Abstract
Purpose: It is well known that implanting a bioactive scaffold into a cartilage defect site can enhance cartilage repair after bone marrow stimulation (BMS). However, most of the current scaffolds are derived from xenogenous tissue and/or artificial polymers. The implantation of these scaffolds adds risks of pathogen transmission, undesirable inflammation, and other immunological reactions, as well as ethical issues in clinical practice. The current study was undertaken to evaluate the effectiveness of implanting autologous bone marrow mesenchymal stem cell–derived extracellular matrix (aBMSC-dECM) scaffolds after BMS for cartilage repair.
Methods: Full osteochondral defects were performed on the trochlear groove of both knees in 24 rabbits. One group underwent BMS only in the right knee (the BMS group), and the other group was treated by implantation of the aBMSC-dECM scaffold after BMS in the left knee (the aBMSC-dECM scaffold group).
Results: Better repair of cartilage defects was observed in the aBMSC-dECM scaffold group than in the BMS group according to gross observation, histological assessments, immunohistochemistry, and chemical assay. The glycosaminoglycan and DNA content, the distribution of proteoglycan, and the distribution and arrangement of type II and I collagen fibers in the repaired tissue in the aBMSC-dECM scaffold group at 12 weeks after surgery were similar to that surrounding normal hyaline cartilage.
Conclusions: Implanting aBMSC-dECM scaffolds can enhance the therapeutic effect of BMS on articular cartilage repair, and this combination treatment is a potential method for successful articular cartilage repair.
Introduction
Cartilage defects are one of the most common causes of arthritis and are present in ∼60% of patients who undergo knee arthroscopy, a procedure that has been widely performed in the world.1,2 Due to the acellular and avascular nature of mature cartilage tissue, cartilage defects have very limited self-healing capacity. Therefore, the treatment of articular cartilage injury is of major interest in clinical practice.3,4
It is well known that bone marrow stimulation (BMS) techniques are generally performed as the first-line and standard treatment for cartilage repair, as BMS techniques are considered to offer an easy, rapid, and relatively economical way to restore the damaged cartilage.5 BMS techniques, such as drilling, abrasion, and microfracture, are adopted to allow the migration of endogenous bone marrow mesenchymal cells (BMSCs) into the damaged area, thereby stimulating cartilage repair.6 Although various studies have shown that BMS techniques can be helpful in improving short-term clinical symptoms, longer follow-up studies have shown that the symptoms worsen over time and patients experience consistent functional decline.7 Cartilage defects treated by BMS techniques are repaired with only fibrous tissue or fibrocartilage, which are often unstable and have a low mechanical stress resistance.7 One of the most probable reasons is the limited number of BMSCs available due to the loss and dilution of bone marrow caused by the synovial fluid.8–10
To address this problem, an autologous matrix-induced chondrogenesis (AMIC) technique was developed to enhance the clinical outcomes of BMS techniques.11 In the original AMIC technique, a type I/III collagen scaffold was implanted into a cartilage defect after the BMS technique. Improved long-term outcomes after use of the AMIC technique have been previously noted by many researchers.12 The results may be attributable to the implantation of a scaffold to host endogenous BMSCs, which helps to stabilize the blood clot and to prevent the blood clot from leaking into the joint fluid. Furthermore, a bioactive scaffold can improve local cell proliferation, differentiation, and matrix production, guiding the tissue toward a more hyaline-like histological appearance.13 Several scaffolds have been investigated for this role, such as type I/III collagen scaffolds (Chondro-Gide®; Geistlich Biomaterials),14 Chitosan (Piramal Healthcare, Inc.),15 Chondrotissue (Bio Tissue AG),16 and Gelrin C (Regentis Biomaterials).17 It is worth noting that most of the current scaffolds are derived from xenogenous tissue. The implantation of these scaffolds adds risks of pathogen transmission, undesirable inflammation, and other immunological reactions, as well as ethical issues in clinical practice.18,19 It is widely reported that autologous scaffolds can effectively overcome this disadvantage.20,21 However, to our knowledge, few studies have investigated the use of autologous biomaterials combined with a BMS technique for cartilage repair.
Recently, we successfully developed a novel autologous bone marrow mesenchymal stem cell–derived extracellular matrix (aBMSC-dECM) scaffold, which can provide a favorable environment for chondrocytes and promote hyaline cartilage-like tissue regeneration.22 Our current study attempts to assess the feasibility of implanting this scaffold into cartilage defect sites after BMS and further focuses on investigating the therapeutic effectiveness of this technique in a rabbit model.
Materials and Methods
The use of animals in this experiment was approved by the Institutional Animal Experiment Committee of Nanjing Medical University, and animals were treated according to the US National Institutes of Health guidelines. Twenty-four New Zealand white rabbits with an average weight of 3.0–3.5 kg and more than 6 months old were used in this study. All animals underwent a veterinary examination to evaluate general health status. All experimental procedures, including bone marrow aspiration, limb preparation, and surgery, were performed under general anesthesia with a mixture (1 mL/kg body weight) of ketamine and xylazine (ratio 3.5:1.5).
Preparation of aBMSC-dECM scaffolds
The aBMSC-dECM scaffolds were prepared from autologous BMSCs as described in our previous study.22 In brief, the autologous BMSCs from iliac crest were first expanded in monolayer culture. Once the primary BMSCs reached 70–80% confluence, 50 μg/mL L-ascorbic acid (Sigma) was added into the culture medium to stimulate ECM deposition. After 4 weeks, the ECM membrane was separated carefully and freeze-dried (Sihuan) at −70°C under 1 Pa for 48 h. The freeze-dried specimen was crosslinked by 50 mM 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) and 50 mM N-hydroxysuccinimide (NHS) to improve mechanical strength, and freeze-dried again. The final form of an aBMSC-dECM scaffold was obtained by cutting with a biopsy punch (5 mm in diameter) and trimming off the surface layer by ∼2 mm in thickness with a clean razor blade.
The scaffolds were sterilized with ethylene oxide gas before implantation. We previously showed that the scaffolds exhibit uniform porosity and a highly interconnected structure, with a pore size of 304.4±108.2 μm, a porosity of 93.3%±4.5%, and a compressive modulus of 6.8±1.5 kPa.
Surgical treatment
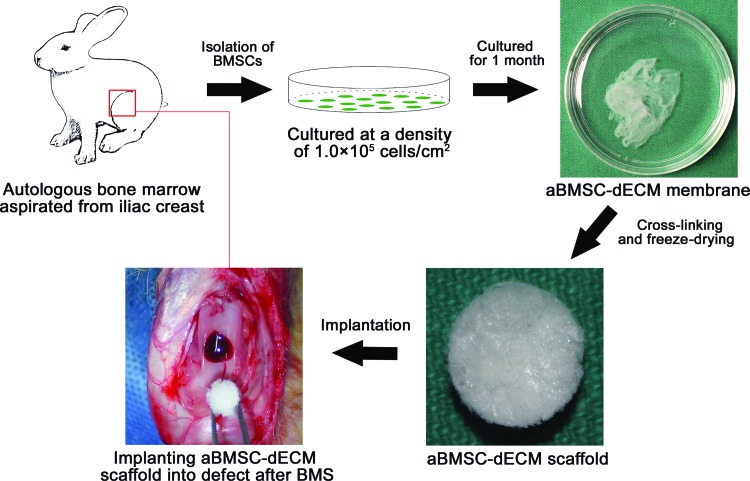
Both knee joints of each rabbit were operated on in this study. An arthrotomy was performed through a midline longitudinal incision on a medial parapatellar. The patella was dislocated laterally to expose the articular capsule. A full-thickness cylindrical osteochondral defect (2 mm in depth and 5 mm in diameter) was created in the trochlear groove using a 5-mm drill. After making the osteochondral defect, the defect of the left knee was treated with BMS using an 18-gauge needle, followed by implantation of the aBMSC-dECM scaffold by pressfitting (the aBMSC-dECM scaffold group, Fig. 1). The defect of the right knee was treated only with BMS as described above (the BMS group). In each group, 12 rabbits were sacrificed at 6 and 12 weeks after surgery for further analysis.
FIG. 1.
Procedure for preparation and implantation of autologous bone marrow mesenchymal stem cell–derived extracellular matrix (aBMSC-dECM) scaffolds. Bone marrow was aspirated from the iliac crest. Then, the isolated mononuclear cells were cultured at high density for 1 month. The aBMSC-dECM membrane was collected from the bottom of the culture plate. The aBMSC-dECM scaffold was fabricated by crosslinking and freeze-drying, and then implanted into a cartilage defect after bone marrow stimulation (BMS). Color images available online at www.liebertpub.com/tea
Macroscopic and histological evaluation
Distal femurs of both knees were harvested after removing all periarticular soft tissues. Articular cartilage samples were imaged with a high-resolution camera (Canon) to reveal surface fissures and defects.23
For histological evaluation, the samples (n=6 in each group) were fixed with 4% formaldehyde for 24 h, dehydrated with 5% nitric acid for 48 h, embedded in paraffin, and then sectioned at 4 μm. The sections were then stained with Safranin O, Masson's Trichrome, or Sirius Red staining, respectively.
Immunohistochemical staining
Type II collagen and type I collagen were determined by immunohistochemistry using the avidin–biotin–peroxidase complex technique with the mouse monoclonal anti-rabbit type II collagen antibody (Acris) and mouse monoclonal anti-rabbit type I collagen antibody (Sigma-Aldrich), as described previously.22 Briefly, the sections were treated sequentially with 3% H2O2 and 50 μg/mL proteinase K and incubated with the primary antibodies (1:100) for 90 min at room temperature. As a negative control, preimmune serum was substituted for the primary antibody. Sections were then incubated sequentially with a biotinylated secondary antibody against mouse IgG (Maixin) at a 1:200 density for 10 min and peroxidase-conjugated streptavidin solution (Maixin) for 10 min at room temperature. Sections were finally counterstained with hematoxylin and mounted for microscopic observation (Olympus).24
Histological score
The quality of the repaired articular cartilage in the defect was evaluated with a modified version of the International Cartilage Research Society (ICRS) grading scale consisting of seven categories and score assignments ranging from 0 to 18 points, as described previously.23 Three researchers independently evaluated each sample to minimize the effects of subjective bias.
Chemical measurement
For chemical assay, repaired tissue samples were harvested from the defect by a surgical knife and curette. All samples were dried at 37°C for 48 h and then digested with a papain solution (5 mM L-cysteine, 100 mM Na2HPO4, 5 mM EDTA, 125 μg/mL papain, and pH 6.4) at 60°C for 24 h, followed by centrifugation at 12,000 g for 10 min. The supernatant was used for assays.25 The surrounding native hyaline cartilage was used as a positive control.
Total DNA content was determined using the Quit-iT dsDNA kit (Invitrogen) using salmon testes DNA (Sigma) to generate a standard curve. Briefly, the papain-digested solution and standard solution were reacted with the Hoechst dye 33258 (0.25 μL/mL) for 30 min in the dark. Fluorescence intensity was measured with a 96-well plate reader (excitation at 360 nm and emission at 460 nm, Perkin-Elmer LS-55).
The glycosaminoglycan (GAG) content was measured by the dimethylmethylene blue (DMMB) colorimetric assay using chondroitin sulfate from shark cartilage (Sigma) to generate a standard curve. Briefly, the papain-digested solution and standard solution were mixed with the DMMB solution to bind GAG for 30 min at room temperature. The GAG-dye complex was collected by centrifugation at 12,000 g for 10 min. Absorbance at 530 nm was measured using a Benchmark plus microplate spectrophotometer (Bio-Rad).
Statistical analysis
All data are expressed as the mean±standard deviation (SD). The ICRS histological score differences for repaired cartilage between the BMS group and the aBMSC-dECM scaffold group were compared by the Wilcoxon signed-rank test. The DNA content and GAG content of repaired cartilage at 6 and 12 weeks within each group was compared by Student's t test. The ICRS histological score at 6 and 12 weeks in each group was compared by the Mann–Whitney U test. The differences of DNA content and GAG content among the BMS group, the aBMSC-dECM scaffold group, and the normal cartilage group at each time point were analyzed by one-way analysis of variance followed by post hoc testing (Student–Newman–Keuls). The p-value was two sided, and values less than 0.05 were considered statistically significant. All statistical analyses were carried out using SPSS 13.0 (SPSS, Inc.).
Results
Gross observation
At 6 weeks after surgery, a macroscopic evaluation showed that repaired cartilage tissue partially covered the defect site in both the BMS group (Fig. 2A) and the aBMSC-dECM scaffold group (Fig. 2B). The tissue appeared whitish and was visibly distinguishable from the surrounding native hyaline cartilage. At 12 weeks, the repaired tissue was replaced with white glistening tissues in both groups. However, in the aBMSC-dECM scaffold group (Fig. 2D), the area of the repaired tissue was wider and the repaired tissue showed better integration with the surrounding normal articular cartilage.
FIG. 2.
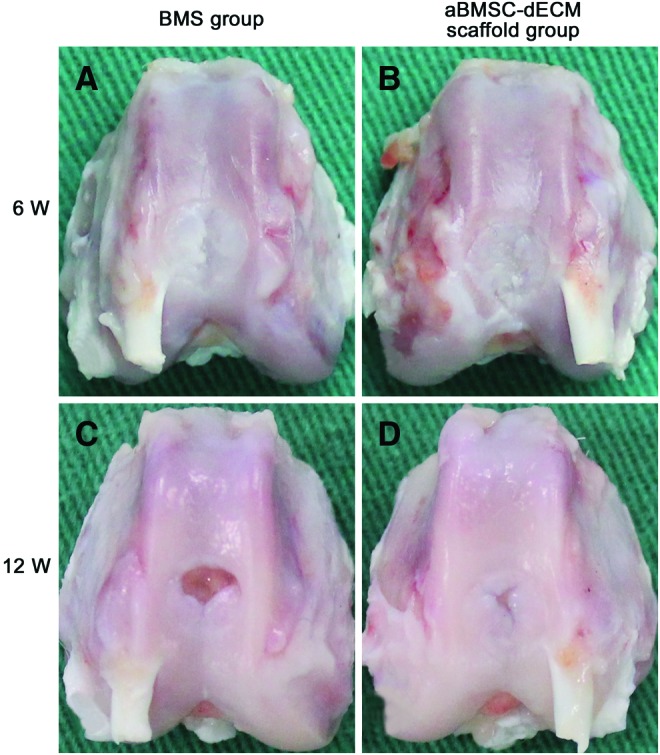
Macroscopic evaluation of the repaired cartilage tissue in both the BMS group and the aBMSC-dECM scaffold group at 6 and 12 weeks after surgery. At 6 weeks, the repaired tissue of the BMS group (A) and the aBMSC-dECM scaffold group (B) partially covered the cartilage defect. At 12 weeks, compared with the BMS group (C), the area of repaired tissue in the aBMSC-dECM scaffold group (D) was wider. Color images available online at www.liebertpub.com/tea
Histological evaluation
The Safranin-O and Masson's Trichrome staining showed that all defects were mostly filled with fibrous tissue in the BMS group (Fig. 3A–C) and the aBMSC-dECM scaffold group (Fig. 3D–F) at 6 weeks after surgery. Most repaired tissue exhibited metachromatic staining in the defect site, but its surface was rough and cells generally lacked orientation. None of the groups showed subchondral bone remodeling. At 12 weeks, the repaired tissues did not exhibit metachromatic staining at the defect site in the BMS group (Fig. 3G–I), whereas most repaired tissues show pale, metachromatic stains in the aBMSC-dECM scaffold group (Fig. 3J–L). The strength of metachromatic staining in the aBMSC-dECM scaffold group resembled that in normal cartilage, and chondrocytes formed mature lacunas and were perpendicularly oriented to the subchondral bone. In addition, the newly formed tissue also formed compact bone and osteoid matrix in most zones of defect sites in the aBMSC-dECM scaffold group.
FIG. 3.
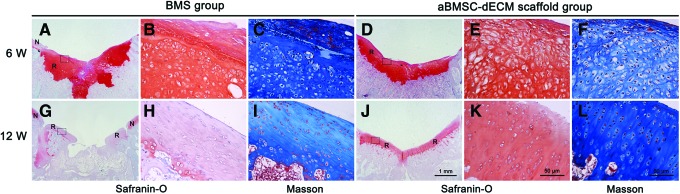
Safranin-O (A, B, D, E, G, H, J, K) and Masson's Trichrome (C, F, I, L) staining of the repaired cartilage tissue in both the BMS group and the aBMSC-dECM scaffold group at 6 and 12 weeks after surgery. All defects were filled mostly with nonhyaline cartilage tissue in the BMS group (A–C) and the aBMSC-dECM scaffold group (D–F) at 6 weeks. However, after 12 weeks, the strength of metachromatic staining in the aBMSC-dECM scaffold group (J–L) resembled that of normal cartilage, and chondrocytes formed mature lacunas and were perpendicularly aligned with subchondral bone. A, D, G, and J:×10, B, C, E, F, H, I, K, and L:×400. N, native cartilage; R, repaired cartilage. Color images available online at www.liebertpub.com/tea
The distribution and arrangement of collagen fibers were observed by Sirius Red staining. At 6 weeks, only small amounts of collagen fibers and no oriented pattern could be found in the BMS group (Fig. 4C, I) or in the aBMSC-dECM scaffold group (Fig. 4F, L). However, at 12 weeks, the amount of collagen fibers was increased in each group. Collagen fibers in the aBMSC-dECM scaffold group (Fig. 4R, X) were distributed more evenly and were well aligned vertically, similar to normal cartilage, whereas the collagen fibers in the BMS group were distributed irregularly and randomly in various directions (Fig. 4O, U).
FIG. 4.
The distribution and arrangement of collagen fibers were observed by immunohistochemical analysis of type II and I collagen, and Sirius red staining. At 6 weeks after surgery, no organized orientation pattern could be found in either the BMS group (A–C, G–I) or the aBMSC-dECM scaffold group (D–F, J–L). However, at 12 weeks, the distribution and arrangement of collagen fibers in the aBMSC-dECM scaffold group (P–R, V–X) were distributed more evenly and vertically aligned similar to normal cartilage. A–F and M–R:×100; G–L and S–X:×400. N, native cartilage; R, repaired cartilage. Color images available online at www.liebertpub.com/tea
ICRS histological scoring
The ICRS histological scores are illustrated in Table 1. The score gradually increased with time in the aBMSC-dECM scaffold group. The score in the aBMSC-dECM scaffold group was significantly higher than that in the BMS group at 6 and 12 weeks after surgery (p=0.041 and p=0.027, respectively).
Table 1.
Result of International Cartilage Research Society Score at 6 and 12 Weeks After Surgery
| Time point | BMS group | aBMSC-dECM scaffold group | p-Value |
|---|---|---|---|
| 6 W | 5.0±2.1 | 8.7±2.1 | 0.041 |
| 12 W | 5.5±2.7 | 9.7±2.3 | 0.027 |
aBMSC-dECM, autologous bone marrow mesenchymal stem cell–derived extracellular matrix; BMS, bone marrow stimulation.
Expression of type II collagen and type I collagen
At 6 weeks after surgery, type II collagen was diffusely distributed in the pericellular area in the repaired tissue of the BMS group (Fig. 4A, G) and the aBMSC-dECM scaffold group (Fig. 4D, J). Over time, the expression of type II collagen was observed in zonal structures and converged in the pericellular area along with chondrocytes in the aBMSC-dECM scaffold group (Fig. 4P, V), but not in the BMS group (Fig. 4M, S).
The expression of type I collagen was mainly observed at the pericellular area in the surface of repaired tissue in both the BMS group (Fig. 4B, H) and the aBMSC-dECM scaffold group (Fig. 4E, K) at 6 weeks after surgery. At 12 weeks, expression of type I collagen was enriched in the whole defect of the BMS group (Fig. 4N, T), which might explain the overall reduction of type II collagen expression. In contrast, the aBMSC-dECM scaffold group (Fig. 4Q, W) showed relatively weak expression of type I collagen in the peripheral area.
Chemical assay of repaired cartilage
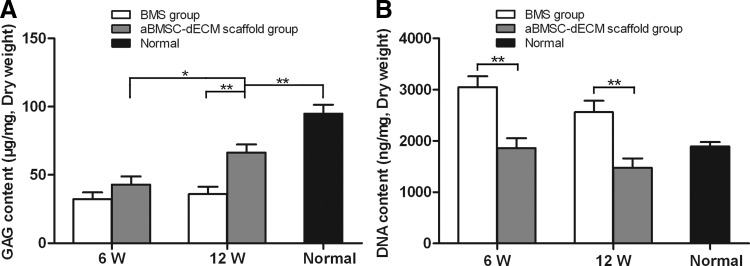
The GAG contents of the repaired tissues were 43±14.4 μg/mg and 66.5±14.3 μg/mg in the aBMSC-dECM scaffold group and 32.3±12.0 μg/mg and 36±13.1 μg/mg in the BMS group at 6 and 12 weeks after surgery, respectively. The GAG contents in the aBMSC-dECM scaffold group rapidly increased with time (p=0.018). Twelve weeks after surgery, the GAG contents of the repaired tissue in the aBMSC-dECM scaffold group reached 70% of normal hyaline cartilage (94.9±16.1 μg/mg) (p<0.05) and were significantly higher compared with the BMS group (p<0.05) (Fig. 5A).
FIG. 5.
The glycosaminoglycan (GAG) (A) and DNA (B) contents of repaired cartilage tissue in both the BMS group and the aBMSC-dECM scaffold group. The GAG contents in the aBMSC-dECM scaffold group rapidly increased with time. At 12 weeks after surgery, the GAG content of repaired tissue in the aBMSC-dECM scaffold group reached 70% that of normal hyaline cartilage and was significantly higher than that in the BMS group. The DNA content in the aBMSC-dECM scaffold group was similar to that of normal hyaline cartilage at 6 and 12 weeks after surgery. Results are presented as mean±SD. *p<0.05, **p<0.01.
The DNA contents in the aBMSC-dECM scaffold group (1861.5±474.2 and 1473.9±452.8 ng/mg) were lower than those of the BMS group (3053.8±512 and 2562.5±548.7 ng/mg) at 6 and 12 weeks after surgery (p<0.05 and p<0.05, respectively). On the other hand, the repaired tissues of the aBMSC-dECM scaffold group at 6 and 12 weeks did not appear significantly different from normal hyaline cartilage (p>0.05 and p>0.05, respectively) (Fig. 5B).
Discussion
In the present study, aBMSC-dECM scaffolds were implanted into defect sites for cartilage repair after BMS. The results showed that implanting an aBMSC-dECM scaffold can effectively improve the outcome of a BMS technique for cartilage defect repair. The repaired tissue within the cartilage defect sites was better remodeled in the aBMSC-dECM scaffold group than in the BMS group.
It has generally been recognized that the AMIC technique can enhance the cartilage repair potential of a BMS technique. Erggelet et al. used a polyglycolic acid scaffold, hyaluronan, and autologous serum to cover full-thickness ovine cartilage defects after microfracture. The implantation of the scaffold induced cartilaginous repair tissue rich in proteoglycan and type II collagen.26 Li et al. used a porcine chondrocyte-derived extracellular matrix membrane to protect blood clots after BMS. The results showed that the treatment could generate more hyaline-like cartilage according to histology and chemical analyses and achieve a higher ICRS score than BMS alone.27 Moreover, compared with the BMS technique, the midterm clinical results for the AMIC technique showed that most patients were subjectively highly satisfied with AMIC and experienced significant improvements in reliable clinical outcome scores.28 The results of our study were consistent with the results of these previous studies. We propose that the aBMSC-dECM scaffold can provide a uniformly distributed and highly interconnected porous structural environment, which can effectively host more BMSCs therein and better stabilize the blood clot after BMS. This allows BMSCs to maintain higher numbers and biological function in the cartilage defect site. Overall, the aBMSC-dECM scaffolds successfully enhanced hyaline cartilage tissue formation in the defect site.
It is worthwhile to mention that the GAG and DNA contents, the distribution of proteoglycan, and the distribution and arrangement of type II collagen fibers of repaired tissue in the aBMSC-dECM scaffold group at 12 weeks after surgery were similar to these characteristics of surrounding normal hyaline cartilage. Furthermore, subchondral bone remodeling and tidemarks were also observed in most zones of the defect sites. In the previous study of Milano et al., an irregular surface was observed on the repaired tissue in a sheep model at 6 months after AMIC treatment. Histological analysis revealed that none of the experimental treatments produced hyaline cartilage. Moreover, subchondral lamina and bone changes can also be found after 6 months.29 These findings are comparable with those published by Dorotka et al.30 and Chevrier et al.31 Our results show that the aBMSC-dECM scaffolds are more promising than previously developed scaffolds. This may be due to the following three reasons: First, the aBMSC-dECM scaffold was made from autologous BMSCs and their ECM components. The scaffold could provide a biocompatible environment, which was beneficial for BMSC homing, adhesion, and proliferation.32 Second, natural extracellular matrix (ECM) biomaterials can provide not only structural guidance and tissue morphogenesis but also abundant biological signaling molecules, including growth factors, cytokines, and other functional proteins.33 Many ECM scaffolds, such as porcine small intestinal submucosa, fetal bovine skin, and bovine pericardium, have already been commercially used for the reconstruction of the urethra, cranium, rotator cuff, and spinal dura. It is plausible that component growth factors such as basic fibroblast growth factor and transforming growth factor beta are released during aBMSC-dECM scaffold degradation and exert their biologic effects, such as regulations of cellular proliferation and chondrogenesis. Third, Pei et al. demonstrated that the stem cell-derived ECM can prevent stem cells from undergoing oxidative stress-induced cell senescence, allowing for cell proliferation and chondrogenic differentiation.34 Because most patients with cartilage defects have diseased joints with oxidative stress, we propose that the aBMSC-dECM scaffold is a promising cell expansion system for providing a large number of high-quality stem cells for cartilage repair.
The most important finding of the present study is that we can successfully collect autologous cell-derived ECM membranes, fabricate them into a three-dimensional porous ECM scaffold, and combine the ECM scaffold with BMS for cartilage repair. This technique could effectively avoid the risks of pathogen transmission undesirable inflammation and other immunological reactions, and ethical issues related to the application of allogenous or xenogenous materials and artificial polymers. Therefore, the aBMSC-dECM scaffold may be a bio-safe scaffold for clinical application.
Our study has some limitations. First, although the rabbit model is well accepted in research, it is structurally different from humans. The thickness of the hyaline articular cartilage of mature rabbits is ∼300–400 μm. Therefore, larger animals, such as goats, pigs, and dogs, might make better models for evaluating the cartilage regeneration achieved using this technique.23 Second, the compressive modulus is an important criterion for evaluation engineered cartilage. Our analysis methods may not be enough to offer a comprehensive evaluation.35 Finally, examination of more animals is needed to confirm the reproducibility of our study.
Conclusions
We observed that implantation of aBMSC-dECM scaffolds can enhance the therapeutic effect of BMS on articular cartilage repair. We propose that the aBMSC-dECM scaffold played an important role in holding and stabilizing BMSCs in the defect site and enhanced cell proliferation, chondrogenic differentiation, and matrix production. Because the method employed in this study is low cost, bio-safe, and easy, it represents a feasible candidate method for articular cartilage repair.
Acknowledgment
This research was supported by the Chinese National Nature Sciences Foundation (31070861, 81171745).
Disclosure Statement
No competing financial interests exist.
References
- 1.Chang C.H., Kuo T.F., Lin F.H., Wang J.H., Hsu Y.M., Huang H.T., et al. . Tissue engineering-based cartilage repair with mesenchymal stem cells in a porcine model. J Orthop Res 29,1874, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lee C.H., Cook J.L., Mendelson A., Moioli E.K., Yao H., and Mao J.J.Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376,440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomoll A.H., Farr J., Gillogly S.D., Kercher J., and Minas T.Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am 92,2470, 2010 [PubMed] [Google Scholar]
- 4.Mithoefer K., McAdams T.R., Scopp J.M., and Mandelbaum B.R.Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med 28,25, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Kalson N.S., Gikas P.D., and Briggs T.W.Current strategies for knee cartilage repair. Int J Clin Pract 64,1444, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Gomoll A.H.Microfracture and augments. J Knee Surg 25,9, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., and Mandelbaum B.R.Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37,2053, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Gunes T., Sen C., Erdem M., Koseoglu R.D., and Filiz N.O.Combination of microfracture and periosteal transplantation techniques for the treatment of full-thickness cartilage defects. Acta Orthop Traumatol Turc 40,315, 2006 [PubMed] [Google Scholar]
- 9.Breinan H.A., Martin S.D., Hsu H.P., and Spector M.Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res 18,781, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Jin L.H., Choi B.H., Kim Y.J., Park S.R., Jin C.Z., and Min B.H.Implantation of bone marrow-derived buffy coat can supplement bone marrow stimulation for articular cartilage repair. Osteoarthritis Cartilage 19,1440, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Gille J., Behrens P., Volpi P., de Girolamo L., Reiss E., Zoch W., et al. . Outcome of autologous matrix induced chondrogenesis (Amic) in cartilage knee surgery: data of the Amic registry. Arch Orthop Trauma Surg 133,87, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benthien J.P., and Behrens P.Autologous matrix-induced chondrogenesis (Amic). a one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg 76,260, 2010 [PubMed] [Google Scholar]
- 13.Benthien J.P., and Behrens P.The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (Amic): method description and recent developments. Knee Surg Sports Traumatol Arthrosc 19,1316, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Dhollander A.A., De Neve F., Almqvist K.F., Verdonk R., Lambrecht S., Elewaut D., et al. . Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc 19,536, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Hoemann C.D., Sun J., McKee M.D., Chevrier A., Rossomacha E., Rivard G.E., et al Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage 15,78, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Patrascu J.M., Freymann U., Kaps C., and Poenaru D.V.Repair of a post-traumatic cartilage defect with a cell-free polymer-based cartilage implant: a follow-up at two years by mri and histological review. J Bone Joint Surg Br 92,1160, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Gonen-Wadmany M., Oss-Ronen L., and Seliktar D.Protein-polymer conjugates for forming photopolymerizable biomimetic hydrogels for tissue engineering. Biomaterials 28,3876, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Schussler O., Shen M., Shen L., Carpentier S.M., Kaveri S., and Carpentier A.Effect of human immunoglobulins on the immunogenicity of porcine bioprostheses. Ann Thorac Surg 71,S396, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kim H.L., Do J.Y., Cho H.J., Jeon Y.C., Park S.J., Ma H.I., et al Dura mater graft-associated Creutzfeldt-Jakob disease: the first case in Korea. J Korean Med Sci 26,1515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H., Hoshiba T., Kawazoe N., and Chen G.Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 32,2489, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Zeitouni S., Krause U., Clough B.H., Halderman H., Falster A., Blalock D.T., et al Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med 4,132ra55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang C., Xu Y., Jin C., Min B.H., Li Z., Pei X., et al. . Feasibility of autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffold for cartilage tissue engineering. Artif Organs 37,E179, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Jin C.Z., Cho J.H., Choi B.H., Wang L.M., Kim M.S., Park S.R., et al The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect. Tissue Eng Part A 17,3057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin C.Z., Choi B.H., Park S.R., and Min B.H.Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A 92,1567, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Jin C.Z., Park S.R., Choi B.H., Park K., and Min B.H.In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artif Organs 31,183, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Erggelet C., Endres M., Neumann K., Morawietz L., Ringe J., Haberstroh K., et al. . Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res 27,1353, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Li T.Z., Jin C.Z., Choi B.H., Kim M.S., Kim Y.J., Park S.R., et al Using cartilage extracellular matrix (CECM) membrane to enhance the reparability of the bone marrow stimulation technique for articular cartilage defect in canine model. Adv Funct Mater 22,4292, 2012 [Google Scholar]
- 28.Gille J., Schuseil E., Wimmer J., Gellissen J., Schulz A.P., and Behrens P.Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 18,1456, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Milano G., Sanna Passino E., Deriu L., Careddu G., Manunta L., Manunta A., et al. . The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage 18,971, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Dorotka R., Windberger U., Macfelda K., Bindreiter U., Toma C., and Nehrer S.Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials 26,3617, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Chevrier A., Hoemann C.D., Sun J., and Buschmann M.D.Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage 15,316, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Badylak S.F.The extracellular matrix as a biologic scaffold material. Biomaterials 28,3587, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Badylak S.F.Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12,367, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Pei M., Zhang Y., Li J., and Chen D.Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev 22,889, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Yang F., Liu K., Shen H., Zhu Y., Zhang W., et al. . The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 33,2926, 2012 [DOI] [PubMed] [Google Scholar]