Abstract
Purpose
Taxane antineoplastic agents are extensively taken up into hepatocytes by OATP1B-type transporters prior to metabolism and excretion. Because the biodistributional properties imposed upon these agents by different solubilizers drive clinically-important pharmacodynamic endpoints, we tested the hypothesis that the in vitro and in vivo interaction of taxanes with OATP1B transporters is affected by the choice of drug delivery system.
Experimental Design
Transport of paclitaxel, docetaxel, and cabazitaxel was studied in vitro using various cell lines transfected with OATP1B1, OATP1B3, or the rodent equivalent Oatp1b2. Pharmacokinetic studies were done in wildtype and Oatp1b2-knockout mice in the presence or absence of polysorbate 80 (PS80) or Kolliphor EL (formerly Cremophor EL; CrEL).
Results
Paclitaxel and docetaxel, but not cabazitaxel, were transported substrates of OATP1B1, OATP1B3, and Oatp1b2, and these transport processes were strongly reduced in the presence of clinically-relevant concentrations of PS80 and CrEL. In the absence of solubilizers, deficiency of Oatp1b2 in mice was associated with a significantly decreased taxane clearance due to a liver distribution defect (P<0.00001), but these kinetic changes were masked in the presence of PS80 or CrEL (P>0.05).
Conclusions
Our findings confirm the importance of OATP1B-type transporters in the hepatic elimination of taxanes, and that this process can be inhibited by PS80 and CrEL. These results suggest that the likelihood of drug-drug interactions mediated by these transporters is strongly dependent on the selected taxane solubilizer.
Keywords: Oatp1b2, OATP1B1/3, paclitaxel, pharmacokinetics, Cremophor EL
INTRODUCTION
The nonionic surfactants Kolliphor EL (formerly Cremophor EL; CrEL) and polysorbate 80 (Tween 80; PS80) are widely used as drug solubilizers, including for the taxane-based antineoplastic agents paclitaxel (1), docetaxel (2), and cabazitaxel (3). A wealth of experimental data has indicated that these solubilizers are biologically and pharmacologically active compounds, and their use as drug formulation vehicles has been implicated in clinically important toxic side effects such as acute hypersensitivity reactions (4). CrEL and PS80 have also been found to influence the disposition of solubilized drugs administered intravenously (5). This is particularly striking in the case of paclitaxel formulated in CrEL, where the overall resulting effect is a highly increased systemic exposure to paclitaxel (6), which is dependent on the dose and time-varying blood concentrations of the solubilizer (7). Kinetic experiments (8, 9) and model-based predictions (10, 11) have revealed that paclitaxel undergoes reversible partitioning into a circulating CrEL microemulsion that acts as a nano-sink and reduces the fraction of free drug available for extravascular distribution.
In line with these predictions, it was demonstrated that CrEL can inhibit the hepatic elimination of paclitaxel in the isolated perfused rat liver, the main organ of elimination (12), by preventing the drug from reaching sites of metabolism and excretion (13). This process is believed to be primarily mediated by the organic anion-transporting polypeptides OATP1B3 (in humans) (14, 15) and Oatp1b2 (in rodents) (16, 17), which are uptake transporters localized to the basolateral membrane of hepatocytes (18). To add further to the complexity of the carrier-mediated disposition properties of paclitaxel, CrEL was found to strongly inhibit the uptake of OATP1B3 substrates in vitro into cells overexpressing the transporter (19). However, the mechanistic basis underlying this observation, as well as its potential in vivo relevance, remains unclear.
Because the biodistributional properties imposed upon taxanes by different solubilizers drive clinically-important pharmacodynamic endpoints that further depend, at least in part, on whether or not the pharmacokinetics of carrier-released free drug is formulation-dependent (10), we here tested the hypothesis that the in vitro and in vivo interaction of paclitaxel with OATP1B-type transporters is affected by the choice of a particular drug delivery system.
MATERIALS AND METHODS
In vitro transport studies
Xenopus laevis oocytes injected with OATP1B1, OATP1B3, or rat Oatp1b2 cRNA along with water-injected controls were obtained from BD Biosciences. Human embryonal kidney (HEK293) cells overexpressing OATP1B1, OATP1B3, or Oatp1b2 (20) and Chinese hamster ovary (CHO) cells expressing OATP1B1 or OATP1B3 (21) have been described previously. Overexpression of transporters was confirmed using TaqMan probes (Applied Biosystems). The cell culture conditions and details of intracellular accumulation experiments for [3H]paclitaxel (specific activity, 25.6 Ci/mmol; Vitrax) and [3H]docetaxel (specific activity, 60.0 Ci/mmol; American RadioChemicals) were described earlier (20), and based on liquid scintillation counting using a LS 6500 Multipurpose Scintillation Counter (Beckman). Intracellular concentrations of cabazitaxel were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS), as described (22). Total protein was measured using a Pierce BCA Protein Assay Kit (Thermo Scientific) and quantified using a Biotek µQuant microplate spectrophotometer. Drug uptake results were normalized to total protein content and then to data obtained in cells carrying an empty vector plasmid, which was set to 100.
Animal experiments
Wildtype and Oatp1b2 knockout [Oatp1b2(−/−)] mice, both on a DBA/1lacJ background, between 8 and 12 weeks of age, were housed in a temperature-controlled environment with a 12-hour light cycle. Experiments were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital. All mice received a standard diet and water ad libitum and fasted 3 hours before drug administration. Paclitaxel was formulated in CrEL-ethanol (1:1, v/v), PS80-ethanol (1:1, v/v) or as an albumin-bound nanoparticle (nabpaclitaxel; ABI-007; Abraxane; Celgene) without CrEL or PS80. Solutions were diluted in normal saline and administered by i.v. bolus in the tail vain at a dose of 10 mg/kg. Docetaxel and cabazitaxel Sanofi-Aventis) were formulated in PS80-ethanol (1:1, v/v), diluted in saline, and also administered by tail vein injection at a dose of 10 mg/kg.
At select time points after drug administration, blood samples (30 µL each) were taken from individual mice at 3.5, 7.5, and 15 min from the submandibular vein using a lancet, and at 30 and 60 min from the retro-orbital venous plexus using a capillary. A final blood draw was obtained at 120 min by a cardiac puncture using a syringe and needle. The total blood volume collected during the procedure from each mouse was 150 µL. All blood samples were centrifuged at 1500 × g for 5 min, and plasma was separated and stored at −80°C until analysis. Livers, kidneys, and small intestines were collected from the same animals at 120 min. A separate group of mice was euthanized by CO2 asphyxiation and the same tissues were immediately collected and flash-frozen on dry ice or in liquid nitrogen. All tissue specimens were stored at −80°C until further processing, as described (20). Samples were analyzed by LC-MS/MS (see Supplementary Methods for details) (23), and non-compartmental parameters were calculated using WinNonlin 6.2 software (Pharsight). Concentrations in tissue were corrected for contaminating plasma (24).
Statistical considerations
All data are presented as mean ± SD. Statistical analyses were done using SPSS version 20 (SPSS), and P < 0.05 was regarded as statistically significant. Student’s t-test (2 groups) or one-way ANOVA (>2 groups) was used for statistical analysis on in vitro uptake data, plasma pharmacokinetic parameters, and tissue concentrations.
RESULTS
Paclitaxel transport in vitro
Although previous in vitro studies have consistently identified paclitaxel as a potent inhibitor of OATP1B1- (25, 26) and OATP1B3-mediated transport (25, 27, 28), the actual transport of paclitaxel itself by these transporters remains controversial (14, 28–30). In line with these conflicting data, we found that the interaction of paclitaxel with human OATP1B1 and OATP1B3 was dependent on cell context, with both proteins being able to take up docetaxel when expressed in HEK293 cells or CHO cells, but no noticeable transport occurring by OATP1B1 expressed in Xenopus laevis oocytes (Fig. 1A). Paclitaxel was also found to be transported into cells expressing mouse Oatp1b2 or rat Oatp1b2 (Fig. 1B), as predicted from studies done in isolated rate hepatocytes (31).
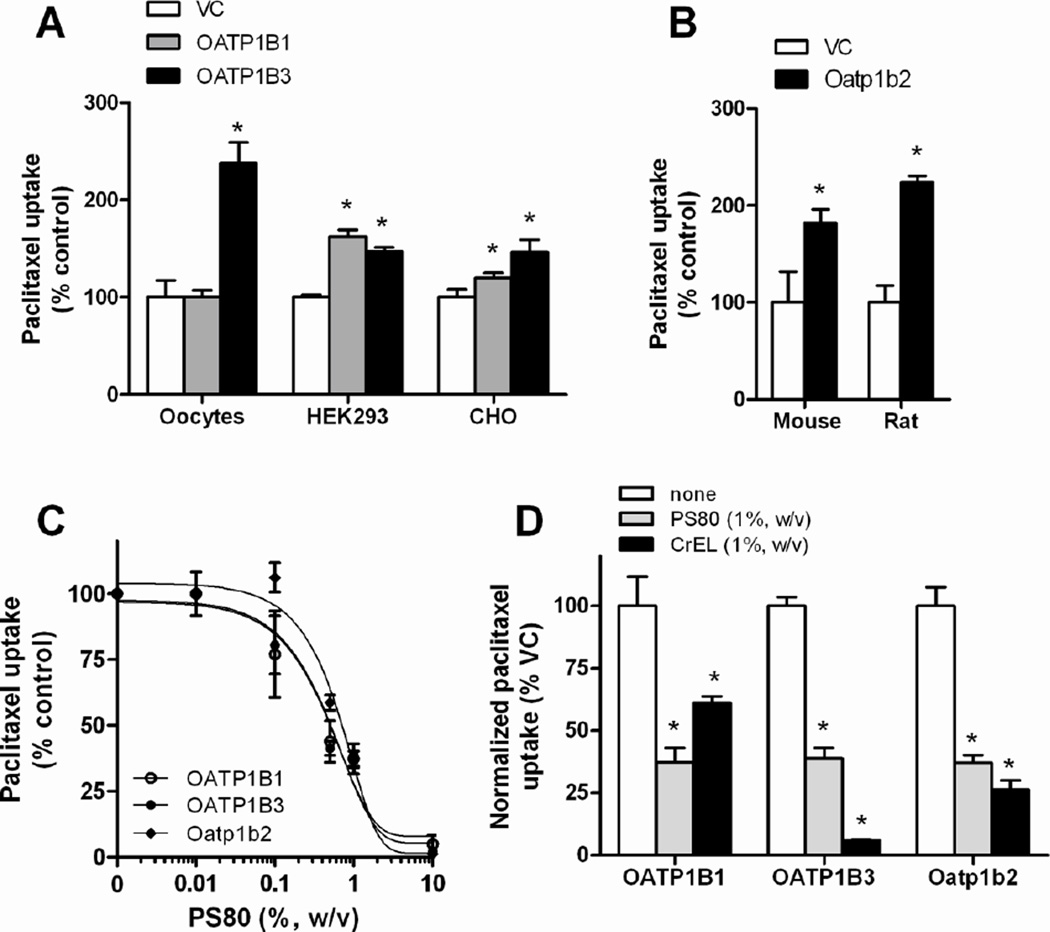
Figure 1.
In vitro transport studies of paclitaxel. (A) The uptake of paclitaxel by human OATP1B1 and human OATP1B3 was assessed in OATP1B1 and OATP1B3-injected Xenopus laevis oocytes (paclitaxel concentration, 2 µM; 30-min incubations), HEK293 cells (2 µM; 30-min) and CHO cells (1 µM; 2-min) and their respective vector controls (VC). (B) The uptake of paclitaxel by mouse Oatp1b2 transfected in HEK293 cells (0.1 µM; 15-min) or rat Oatp1b2 transfected in Xenopus laevis oocytes (2 µM; 30-min). (C) The uptake of paclitaxel by human OATP1B1, human OATP1B3 in CHO cells (1 µM; 2-min), or mouse Oatp1b2 in HEK293 cells (0.1 µM; 15-min) was evaluated in the presence of different concentrations of PS80. (D) The uptake of paclitaxel by human OATP1B1, human OATP1B3 in CHO cells (1 µM; 2-min), or mouse Oatp1b2 in HEK293 cells (0.1 µM; 15-min) was evaluated in the presence of either CrEL (1%, w/v) or PS80 (1%, w/v), with results normalized to transporter-specific uptake. Data are presented as the mean percentage of uptake values in the VC cells (bars) for 4–16 observations per condition, along with SD (error bars). The star (*) indicates a statistically significant difference compared to VC (P < 0.05).
The transport of paclitaxel into CHO cells transfected with OATP1B1 or OATP1B3 was found to be time-dependent and saturable with a Michaelis-Menten constant (Km) of 0.408±0.190 µM and 2.36±1.40 µM, respectively, and a maximum velocity (Vmax) of 22.1±3.20 pmol/mg/min and 14.2±5.26 pmol/mg/min, respectively (Supplementary Fig. S1), which values are similar to those reported previously for docetaxel (20).
Next, we evaluated the ability of CrEL and PS80 to inhibit the intracellular accumulation of paclitaxel into CHO cells overexpressing human OATP1B1, human OATP1B3, or mouse Oatp1b2. Similar to findings reported recently for CrEL (19), PS80 inhibited this process in a concentration-dependent manner (Fig 1C). After correcting for non-specific inhibition due to drug trapping in solubilizer micelles, CrEL directly inhibited the transporters in decreasing order of potency OATP1B3 > Oatp1b2 > OATP1B1, whereas PS80 inhibited all transporters to a similar extent (Fig 1D).
Paclitaxel tissue distribution studies in vivo
We next evaluated the possible importance of these transporters for paclitaxel in mice with a genetic deletion of Oatp1b2 [Oatp1b2(−/−) mice]. Early after administration (5 min) of paclitaxel as an albumin-bound nanoparticle (nab-paclitaxel) in the absence of a solubilizer, uptake into the liver was dramatically decreased in Oatp1b2(−/−) mice (Fig 2A). This finding is consistent with the notion that immediately after infusion, the amorphous nab-paclitaxel nanoparticles rapidly dissolve into soluble albumin-paclitaxel complexes with a size similar to that of native albumin, with no nanoparticles detected at any time point post infusion (32). Therefore, phagocytosis-based uptake mechanisms involving the reticuloendothelial system that are relevant to the biodistribution of paclitaxel nanoparticles that remain stable in the circulation (33) are not contributing in the case of nab-paclitaxel.
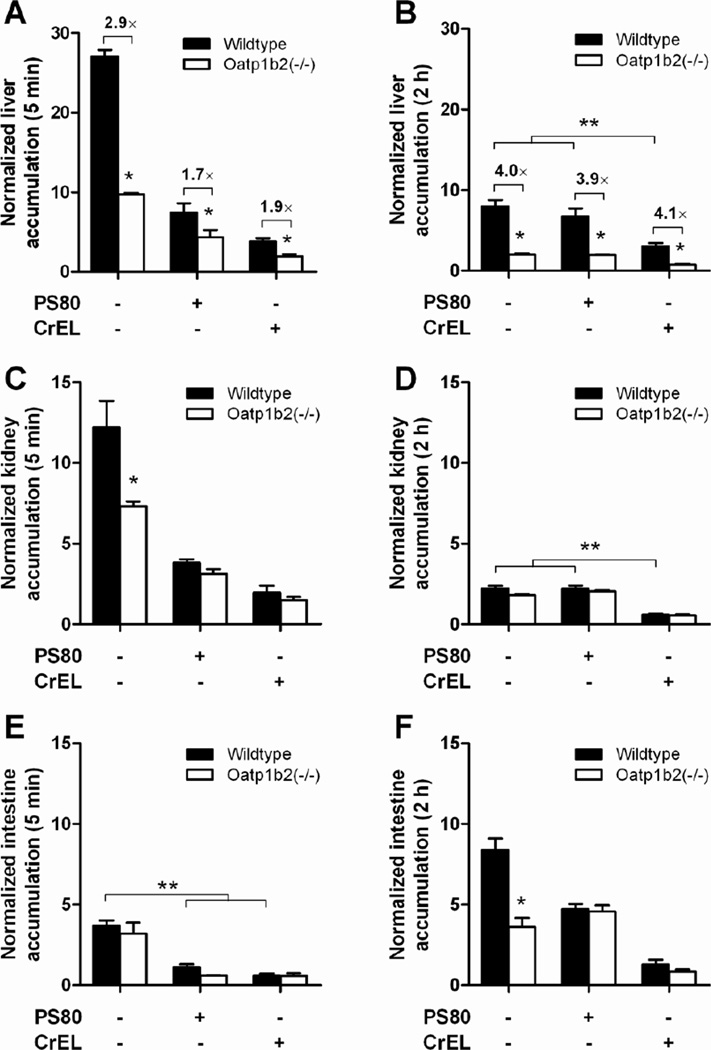
Figure 2.
Influence of paclitaxel formulation and Oatp1b2-deficiency on paclitaxel distribution. Paclitaxel was formulated as nab-paclitaxel in the absence of PS80 or CrEL, in PS80, or in CrEL (see Methods for details), and total paclitaxel levels were determined in wildtype and Oatp1b2(−/−) mice at 5 min and 2 hours after i.v. injection in liver (A–B), kidney (C–D), and intestine (E–F). Tissue levels were normalized to the corresponding plasma concentration (5 min data) or the AUC from time zero to 2 hours (2-hour data). Data are presented as the mean (bars) of 4 observations per condition per time point, along with SD (error bars). The star (*) indicates a statistically significant difference compared to the corresponding wildtype group (P < 0.05), and the double star (**) indicates a statistically significant difference compared to the other formulations (P < 0.05).
As predicted based on the in vitro inhibition data, the differences in uptake of paclitaxel into the liver between wildtype and Oatp1b2(−/−) mice were much less pronounced in the presence of PS80 or CrEL. In particular, the liver uptake in wildtype mice receiving paclitaxel formulated in PS80 was similar to that observed in Oatp1b2(−/−) mice receiving the drug without solubilizers, whereas uptake was further reduced by about 2-fold in the presence of CrEL (Fig 2A). Over time, the formulation-dependent differences in liver uptake normalized to control levels for the PS80 group (Fig 2B), but remains noticeably reduced in the presence of CrEL. This is consistent with the fact that PS80 is very rapidly cleared, with plasma levels becoming undetectable within 15 min after i.v. administration (34), whereas the half-life of CrEL amounts to >17 hours (6).
Interestingly, a similar solubilizer-, time-, and genotype-dependent distribution of paclitaxel was observed for uptake into kidney (Fig 2C–D) and intestine (Fig 2E–F). The significant reduction of paclitaxel administered without a solubilizer in the kidneys of Oatp1b2(−/−) mice at 5 min after drug administration is possibly the result of low expression of Oatp1b2 in renal cells of mice (35), and a similar phenotype has been reported previously for the Oatp1b2 substrate, hydroxyurea (36). The concentrations of paclitaxel in the intestine were not dependent on Oatp1b2 genotype at the early time point, where appearance of the drug likely reflects direct intestinal secretion (37). At the 2-hour time point, the higher levels in the intestine are presumably the result of hepatobiliary secretion becoming an increasingly dominant contributor to the elimination of paclitaxel.
Effects of formulation and transport on taxane clearance in vivo
As anticipated from the tissue distribution findings, the plasma concentration-time profiles of paclitaxel were inversely related to corresponding drug levels in liver, and concentrations in plasma were consistently higher by 5–7 fold for the CrEL-containing formulation compared with the other groups (Fig 3A–B). The notion that the slow clearance of paclitaxel administered in CrEL is due to a distribution defect rather than an event occurring in the terminal elimination phase is also consistent with the observed terminal half-lives of paclitaxel that were not significantly dependent on the formulation or genotype (Table 1).
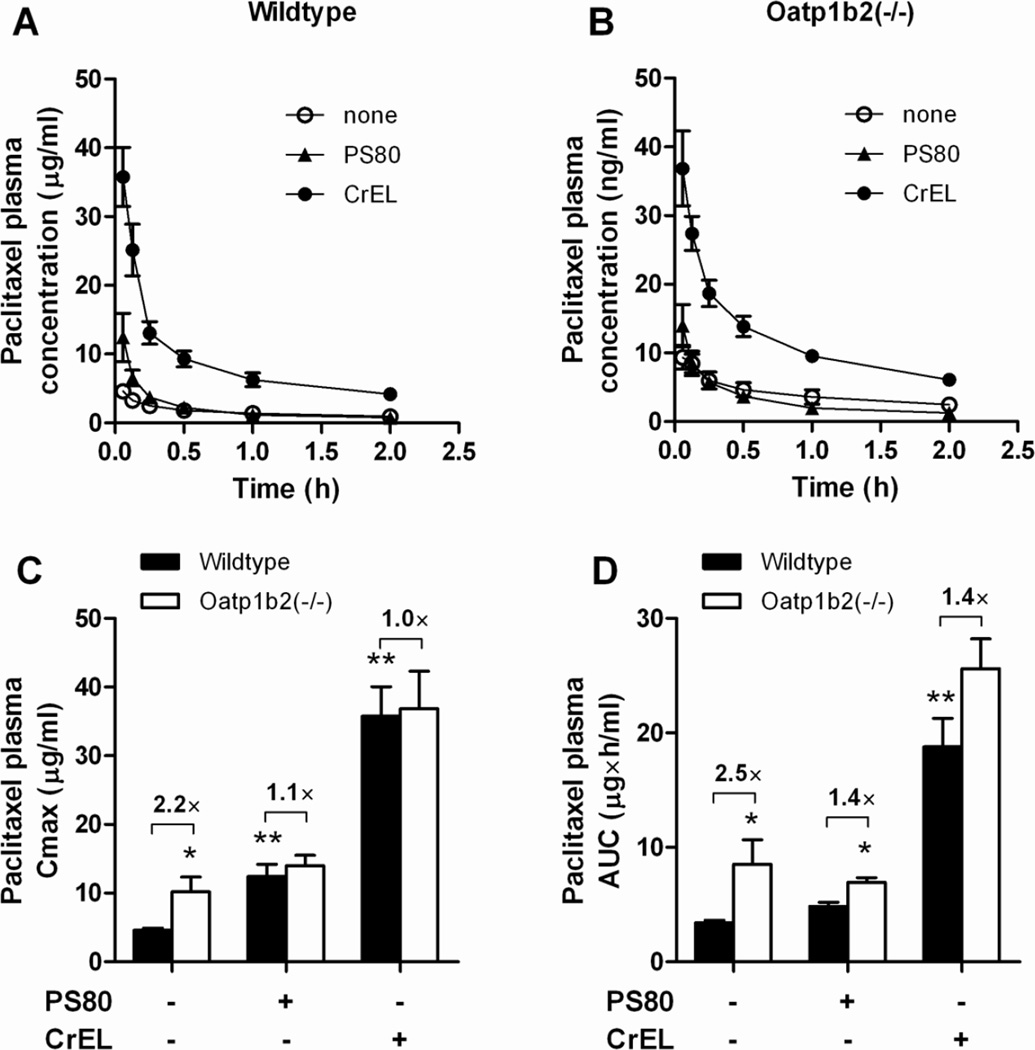
Figure 3.
Influence of formulation and Oatp1b2-deficiency on paclitaxel plasma pharmacokinetics. Paclitaxel was formulated as nab-paclitaxel in the absence of PS80 or CrEL, in PS80, or in CrEL (see Methods for details), and total paclitaxel levels were determined in wildtype (A) and Oatp1b2(−/−) mice (B) at in plasma samples taken at serial time points after dosing (5–120 min). The resulting concentration-time profiles were used to derived peak plasma concentrations (Cmax) (C) and area under the curve (AUC) (D). Data are presented as the mean (symbols or bars) of 4 observations per condition per time point, along with SD (error bars). The star (*) indicates a statistically significant difference compared to the corresponding wildtype group (P < 0.05), and the double star (**) indicates a statistically significant difference compared to the other formulations (P < 0.05).
Table 1.
Plasma pharmacokinetic parameters of paclitaxel (10 mg/kg) in mice using different formulations.
| Solubilizer | Mouse genotype |
AUC (µg×h/ml) |
CL (L/h/kg) |
T1/2 (h) |
Cmax (µg/ml) |
|---|---|---|---|---|---|
| None | Oatp1b2(−/−) | 8.51 ± 4.32 | 1.36 ± 0.493 | 1.64 ± 0.668 | 9.37 ± 3.46 |
| None | Wildtype | 3.44 ± 0.394 | 2.38 ± 1.26 | 1.70 ± 0.427 | 4.61 ± 0.608 |
| PS80 | Oatp1b2(−/−) | 6.93 ± 0.832 | 1.26 ± 0.454 | 1.06 ± 0.265 | 14.0 ± 3.12 |
| PS80 | Wildtype | 4.83 ± 0.761 | 1.94 ± 0.479 | 1.07 ± 0.230 | 12.4 ± 3.53 |
| CrEL | Oatp1b2(−/−) | 25.6 ± 5.23 | 0.406 ± 0.0962 | 1.39 ± 0.294 | 36.9 ± 10.9 |
| CrEL | Wildtype | 18.8 ± 4.90 | 0.565 ± 0.174 | 1.30 ± 0.365 | 35.8 ± 8.60 |
Abbreviations: AUC, area under the curve; CL, systemic clearance; T1/2, half life of the terminal phase; Cmax, peak plasma concentration
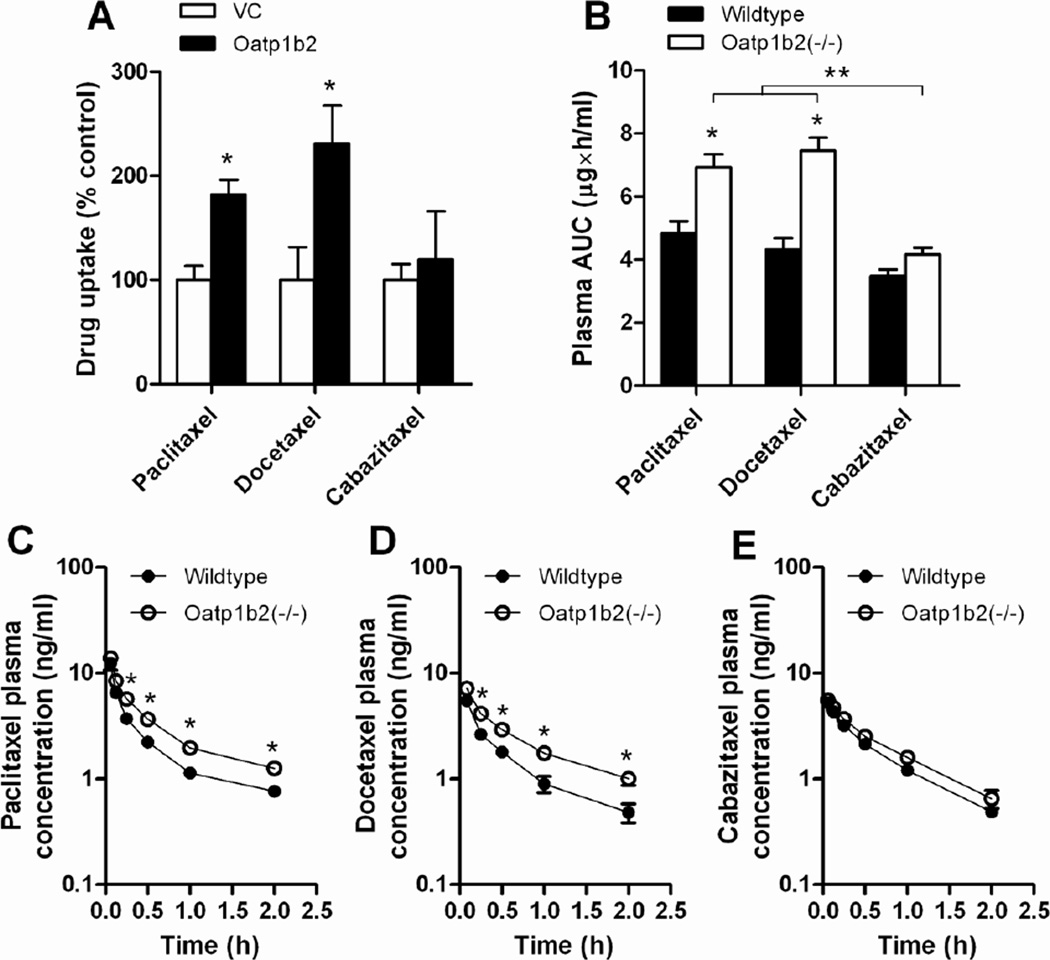
Without any solubilizer, the peak plasma concentration of paclitaxel was significantly increased in Oatp1b2(−/−) mice, and this genotype-dependence was nullified in the presence of PS80 or CrEL (Fig 3C). Based on total area under the curve (AUC), a significant but blunted influence of genotype was still noted for PS80-based formulation, but not for CrEL (Fig 3D). Since the same PS80 formulation is used in the clinical preparation of docetaxel and cabazitaxel, we also evaluated the comparative plasma pharmacokinetic properties of the two other approved taxanes in the same mouse model. Studies performed in transfected cells confirmed that, like paclitaxel, docetaxel is a transported substrate of Oatp1b2 (20), but this was not noted under the experimental conditions applied for cabazitaxel (Fig 4A). The lack of transport of cabazitaxel by Oatp1b2 is somewhat surprising considering its structural similarity with docetaxel, both having a 10-deacetylbaccatin III backbone (3), and because it has been reported to inhibit OATP1B1 and OATP1B3, albeit at relatively high concentrations (Jevtana prescribing information, see: http://products.sanofi.us/jevtana/jevtana.pdf). As expected based on the in vitro studies, the AUC (Fig 4B) and plasma levels at the time points evaluated were modestly but significantly increased in the absence of Oatp1b2 for paclitaxel (Fig 4C) and docetaxel (Fig 4D), but not for cabazitaxel (Fig 4E).
Figure 4.
Comparative effects of formulation and Oatp1b2-deficiency on transport of paclitaxel, docetaxel, and cabazitaxel. (A) The uptake of paclitaxel, docetaxel, and cabazitaxel by mouse Oatp1b2 transfected was evaluated in transfected HEK293 cells (0.1 µM; 15-min). Data are presented as the mean percentage of uptake values in the VC cells (bars) for 4–11 observations per condition, along with SD (error bars). The star (*) indicates a statistically significant difference compared to VC (P < 0.05). (B) Area under the curve (AUC) of paclitaxel in wildtype and Oatp1b2(−/−) mice following administration of paclitaxel, docetaxel, or cabazitaxel, all formulated in PS80 (see Methods for details), along with the corresponding plasma concentration-time profiles for paclitaxel (C), docetaxel (D), and cabazitaxel (E). Data are presented as the mean (bars or symbols) of 4 observations per condition per time point, along with SD (error bars). The star (*) indicates a statistically significant difference compared to the corresponding wildtype group (P < 0.05), and the double star (**) indicates a statistically significant difference compared to the other formulations (P < 0.05).
DISCUSSION
The current study add to a growing body of knowledge that solute carriers belonging to the OATP1B family can have a dramatic impact on the hepatocellular accumulation and systemic clearance of structurally diverse anticancer drugs. Employing an array of in vitro transport assays, including intracellular accumulation studies in multiple transfected model systems, paclitaxel was confirmed to be a high-affinity substrate for both OATP1B1 and OATP1B3. We found that the interaction of paclitaxel with OATP1B1 and OATP1B3 was strongly dependent on cell context, and this has obvious implications for future screening strategies aimed at identifying novel substrates for these transporters. The relatively low Km observed for paclitaxel transport by OATP1B1 suggests that this route of entry into hepatocytes may be saturated first, and this is consistent with results obtained in humanized transgenic mice indicating that OATP1B1 does not substantially contribute to paclitaxel transport in vivo (15). Indeed, the Km for OATP1B1 of 0.408 µM is substantially lower than the peak plasma concentration of unbound paclitaxel in patients receiving nab-paclitaxel at the recommended dose of 260 mg/m2 (on average, 1.50 µM), but much higher than that observed for paclitaxel in CrEL at 175 mg/m2 (on average, 0.143 µM) (38). This suggests that the contribution of OATP1B1 to the disposition of paclitaxel in patients is likely to be dependent on the prescribed product, in addition to the total dose and duration of infusion.
Our in vitro studies also confirmed that paclitaxel and docetaxel, but not cabazitaxel, are transported substrates of mouse Oatp1b2 and, for paclitaxel, rat Oatp1b2. The rodent Oatp1b2 transporters share more than 60% amino acid sequence homology to the two human isoforms, and on the basis of their shared basolateral localization in hepatocytes and overlapping substrate specificity (39), it is possible that, in the context of paclitaxel, the rodent Oatp1b2 fulfills the same function in the liver as OATP1B1 and OATP1B3 in humans. Based on this premise, we evaluated the pharmacokinetic properties of paclitaxel in a mouse model with a genetic deletion of Oatp1b2. One possible limitation of this model is that fact that, unlike in humans, mouse hepatocytes express multiple members of Oatp1a, a related subfamily of transporters that can potentially provide compensatory restoration of function when Oatp1b2 is lost (40). Despite this limitation, compared to wildtype mice, the systemic exposure to paclitaxel, administered without PS80 or CrEL, in the Oatp1b2(−/−) mice was increased by more than 4-fold. Our previously reported gene expression profiling and enzyme activity measurements in liver samples exclude alterations in alternate transport mechanisms or metabolic pathways as a likely cause of the delayed clearance phenotype in Oatp1b2(−/−) mice. Thus, these findings suggest that Oatp1b2-mediated transport of paclitaxel is an important process in the elimination of this drug in mice, depending on the solubilizer used for drug formulation.
We previously reported that the presence of PS80, the pharmaceutical vehicle used to solubilize docetaxel in clinical preparations, even in relatively low amounts, can completely nullify the genotype-dependent transport of docetaxel by OATP1B1 (20), and similar findings have been reported for CrEL, used in one of the clinical preparations of paclitaxel (19). Based on our current in vitro and subsequent confirmatory in vivo studies, it appears that the interaction of paclitaxel with Oatp1b2 is strongly diminished in the presence of PS80 and CrEL in a fashion that is consistent with the known disposition properties of these respective solubilizers. It is interesting to note that a previous study demonstrated that mice deficient in all Oatp1a and Oatp1b genes display a rather modest increase (<2-fold) in concentrations of paclitaxel in plasma following i.v. administration of a PS80-based formulation that is very similar to our present findings (17). These authors speculated that the lack of differences in plasma levels of paclitaxel early after its administration (up to 3.5-min) may be due to saturation of Oatp1a/Oatp1b-mediated liver uptake, and that this distribution process is predominantly dependent on other uptake mechanisms. Our current findings now provide an alternative explanation, where the initially high levels of PS80 in plasma can cause both temporary partitioning into a circulating PS80 microemulsion as well as directly inhibit the transporters required for hepatocellular uptake. These two mechanisms combined likely also explain the results obtained for paclitaxel in the presence of CrEL, although here the former mechanism clearly remains the dominant contributor to the overall disposition phenotypes.
The present observation that PS80 and CrEL can directly inhibit OATP1B-type transporters suggests that the pharmacokinetic profile of carrier-released (free) paclitaxel is not formulation-independent. This finding contrasts previously made assumptions (10), and may have important ramification for the proper interpretation of the clinical pharmacology of paclitaxel. Firstly, we previously found that several common, naturally-occurring genetic variants in OATP1B3 with impaired function were not associated with the pharmacokinetics of paclitaxel in a cohort of 90 cancer patients receiving the drug in a CrEL-based formulation (16). This somewhat unexpected observation is consistent with our current findings in that the interaction of paclitaxel with OATP1B3 may be masked by CrEL irrespective of an individual’s genotypic constitution. It also suggests that the impact of reduced function variants of OATP1B1 and/or OATP1B3 on the clearance of paclitaxel may be much more pronounced for CrEL-free formulations of the drug, such as nab-docetaxel.
Secondly, it can be postulated that intrinsic physiologic and environmental variables influencing OATP1B1- or OATP1B3-mediated uptake of paclitaxel into hepatocytes may have a more profound influence on drug clearance for formulations lacking solubilizers. For example, substrates of OATP1B1 and OATP1B3 for which the liver is the main organ of elimination are highly liable to drug interactions associated with these transporters (41, 42). Although formal drug interaction studies have not been performed to date with nab-paclitaxel, our present findings strongly suggest that drug-drug interactions at the level of hepatocellular uptake mechanisms would be exacerbated with a formulation like nab-paclitaxel. Conversely, CrEL may act as a perpetrator in known pharmacokinetic interactions involving other OATP1B substrates co-administered with paclitaxel [reviewed in (4)], such as etoposide (43), docetaxel (44), oxaliplatin (45), and SN-38 (46).
Thirdly, OATP1B1 is expressed as relatively high levels in tumors of the colon, endometrium, esophagus, lung, prostate, stomach, testis, and bladder (45), and both OATP1B1 and OATP1B3 contribute to the in vitro cytotoxicity of paclitaxel in ovarian cancer cells (29). Although systemic exposure to free paclitaxel is believed to be the dominant driver of drug-induced cytotoxicity at tumor sites (10), it is conceivable that these transporters can contribute directly to tumoral uptake and that this process can be inhibited by solubilizers such as PS80 and CrEL. This possibility would be consistent with available clinical data on the comparative efficacy of the various paclitaxel formulations [reviewed in (10)], and with preclinical findings suggesting that (i) the absorption rate constant of paclitaxel uptake into tumors is dramatically decreased in the presence of CrEL compared to nab-paclitaxel (32), and (ii) a tumor-delivery mechanism exists for nab-paclitaxel that is independent of SPARC (47), a matricellular glycoprotein produced by tumors and/or neighboring stroma that facilitates the intracellular accumulation of intact albumin nanoparticles (48).
Collectively, our findings demonstrate the importance of OATP1B-type solute carriers in the hepatic elimination of paclitaxel, and that solubilizers used in clinical preparations of this agents can inhibit this process in a time-dependent fashion. These results offer a mechanistic basis for previously reported interrelationships of taxane disposition with PS80 and CEL, and suggest that the likelihood of drug-drug interactions mediated by these transporters is strongly dependent on the selected paclitaxel formulation.
Supplementary Material
Translational Relevance.
Taxane-based antineoplastic agents have been widely used for the treatment of multiple solid tumors for over two decades, but elemental information on how these agents are eliminated remains incompletely understood. We hypothesized that the pharmacokinetic profiles of the taxanes paclitaxel, docetaxel and cabazitaxel are dependent on a process that is initiated by hepatocellular uptake by organic anion transporting polypeptides (OATPs) in a manner that depends on the presence or absence of polymeric solubilizers such as Kolliphor EL (formerly Cremophor EL; used for paclitaxel) or polysorbate 80 (used for docetaxel and cabazitaxel). Using an array of in vitro and in vivo model systems, we found that the clearance of taxanes is strongly dependent on OATP-mediated hepatic uptake, and that solubilizers used in clinical preparations can inhibit this process in a time-dependent fashion. These results offer a mechanistic basis for previously reported interrelationships of taxane disposition with Kolliphor EL and polysorbate 80, and suggest that the likelihood of drug-drug interactions mediated via OATPs is strongly dependent on the selected formulation of these drugs.
Acknowledgments
We would like to thank Richard Kim and Jeffrey Stock for providing the Oatp1b2 (−/−) mice, and Cynthia Lancaster, Aksana Vasilyeva, Aisha Walker, and Eric Zimmerman for their assistance with performing the animal experiments.
Acknowledgment of research support: This study was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), USPHS Cancer Center Support Grant P30CA021765 and by an Academic Internship Grant from the Dutch Cancer Society.
Footnotes
Disclosure of Potential Conflict of Interest
The authors declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- 1.Zhang Z, Mei L, Feng SS. Paclitaxel drug delivery systems. Expert Opin Drug Deliv. 2013;10:325–340. doi: 10.1517/17425247.2013.752354. [DOI] [PubMed] [Google Scholar]
- 2.Tan Q, Liu X, Fu X, Li Q, Dou J, Zhai G. Current development in nanoformulations of docetaxel. Expert Opin Drug Deliv. 2012;9:975–990. doi: 10.1517/17425247.2012.696606. [DOI] [PubMed] [Google Scholar]
- 3.Vrignaud P, Semiond D, Lejeune P, Bouchard H, Calvet L, Combeau C, et al. Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2973–2983. doi: 10.1158/1078-0432.CCR-12-3146. [DOI] [PubMed] [Google Scholar]
- 4.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles : implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17:735–749. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 6.Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996;56:2112–2115. [PubMed] [Google Scholar]
- 7.van Zuylen L, Karlsson MO, Verweij J, Brouwer E, de Bruijn P, Nooter K, et al. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol. 2001;47:309–318. doi: 10.1007/s002800000215. [DOI] [PubMed] [Google Scholar]
- 8.Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–1457. [PubMed] [Google Scholar]
- 9.van Tellingen O, Huizing MT, Panday VR, Schellens JH, Nooijen WJ, Beijnen JH. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br J Cancer. 1999;81:330–335. doi: 10.1038/sj.bjc.6690696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ait-Oudhia S, Straubinger RM, Mager DE. Meta-analysis of nanoparticulate paclitaxel delivery system pharmacokinetics and model prediction of associated neutropenia. Pharm Res. 2012;29:2833–2844. doi: 10.1007/s11095-012-0775-8. [DOI] [PubMed] [Google Scholar]
- 11.Henningsson A, Karlsson MO, Vigano L, Gianni L, Verweij J, Sparreboom A. Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol. 2001;19:4065–4073. doi: 10.1200/JCO.2001.19.20.4065. [DOI] [PubMed] [Google Scholar]
- 12.Rochat B. Role of cytochrome P450 activity in the fate of anticancer agents and in drug resistance: focus on tamoxifen, paclitaxel and imatinib metabolism. Clin Pharmacokinet. 2005;44:349–366. doi: 10.2165/00003088-200544040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ellis AG, Webster LK. Inhibition of paclitaxel elimination in the isolated perfused rat liver by Cremophor EL. Cancer Chemother Pharmacol. 1999;43:13–18. doi: 10.1007/s002800050857. [DOI] [PubMed] [Google Scholar]
- 14.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 15.van de Steeg E, van Esch A, Wagenaar E, Kenworthy KE, Schinkel AH. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin Cancer Res. 2013;19:821–832. doi: 10.1158/1078-0432.CCR-12-2080. [DOI] [PubMed] [Google Scholar]
- 16.Smith NF, Marsh S, Scott-Horton TJ, Hamada A, Mielke S, Mross K, et al. Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin Pharmacol Ther. 2007;81:76–82. doi: 10.1038/sj.clpt.6100011. [DOI] [PubMed] [Google Scholar]
- 17.van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, et al. High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res. 2011;17:294–301. doi: 10.1158/1078-0432.CCR-10-1980. [DOI] [PubMed] [Google Scholar]
- 18.Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 19.Engel A, Oswald S, Siegmund W, Keiser M. Pharmaceutical excipients influence the function of human uptake transporting proteins. Mol Pharm. 2012;9:2577–2581. doi: 10.1021/mp3001815. [DOI] [PubMed] [Google Scholar]
- 20.de Graan AJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, et al. Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res. 2012;18:4433–4440. doi: 10.1158/1078-0432.CCR-12-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruijn P, Moghaddam-Helmantel IM, Loos WJ, Mathijssen RH, Wiemer EA. The issue of non-specific binding of cabazitaxel. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;932:74–75. doi: 10.1016/j.jchromb.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Engels FK, Mathot RA, Loos WJ, van Schaik RH, Verweij J. Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther. 2006;5:833–839. doi: 10.4161/cbt.5.7.2839. [DOI] [PubMed] [Google Scholar]
- 24.Kaliss N, Pressman D. Plasma and blood volumes of mouse organs, as determined with radioactive iodoproteins. Proc Soc Exp Biol Med. 1950;75:16–20. doi: 10.3181/00379727-75-18083. [DOI] [PubMed] [Google Scholar]
- 25.Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gui C, Wahlgren B, Lushington GH, Hagenbuch B. Identification, Ki determination and CoMFA analysis of nuclear receptor ligands as competitive inhibitors of OATP1B1-mediated estradiol-17beta-glucuronide transport. Pharmacol Res. 2009;60:50–56. doi: 10.1016/j.phrs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, et al. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260:163–169. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, et al. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother. 2011;65:417–426. doi: 10.1016/j.biopha.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Takano M, Otani Y, Tanda M, Kawami M, Nagai J, Yumoto R. Paclitaxel-resistance conferred by altered expression of efflux and influx transporters for paclitaxel in the human hepatoma cell line, HepG2. Drug Metab Pharmacokinet. 2009;24:418–427. doi: 10.2133/dmpk.24.418. [DOI] [PubMed] [Google Scholar]
- 31.Tanino T, Nawa A, Nakao M, Noda M, Fujiwara S, Iwaki M. Organic anion transporting polypeptide 2-mediated uptake of paclitaxel and 2'-ethylcarbonate-linked paclitaxel in freshly isolated rat hepatocytes. J Pharm Pharmacol. 2009;61:1029–1035. doi: 10.1211/jpp/61.08.0006. [DOI] [PubMed] [Google Scholar]
- 32.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 33.Yeh TK, Lu Z, Wientjes MG, Au JL. Formulating paclitaxel in nanoparticles alters its disposition. Pharm Res. 2005;22:867–874. doi: 10.1007/s11095-005-4581-4. [DOI] [PubMed] [Google Scholar]
- 34.van Tellingen O, Beijnen JH, Verweij J, Scherrenburg EJ, Nooijen WJ, Sparreboom A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5:2918–2924. [PubMed] [Google Scholar]
- 35.Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, et al. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–329. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker AL, Lancaster CS, Finkelstein D, Ware RE, Sparreboom A. Organic anion transporting polypeptide 1B (OATP1B) transporters modulate hydroxyurea pharmacokinetics. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci. 2012;33:100–108. doi: 10.1016/j.tips.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Muller F, Fromm MF. Transporter-mediated drug-drug interactions. Pharmacogenomics. 2011;12:1017–1037. doi: 10.2217/pgs.11.44. [DOI] [PubMed] [Google Scholar]
- 42.Zolk O, Fromm MF. Transporter-mediated drug uptake and efflux: important determinants of adverse drug reactions. Clin Pharmacol Ther. 2011;89:798–805. doi: 10.1038/clpt.2010.354. [DOI] [PubMed] [Google Scholar]
- 43.Fahrmayr C, Konig J, Auge D, Mieth M, Fromm MF. Identification of drugs and drug metabolites as substrates of multidrug resistance protein 2 (MRP2) using triple-transfected MDCK-OATP1B1-UGT1A1-MRP2 cells. Br J Pharmacol. 2012;165:1836–1847. doi: 10.1111/j.1476-5381.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo MA, Garcia M, Ponton JL, Martinez M, Valenti V, Navarro M, et al. A phase I clinical and pharmacokinetic study of paclitaxel and docetaxel given in combination in patients with solid tumours. Eur J Cancer. 2006;42:1789–1796. doi: 10.1016/j.ejca.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther. 2013;12:1537–1544. doi: 10.1158/1535-7163.MCT-12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita KI, Sugiura T, Okumura H, Umeda S, Nakamichi N, Watanabe Y, et al. Direct Inhibition and Down-regulation by Uremic Plasma Components of Hepatic Uptake Transporter for SN-38, an Active Metabolite of Irinotecan, in Humans. Pharm Res. 2013 doi: 10.1007/s11095-013-1153-x. [DOI] [PubMed] [Google Scholar]
- 47.Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2013 doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170:365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.