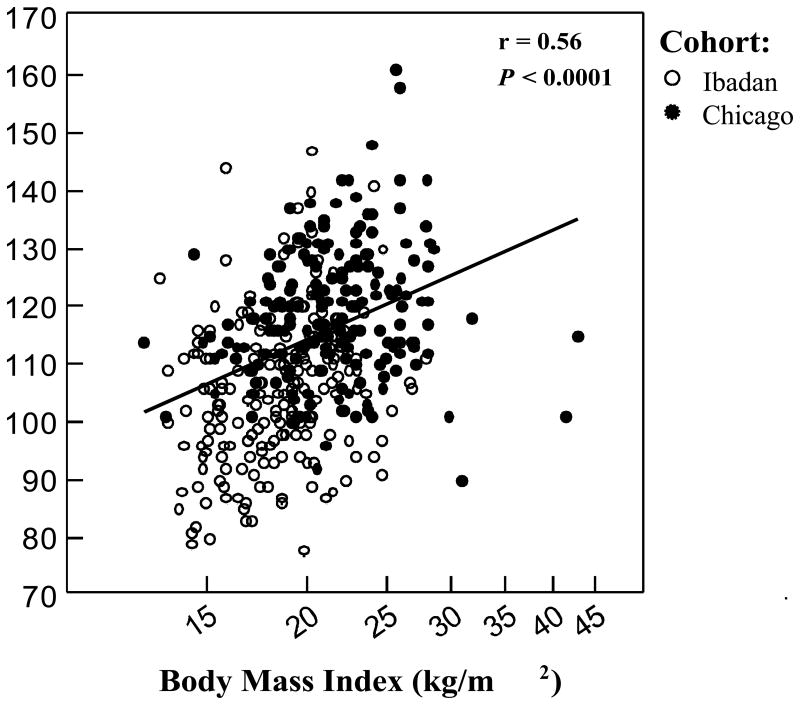
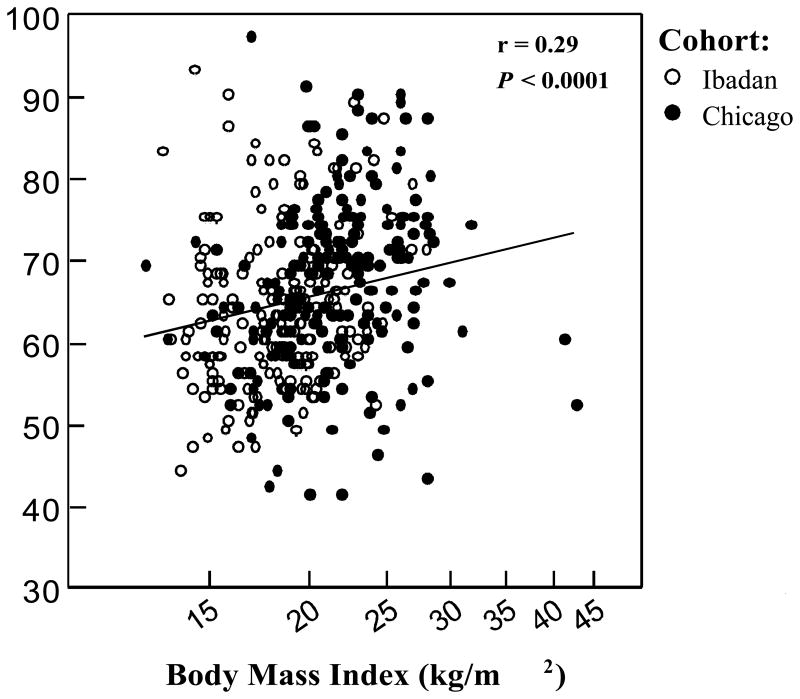
Among the combined cohorts, the BMI was associated with both systolic (Figure 3A) and diastolic BPs (Figure 3B) after adjusting for age, gender, HU status, and cohort. On univariate analyses, an elevated BP (defined as pre hypertension or hypertension) was associated with greater age, higher Hb concentration, higher BMI, male gender, and a history of stroke (p<0.006). On multivariate analysis, an elevated BP was associated with male gender [odds ratio (OR) 4.9, 95% confidence interval (CI): 2.8-8.5; p<0.0001], being from the Chicago cohort (OR 3.6, 95% CI: 2.0-6.5; p<0.0001), higher BMI (natural log OR 7.0, 95% CI: 1.3-38.4; p = 0.025) and greater age (5-year OR 1.5, 95% CI: 1.1-2.1; p = 0.004).
Figure 3.
(A) Body mass index and systolic BP. On linear regression analysis, the BMI was independently associated with systolic BP with adjustment for age, gender, HU therapy, and cohort (systolic BP = 12.2 (natural log BMI, p = 0.0023) + 0.4 (age, p = 0.00047) + 8.1 (male gender, p <0.0001) – 10.0 (Nigeria cohort, p <0.0001) + 1.3 (HU therapy, p = 0.4). (B) Body mass index and diastolic BP. On linear regression analysis, BMI was independently associated with diastolic BP with adjustment for age, gender, HU therapy, and cohort (diastolic BP = 7.7 (natural log BMI, p = 0.01) + 0.2 (age, p = 0.02) – 0.7 (male gender, p = 0.4) – 0.4 (Nigeria cohort, p = 0.7) + 2.5 (HU therapy, p = 0.051).
Stroke history was associated with higher systolic BP and BMI, lower Hb F%, and being from the Chicago cohort on univariate analyses (p<0.006). On multivariate analysis, after adjusting for age, gender, and HU status, stroke history was associated with the natural log of Hb F% (OR 0.4, 95% CI: 0.3-0.6; p<0.0001), being from the Chicago cohort (OR 8.9, 95% CI: 3.4-23.4; p<0.0001), and being overweight or obese (OR 2.7, 95% CI: 1.2-6.0; p = 0.019). Acute chest syndrome history was associated with a higher systolic BP and weight status (p<0.006) on univariate analyses. After adjusting for age, gender, HU status and cohort, neither variable remained significantly associated with a history of ACS. A history of ≥3 vaso-occlusive crises per year requiring medical attention was associated with a higher absolute granulocyte count (4.1 vs. 3.3 × 103/μL, p = 0.045) and lower Hb F% (4.7 vs. 6.5%, p = 0.017), although the associations were not significant after Bonferroni correction. On multivariate analysis, a history of ≥3 vaso-occlusive crises per year requiring medical attention was associated with the natural log of Hb F% (OR 0.7, 95% CI: 0.5-0.9; p = 0.0067) after adjusting for age, gender, HU status, and cohort.
Discussion
In this study, we compared the clinical and laboratory features between two cohorts of sickle cell anemia patients from Ibadan, Oyo, Nigeria and Chicago, IL, USA in light of known population differences, to better understand the potential role for environmental and genetic influences on sickle cell anemia phenotypes. We observed higher rates of overweight or obesity, and elevated BP in the Chicago cohort of sickle cell anemia patients and lower rates of pneumococcal vaccination and HU use in the Ibadan cohort. These differences may reflect environmental effects such as access to care, diet, and activity levels as well as potential differences in genetic modifiers of metabolic indicators affecting the clinical course of sickle cell anemia.
Overweight and obesity are risk factors for elevated BP and cardiovascular sequelae in the general population (20). Similarly, increased BMI in patients with sickle cell anemia has been shown to be associated with elevated BP and hypertension (8,11-13). In turn, elevated BP has been associated with complications in sickle cell anemia including stroke, kidney disease and early mortality (8-10,21). We observed a significantly higher proportion of sickle cell anemia patients who were overweight or obese in the Chicago vs. Ibadan cohort (age <18: 6.0 vs. 3.0%, age ≥18: 25.0 vs. 3%, respectively) and increasing BMI was independently associated with increasing systolic BP, relative hypertension, and a history of stroke. Corresponding with a higher BMI in the Chicago cohort, sickle cell anemia patients from Chicago also had higher systolic BP levels and higher prevalence rates of pre hypertension and hypertension than the Ibadan cohort. Public health measures such as dietary counseling to address weight status in Chicago may help mitigate hypertension and stroke risk in sickle cell anemia patients. Other genetic factors that modulate weight status and BP are suggested based on our findings of an independent association of being from the Chicago cohort with relative hypertension. Future studies to identify these genetic modifiers may help guide the identification and therapeutic development for metabolic abnormalities in both sickle cell anemia patients as well as the general population.
Patients with sickle cell anemia are functionally asplenic and infections by encapsulated bacteria such as Streptococcus pneumoniae are an important cause of morbidity and mortality in children with sickle cell anemia. Penicillin prophylaxis in early childhood and pneumococcal vaccination can reduce the rate of systemic pneumococcal infections (22-24). In our analysis, pneumococcal vaccination was underutilized in sickle cell anemia patients from Ibadan and future strategies to increase pneumococcal vaccination are warranted.
Hydroxyurea therapy is currently the only therapy that has been shown, in randomized studies, to improve the clinical course of sickle cell anemia (7). Hydroxyurea reduces the rate of vaso-occlusive crises, ACS, and RBC transfusion requirements and recent data suggests improved survival with long-term HU therapy use (7,25,26). However, HU can cause myelotoxicity and the cost of frequent complete blood count monitoring and purchasing the medication may be prohibitive in Nigeria (27). Correspondingly, we observed a significantly lower rate of HU use in the cohort of sickle cell anemia patients from Ibadan compared to Chicago. This highlights the importance for future investigations to overcome barriers to the use of HU and evaluate potential risks for HU therapy such as infections specific to Nigeria.
The prevalence of complication rates between the Ibadan and Chicago adult cohorts were similar for vaso-occlusive crises ≥3 per year requiring medical attention, while a higher frequency of adult patients from the Chicago cohort had a history of stroke or ACS. These differences may reflect actual differences in patterns for these complications between the two cohorts, although differences in survival and detection rates will need to be elucidated in future prospective studies. Consistent with the literature in both Nigerians (28-30) and African Americans (4,31) with sickle cell anemia, the Hb F% was independently associated with a history of stroke or vaso-occlusive crises ≥3 per year, highlighting the importance of Hb F% in modulating the clinical phenotype of sickle cell anemia in both cohorts. We observed a lower proportion of patients with a history of >5 units RBC in the Ibadan cohort and this may reflect inherent problems with availability of units as well as the inability of many patients to afford transfusions that can cost the patient upwards of US$40 per unit.
There were several limitations to our study. This comparison was a cross-sectional analysis that introduced the potential for survival bias contributing to the observed differences in complications between the Ibadan and Chicago cohort. However, the prevalence of stroke history in our Ibadan cohort was comparable with the pediatric and adult literature from Nigeria (28,32,33). Another limitation was that stroke history was determined by chart review and medical interview and did not include silent strokes by magnetic resonance imaging. Differences in rates of detection and recall bias may also explain differences between the two cohorts. The associations between clinical variables such as BMI and elevated BP with clinical outcomes such as a history of stroke do not infer causality in a cross-sectional analysis and these biases will need to be addressed in future prospective studies. Different methodologies for measuring the CBC parameters and Hb F% between the two cohorts limited our ability to compare these variables between the two cohorts and comparisons with standardized methods are warranted in future studies. Another potential limitation was that steady-state was defined as not being examined while suffering a vaso-occlusive crisis and it being at least 4 weeks after an RBC transfusion or vaso-occlusive crisis requiring medical attention. This definition does not include other illnesses such as a viral infection with fever and it is also unclear whether 4 weeks after a vaso-occlusive crisis or RBC transfusion is sufficient time for a patient to return to a steady-state status.
In conclusion, we observed higher rates of overweight or obesity and elevated BP in sickle cell anemia patients from Chicago and lower rates of HU and pneumococcal vaccination use in sickle cell anemia patients from Ibadan. Life-style modifications such as a healthy diet and moderate exercise should be incorporated into treatment guidelines for sickle cell anemia patients from Chicago to improve increased weight status and elevated BP. Strategies to better implement pneumococcal vaccination to sickle cell anemia patients from Ibadan are necessary to prevent infection in children with sickle cell anemia. Future studies to assess the feasibility and safety of HU in Nigeria and studies to understand potential genetic modifiers and environmental risk factors for the metabolic abnormalities in sickle cell anemia patients from the USA are also warranted.
Acknowledgments
The project described was supported in part by grants from the National Institutes of Health (NIH), Bethesda, MD, USA (5R01HL053353 and 5R03TW008695), by the National Center for Advancing Translational Sciences, NIH, Bethesda, MD, USA through Grant KL2TR000048, and by the Doris Duke Charitable Foundation (DDCF), New York, NY, USA through Grant #2013140.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the DDCF.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Serjeant GR, Serjeant BE. Sickle Cell Disease. 3rd. Oxford: Oxford University Press; 2001. [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 5.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84(2):643–649. [PubMed] [Google Scholar]
- 6.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 7.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 8.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: Risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102(2):171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 9.Gordeuk VR, Sachdev V, Taylor JG, et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83(1):15–18. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 11.Lamarre Y, Lalanne-Mistrih ML, Romana M, et al. Male gender, increased blood viscosity, body mass index and triglyceride levels are independently associated with systemic relative hypertension in sickle cell anemia. PloS One. 2013;8(6):e66004. doi: 10.1371/journal.pone.0066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai PC, Deal AM, Brittain JE, et al. Decades after the cooperative study: A re-examination of systemic blood pressure in sickle cell disease. Am J Hematol. 2012;87(10):E65–E68. doi: 10.1002/ajh.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oguanobi NI, Onwubere BJ, Ibegbulam OG, et al. Arterial blood pressure in adult Nigerians with sickle cell anemia. J Cardiol. 2010;56(3):326–331. doi: 10.1016/j.jjcc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Cooper R, Rotimi C, Ataman S, et al. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87(2):160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal J, Rodewald L, McCauley M, et al. Immunization coverage levels among 19- to 35-month-old children in 4 diverse, medically underserved areas of the United States. Pediatrics. 2004;113(4):e296–e302. doi: 10.1542/peds.113.4.e296. [DOI] [PubMed] [Google Scholar]
- 16.Fatiregun AA, Adebowale AS, Ayoka RO, Fagbamigbe AF. Assessing full immunisation coverage using lot quality assurance sampling in urban and rural districts of southwest Nigeria. Trans Roy Soc Trop Med Hyg. 2013;107(11):731–740. doi: 10.1093/trstmh/trt079. [DOI] [PubMed] [Google Scholar]
- 17.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 18.The National Institute of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. [Date accessed October 17, 2013];National Institutes of Health, US Department of Health and Human Services. 1998 http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. [PubMed]
- 19.The National Institute of Health. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. [Date accessed October 17, 2013];National Institutes of Health, US Department of Health and Human Services. 2003 http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf.
- 20.Wilson PW, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Internal Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 21.Abo-Zenah H, Moharram M, El Nahas AM. Cardiorenal risk prevalence in sickle cell hemoglobinopathy. Nephron Clin Pract. 2009;112(2):c98–c106. doi: 10.1159/000213897. [DOI] [PubMed] [Google Scholar]
- 22.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 23.Ammann AJ, Addiego J, Wara DW, et al. Polyvalent pneumococcal-polysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med. 1977;297(17):897–900. doi: 10.1056/NEJM197710272971701. [DOI] [PubMed] [Google Scholar]
- 24.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010;85(6):403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: Results of a 17-year, single-center trial (LaSHS) Blood. 2010;115(12):2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 27.Galadanci N, Wudil BJ, Balogun TM, et al. Current sickle cell disease management practices in Nigeria. Int Health. 2014;6(1):23–28. doi: 10.1093/inthealth/iht022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yetunde A, Anyaegbu CC. Profile of the Nigerian sickle cell anaemia patients above 30 years of age. Cent Afr J Med. 2001;47(4):108–111. [PubMed] [Google Scholar]
- 29.Enosolease ME, Ejele OA, Awodu OA. The influence of foetal haemoglobin on the frequency of vaso-occlusive crisis in sickle cell anaemia patients. Niger Postgrad Med J. 2005;12(2):102–105. [PubMed] [Google Scholar]
- 30.Fatunde OJ, Scott-Emuakpor AB. Foetalhaemoglobin in Nigerian children with sickle cell anaemia. Effect on haematological parameters and clinical severity. Trop Geogr Med. 1992;44(3):264–266. [PubMed] [Google Scholar]
- 31.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63(4):921–926. [PubMed] [Google Scholar]
- 32.Lagunju IA, Brown BJ. Adverse neurological outcomes in Nigerian children with sickle cell disease. Int J Hematol. 2012;96(6):710–718. doi: 10.1007/s12185-012-1204-9. [DOI] [PubMed] [Google Scholar]
- 33.Fatunde OJ, Adamson FG, Ogunseyinde O, et al. Stroke in Nigerian children with sickle cell disease. Afr J Med Med Sci. 2005;34(2):157–160. [PubMed] [Google Scholar]