Abstract
Objective
To examine prepregnancy cardiometabolic and inflammatory markers and subsequent risk of hypertensive disorders of pregnancy.
Study Design
A retrospective cohort study of 3,380 women who took part in a comprehensive multiphasic health checkup (MHC) exam between 1984 and 1996 and had a subsequent delivery in Kaiser Permanente Northern California.
Results
205 women were diagnosed with a hypertensive disorder of pregnancy. Prepregnancy measurements of overweight/obesity (BMI ≥ 25.0 kg/m2), pre-hypertension and inflammation (leukocytes ≥ 7.2 103/µL) were independently associated with hypertensive disorder of pregnancy risk [odds ratios (95% confidence intervals): 1.6 (1.2–2.3), 2.1(1.5–2.8) and 1.6 (1.1–2.3), respectively]. Being overweight/obese and having pre-hypertension before pregnancy was associated with a 3.5-fold increased risk of hypertensive disorder of pregnancy compared with women with normal levels of both risk factors.
Conclusion
Prepregnancy cardiometabolic and inflammation risk profile may help clinicians identify high risk women to target for early intervention or management of hypertensive disorder of pregnancy.
Keywords: Cardiometabolic risk factors, gestational hypertension, hypertensive disorder of pregnancy, inflammation, preeclampsia
Introduction
Preeclampsia is a multisystem complication that is characterized by systemic maternal inflammation, endothelial dysfunction and insulin resistance in pregnancy; it is diagnosed by new-onset hypertension and proteinuria after 20 weeks’ gestation.1 Preeclampsia and other hypertensive disorders of pregnancy are the leading contributors to perinatal morbidity and mortality worldwide.2 The second half of normal pregnancy is characterized by a progressive state of insulin resistance, hyperinsulinemia, hyperlipidemia, and increasing inflammatory markers, and it has been suggested that these responses may be exaggerated in women who develop hypertensive disorder of pregnancy.3
Women with a history of hypertensive disorder of pregnancy (including preeclampsia) appear to have a more than 2-fold increased risk of cardiovascular and metabolic diseases later in life.4 Preeclampsia and cardiovascular disease have several common underlying risk factors, including obesity, insulin resistance, dyslipidemia, endothelial dysfunction and inflammation. 4 However, it is unclear whether women who develop pregnancy induced hypertension and/or preeclampsia have altered levels of cardiometabolic and inflammatory markers before pregnancy or whether the conditions arise due to disturbances induced by the pregnancy itself. A better understanding of prepregnancy predictors of hypertensive disorders in pregnancy may increase our understanding of the underlying etiology and enable the identification of women at high risk to target for early intervention or prevention strategies.
We examined the association between prepregnancy cardiometabolic and inflammation risk factors (i.e. serum glucose, total cholesterol, blood pressure, BMI and leukocytes) alone or in combination, and subsequent risk of hypertensive disorders of pregnancy, using a multi-ethnic cohort of women who took part in a multiphasic health checkup (MHC) exam in 1984–1996 and had a subsequent pregnancy at Kaiser Permanente Northern California (KPNC).
Materials and Methods
Study population
KPNC is an integrated health care delivery system providing medical care for about one third of the commercially insured in the San Francisco Bay Area. KPNC subscribers are representative of the region.5
The source population consisted of female KPNC members who attended a voluntary comprehensive Multiphasic Health Checkup (MHC) at the Kaiser Permanente Oakland Medical Center between 1984 and 1996. KPNC members at this facility were invited to participate upon enrollment. The MHC consisted of a clinic visit for completion of questionnaires and clinical measurements of blood pressure, weight, height and serum random glucose, cholesterol, and leukocytes, with the goal of providing health maintenance through early diagnosis.6 Measurements of serum glucose assessed using the hexokinase method, and total cholesterol assessed using a Kodak Ektachem Chemistry analyzer, were performed by the regional laboratory of KPNC. This laboratory participates in the College of American Pathologists' accreditation and monitoring program. Body mass index (BMI) was calculated using standard methods from height measured using a stadiometer and weight using a balance beam scale. Information on age, sex, race/ethnicity, education level, cigarette smoking, alcohol consumption, coffee consumption, and use of medications was collected using self-administered questionnaires previously described.6
Among women of reproductive age (18–45 years old) who participated in the MHC from 1985–1996, we identified all women who subsequently delivered up to 2009 by searching the KPNC Pregnancy Glucose Tolerance Registry.7 This is an active surveillance registry that annually identifies among KPNC members all pregnancies resulting in a livebirth or stillbirth (available since 1991). We selected the first pregnancy and latest serum with complete data for pregravid measurements of interest. When data from more than one MHC visit were available, data from the visit prior to but closest to the index pregnancy were used in the analysis. We excluded women from the study cohort who reported a history of hypertension at the time of the MHC visit (n=237) and who had a diagnosis of hypertension after the MHC visit but before the index pregnancy (n=202) and an additional 95 who had a pregnancy complicated by essential hypertension (ICD-9 code 642.0), hypertension secondary to a chronic underlying condition (ICD-9 codes 642.1–642.2), pre-eclampsia or eclampsia superimposed on pre-existing hypertension (ICD-9 code 642.7) and unspecified hypertension of pregnancy (ICD-9 code 642.9).
Ascertainment of pregnancy hypertensive disorders
We identified a total of 205 women with a hypertensive disorder of pregnancy. Of these, 80 had gestational hypertension according to ICD-9 code 642.3, 106 had preeclampsia or eclampsia according to ICD-9 codes 642.4–642.7, and 19 had codes indicative of both. To assess the accuracy of the electronic diagnosis codes, we had medical chart review data on a subset of 171 women from the study cohort. Our chart review included 11 women with a diagnosis code for gestational hypertension (ICD-9 code 642.3) and 16 women with a diagnosis code for preeclampsia (ICD-9 code 642.4 or 642.5), and the agreement between the electronic diagnosis and the chart review data was 93.8% for preeclampsia and 90.9% for gestational hypertension. The women who did not meet the criteria for gestational hypertension or preeclampsia had two borderline elevated blood pressures with systolic blood pressures of 130 mm Hg or higher. Of the 144 women who we identified as not having a hypertensive disorder of pregnancy, we found 4 (2.8%) had preeclampsia or gestational hypertension according to medical chart review.
Prepregnancy cardiometabolic and inflammation risk factors
Serum glucose, total cholesterol, leukocyte count, diastolic and systolic blood pressure were first categorized into tertiles. In addition, women were categorized according to clinically relevant adverse levels of cardiometabolic risk factors: Overweight/obesity: A BMI ≥ 25 kg/m2 according to the WHO classification of overweight/obesity 8 Pre-hypertension: Systolic blood pressure greater than 120 mm Hg and/or diastolic blood pressure at least 80 mm Hg for diastolic according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines 9; Hypercholesterolemia: A serum total cholesterol ≥ 200 mg/dl, which, according to the National Cholesterol Education Program{ref}, is above the desirable range 10; Mild Hyperglycemia: A prepregnancy random serum glucose ≥ 100 mg/dl but < 200 mg/dl. 53% of women had fasting hyperglycemia (at least a 6 hour fast) and 47% had a random glucose test. We used the cut-off of 100 mg/dl recommended by the American Diabetes Association (ADA) to define impaired fasting glucose given that the 100 mg/dl cut point represented the 95th percentile for our study population due to the young age of our population and we would have had a low prevalence of the risk factor if more stringent criteria were applied. Mild Subclinical Inflammation: A leukocyte count in the highest tertile among the controls was considered mild subclinical inflammation since there is no standard definition based on leukocyte count.
Statistics
Unconditional logistic regression was used to first obtain odds ratios (ORs) as estimates of the relative risk of hypertensive disorder of pregnancy in relation to each individual cardiometabolic and inflammation risk factor separately. We then examined a multivariable model containing all the clinically relevant cardiometabolic and inflammation risk factors defined above to identify independent cardiometabolic predictors of hypertensive disorder of pregnancy. We also looked at associations between increasing number of prepregnancy risk factors and hypertensive disorder of pregnancy. To assess confounding, we entered covariates into logistic regression models one at a time and then compared the adjusted and unadjusted odds ratios. The final adjusted logistic regression models included covariates that altered unadjusted odds ratios by at least 10% for at least one cardiometabolic or inflammation risk factor. Variables evaluated for confounding were: race/ethnicity, prepregnancy BMI (kg/m2) (except for the model with obesity), parity, maternal education in years, alcohol consumption, cigarette smoking, caffeine consumption and 1st degree family history of hypertension. Sensitivity analyses were conducted by restricting to women with preeclampsia to see if the association varied by severity of disease. Given that we were unable to control for history of preeclampsia, we conducted a sensitivity analysis examining cardiometabolic and inflammation risk factors in relation to hypertensive disorder of pregnancy, restricting to nulliparous women to assess if associations were similar among women without a prior hypertensive disorder of pregnancy.
Heterogeneity in associations by time since MHC exam (< 3, 3–5, 6–9, 10–13, and ≥ 14 years) was assessed by inclusion of appropriate cross-product (interaction) terms in regression models. This study was approved by the human subjects committee of the Kaiser Foundation Research Institute.
Results
On average, the MHC exam assessing prepregnancy risk factors was performed 4.6 years before delivery (S.D. ± 3.7, range 1–20 years). Characteristics of the study cohort by increasing number of cardiometabolic and inflammation risk factors at baseline are presented in Table 1. Women with more risk factors were slightly older, less likely to be nulliparous, had fewer years of education and were more likely to be African American or Hispanic, to be a currently smoker and to have a family history of hypertension. Women with more risk factors also had higher levels of BMI, glucose, cholesterol, systolic and diastolic blood pressure and leukocyte count. Table 2 first presents data from separate logistic regression models for each individual prepregnancy cardiometabolic and inflammation risk factor. After adjusting for year of serum, age at delivery, years between serum collection and pregnancy, race/ethnicity, body mass index, maternal education, family history of hypertension, parity, smoking status, alcohol and coffee intake during the year before the exam, compared with women in the first tertile, women in the third tertile had a risk of hypertensive disorder of pregnancy that was 2.6-fold higher for diastolic blood pressure and 2.7-fold higher for systolic blood pressure (ORs: 2.6 (1.8–12.6) and 2.7 (1.7–4.0), respectively). Women in the highest tertile of prepregnancy leukocyte count were 50% more likely to develop a hypertensive disorder in pregnancy after multivariable adjustment (OR: 1.5, 95% CI: 1.0–2.3). Compared with women who were normal weight at the baseline exam, women who were overweight or obese were approximately 2 times as likely to develop a hypertensive disorder during pregnancy [ORs: 1.8 (95% CIs:1.3–2.6) and 2.2 (95% CIs: 1.4–3.4), respectively]. Pre-pregnancy glucose and cholesterol levels were not associated with subsequent risk of hypertensive disorders of pregnancy.
Table 1.
Characteristics of the cohorta
| Characteristic | Number of cardiometabolic risk factorsb | P value | Total cohort (n=3,380) |
|||
|---|---|---|---|---|---|---|
| 0 (n=1,244) | 1 (n=1,266) | 2 (n=647) | ≥ 3 (n=223) | |||
| Age at Multiphasic Health Checkup Examination (y)c | 27.9 ± 5.4 | 27.7 ± 5.4 | 28.2 ± 5.4 | 28.8 ± 5.3 | 0.014d | 27.9 ± 5.4 |
| Age at delivery, yc | 32.7 ± 5.4 | 32.3 ± 5.2 | 32.5 ± 5.4 | 32.6 ± 5.5 | 0.33d | 32.5 ± 5.3 |
| <30 y, n (%) | 383 (30.8) | 420 (33.2) | 208 (32.1) | 76 (34.1) | 0.27e | 1,087 (32.2) |
| 30–34 y, n (%) | 413 (33.2) | 439 (34.7) | 234 (36.2) | 73 (32.7) | 1,159 (34.3) | |
| 35–39 y, n (%) | 335 (26.9) | 319 (25.2) | 151 (23.3) | 50 (22.4) | 855 (25.3) | |
| ≥ 40 y, n (%) | 113 (9.1) | 88 (7.0) | 54 (8.3) | 24 (10.8) | 279 (8.3) | |
| Time between examination and delivery, (y)c | 4.8 ± 3.8 | 4.7 ± 3.7 | 4.2 ± 3.3 | 3.8 ± 3.1 | <.0001 d | 4.6 ± 3.7 |
| Education (y), n (%) | <.0001 e | |||||
| ≤ 12 | 275 (22.1) | 349 (27.6) | 211 (23.6) | 81 (36.3) | 916 (27.1) | |
| 13–15 | 428 (34.4) | 462 (36.5) | 245 (37.9) | 77 (34.5) | 1,212 (35.9) | |
| ≥ 16 | 495 (39.8) | 416 (32.9) | 167 (25.8) | 56 (25.1) | 1,134 (33.6) | |
| Unknown | 46 (3.7) | 39 (3.1) | 24 (3.7) | 9 (4.0) | 118 (3.5) | |
| Race/Ethnicity, n (%) | <.0001e | |||||
| Non-Hispanic White | 477 (38.3) | 406 (32.1) | 166 (25.7) | 68 (30.5) | 1,117 (33.0) | |
| African American | 379 (30.5) | 452 (35.7) | 265 (41.0) | 78 (35.0) | 1,174 (34.7) | |
| Asian | 244 (19.6) | 227 (17.9) | 100 (15.5) | 37 (16.6) | 608 (18.0) | |
| Hispanic | 108 (8.7) | 143 (11.3) | 81 (12.5) | 33 (14.8) | 365 (10.8) | |
| Other | 36 (2.9) | 38 (3.0) | 35 (5.4) | 7 (3.1) | 116 (3.4) | |
| Hypertension complicating pregnancy, n (%) | <.0001 e | |||||
| Pregnancy-induced hypertension (PIH)f | 25 (2.0) | 24 (1.9) | 15 (2.3) | 16 (7.2) | 80 (2.4) | |
| Pre-eclampsia (PE) g or eclampsia (E) h | 27 (2.2) | 38 (3.0) | 30 (4.6) | 11 (4.9) | 106 (3.1) | |
| PIH and PE/E | 5 (0.4) | 7 (0.6) | 5 (0.8) | 2 (0.9) | 19 (0.6) | |
| Neither PIH nor PE/E | 1,187 (95.4) | 1,197 (94.5) | 597 (92.3) | 194 (87.0) | 3,175 (93.9) | |
| Parity, n (%) | 0.0003 e | |||||
| 0 | 525 (42.2) | 531 (41.9) | 219 (33.8) | 90 (40.4) | 1,365 (40.4) | |
| 1 | 199 (16.0) | 218 (17.2) | 129 (19.9) | 43 (19.3) | 589 (17.4) | |
| 2+ | 125 (10.0) | 133 (10.5) | 102 (15.8) | 33 (14.8) | 393 (11.7) | |
| Unknown | 395 (31.8) | 384 (30.3) | 197 (30.4) | 57 (25.6) | 1,033 (30.6) | |
| Alcohol, n (%) | 0.54e | |||||
| None | 245 (19.7) | 235 (18.6) | 116 (17.9) | 50 (22.4) | 646 (19.1) | |
| Occasional or more drinks/d | 765 (61.5) | 806 (63.7) | 414 (64.0) | 127 (56.9) | 2112 (62.4) | |
| Unknown | 234 (18.8) | 225 (17.8) | 117 (18.1) | 46 (20.6) | 622 (18.4) | |
| Smoking, n (%) | 0.0005e | |||||
| Never | 715 (57.5) | 686 (54.2) | 330 (51.0) | 108 (48.4) | 1,839 (54.4) | |
| Former | 183 (14.7) | 197 (15.6) | 89 (13.8) | 27 (12.1) | 496 (14.7) | |
| Current | 132 (10.6) | 174 (13.7) | 114 (17.6) | 43 (19.3) | 463 (13.7) | |
| Unknown | 214 (17.2) | 209 (16.5) | 114 (17.6) | 45 (20.2) | 582 (17.2) | |
| Coffee consumption, n (%) | 0.89e | |||||
| None | 254 (20.4) | 236 (18.6) | 116 (17.9) | 43 (19.3) | 649 (19.2) | |
| Occasional | 343 (27.6) | 383 (30.3) | 199 (30.8) | 68 (30.5) | 993 (29.4) | |
| ≥ 1 cup/day | 381 (30.6) | 381 (30.1) | 193 (29.8) | 68 (30.5) | 1,023 (30.3) | |
| Unknown | 266 (21.4) | 266 (21.0) | 139 (21.5) | 44 (19.7) | 715 (21.2) | |
| Family history of 1st-degree hypertension, n (%) | 0.006e | |||||
| Yes | 367 (29.5) | 407 (32.1) | 231 (35.7) | 91(40.8) | 1,096 (32.4) | |
| No | 517 (41.6) | 473 (37.4) | 236 (36.5) | 77 (34.5) | 1,303 (38.6) | |
| Unknown | 360 (28.9) | 386 (30.5) | 180 (27.8) | 55 (24.7) | 981 (29.0) | |
| Body mass index, kg/m2 c | 21.1 ± 2.0 | 23.3 ± 4.1 | 26.5 ± 5.6 | 29.9 ± 5.9 | <.0001 d | 23.6 ± 4.8 |
| Serum glucose, mg/dLc | 83.4 ± 7.3 | 84.1 ± 8.5 | 86.4 ± 14 | 93.1 ± 22.2 | <.0001 d | 84.9 ± 11.1 |
| Serum cholesterol, mg/dLc | 162.2 ± 21.4 | 175.5 ± 32.9 | 194.4 ± 36.4 | 213 ± 38.7 | <.0001 d | 176.7 ± 33.9 |
| Systolic blood pressure, mm Hgc | 108.7 ± 10.7 | 112.8 ± 12.8 | 118.6 ± 14.2 | 126.4 ± 15.3 | <.0001 d | 113.3 ± 13.5 |
| Diastolic blood pressure, mm Hgc | 65.4 ± 7.5 | 67.3 ± 8.5 | 70.3 ± 9.3 | 74 ± 10.9 | <.0001 d | 67.6 ± 8.8 |
| White blood cell count, 103/mm3 c | 5.5 ± 1.0 | 6.9 ± 2 | 7.6 ± 1.9 | 8.4 ± 1.7 | <.0001 d | 6.6 ± 1.9 |
Anyone with past medical history of hypertension at time of serum collection was excluded.
Cardiometabolic risk factors include: 1) serum glucose ≥ 100 mg/dL; 2) serum cholesterol ≥ 200 mg/dL; 3) systolic blood pressure ≥ 135 mm Hg or diastolic blood pressure ≥ 85 mm Hg; 4) body mass index ≥ 25 kg/m2; 5) white blood cell count ≥ 7.2 × 103 per mm3 (highest tertile for cohort)
Data are given as mean ± standard deviation
F-test from Analysis of Variance (ANOVA)
Chi-square test
Pregnancy-induced hypertension (ICD-9 codes 643.3x),
pre-eclampsia (ICD-9 codes 642.4x, 642.5x), and/or
eclampsia (ICD-9 code 642.6x)
Table 2.
Pregravid cardiometabolic risk factors and hypertensive disorders of pregnancy
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Pregravid risk factor | Cases, n %)* | Comparison Group, n(%)† |
Minimally adjusted‡ |
Multivariable adjusted§ |
| Separate logistic regression models | ||||
| Random glucose, mg/dL | ||||
| < 81 | 71 (34.6) | 982 (30.9) | 1.0 | 1.0 |
| 81–86 | 63 (30.7) | 1,000 (31.5) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) |
| ≥ 87 | 71 (34.6) | 1,193 (37.6) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) |
| Total cholesterol, mg/dL | ||||
| < 160 | 60 (29.3) | 1,040 (32.8) | 1.0 | 1.0 |
| 160–187 | 78 (38.0) | 1,064 (33.5) | 1.3 (0.9–1.9) | 1.2 (0.8–1.7) |
| ≥ 188 | 67 (32.7) | 1,071 (33.7) | 1.1 (0.8–1.7) | 1.0 (0.7–1.5) |
| Diastolic blood pressure, mm Hg | ||||
| < 63 | 40 (19.5) | 1,011 (31.8) | 1.0 | 1.0 |
| 63–70 | 55 (26.8) | 1,118 (35.2) | 1.3 (0.9–2.0) | 1.3 (0.8–2.0) |
| ≥ 71 | 110 (53.7) | 1,046 (32.9) | 2.9 (2.0–4.3) | 2.6 (1.8–3.9) |
| Systolic blood pressure, mm Hg | ||||
| < 107 | 38 (18.5) | 1,071 (33.7) | 1.0 | 1.0 |
| 107–117 | 65 (31.7) | 1,039 (32.7) | 1.9 (1.2–2.8) | 1.7 (1.1–2.6) |
| ≥ 118 | 102 (49.8) | 1,065 (33.5) | 3.2 (2.1–4.8) | 2.7 (1.7–4.0) |
| Body mass index, kg/m2 | ||||
| < 25.0 | 124 (60.5) | 2,348 (74.0) | 1.0 | 1.0 |
| 25.0–29.9 | 50 (24.4) | 536 (16.9) | 1.8 (1.3–2.6) | 1.8 (1.3–2.6) |
| ≥ 30.0 | 31 (15.1) | 291 (9.2) | 2.1 (1.3–3.3) | 2.2 (1.4–3.4) |
| White blood cell count, 103/μL | ||||
| <5.6 | 49 (23.9) | 983 (31.0) | 1.0 | 1.0 |
| 5.6–7.1 | 70 (34.1) | 1,127 (35.5) | 1.2 (0.8–1.8) | 1.2 (0.8–1.7) |
| ≥ 7.2 | 86 (42.0) | 1,065 (33.5) | 1.7 (1.2–2.4) | 1.5 (1.0–2.3) |
| Single multivariate model | ||||
| Hyperglycemia (random glucose mg/dL) | ||||
| < 100 | 193 (94.1) | 3,306 (95.6) | 1.0 | 1.0 |
| ≥ 100 | 12 (5.9) | 139 (4.4) | 1.3 (0.7–2.4) | 1.3 (0.7–2.4) |
| Hypercholesterolemia (total cholesterol mg/dL) | ||||
| <200 | 159 (77.6) | 2,459 (77.4) | 1.0 | 1.0 |
| ≥ 200 | 46 (22.4) | 716 (22.6) | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) |
| Blood pressure | ||||
| Normal║ | 98 (47.8) | 2,123 (66.9) | 1.0 | 1.0 |
| Pre-/hypertension¶ | 107 (52.2) | 1,052 (33.1) | 2.1 (1.6–2.9) | 2.1 (1.5–2.8) |
| Overweight/obese (BMI, kg/m2) | ||||
| < 25 | 124 (60.5) | 2,348 (74.0) | 1.0 | 1.0 |
| ≥ 25 | 81 (39.5) | 827 (26.0) | 1.6 (1.2–2.2) | 1.6 (1.2–2.3) |
| Mild inflammation (tertile of WBC, 103/μL) | ||||
| <5.6 | 49 (23.9) | 983 (31.0) | 1.0 | 1.0 |
| 5.6–7.1 | 70 (34.1) | 1,127 (35.5) | 1.2 (0.8–1.8) | 1.2 (0.8–1.8) |
| ≥ 7.2 | 86 (42.0) | 1,065 (33.5) | 1.5 (1.0–2.2) | 1.6 (1.1–2.3) |
CI, confidence interval
n = 205 women
n =3,175 women
Adjusted for year of serum, age at delivery, years between serum collection and pregnancy, and race/ethnicity
Adjusted for year of serum, age at delivery, years between serum collection and pregnancy, race/ethnicity, body mass index (except separate model with body mass index), maternal level of educational attainment, family history of hypertension, parity, cigarette smoking status, alcohol and coffee intake during year prior to exam
Systolic blood pressure (SBP) < 80 mm Hg and diastolic blood pressure (DBP) < 120 mg Hg
Based on JNC-7 classification: pre-hypertension is SBP 120–139 mm Hg or DBP 80–89 mm Hg
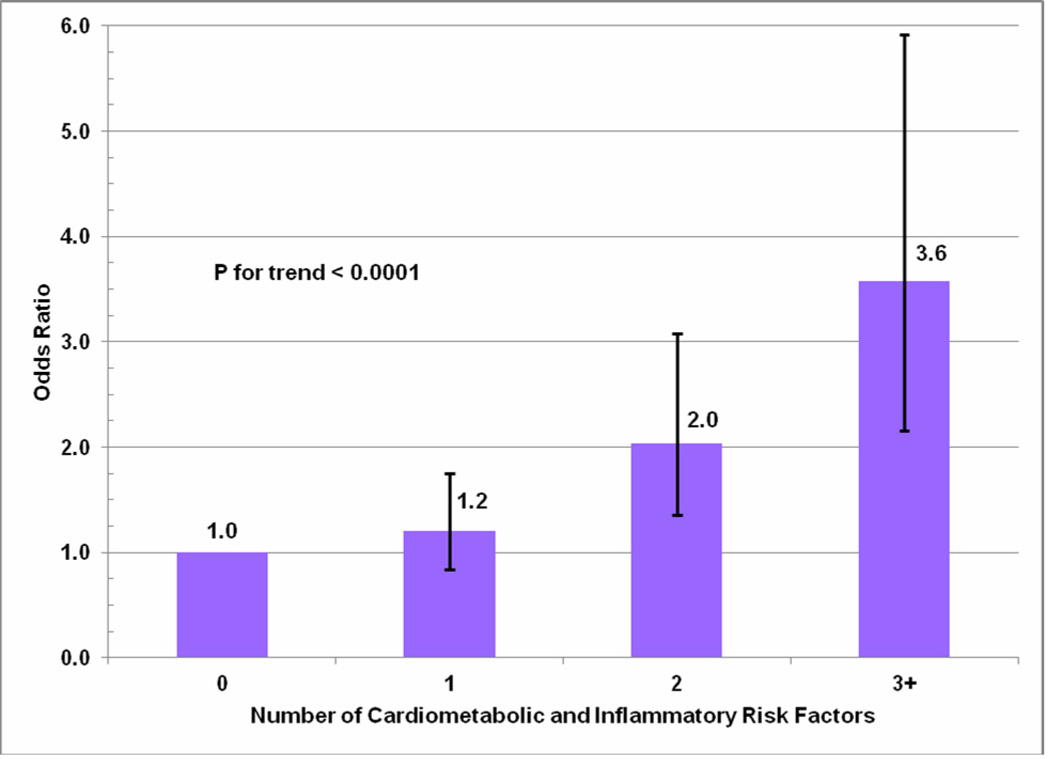
A fully adjusted single multivariate model containing all pregravid cardiometabolic and inflammatory risk factors found that prepregnancy prehypertension (SBP>120 or DBP>80), overweight/obesity (BMI ≥ 25 kg/m2) and high leukocyte count (≥ 7.2 counts per 103) were all independently associated with increased risk of hypertensive disorder of pregnancy (ORs 2.1 [95% CI: 1.5–2.8], 1.6 [95% CI: 1.2–2.3], and 1.6 [95% CI: 1.1–2.3], respectively) (Table 2). There was some suggestion that hyperglycemia was also associated with a 30% increased risk of hypertensive disorder of pregnancy, although this finding did not reach statistical significance. There was no association with hypercholesterolemia. The risk of hypertensive disorder of pregnancy increased with the number of adverse prepregnancy cardiometabolic risk factors, and women with 3 or more risk factors had an almost four-fold increased risk of gestational diabetes mellitus (GDM) compared with women with no risk factors (p-trend <0.001) (Figure 1).
Figure 1.
Odds ratios and 95% confidence intervals for hypertensive disorder of pregnancy associated with number of prepregnancy cardiometabolic and inflammatory risk factors.
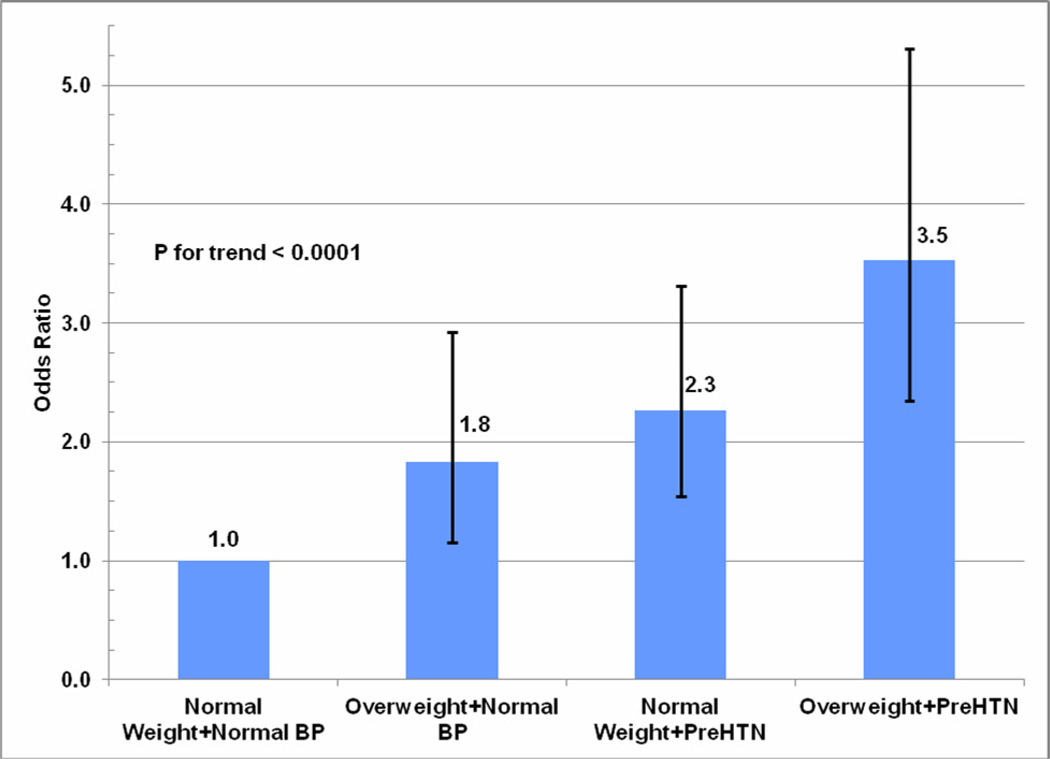
The independent and joint effects of adverse levels of blood pressure and BMI, the two strongest predictors of hypertensive disorder of pregnancy, are reported in Figure 2. Compared to women with prepregnancy BMI < 25 kg/m2 and normal blood pressure (SBP<120 and DBP<80), having a BMI ≥ 25 kg/m2 alone was associated with a 80% increased risk, having prehypertension/hypertension alone was associated with a 2.3-fold increased risk, and having adverse levels of both BMI and blood pressure was associated with a 3.5-fold increased risk of hypertensive disorder of pregnancy (p-trend<0.001).
Figure 2.
Odds ratios and 95% confidence intervals for hypertensive disorder of pregnancy associated with prepregnancy body mass index and blood pressure levels.
There was evidence that the association between overweight/obesity and preeclampsia risk varied by time since exam. The odds ratios for overweight/obesity increased with longer time since MHC exam from 2.7 (95% CI: 1.4–5.2) for 6–9 years since exam, 3.7 (95% CI: 1.6–8.4) for 10–13 years since exam, and 5.7 (95% CI: 1.7–19.5) for 14+ years since exam (overall p-value = 0.04).
In sub-analyses restricted to preeclampsia and eclampsia cases (ICD-9 codes 642.4–642.6), results were similar (data not shown). Results were also similar for the sensitivity analysis restricting to nulliparous women (data not shown).
Comment
In this study, adverse levels of cardiometabolic and inflammation risk factors assessed on average 5 years before pregnancy were associated with an increased risk of subsequent hypertensive disorder of pregnancy. Prepregnancy prehypertension, overweight/obesity and elevated leukocytes (a marker of inflammation) were independently associated with increased risk of hypertensive disorder of pregnancy. In a multivariate model, prepregnancy prehypertension (SBP>120 or DBP>80) was the strongest predictor of subsequent hypertensive disorder of pregnancy. Women who were both overweight and had prehypertension at the clinical exam performed five years before pregnancy had a 3.5-fold increased risk of hypertensive disorder of pregnancy compared with women with neither risk factor. There was also a trend of increasing risk of hypertensive disorder of pregnancy with increasing number of cardiometabolic risk factors present before pregnancy, and women with 3 or more risk factors had a 3.6-fold increased risk of GDM.
Data with prospective measurements of cardiovascular disease and inflammation risk factors and subsequent information on pregnancy outcomes are sparse. Our results are generally consistent with previous studies that examined individual prepregnancy components of metabolic risk and preeclampsia. Studies have found that prepregnancy obesity and chronic hypertension individually are associated with subsequent preeclampsia. 9;11–14 Our findings are generally consistent with one prior study that examined prepregnancy cardiovascular risk factors as predictors of preeclampsia in Norway.11 The Norwegian study found that systolic blood pressure above 121 mmHg and diastolic blood pressure above 71 mmHg were both associated with a four-fold increased risk of preeclampsia, and a BMI greater than 27.08 was associated with an almost twofold increased risk. In addition, their study also found prepregnancy serum levels of triglycerides, cholesterol and LDL-cholesterol were all associated with increased risk of preeclampsia, whereas no association was observed with prepregnancy glucose and HDL. This study did not examine markers of inflammation. The lack of association between prepregnancy hypercholesterolemia and hypertensive disorder of pregnancy observed in our study may be due to the fact that we lacked information on type of cholesterol (HDL versus LDL).
To our knowledge, no other study has examined the association between prepregnancy leukocyte count and risk of pregnancy hypertensive disorders. Data from the Kaiser Permanente MHC were the first to show that inflammation as measured by high leukocytes is predictive of hypertension and myocardial infarction.15;16 While the potential mechanism underlying this potential association is unclear, it has been shown that increased adiposity leads to increased circulating levels of basal interleukin (IL)-6 secretion, 17;18 and the acute phase response that is stimulated by IL-6 19 includes increased leukocyte counts and decreased insulin sensitivity.20 Wolf et al found in a prospective cohort that women with leukocyte counts from 7.9–9.0 103/mm3 during the first trimester of pregnancy had a two-fold increased risk of GDM compared with women with leukocyte counts below 7.9, independent of BMI, and there was a trend of increasing risk of GDM with increasing quartile of leukocytes. 21 Women with gestational hypertension and/or preeclampsia are 50% more likely to develop GDM, suggesting that the two disorders may have some common underlying pathophysiology.22 There is also evidence from outside of pregnancy that elevated leukocyte counts within the normal range are associated with metabolic disorders in women. The Women’s Health Initiative Observational Study, a cohort study of 72 242 postmenopausal women, found that leukocyte count was an independent predictor of cardiovascular events, stroke, and all-cause mortality, and a leukocyte count of greater than 6.7 103/mm3 was the threshold of elevated risk, after adjusting for other known cardiovascular risk factors. 23 While our findings suggest that slightly elevated leukocyte counts (7.8 103/mm3 and higher) may be modestly associated with the development of pregnancy induced hypertensive disorder, we recognize that leukocyte count is a relatively nonspecific marker of inflammation that is influenced by several factors, including infection and certain medications. 24 Future studies with more precise measurements of prepregnancy subclinical inflammation such as high sensitivity c-reactive protein (CRP) are needed.
A systematic review and meta-analysis found that women with a history of preeclampsia/eclampsia had a more than two-fold increased risk of cardiac disease, as well as an increased risk of cerebrovascular disease, peripheral arterial disease, and cardiovascular mortality. 25 The potential mechanisms linking hypertensive disorders in pregnancy with future cardiovascular disease are not well understood. These conditions appear to share common underlying etiologies such as obesity, dyslipidemia, insulin resistance and endothethlial dysfunction.11–13 Our study had the unique ability to assess risk factors before pregnancy, and we found that many women who subsequently develop hypertensive disorders of pregnancy actually have underlying cardiometabolic and inflammatory risk factors even before pregnancy. However, despite the fact that adverse cardiometabolic and inflammatory risk profiles were associated with subsequent hypertensive disorder of pregnancy, 27.8% of women who developed a hypertensive disorder in pregnancy had no cardiometabolic or inflammatory risk factors before pregnancy. These data suggest that perhaps there are other prepregnancy biomarkers that may play a role predicting who will develop hypertensive disorders of pregnancy, or there are additional factors during pregnancy which influence the development of these disorders.
We found evidence that the association between BMI measured at the time of the MHC exam and risk of preeclampsia was stronger among women who had a longer duration of time between the MHC exam and the index pregnancy. It is likely that most women who were overweight/obese at the time of the exam remained overweight/obese until the index pregnancy. Therefore, women with a longer duration of being overweight/obese likely had an increased state of insulin resistance at the time of the index pregnancy, which resulted in their elevated risk of preeclampsia being stronger compared to women whose exam was closer to the index pregnancy and therefore whose duration of being overweight/obese may have been shorter. Unfortunately, we did not have information on body mass index and covariate data at the time of the index pregnancy to examine whether these factors changed over time.
This study had the unique ability to assess the association between cardiometabolic and inflammatory risk factors measured before pregnancy and subsequent risk of preeclampsia in a multi-ethnic population. However, several limitations of the present study merit consideration. There was variation in the time interval between the MHC exam and the subsequent pregnancy; however, we controlled for this in our analysis and also examined effect modification by time since MHC exam to help clarify any variations in associations with time. We were missing data on a relatively large percentage of some potential confounders (especially parity and family history of hypertension), and we had a relatively small number of women with hypertensive disorder of pregnancy. We did not have fasting measures of glucose and total cholesterol and we were not able to use the standard definition of the “metabolic syndrome” given the lack of information on HDL-cholesterol, triglycerides and waist circumference. However, BMI, random glucose, cholesterol and blood pressure are common and inexpensive clinical measures that may be readily available in medical charts and could be useful in identifying women at risk of hypertensive disorder of pregnancy. We did not have a very specific measurement of subclinical inflammation, such as high sensitivity CRP.
We found that the presence of cardiometabolic risk factors and high leukocyte count before pregnancy increases the risk of developing preeclampsia and may help identify women at high risk for preeclampsia, to target for early intervention or possibly prevention strategies. Clinicians should be aware that patients with an increasing number of cardiometabolic and inflammation risk factors (especially overweight patients with higher blood pressure and leukocyte counts) before pregnancy may be at particularly high risk of developing hypertensive disorder of pregnancy and might benefit from early intervention to prevent the development of hypertensive disorders of pregnancy. More information is needed to develop prevention strategies.
Acknowledgements
The authors would like to acknowledge Monica Highbaugh and Mamie Ford for their work reviewing medical records.
This research was supported by R01HD065904 and a community benefit grant from Kaiser Permanente.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data were presented as an oral presentation at the 24rd Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER) in Montreal, Quebec, Canada, on June 21, 2011.
Disclosure: ‘None of the authors have a conflict of interest.'
Reference List
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von DP, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 6.Collen MF. Multiphasic health testing services. New York: John Wiley & Sons; 1978. [Google Scholar]
- 7.Ferrara A, Kahn HS, Quesenberry C, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103(3):526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. BMI Classification. 4-6-2012. Ref Type: Online Source.
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of the High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary. 2001 4-5-2012. Ref Type: Online Source.
- 11.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey SG, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. High body mass index and hypercholesterolemia: risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(4):543–550. doi: 10.1016/s0029-7844(99)00400-7. [DOI] [PubMed] [Google Scholar]
- 13.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175(2):189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290(23):1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD, Selby JV, Quesenberry CP., Jr The leukocyte count: a predictor of hypertension. J Clin Epidemiol. 1990;43(9):907–911. doi: 10.1016/0895-4356(90)90074-y. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 18.Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(3):859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 19.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(2):455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Real JM, Broch M, Vendrell J, Gutierrez C, Casamitjana R, Pugeat M, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49(3):517–520. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Sauk J, Shah A, Vossen SK, Jimenez-Kimble R, Ecker JL, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27(1):21–27. doi: 10.2337/diacare.27.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158(12):1148–1153. doi: 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 23.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165(5):500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 24.Kuhnert M, Schmidt S. Changes in lymphocyte subsets during pregnancy and post-partum in cases of beginning eclampsia. J Perinat Med. 2000;28(5):389–398. doi: 10.1515/JPM.2000.050. [DOI] [PubMed] [Google Scholar]
- 25.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579–584. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]