Abstract
Novel substituted pteridine-derived inhibitors of monocarboxylate transporter 1 (MCT1), an emerging target for cancer therapy, are reported. The activity of these compounds as inhibitors of lactate transport was confirmed using a 14C-lactate transport assay, and their potency against MCT1-expressing human tumor cells was established using MTT assays. The four most potent compounds showed substantial anticancer activity (EC50 37–150 nM) vs MCT1-expressing human Raji lymphoma cells.
Introduction
In the 1920s, the German biochemist Otto Warburg described metabolic differences between cancerous and normal cells, where he noted that tumor cells rely upon a high rate of aerobic glycolysis rather than oxidative phosphorylation to produce energy for maintenance of cellular functions.1,2 Indeed, cancer cells have up to a 60-fold enhanced rate of glycolysis relative to normal cells, even with sufficient oxygen.1 This dependence upon glycolysis, and its consequences, is termed “the Warburg effect”.2
Highly glycolytic cells produce excessive amounts of lactate, the end product of glycolysis, which is actively transported out of the cell to normalize intracellular pH levels. Lactate homeostasis is maintained via a family of 12-membrane pass cell surface proteins coined monocarboxylate transporters (MCTs; also known as the SLC16a transporter family). Fourteen MCTs are known, but only MCT1, MCT2, MCT3, and MCT4 transport small monocarboxylates such as lactate, pyruvate, and ketone bodies (acetoacetate and β-hydroxybutyrate) across plasma membranes in a proton-linked exchange.3
Expression profiling studies have established that most aggressive tumor types express markedly elevated levels of MCT1, MCT4, or both. Notably, the expression of MCT1 and MCT4 is regulated by two major oncogenic transcription factors, MYC and hypoxia inducible factor-1α (HIF-1α), respectively,4,5 that direct marked increases in the production of key proteins that support aerobic glycolysis, including amino acid transporters and enzymes involved in the catabolism of glutamine and glucose.6 Malignancies having MYC involvement and hypoxic tumors are generally resistant to current frontline therapies, with high rates of treatment failure, relapse, and high patient mortality.7,8 Importantly, inhibition of MCT1 or MCT4 can kill tumor cells ex vivo and provoke tumor regression in vivo,4,9 and their potency is augmented by agents such as metformin that force a glycolytic phenotype upon the cancer cell.4
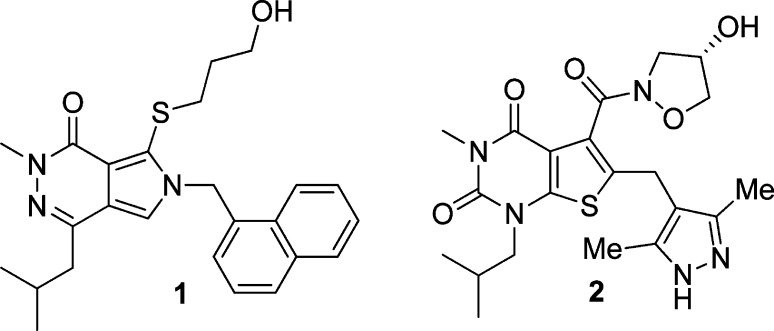
Many weak MCT1 inhibitors (i.e., those effective at high micromolar levels) have been described, including α-cyano-4-hydroxycinnamate,10,11 stilbene disulfonates,12 phloretin,13 and related flavonoids.14 Coumarin-derived covalent MCT inhibitors have also recently been disclosed.15,16 The most potent known MCT1 inhibitors are the pyrrolopyridazinones and the thienopyrimidine diones (e.g., compounds 1–2, Figure 1).17−22 Indeed, compound 2 has advanced into phase I clinical trials for treating some human malignancies.23,24 These compounds, and to our knowledge all MCT1 inhibitors yet described, are dual MCT1/MCT2 inhibitors. MCT2 has very high sequence homology with MCT1, yet it likely has a lesser role than MCT1 and MCT4 for monocarboxylate transport in human cancers based upon expression studies. However, MCT2 inhibition may play a role in potential off-target effects of current agents that could arise from blocking lactate transport in normal cells.
Figure 1.
Potent MCT1 inhibitors.
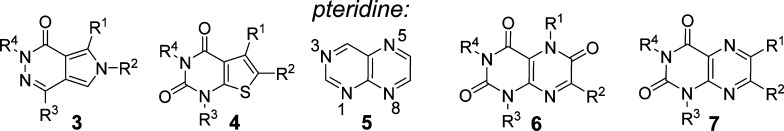
Improved MCT1 inhibitors could be accessed by performing additional structure activity relationship (SAR) studies around scaffold 1. Indeed, toward this goal we have made some refinements in Astra-Zeneca’s original synthetic strategy for 1.25 However, a more desirable approach is to seek alternative scaffolds for MCT1 inhibition that are readily synthesized and that may form similar transporter contacts as do compounds 1–2. We considered [6,6] heterocyclic ring systems as alternatives to the [6,5] ring systems present in compounds 1–2, expecting divergence of SAR and possibly favoring side chains and substituents that would positively alter the physical properties of the resulting MCT1 inhibitors. A core structure of particular interest was the pteridine scaffold 5, a heterocyclic core that is present in many natural products26−29 and that has been widely used in drug discovery efforts.30−32 Accordingly, we targeted appropriately substituted pteridine trione/dione scaffolds 6 and 7 (Figure 2). Routes to 6,7-disubsituted pteridines have been reported,33−38 but to our knowledge the synthetic chemistry of substituted pteridinone scaffolds 6–7 has not been explored. Here we report the synthesis of these substituted pteridinone scaffolds and their activity as MCT1-specific lactate transport inhibitors that selectively block the growth of MCT1-expressing human lymphoma cells.
Figure 2.
Possible MCT inhibitor scaffolds 6 and 7.
Results
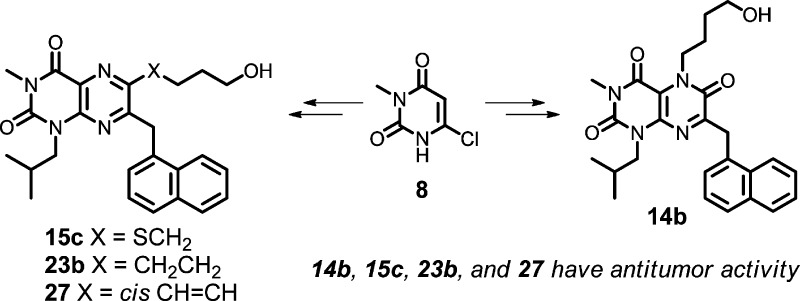
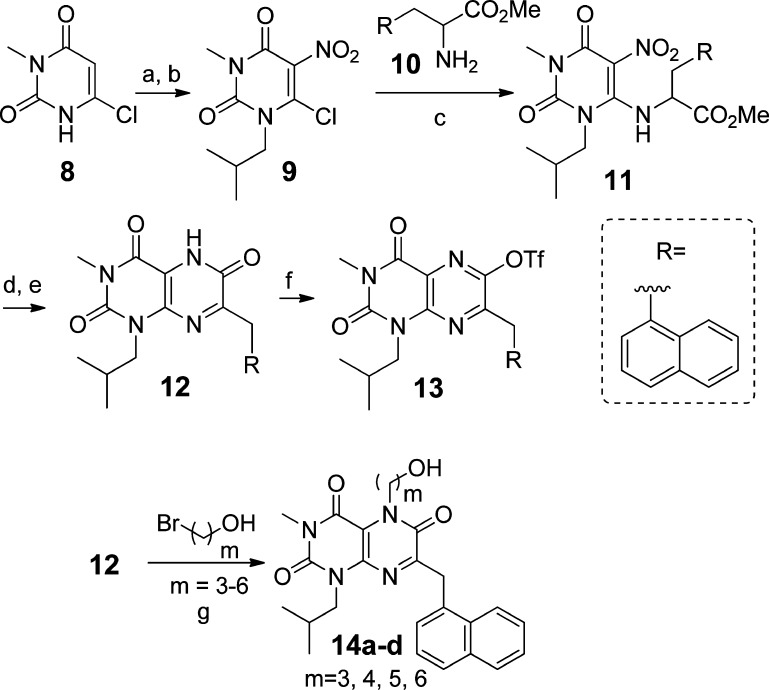
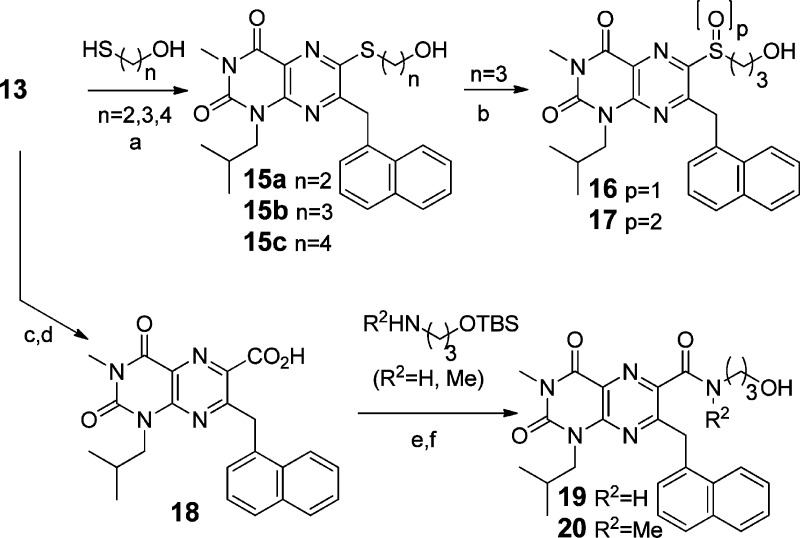
From an efficiency standpoint, it is desirable to introduce structural diversity (e.g., R1 and/or R2 in scaffolds 6–7) late in a synthetic sequence. Our synthesis of these scaffolds began with the commercially available chloride 8 (Scheme 1). Alkylation of 8 using isobutyl iodide gave an inseparable 5:1 mixture of N- and O-alkylated products in 87% yield. After nitration,39 the N-alkyl regioisomer 9 was isolated in 75% yield after column chromatography. Treatment of 9 with protected naphthylalanine 10(40) under basic conditions gave 11 in 67% yield. Nitro group reduction gave an intermediate lactam that readily aromatized upon treatment with DDQ, giving pteridine 2,4,6-trione 12 in 66% yield. The trione was nearly quantitatively converted to triflate 13 using triflic anhydride. Alternatively, alkylation of 12 with ω-bromoalcohols to give R1-substituted compounds in scaffold 6, afforded the expected N5-alkylated pteridine 2,4,6-trione derivatives 14a–d, the first members of the targeted core scaffold 6 available for biological evaluation.
Scheme 1. Synthesis of Test Compounds 14a–d.
Reagents and conditions: (a) (CH3)2CHCH2I, K2CO3, DMSO, 60 °C, 24 h, 87%; (b) HNO3, H2SO4, 0 °C, 2 h, 75%; (c) Na2CO3, DMF, 65 °C, 1 h, 67%; (d) Zn, AcOH, 80 °C, 1 h, 100%; (e) DDQ, CH3CN, room temperature, 1 h, 66%; (f) Tf2O, TEA, CH2Cl2, 0 °C, 30 min, 98%; (g) K2CO3, butanone, reflux.
We recognized that the choice of the naphthylalanine ester 10 and isobutyl iodide in the syntheses of 13 and 14a–d (Scheme 1) limited our early SAR studies to lipophilic analogues in scaffolds 6 and 7, with R2 = 1-naphthylmethyl and R3 = isobutyl (see Figure 2). The high potency of the MCT1 inhibitor 1 guided this selection. Strategically, once the C5 group (R1 in scaffold 6) or the C6 group (R1 in scaffold 7) was optimized, we would then use more polar amino acid esters in place of reagent 10 to give analogues having less lipophilic R2 groups. R3 could then also be varied by choosing different alkylating agents in the first step of Scheme 1.
Access to triflate 13 allowed for the rapid syntheses of C6-substituted compounds (varying R1) in the core scaffold 7. Treatment of 13 with ω-mercaptoalcohols gave thioethers 15a–c via SNAr substitution (Scheme 2). These thioethers were partially or fully oxidized, selectively, to form the racemic sulfoxide 16 and the sulfone 17. Pd-catalyzed cyanation41 of 13 and nitrile hydrolysis gave acid 18, which was then coupled with TBS-protected amino alcohols to give, after deprotection, amides 19–20.
Scheme 2. Synthesis of Test Compounds 15–20.
Reagents and conditions: (a) Et3N, MeOH, rt; (b) m-CPBA, DCE, 0 °C or DCM, rt; (c) Zn(CN)2, Pd(PPh3)4, Zn, NMP, 105 °C; (d) aq H2SO4 1,4-dioxane; (e) HOBt, EDC, DMF–DCM, rt; (f) (n-Bu)4NF, THF, rt.
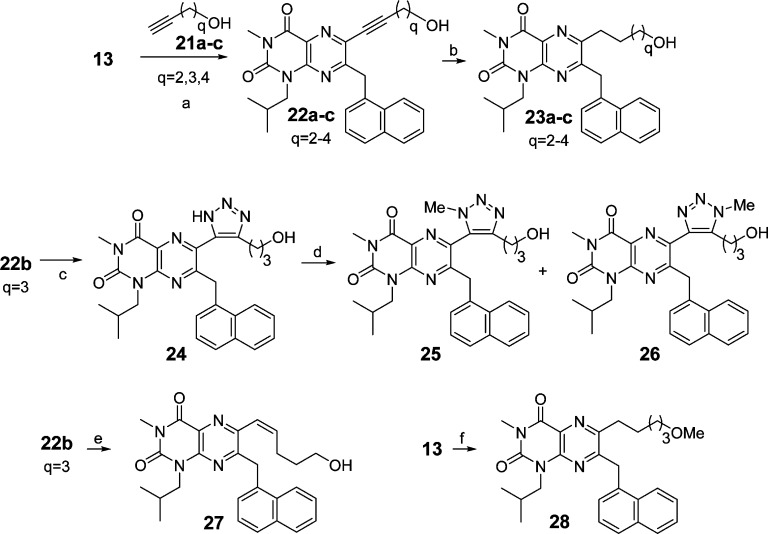
Introduction of a C6 side chain via Sonogashira coupling42 of 13 with alkyne alcohols 21a–c afforded alkynes 22a–c (Scheme 3), which were reduced to compounds 23a–c by hydrogenation. Compound 22b (q = 3) was also converted to triazole 24 by Huisgen–Sharpless 1,3-dipolar cycloaddition.43 Triazole 24 was further transformed to compounds 25 and 26 (as a separable mixture of regioisomers) via N-methylation. Alkyne 22b was also converted by semireduction to olefin 27. Triflate 13 was readily converted to the methyl ether 28 by a two-step sequence.
Scheme 3. Synthesis of Test Compounds 23–28.
Reagents and conditions: (a) Pd(PPh3)4, CuI, Et3N, DMF, microwave, 175 °C, 20 min; (b) Pd/C, H2, MeOH, rt; (c) NaN3, Cu cat. DMF, 120 °C; (d) MeI, K2CO3, acetone, reflux, 5 h; (e) Lindlar’s catalyst, H2, THF; (f) HC≡C(CH2)3OMe, Pd(PPh3)4, CuI, Et3N, DMF, microwave, 175 °C, 20 min; Pd/C, H2, MeOH, THF.
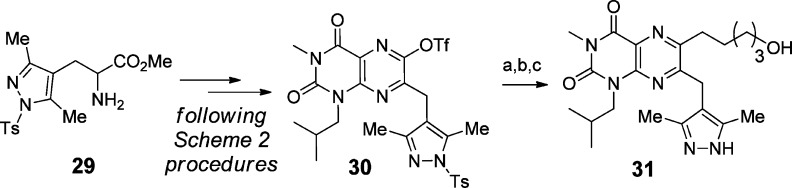
The above methods gave a substantial test set of compounds with differing R1 substituents in scaffolds 6–7. In parallel, we took cues from the clinical MCT1/2 inhibitor 2 and installed a dimethylpyrazole-containing side chain as the R2 group in scaffold 7. The same methods used in Scheme 2 were followed (Scheme 4) to convert the pyrazole-containing amino ester 29(44,45) to the triflate 30 and then to the test compound 31.
Scheme 4. Synthesis of Dimethylpyrazole 31.
Reagents and conditions: (a) compound 21b, Pd(PPh3)4, CuI, DMF, microwave, 175 °C, 20 min; (b) Pd/C, H2, MeOH; (c) LiOH, MeOH.
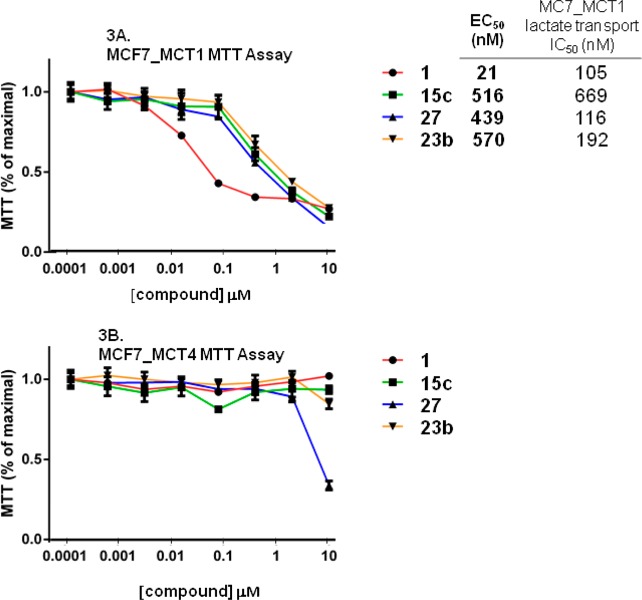
In our previous report that focused on biological studies of MCT1/MCT2 inhibitors 1–2,4 we showed that these compounds rapidly blocked the growth of MCT1-expressing tumor cells. In particular, they halted growth of Burkitt B cell lymphomas, which express high levels of MYC, by virtue of immunoglobulin/MYC gene translocations, and thus a high level of MCT1, which is a direct transcription target induced by MYC.4 Raji Burkitt lymphoma cells express high level of MCT1 mRNA and protein but not mRNA and protein of other MCTs, including MCT4.4 Thus, the proliferation of these cells, as measured by an MTT assay, is a specific marker for MCT1 inhibition. We used a 96 h MTT assay to test the ability of pteridinones to block the growth of human Raji lymphoma cells. To test for potential off-target toxicity in the MTT assay, we also assessed the ability of compounds to impede transport of 14C-lactate in human estrogen receptor-positive MCF7 breast cancer cells using established methods.46 As expected, in the MTT assay inhibitors 1 and 2 dramatically impeded tumor cell growth, with EC50 = 0.5 and 18 nM, respectively, in general agreement with published data.18,19 In the lactate transport inhibition assay, compound 1 had an IC50 of 105 nM. Curiously, all MCT1 inhibitors that we have tested are substantially more potent in the inhibition of Raji cell proliferation assay than in blocking lactate transport in MCF7 cells. While this might suggest shared off-target effects of all classes of MCT1 inhibitors, it more likely reflects the very different conditions, time frames, end points, and cell types used in these assays.47
To confirm that the antiproliferative effects seen in the Raji lymphoma-based MTT assays were indeed due to selective effects on MCT, we also assessed their activity in MCF7 breast cancer cells engineered to overexpress mouse MCT1 (termed MCF7_MCT1 cells, which were the same cells used in lactate transport assays) or human MCT4 (termed MCF7_MCT4 cells).4 We then measured the effect of compound 1 on 14C lactate uptake using each of these cell lines. MCF7_MCT1 cells showed dramatically increased 14C lactate uptake compared to parental MCF7 cells, and this was abolished by treatment with compound 1. In contrast, MCF7_MCT4 cells also showed increased 14C lactate uptake that was not inhibited by compound 1. Finally, in MTT assays, MCF7_MCT1 cells were highly sensitive to compound 1 (growth inhibition EC50 = 21 nM), whereas MCF7_MCT4 cells were resistant to compound 1 (growth inhibition EC50 >10 μM, Figure 3).
Figure 3.
MTT assays in MCF7_MCT1 and MCF7_MCT4 cell lines.
Thus, studies using MCF7 breast cancer cells and Raji lymphoma cells indicate that (1) the vast majority of 14C lactate uptake is mediated by transport through MCT1 in MCF7_MCT1 cells, (2) inhibition of lactate uptake is indicative of MCT1 inhibition, (3) the lack of an effect of compounds on the growth of MCF7_MCT4 cells in MTT assays is a marker for non-MCT mediated cytotoxicity, and (4) the robust MTT assay using the Raji lymphoma cell line is well-suited to served as a front-line screen for assessing the activity of test MCT1 inhibitors. Actives from this screen are then profiled for 14C lactate uptake in MCF7_MCT1 cells and are finally cross-checked for cytoxicity in an MTT assay using MCF7_Mct4 cells.
The antiproliferative effects of our compounds in Raji lymphoma cells are shown in the left column of Table 1. Compound 12, which lacks an R1 group, was inactive, whereas the C5-alkylated pteridine triones 14a–c blocked lymphoma cell proliferation, although 14d was also inactive. The potency of the compounds varies with the R1 chain length, where a four-methylene spacer (m = 4) is optimal (see 14b, EC50 = 150 ± 16 nM). For thioethers 15a–c, the compound with a five-atom tether, R1 = a sulfur atom and four methylene groups (n = 4) was most potent (15c, EC50 = 37 ± 10 nM). The racemic sulfoxide 16 and the sulfone 17, possible thioether oxidative metabolites of thioether 15b,48 were less potent. The amide-linked compounds 19–20 were inactive in blocking Raji lymphoma cell proliferation.
Table 1. Assay Results, Test Compounds.
| compd | EC50 (nM)a MTT | IC50 (nM)b lactate transport |
|---|---|---|
| 12 | >10000c | ntd |
| 14a (m = 3) | 1547 ± 192e | >2000 |
| 14b (m = 4) | 150 ± 16e | 548 |
| 14c (m = 5) | 1420c | nt |
| 14d (m = 6) | >10000c | nt |
| 15a (n = 2) | 2848c | nt |
| 15b (n = 3) | 151 ± 32e | 858 |
| 15c (n = 4) | 37 ± 10e | 669 |
| 16 (p = 1) | 1986c | nt |
| 17 (p = 2) | 3752c | nt |
| 19 | >10000c | nt |
| 20 | >10000c | nt |
| 23a (q = 2) | 285 ± 41e | >2000 |
| 23b (q = 3) | 58 ± 19e | 192 |
| 23c (q = 4) | 1687c | nt |
| 24 | 6016c | nt |
| 25 | >10000c | nt |
| 26 | >10000c | nt |
| 27 | 70 ± 12e | 116 |
| 28 | 5777c | nt |
| 31 | >10000c | nt |
| 1 | 0.5 ± 0.1e | 105 |
| 2 | 18 ± 2e | nt |
EC50 values (cell-based) were determined using an MTT assay of human Raji lymphoma cell proliferation.
IC50 values were determined by a labeled lactate transport assay using MCF7 breast cancer cells engineered to overexpress MCT1.
Value from one cell growth experiment, run in triplicate
“nt” = not tested
With standard error of mean, from at least three independent Raji MTT experiments, each run in triplicate.
The remaining compounds presented in Table 1 are the 6,7-substituted pteridine diones. Compound 23b, which like compound 15c also has R1 with a five-atom tether to the primary hydroxyl group (q = 3), showed strong growth inhibition in the MTT assay (EC50 = 58 ± 10 nM). Compound 23a, with one less methylene group, was less active (EC50 = 285 ± 41 nM), while compound 23c, with one more methylene group, had even lower activity (EC50 ∼ 1.7 μM). These findings mirror the results of compounds 14a–d and 15a–c, where proper positioning of the side chain hydroxyl group in R1 is critical for maximizing activity. Incorporation of an NH or NMe triazole moiety at the 6-position led to a loss in potency (compounds 24–26). The cis olefin 27 (EC50 = 70 ± 12 nM) was roughly equipotent to its saturated analogue 23b. The methyl ether analogue 28 was largely inactive; thus, the H-bond donating hydroxyl group is an essential component of the MCT1 inhibitor pharmacophore.
Compound 31 was designed based upon clinical compound 2, so quite to our surprise it was inactive in the Raji lymphoma cell proliferation assay. Thus, different binding preferences exist for the pteridine dione scaffold 7 versus the thienopyrimidine diones (e.g., compound 2).49 Another scaffold difference is that the sulfur atom in compound 1 cannot be replaced with a methylene group and retain potency (not shown), whereas compound 23b, with an all-carbon link to the hydroxyl group, has similar potency to its thioether analogue 15c. Finally, data for compounds 1–2 are shown in Table 1 for comparison. Our most potent new compounds in the MTT assay are compounds 15c, 23b, and 27, which displayed a ca. 2–4-fold higher EC50 than did the clinical compound 2, which, although less potent than compound 1, is presumably preferable due to DMPK and drug-likeness50 criteria.
Compounds showing activity in the MTT assay were then tested for their ability to inhibit the transport of 14C-lactate in MCF7 breast cancer cells engineered to overexpress MCT14 (right column, Table 1). The antiproliferative effects of test compounds, including potent inhibitors 14b, 15b, 15c, 23b, 27, and 1, on MCT1-expressing tumor cells generally correlates with their ability to inhibit lactate transport, supporting the proposed mode of action for compounds in these distinct chemical series.47 Our most potent compound in the lactate transport assay is 27, which is roughly equal in IC50 to compound 1, the compound in Table 1 that is most potent in both of the assays shown. When our new compounds tested in both of these assays are ranked by potency in the lactate transport assay, the order is 27 > 23b > 14b ≈ 15c ≈ 15b > 23a ≈ 14a. When the same compounds are instead ranked by potency in the MTT assay, the order is 15c ≈ 23b ≈ 27 > 14b ≈ 15b > 23a > 14a. Thus, rank orders generally correlate, with the exception being the high potency of compound 15c in the MTT assay. While rank ordering for compound 27 differs, it is quite effective in both assays.
The results of MTT assays in MCF7_MCT1 and MCF7_MCT4 cells using a set of top-performing compounds are shown in Figure 3. As shown in panel A, all three new compounds were cytotoxic. Compound 1 was more effective, although the potency (EC50 value) was reduced in MCF7_MCT1 cells versus Raji lymphoma, with values that were more similar to IC50 values obtained in the MCF7_MCT1 lactate transport assay. As shown in panel B, MCF7_MCT4 cells were unaffected by all test compounds, except perhaps compound 27 at the highest dose tested (10 μM). Thus, these compounds selectively inhibit MCT1 but not MCT4 and off-target effects do not contribute to the performance of compound 15c in the Raji MTT assay.
Discussion and Conclusions
Our SAR studies have established that several newly designed pteridinones impair Raji lymphoma cell proliferation at submicromolar doses and that their potency generally correlates with their ability to inhibit lactate transport. Notably, our SAR analyses indicate that (i) side chains at either C5 or C6 (R1 in scaffolds 6–7) bearing an appropriately positioned hydroxyl group are essential for potent MCT1 inhibition in these scaffolds, and (ii) compounds having the hydroxyl group present in alkyl and thioether-containing tethers are also active. In contrast, related sulfoxides, sulfones, amides, and triazoles are weakly active or inactive.
Previous potent anti-MCT1 scaffolds (compounds 1–2) have [6,5]-fused ring systems. The new compounds reported here (see Table 1) with a [6,6]-fused ring system are novel. These small molecules, like other compounds described as MCT1 inhibitors, are likely dual MCT1/MCT2 inhibitors, although we have not specifically measured activity at MCT2. Further improvements in the series are needed to augment their potency and to enhance their physical properties. The SAR trends in the [6,5]- and [6,6]-systems diverge, and these altered substituent effects may provide new opportunities in MCT inhibitor design. Importantly, the synthetic methods shown are also versatile and will allow additional SAR development: the R1, R2, R3, and R4 groups in scaffolds 6–7 can be selectively and systematically altered.
Efforts to revise the synthesis strategy shown herein are underway and will permit more extensive investigation of SAR. Analyses of additional highly biologically active compounds in these scaffolds, including new synthetic methodologies, DMPK studies,51 animal efficacy studies, and computational efforts to define the mode of interaction of these inhibitors with MCT1, as well as the design of MCT4 inhibitors, are a focus of our current investigations.
Experimental Section
Chemistry Methods
Reactions were performed in flame-dried glassware under positive pressure of N2 or Ar. Anhydrous THF, Et2O, DMF, CH3CN, and DCM were purchased from Aldrich and used as received. Commercial reagents were used without further purification. Flash chromatography was performed on 40–63 μm silica. All reported compounds passed a purity standard of ≥95% (HPLC). Analytical HPLC was performed on Agilent Technologies 1200 series instruments with CH3CN/water gradient mixtures as eluent and 0.1% TFA as modifier. The targeted products were detected by UV in the detection range of 215–310 nm. NMR spectra were recorded at on Brüker instruments at 400 MHz (1H) or 100 MHz (13C) in CDCl3, unless otherwise stated. Preparative and analytical HPLC and LCMS analysis and microwave-promoted reactions were performed using standard techniques and commercial instruments, listed in the Supporting Information, which provides complete experimental details. Methods for synthesis of specific key compounds include:
Methyl-2-((3-Isobutyl-1-methyl-5-nitro-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)amino)-3-(naphthalene-1-yl)propanoate (11)
A mixture of 9 (1.44 g, 5.51 mmol), 10 (1.54 g, 5.78 mmol), and Na2CO3 (1.46 g, 13.8 mmol) in DMF (11.0 mL) was heated to 65 °C for 2 h, cooled to room temperature, quenched with saturated NH4Cl, and extracted with ethyl acetate (EA). The combined extracts were washed with brine 3× and dried over Na2SO4. The solvent was removed and the residue was purified by flash column chromatography (hexanes:EA = 3:2) to afford 1.69 g (67%) of 11 as a yellow oil. Rf = 0.50 (hexanes:EA = 1:1). LC-MS (ESI): m/z 455 [M + 1]+. 1H NMR δ (ppm) 0.36 (d, J = 6.6 Hz, 3H), 0.44 (d, J = 6.6 Hz, 3H), 1.55 (sep, J = 6.6 Hz, 1H), 2.85–2.91 (m, 1H), 3.24 (s, 3H), 3.33–3.37 (m, 2H), 3.86 (s, 3H), 3.98–4.03 (m, 1H), 4.58–4.66 (m, 1H), 7.34 (d, J = 6.8 Hz, 1H), 7.42–7.46 (m, 1H), 7.53–7.61 (m, 2H), 7.83 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.0 Hz, 1H), 8.02 (d, J = 8.0 Hz, 1H). 13C NMR δ (ppm) 19.0, 19.1, 26.8, 28.4, 36.1, 53.4, 53.6, 59.8, 116.5, 122.5, 125.5, 126.2, 126.9, 128.4, 128.7, 129.2, 130.8, 131.4, 133.9, 149.0, 154.9, 155.2, 170.6.
1-Isobutyl-3-methyl-7-(naphthalene-1-ylmethyl)-2,4-dioxo-1,2,3,4-tetrahydropterdin-6-yl Trifluoromethanesulfonate (13)
A suspension of 12 (2.3 g, 5.9 mmol) in DCM (60 mL) was cooled to 0 °C under N2. Tf2O (3.0 g, 10.6 mmol) was added, followed by Et3N (1.8 g, 17.6 mmol). The mixture was stirred 1h at 0 °C, quenched with saturated NH4Cl, extracted with DCM, washed with brine, and dried over Na2SO4. The solvent was removed, and the residue was purified by flash column chromatography (hexanes:EA = 4:1 to 2:1) to afford 3.01 g (98%) of 13 as a yellow solid. Rf = 0.25 (hexanes:EA = 4:1). LC-MS (ESI): m/z 523 [M + 1]+. 1H NMR δ (ppm) 0.50 (d, J = 6.8 Hz, 6H), 1.89 (sep, J = 6.8 Hz, 1H), 3.47 (s, 3H), 3.65 (d, J = 7.6 Hz, 2H), 4.80 (s, 2H), 7.47–7.52 (m, 4H), 7.85–7.93 (m, 3H). 19F NMR δ (ppm) −71.8. 13C NMR δ (ppm) 19.4, 26.6, 29.0, 36.3, 49.9, 117.0, 120.2, 122.3, 123.8, 125.5, 125.9, 126.5, 128.5, 129.0, 131.0, 132.0, 134.0, 146.1, 147.1, 150.2, 153.5, 158.2.
1-Isobutyl-3-methyl-7-(naphthalene-1-ylmethyl)-2,4-dioxo-1,2,3,4-tetrahydropteridine-6-carboxylic Acid (18)
Step 1: A mixture of 13 (314 mg, 0.60 mmol), Zn(CN)2 (70 mg, 0.60 mmol), and Zn (8.0 mg, 0.12 mmol) in NMP (6.0 mL) was degassed. Pd(PPh3)4 (69 mg, 0.06 mmol) was added, and the mixture was stirred at 100 °C for 24 h, cooled to rt, quenched with NH4Cl, and extracted with EA. The combined extracts were washed with brine, dried over Na2SO4, concentrated, and purified by flash column chromatography (hexanes:EA = 3:1) to give 151 mg (63%) of 1-isobutyl-3-methyl-7-(naphthalene-1-ylmethyl)-2,4-dioxo-1,2,3,4-tetrahydropteridine-6-carbonitrile as a yellow solid. LC-MS (ESI): m/z 400 [M + 1]+. 1H NMR δ (ppm) 0.55 (d, J = 6.8 Hz, 6H), 1.66 (sep, J = 6.8 Hz, 1H), 3.50 (s, 3H), 3.72 (d, J = 7.6 Hz, 2H), 4.94 (s, 2H), 7.47–7.56 (m, 4H), 7.85–8.01 (m, 3H). Step 2: A solution of 1-isobutyl-3-methyl-7-(naphthalene-1-ylmethyl)-2,4-dioxo-1,2,3,4-tetrahydropteridine-6-carbonitrile (145 mg, 0.363 mmol) in 1,4-dioxane (18 mL) was treated with H2SO4 (11 mL, 60%). The resultant mixture was stirred at 110 °C for 60 h, cooled to room temperature, diluted with H2O, and extracted with EA. The combined organic extracts were washed with brine and dried over Na2SO4. The solution was concentrated to afford 137 mg (90%) of 18 as a brown solid. LC-MS (ESI): m/z 419 [M + 1]+. 1H NMR (DMSO-d6) δ (ppm) 0.34 (d, J = 6.8 Hz, 6H), 1.45 (sep, J = 6.8 Hz, 1H), 3.26 (s, 3H), 3.41 (d, J = 7.6 Hz, 2H), 3.41 (t, J = 7.6 Hz, 2H), 5.05 (s, 2H), 7.41–7.52 (m, 4H), 7.86–7,98 (m, 3H).
6-(5-(Hydroxypent-1-yn-1-yl)-1-isobutyl-3-methyl-7-(naphthalen-1-ylmethyl)pteridine-2,4-(1H,3H)-dione (22b)
A mixture of 13 (261 mg, 0.50 mmol), 21b (50 mg, 0.60 mmol), and CuI (9.5 mg, 0.05 mmol) in DMF (7.5 mL) was degassed. Pd(PPh3)4 (29 mg, 0.025 mmol) was added, followed by Et3N (506 mg, 5.0 mmol). The mixture was again degassed and heated by microwave to 175 °C for 20 min. The reaction mixture was diluted with EA, washed with brine, dried over Na2SO4, concentrated, and purified by flash column chromatography (hexanes:EA = 2:3) to give 105 mg (46%) of 22b as a yellow solid. Rf = 0.10 (hexanes:EA = 1:1). LC-MS (ESI): m/z 457 [M + 1]+. 1H NMR δ (ppm) 0.39 (d, J = 6.8 Hz, 6H), 1.52 (sep, J = 6.8 Hz, 1H), 1.81–1.87 (m, 2H), 2.62 (t, J = 7.0 Hz, 2H), 3.38 (s, 3H), 3.57 (d, J = 7.6 Hz, 2H), 3.70–3.74 (m, 2H), 4.77 (s, 2H), 7.31–7.40 (m, 4H), 7.71–7.84 (m, 3H). 13C NMR δ (ppm) 16.3, 19.4, 26.6, 29.0, 30.8, 39.0, 49.1, 61.3, 77.3, 98.4, 124.1, 124.5, 125.4, 125.7, 126.2, 127.9, 128.0, 128.8, 132.1, 133.2, 133.9, 134.6, 145.4, 150.4, 159.6, 161.8.
6-(5-(Hydroxypentyl)-1-isobutyl-3-methyl-7-(naphthalen-1-ylmethyl)pteridine-2,4-(1H,3H)-dione (23b)
A solution of 22b (20 mg, 0.044 mmol) in THF (2.0 mL) was treated with 10% Pd/C (5.0 mg). The mixture was stirred 5 h under H2, filtered, concentrated, purified by flash column chromatography (hexanes:EA = 2:3), and then by preparative HPLC to afford 10 mg of 23b as a white solid. A >97% purity was determined by analytical HPLC. LC-MS (ESI): m/z 461 [M + 1]+. 1H NMR δ (ppm) 0.37 (d, J = 6.8 Hz, 6H), 1.43–1.57 (m, 4H), 1.78–1.81 (m, 3H), 3.01 (t, J = 7.4 Hz, 2H), 3.39 (s, 3H), 3.52 (d, J = 7.6 Hz, 2H), 3.60–3.70 (m, 2H), 4.63 (s, 2H), 7.14 (d, J = 6.8 Hz, 1H), 7.33–7.43 (m, 3H), 7.69–7.83 (m, 3H).
Biological Assay Methods
Raji Lymphoma Cell MTT Assay
Methods used were as previously described.4 In brief, human Raji Burkitt lymphoma cells were seeded in 96-well plates at 20000 cells/well and were cultured ± compounds generated herein, or with vehicle, in 5% CO2 incubator for 4 days. MTT reagents (Millipore CT0-A) were added at 10 μL per well, and cells were incubated for 5 h. Then 100 μL of 2-propanol/0.04 N HCl was added to each well, pipetted to mix, and plates were read using Biotek Synergy II plate reader at 570 and 630 nm. Experiments were performed in triplicate, and assays of the most active compounds were repeated three times. Representative data are shown in figures. EC50 values were determined using GraphPad Prism software.
MCF7_MCT1 14C-Lactate Transport Assay
Methods used were as previously described.4 Briefly, MCF7 breast cancer cells engineered to overexpress MCT1 were cultured at 35000 cell/well in 24-well plates for 2 days.4 Assays were performed on ice by removing medium and washing once with cold buffer (150 mM NaCl, 10 mM Hepes, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 0.2% BSA at pH 7.4) containing compound (1 nM to 1 μM) or DMSO for 5 min. Cells were incubated in 200 μL of cold buffer containing 0.5 μCi/well l-14C(U)-lactic acid, sodium salt (PerkinElmer) and compounds for 10 min on ice. Cells were washed three times in cold buffer containing 0.1 mM Pholoretin (Sigma) and lysed with 200 μL of 0.1 M NaOH for 30 min at room temperature. Radioactivity was measured by scintillation counting. Samples were done in triplicate, and the log of compound concentration versus CPM incorporated was plotted using GraphPad Prism and used to calculate the IC50 for each compound.
MCF7_MCT1 and MCF7_MCT4MTT Assays
MCF7_MCT1 and MCF7_MCT4 cells4 were seeded in a 96-well plate at 2000/well. The following day, compounds were added and cultured for 4 days. All methods for culture and cell-handling followed the procedure that was described above for the Raji lymphoma-based MTT assays.
Acknowledgments
This research is supported in part by a grant from the NIH (R01CA154739). We thank Dr. Jitendra Mishra, Dr. Reji Nair, and Dr. Andy Tsai for the synthesis of reference compounds 1 and 2.
Glossary
Abbreviations Used
- DCE
dichloroethane
- DCM
dichloromethane
- DDQ
2,3-dichloro-5,6-dicyano-1,4-benzoquinone
- DMF
dimethylformamide
- DMPK
drug metabolism and pharmacokinetics
- EA
ethyl acetate
- h
hours
- MCF7
Michigan Cancer Foundation-7
- MCT
monocarboxylate transporter
- min
minutes
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NMP
N-methyl 2-pyrrolidone
- rt
room temperature
- SAR
structure–activity relationships
- SLC
solute carrier
- TEA
triethylamine
- Tf
trifluoromethanesulfonyl
- TFA
trifluoroacetic acid
Supporting Information Available
Additional experimental details and NMR spectra assignments for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
§ C.Y. and J.L.C.: Department of Tumor Biology, Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa, Florida 33612, United States.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Warburg O. On the origin of cancer cells. Science 1956, 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H.; Bounds P. L.; Dang C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature Rev. Cancer 2011, 11, 325–327. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The SLC16 gene family—structure, role and regulation in health and disease. Mol. Aspects Med. 2013, 34, 337–349. [DOI] [PubMed] [Google Scholar]
- Doherty J. R.; Yang C.; Scott K.; Cameron M. D.; Fallahi M.; Li W.; Hall M. A.; Amelio A. L.; Mishra J. K.; Li F.; Tortosa M.; Genau H. M.; Rounbehler R. J.; Yungi L.; Dang C. V.; Kumar K. G.; Butler A. A.; Bannister T. D.; Hooper A. T.; Unsal-Kacmaz K.; Roush W. R.; Cleveland J. L. Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014, 74, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M. S.; Davies A. J.; Halestrap A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [DOI] [PubMed] [Google Scholar]
- Dang C. V. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found. Symp. Proc. 2007, 35–53. [DOI] [PubMed] [Google Scholar]
- Vaupel P.; Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [DOI] [PubMed] [Google Scholar]
- Kizaka-Kondoh S.; Inoue M.; Harada H.; Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003, 94, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floch R.; Chiche J.; Marchiq I.; Naiken T.; Ilk K.; Murray C. M.; Critchlow S. E.; Roux D.; Simon M. P.; Pouyssegur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P.; Vegran F.; Schroeder T.; Wergin M. C.; Verrax J.; Rabbani Z. N.; De Saedeleer C. J.; Kennedy K. M.; Diepart C.; Jordan B. F.; Kelley M. J.; Gallez B.; Wahl M. L.; Feron O.; Dewhirst M. W. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008, 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S.; Schneider H.; Broer A.; Rahman B.; Hamprecht B.; Deitmer J. W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V. N.; Halestrap A. P. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J. Biol. Chem. 1996, 271, 861–868. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Itagaki S.; Hirano T.; Iseki K. mechanism of l-lactic acid transport in L6 skeletal muscle cells. Drug Metab. Pharmacokinet. 2004, 19, 363–368. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Morris M. E. Flavonoids modulate monocarboxylate transporter-1-mediated transport of γ-hydroxybutyrate in vitro and in vivo. Drug Metab. Dispos. 2007, 35, 201–208. [DOI] [PubMed] [Google Scholar]
- Draoui N.; Schicke O.; Fernandes A.; Drozak X.; Fady N.; Dumont A.; Douxfils J.; Hermans E.; Dogné J.-M.; Corbau R.; Marchand A.; Chaltin P.; Sonveaux P.; Feron O.; Riant O. Synthesis and pharmacological evaluation of carboxycoumarins as a new antitumor treatment targeting lactate transport in cancer cells. Bioorg. Med. Chem. 2013, 21, 7107–7117. [DOI] [PubMed] [Google Scholar]
- Mereddy V. R.; Drewes L. R.; Alam M. A.; Jonnalagadda S. K.; Gurrapu S.. Preparation of benzopyran derivatives and related compounds as MCT1 inhibitors. PCT Int. Appl. WO2013109972 A2, 2013.
- Murray C. M.; Hutchinson R.; Bantick J. R.; Belfield G. P.; Benjamin A. D.; Brazma D.; Bundick R. V.; Cook I. D.; Craggs R. I.; Edwards S.; Evans L. R.; Harrison R.; Holness E.; Jackson A. P.; Jackson C. G.; Kingston L. P.; Perry M. W. D.; Ross A. R. J.; Rugman P. A.; Sidhu S. S.; Sullivan M.; Taylor-Fishwick D. A.; Walker P. C.; Whitehead Y. M.; Wilkinson D. J.; Wright A.; Donald D. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nature Chem. Biol. 2005, 1, 371–376. [DOI] [PubMed] [Google Scholar]
- Guile S. D.; Bantick J. R.; Cheshire D. R.; Cooper M. E.; Davis A. M.; Donald D. K.; Evans R.; Eyssade C.; Ferguson D. D.; Hill S.; Hutchinson R.; Ingall A. H.; Kingston L. P.; Marin I.; Martin B. P.; Mohammed R. T.; Murry C.; Perry M. W. D.; Reynolds R. H.; Thorne P. V.; Wilkinson D. J.; Withnall J. Potent blockers of the monocarboxylate transporter MCT1: novel immunomodulatory compounds. Bioorg. Med. Chem. Lett. 2006, 16, 2260–2265. [DOI] [PubMed] [Google Scholar]
- Guile S. D.; Bantick J. R.; Cooper M. E.; Donald D. K.; Eyssade C.; Ingall A. H.; Lewis R. J.; Martin B. P.; Mohammed R. T.; Potter T. J.; Reynolds R. H.; St-Gallay S. A.; Wright A. D. Optimization of monocarboxylate transporter 1 blockers through analysis and modulation of atropisomer interconversion properties. J. Med. Chem. 2007, 50, 254–263. [DOI] [PubMed] [Google Scholar]
- Bueno V.; Binet I.; Steger U.; Bundick R.; Ferguson D.; Murray C.; Donald D.; Wood K. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation 2007, 84, 1204–1207. [DOI] [PubMed] [Google Scholar]
- Ovens M. J.; Davies A. J.; Wilson M. C.; Murray C. M.; Halestrap A. P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem. J. 2010, 425, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow S. E.; Tate L.. Use of a MCT1 inhibitor in the treatment of cancers expressing MCT1 over MCT4. PCT Int. Appl. WO2010089580 A1 20100812, 2010.
- A phase I trial of AZD3965 in patients with advanced cancer; U.S. National Library of Medicine, U.S. National Institutes of Health: Bethesda, MD, 2014; http://clinicaltrials.gov/show/NCT01791595 (Accessed July 25, 2014).
- Polanski R.; Hodgkinson C. L.; Fusi A.; Nonaka D.; Priest L.; Kelly P.; Trapani F.; Bishop P. W.; White A.; Critchlow S. E.; Smith P. D.; Blackhall F.; Dive C.; Morrow C. J. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 2014, 20, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R.; Bannister T. D. Grubbs cross-metathesis pathway for a scalable synthesis of γ-keto α,β-unsaturated esters. J. Org. Chem. 2014, 79, 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Wang G.; Wang C.; Ye W. Three new pteridines from the leech Whitmania pigra. Chem. Lett. 2013, 42, 983–985. [Google Scholar]
- Meyer S. W.; Mordhorst T. F.; Lee C.; Jensen P. R.; Fenical W.; Koeck M. Penilumamide, a novel lumazine peptide isolated from the marine-derived fungus, Penicillium sp. CNL-338. Org. Biomol. Chem. 2010, 8, 2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voerman G.; Cavalli S.; Van der Marel G. A.; Pfleiderer W.; Van Boom J. H.; Filippov D. V. 1,3-Dimethyllumazine derivatives from Limnatis nilotica. J. Nat. Prod. 2005, 68, 938–941. [DOI] [PubMed] [Google Scholar]
- Sato N.; Fukuya S. Studies on pyrazines. Part 37. Synthesis of 6-propionylpteridine-2,4(1H,3H)-dione and its 1- and/or 3-methyl derivatives from marine natural products. J. Chem. Soc., Perkin Trans. 1 2000, 89–95. [Google Scholar]
- Hanaya T.; Yamamoto H. Synthetic studies on natural pterin glycosides. Heterocycles 2012, 85, 2375–2390. [Google Scholar]
- Prins L. H. A.; Petzer J. P.; Malan S. F. Synthesis and in vitro evaluation of pteridine analogues as monoamine oxidase B and nitric oxide synthase inhibitors. Bioorg. Med. Chem. 2009, 17, 7523–7530. [DOI] [PubMed] [Google Scholar]
- Murata S.; Kiguchi K.; Sugimoto T. Recent advances in selective syntheses of 6- and 7-substituted pteridines. Heterocycles 1998, 48, 1255–1274. [Google Scholar]
- Gulevskaya A. V.; Dang S. V.; Tyaglivy A. S.; Pozharskii A. F.; Kazheva O. N.; Chekhlov A. N.; Dyachenko O. A. A novel tandem cyclization of condensed 2,3-dialkynylpyrazines into [1,2,3]triazolo[1′,5′;1,2]pyrazines promoted by sodium azide. Tetrahedron 2010, 66, 146–151. [Google Scholar]
- Zheng L.; Xiang J.; Dang Q.; Guo S.; Bai X. Novel heterocyclic scaffold consisting of indole-fused pteridines. J. Comb. Chem. 2005, 7, 813–815. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Girardet J.; Smith K. L.; Larson G.; Prigaro B.; Lai V. C. H.; Zhong W.; Wu J. Z. Parallel synthesis of pteridine derivatives as potent inhibitors for hepatitis C virus NS5B RNA-dependent RNA polymerase. Bioorg. Med. Chem. Lett. 2005, 15, 675–678. [DOI] [PubMed] [Google Scholar]
- Gulevskaya A. V.; Dang S. V.; Pozharskii A. F. Synthesis and heterocyclizations of 3-alkynyl-6,8-dimethylpyrimido-[4,5-c]pyridazine-5,7-(6H,8H)-diones and their lumazine analogues. J. Heterocycl. Chem. 2005, 42, 413–419. [Google Scholar]
- Tada M.; Asawa Y.; Igarashi M. Synthesis of pteridine derivatives related to folic acid and methanopterin from pyrazine-2,3-dicarbonitrile. J. Heterocycl. Chem. 1997, 34, 973–981. [Google Scholar]
- Suckling C. J. The diversity-oriented synthesis of pteridines—achievements and potential for development. IUBMB Life 2013, 65, 283–299. [DOI] [PubMed] [Google Scholar]
- Liao T. K.; Cheng C. C. 1,3-Dimethyl-5-nitro-6-chlorouracil. J. Heterocycl. Chem. 1964, 1, 212. [Google Scholar]
- Groutas W. C.; Brubaker M. J.; Zandler M. E.; Mazo-Gray V.; Rude S. A.; Crowley J. P.; Castrisos J. C.; Dunshee D. A.; Giri P. K. Inactivation of leukocyte elastase by aryl azolides and sulfonate salts. Structure–activity relationship studies. J. Med. Chem. 1986, 29, 1302–1305. [DOI] [PubMed] [Google Scholar]
- Erhardt S.; Grushin V. V.; Kilpatrick A. H.; Macgregor S. A.; Marshall W. J.; Roe D. C. Mechanisms of catalyst poisoning in palladium-catalyzed cyanation of haloarenes. Remarkably facile C–N bond activation in the [(Ph3P)4Pd]/[Bu4N]+CN– system. J. Am. Chem. Soc. 2008, 130, 4828–4845. [DOI] [PubMed] [Google Scholar]
- Tamao K.; Miyaura N. Introduction to cross-coupling reactions. Top. Curr. Chem. 2002, 219, 1–9. [Google Scholar]
- Kolb H. C.; Sharpless K. B. The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128–1137. [DOI] [PubMed] [Google Scholar]
- Lubell W.; Rapoport H. Surrogates for chiral aminomalondialdehyde. Synthesis of N-(9-phenylfluoren-9-yl)serinal and N-(9-phenylfluoren-9-yl)vinylglycinal. J. Org. Chem. 1989, 54, 3824–3831. [Google Scholar]
- Wei L.; Lubell W. Scope and limitations in the use of N-(PhF)serine-derived cyclic sulfamidates for amino acid synthesis. Can. J. Chem. 2001, 79, 94–104. [Google Scholar]
- Garcia C. K.; Goldstein J. L.; Pathak R. K.; Anderson R. G. W.; Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 1994, 76, 865–873. [DOI] [PubMed] [Google Scholar]
- The low EC50 values seen in the MTT assay relative to the IC50 values seen in the lactate transport assay may arise from several factors. It is important to note that the Raji cells rely heavily on glycolysis for rapid proliferation. Over the 96 h treatment window in the MTT assay, even partial MCT1 inhibition may greatly impede proliferation by preventing cells from meeting their energy needs. Also, in comparing panel A of Figure 3 with the right column in Table 1, when the same cell type is used in both assays, the MTT and 14C-lactate transport assay results are more aligned.
- Uetrecht J. P.; Trager W.. Drug Metabolism: Chemical and Enzymatic Aspects; Informa Healthcare USA, Inc.: New York, 2007; pp 100–101. [Google Scholar]
- Compounds 1 and 2 appear to have complementary SAR. For example we found that hybrids, such as the C7 pyrazole-containing analogue of compound 1 and the C7 naphthylmethyl analogue of compound 2 (structures and data not shown) are highly cytotoxic in the Raji cell/Burkitt B cell lymphoma MTT assay.
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [DOI] [PubMed] [Google Scholar]
- We routinely perform DMPK analyses before initiating animal studies. None are included in this report because no compounds meet the potency criteria that we have established for progression to animal studies: meeting or exceeding all activity criteria of clinical compound 2, although we believe these compounds constitute a significant advance because they define a new MCT1 inhibitor scaffold with divergent SAR.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.