Abstract
Liver-directed gene transfer and gene therapy are rapidly gaining attention primarily because the liver is centrally involved in a variety of metabolic functions that are affected in various inherited disorders. Recombinant adeno-associated virus (rAAV) is a popular gene delivery vehicle for gene therapy and intravenous delivery of some rAAV serotypes results in very efficient transduction of the liver. rAAV-mediated and liver-directed gene transfer can help in creating somatic transgenic animals or disease models and studying the function of various genes and miRNAs. The liver is the target tissue for gene therapy of many inborn metabolic diseases and may also be exploited as a “bio-factory” for the production of coagulation factors, insulin and growth hormones and other non-hepatic proteins. Hence efficient delivery of transgenes and small RNAs to the liver by rAAV vectors has been of long-standing interest to research scientists and clinicians alike.
Keywords: Adeno-associated Virus, Liver, Gene Transfer, Gene therapy
INTRODUCTION
Adeno-associated viruses (rAAVs) are a diverse collection of non-pathogenic, naturally replication-deficient single stranded parvoviruses. They generally persist in primates and were initially discovered as contaminants of adenovirus cultures. Many of the extensive studies on recombinant AAVs (rAAVs) were conducted on the common laboratory variant, AAV serotype 2, until the discovery of over 100 novel serotypes, many of which can be exploited as novel vectors for safe and persistent gene transfer with a variety of cell and tissue tropisms (Gao et al., 2003; Gao et al., 2004; Gao et al., 2005; Gao et al., 2002; Vandenberghe et al., 2009; Xiao et al., 1999). An important consideration in exploiting rAAVs for gene therapy has been that these viruses are capable of persistence in tissues for long durations without activating the host immune system. Viral capsids are major determinants of the characteristics of an rAAV serotype which include their preference for tissue, efficiency of gene transfer and ability to activate immune responses.
One of the major reasons for targeting liver by rAAVs is that the liver is the primary target for systemically delivered rAAVs irrespective of the serotypes used. Extensive studies on liver-tropic rAAVs have revealed that AAV8 derived vector with either a single-stranded or a double stranded viral genome outperformed all other rAAV serotypes (Gao et al., 2005) in transducing hepatocytes in vivo. The transduced rAAV proviral genomes mostly persist episomally in the target cells (Duan et al., 1998; McCarty et al., 2004), therefore leading to unstable transduction after cell division. However, liver is primarily a terminally differentiated organ which would not affect stability of these episomally persistant rAAV genomes, allowing long term transgene expression (Sun et al., 2010). Liver directed gene therapy with rAAV2 was first used in clinical trials (Manno et al., 2006) for hemophilia. The rAAV2-mediated liver-directed hemophilia gene therapy resulted in a transient increase in hepatic enzymes indicating T-cell activation which resulted in the loss of transgene expression (Manno et al., 2006; Mingozzi et al., 2007). This result may be correlated to the fact that adaptive immune responses vary with the dose as well as serotype of AAV capsid used; suggesting that a liver-tropic rAAV may need a lower dose to result in effective liver-directed therapy. This conundrum would be well addressed by the vast repertoire of rAAVs that have emerged in the past decade that vary in biodistribution and tissue and cell tropism. A recent hemophilia B gene therapy trial using rAAV8 vector to target liver that led to sufficient FIX transgene expression to improve the disease phenotype (Nathwani et al., 2011), gives strong support to this theory.
In this unit, we present protocols for delivery of rAAV vectors via the lateral tail vein, retro-orbital sinus and intraportal vein and intrasplenic injections in adult mice and methods to detect and quantify transgene expression in the liver.
BASIC PROTOCOL 1: SAMPLE PREPARATION FOR INJECTION
High quality of rAAV vector formulations for injection should be free of contaminants that might harm animals, such as bacteria, endotoxins, cellular protein and empty virions. Cesium chloride (CsCl) (Zhou and Muzyczka, 1998) or iodixanol (Lock et al., 2010) gradient centrifugation and affinity column purification methods (Gao et al., 2000) are standard protocols for rAAV vector purification. Analysis of rAAV purity by silver-stained SDS-PAGE and electron microscopy is recommended before sample preparation for injection. All solutions used for vector dilution should be tissue culture grade. To avoid contamination, it is important to prepare vector samples in the culture hood dedicated for rAAVs. Calculation of vector dosage is extremely critical for any injection procedure involving rAAVs. For details on calculation of vector genome copy number please see CPMC Unit 14D.1. We have found that intravenous injections in the range of 3×1010 - 4×1011 (optimum 1×1011) vector genomes per animal with most rAAV serotypes results in an efficient transduction of the liver. Since different serotypes have varied tropism to the liver, the final injection amount of vector genomes would need to be optimized by the researcher. To reduce variation, vectors should be diluted in 0.9% saline or Phosphate Buffered Saline (PBS) to keep within the recommended injection volume range during sample preparation and use the same final volume for injection for different study groups. The following table can serve as a guideline for determining dilution criteria for 6–8 week old mice.
| Route of administration | Injection volume range |
|---|---|
| Lateral tail vein | 75–µl |
| Retro-orbital sinus | 50–100µl |
| Portal vein | 20–100µl |
| Intrasplenic injection | 20–100µl |
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
rAAV vectors [rAAVs can be produced in-house (see Unit 14D.1), through a local vector core or via a commercial manufacturer]
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 15 ml conical tubes (Corning Corp.)
Sterile 50 ml conical tubes (Corning Corp.)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
Microcentrifuge (Thermo Sci)
Culture hood (Labconco)
Calculate the amounts of rAAV vector needed in the experiments based on vector dose, vector titer, injection route and animal numbers using the table provided as a guideline. If the vector titer is too high, make serial dilutions of the vector. The final injection volume should be consistent across study groups.
Use sterile tubes of appropriate sizes to dilute the vector in the culture hood dedicated for rAAV. Calculate the volumes and dilute the rAAV vectors in sterile D-PBS appropriately Centrifuge the vector containing vial for a short time before use. Aliquot the dosing vector as needed and keep the vectors on ice.
VECTOR DELIVERY METHODS
The mouse (Mus musculus) is the most commonly used animal model for in vivo gene transfer studies. Intravenous (IV) injections through lateral tail vein, retro-orbital sinus and portal vein as well as intrasplenic injections can deliver the viral vectors efficiently into liver. These techniques are used widely in current gene transfer studies in mice and are discussed in detail in the section below.
BASIC PROTOCOL 2: VECTOR DELIVERY BY LATERAL TAIL VEIN INJECTION
The lateral tail vein injection is the most common and efficient method to deliver viral vectors to the liver. The method requires considerable skill and patience, hence researchers should devote time to practice sessions using PBS as injectate. The maximum volume for injection depends on the size of the mouse ranging from 0.1–0.3 ml for mice weighing 20–40 g. A standard veterinary recommendation is usually to limit the injection to 0.25 ml and most users try to inject the vector for 0.1–0.2 ml. In our experience, it is almost impossible to deliver the vectors into young mice less than 4 weeks old by i.v. injection via tail vein hence alternative methods need to be considered. Details about other injection methods are also available in CPI Unit 1.6.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
rAAV vectors [rAAVs can be produced in-house (see Unit 14D.1), through a local vector core or via a commercial manufacturer ]
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
Circulating warm water heating pad (Kent Scientific Corp.) or Heat lamp (60 W) (OSRAM Sylvania Products Inc.)
1ml syringe with a 28 or 30 G needle (Fisher Sci)
Mouse restraining device (Dan-Kar Corp.)
Obtain Institutional Animal Care and Use Committee (IACUC) approval for the protocol.
Put the experimental mouse in a restrainer on a circulating warm water heating pad and soak the tail in warm water (less than 50°C) or warm the tail for 5 mins using a heat lamp (60 W) to dilate the vessels and increase blood flow to the tail vein.
Swab the tail with gauze dampened with ethanol and identify the lateral tail vein on either side (Fig 1).
Use a 1 ml syringe with a 28 or 30G needle for the injection. Fill syringe with injectate and remove air bubbles. Removal of air bubbles is critical to avoid air embolism. With the bevel of the needle facing upward and the needle almost parallel to the vein, slide the needle about 2 mm into the tail vein and gently pull the plunger. You should see a flash of blood in the hub of the needle.
After confirming the entry of the needle into the vein, slowly inject the rAAV vector suspension (0.1–0.2 ml) into the tail vein. No bleb should form if needle is properly located. If a bleb appears, indicating failure to cannulate the vein, additional attempts may be made proximally. Thus it is helpful to make the first attempt at injection as close to the tip of the tail as possible.
After removing the needle, use gauze to stop the bleeding, if any, before returning the mice to their cages.
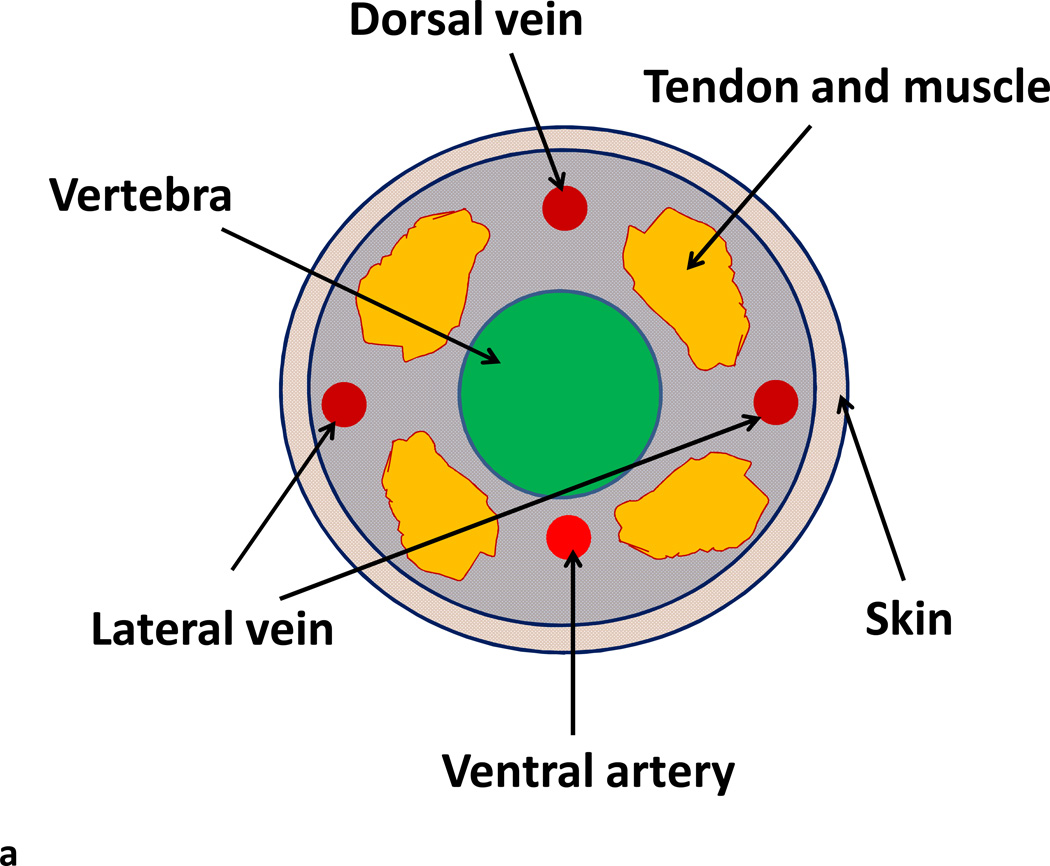
Fig. 1.
Lateral tail vein injection of rAAV vector for liver transduction. (A) Diagram of a transverse sectional view of a mouse tail showing the lateral veins (LV), dorsal vein (DV), and ventral artery (VA). (B) Lateral tail vein injection of rAAV vector into a Balb/c mouse. The mouse was restrained in a mouse holding device. The tail was bent slightly and the approach angle of the needle was almost parallel to the vein with the needle bevel facing upward.
ALTERNATE PROTOCOL 1: VECTOR DELIVERY BY RETRO-ORBITAL SINUS INJECTION
Delivery of vectors into liver by i.v. injection via tail vein or facial vein is technically challenging in young and neonatal mice. The technique of retro-orbital injection of the venous sinus is a very useful alternative method for gene therapy (Yardeni et al., 2011) to administer volumes up to 0.1 ml reliably in adult, young and neonatal mice.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
rAAV vectors [rAAVs can be produced in-house (see Unit 14D.1), through a local vector core or via a commercial manufacturer]
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
1 ml syringe with a 30G needle (Fisher Sci)
Isoflurane (Webster Veterinary Supply, Inc.) [For anesthesia methods please see CPI Unit 1.4]
Adult and young mice injection (Fig. 2)
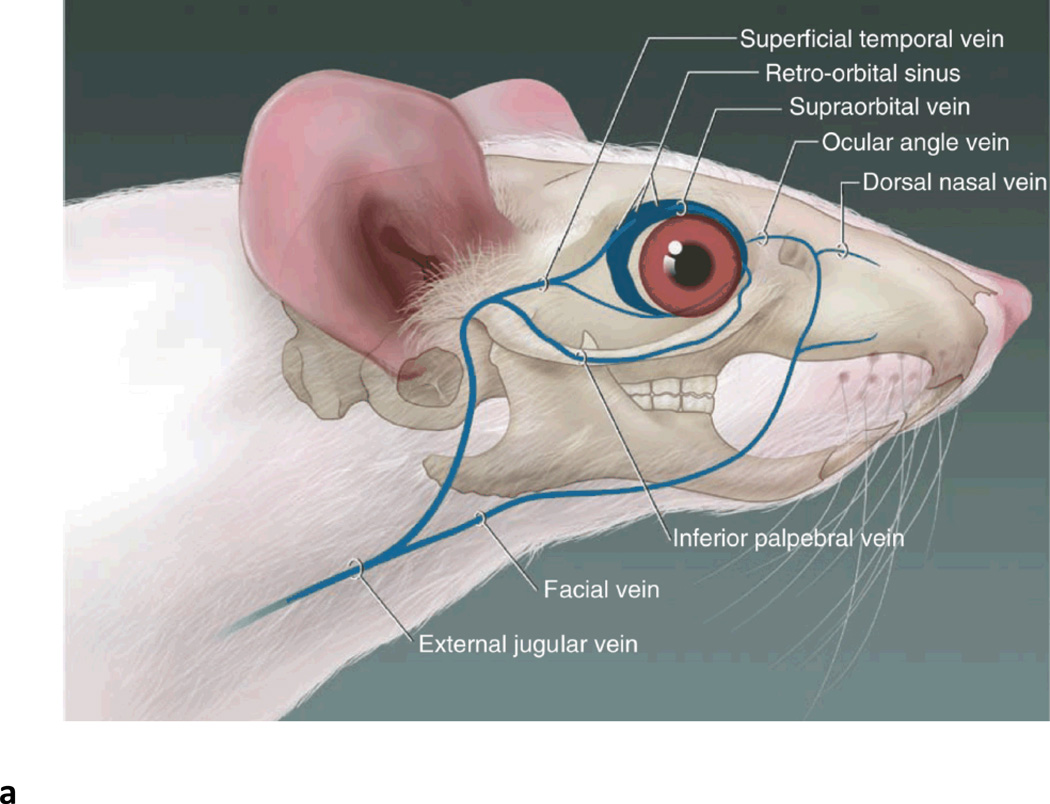
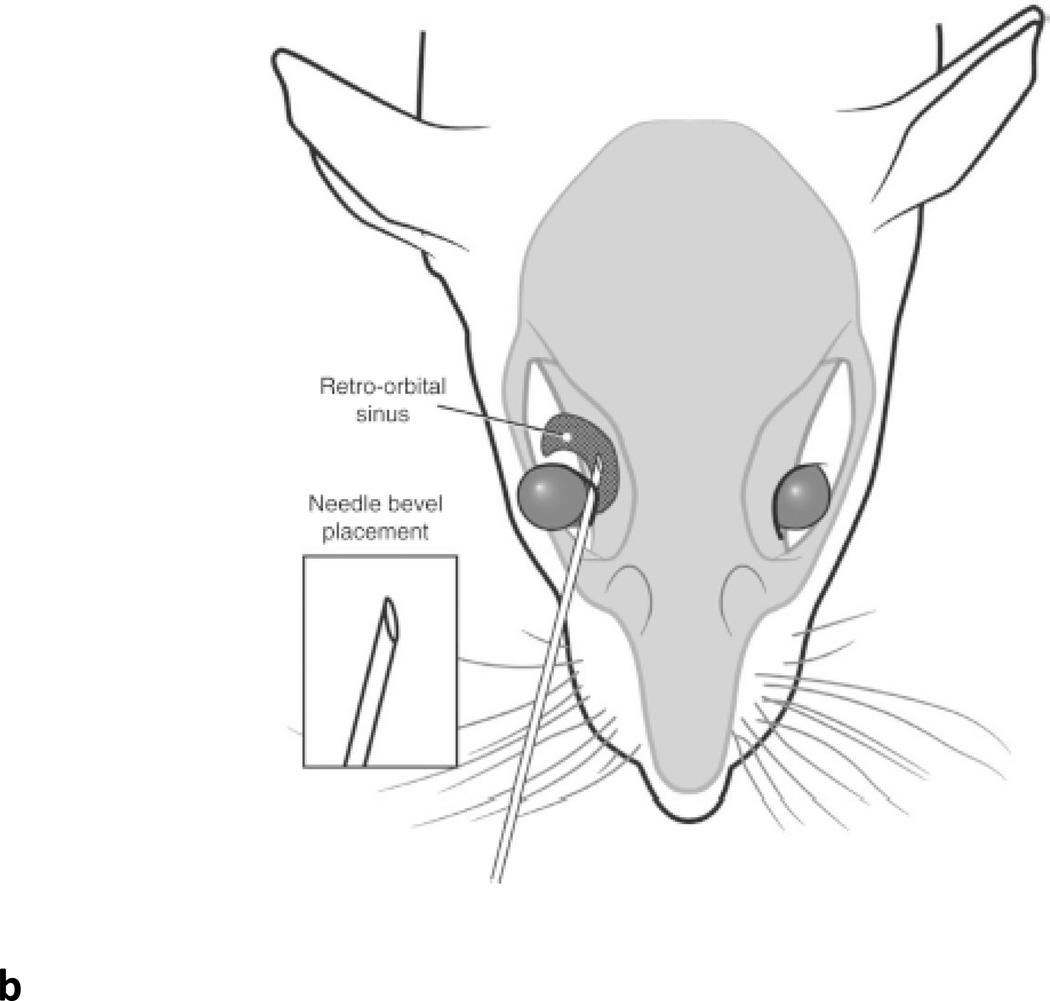
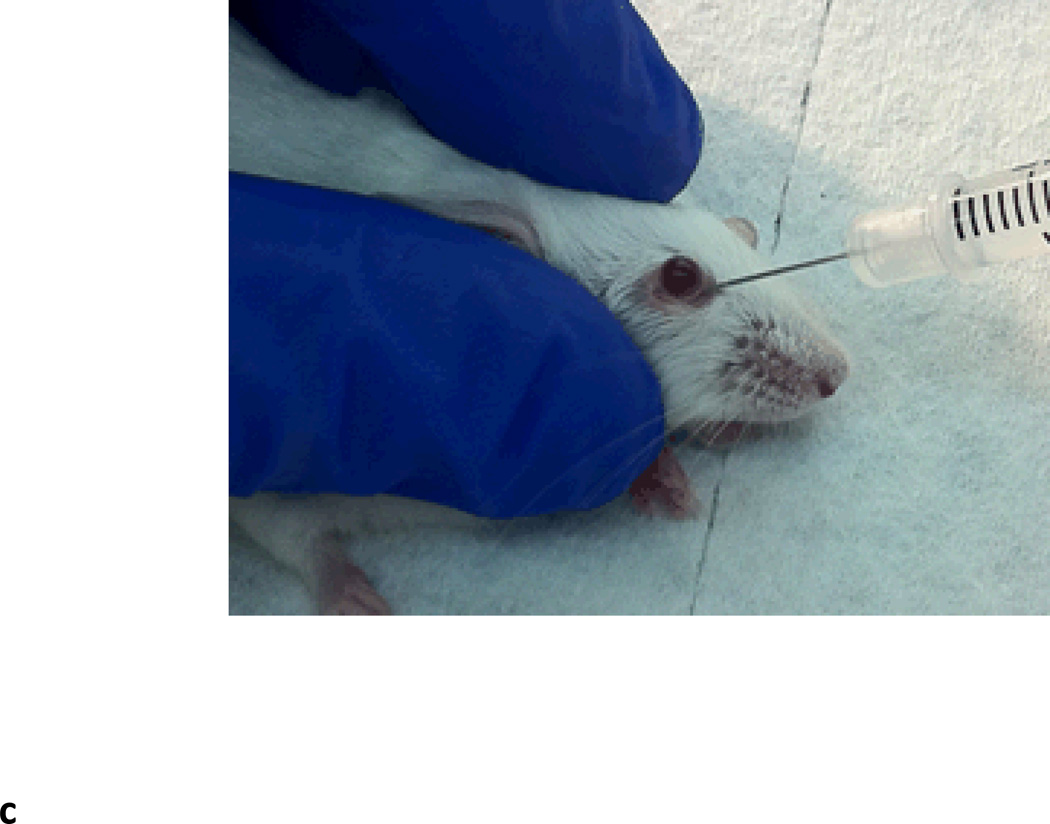
Fig. 2.
Retro-orbital sinus injection of rAAV vector for liver transduction. (A) Diagram of the blood vessels contributing to the retro-orbital sinus of the mouse. (Adapted from Yardeni T et al. Lab Anim (NY)) (Yardeni et al., 2011). (B) Correct placement of the needle relative to the retro-orbital sinus, the eyeball and the back of the orbit. (Adapted from Yardeni T et al. Lab Anim (NY)) (Yardeni et al., 2011). (C) Retro-orbital sinus injection of AAV vector into a Balb/c mouse.
Obtain IACUC approval for the protocol.
Anesthetize recipient mice by subjecting them to gaseous isoflurane in an induction chamber. Place the exhaust line into a non-recirculating fume hood or biosafety cabinet that provides 10–15 air changes/hour. Once unconscious, remove the mice from the chamber, and place a nose cone to administrate 2% isoflurane in medical grade 21% oxygen (flow of 2 liter/min). Assess the depth of anesthesia by monitoring mice for movement and limb withdrawal reflexes to toe pinch. Refer to CPI Unit 1.4 for other anesthesia protocols.
Grasp the mouse so that its back rests on the palm of the left hand (right hand if the person is left-handed) with its head toward the thumb. Place the thumb lateral to the animal's trachea and draw the fur on the animals head into the palm of your hand causing the animal’s eyes to bulge slightly.
Direct a 1 ml syringe with a 30G needle with the bevel facing outward (lateral toward globe), into the medial canthus (junction of eyelids closest to the animal's nose). Insert the needle at a 45° angle into the center of the area to carefully penetrate the retro-orbital sinus. At this time the needle is in mid-sinus.
Inject up to 0.1 ml of vector suspension slowly so that the mouse stays relaxed.
After injection, remove the needle carefully, keeping the bevel outward to protect the mouse’s eye from being scratched.
If blood appears at the injection site, clean it with gauze.
Return the mice immediately to their cages.
Neonatal mice (1–2 d old pups) injection (Yardeni et al., 2011)
Obtain IACUC approval for the protocol.
Remove the neonates from cage and hold the pups in one hand as they can be adequately restrained without anesthesia.
Place the pup in left lateral recumbency (right if the person is left-handed) with its head toward the right. Gently restrain the head between the thumb and forefinger and the rest of body is nestled between the thumb and forefinger.
With the bevel facing outward (lateral toward globe), direct a 30G needle attached to a 1 ml syringe into the medial canthus (junction of eyelids closest to the animal's nose) and insert the needle at a 45° angle into the center of the area to carefully penetrate the retro-orbital sinus. At this time the needle is in mid-sinus.
Up to 0.1 ml of vector suspension will be injected slowly so that the mouse stays relaxed.
After injection, carefully remove the needle, keeping the bevel outward to protect the mouse’s eye from being scratched.
If blood appears at the injection site, clean it with gauze.
Gently rub the pup with soiled bedding before putting it back into the cage to remove extraneous smell and make it more acceptable to the mother,.
Numb the nose of the mother using alcohol to prevent it from killing the pups due to unfamiliar smell.
BASIC PROTOCOL 3: VECTOR DELIVERY BY INTRAPORTAL INJECTION
Delivery of vectors by i.v. injection via portal vein can target the liver directly and lead to higher transgene expression compared to peripheral vein delivery, such as tail vein and retro-orbital sinus injections. Usually up to 0.1 ml of vector is delivered by this method. This method requires survival surgery in the mice, which is a difficult procedure requiring numerous practice sessions since mistakes will result in death of the animal. The inexperienced investigator should first gain this skill in several practice sessions using dead mice to identify the site of injection. If done correctly, the mice tolerate this surgery very well with no mortality.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
rAAV vectors [rAAVs can be produced in-house (see Unit 14D.1), through a local vector core or via a commercial manufacturer]
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
1ml syringe with a 33G needle (Fisher Sci)
Isoflurane (Webster Veterinary Supply, Inc.)
Animal Hair trimmer with clipper Blade size 40 or less (Oster)
Mouse surgical package (Kent Scientific Corp.)
Anesthesia machine (Vetequip) (Colonial Medical Supply Co.)
Obtain IACUC approval for the protocol.
All survival surgery should be performed by using aseptic procedures, including sterile gloves, masks, sterile instruments, and aseptic techniques. The surgical area should be uncluttered and disinfected. (Refer CPI Unit 1.12 for a detailed description of setting up the surgical area. Injection material should be ready and kept on ice before the surgical procedures.
Anesthetize recipient mice by subjecting them to gaseous isoflurane in an induction chamber. Place the exhaust line into a non-recirculating fume hood or biosafety cabinet that provides 10–15 air changes/hour.
Anesthetize the mouse by inhalation of isoflurane in an induction chamber in a ducted hood. Once unconscious, remove the mouse from the chamber, and place a nose cone for inhalation of 2% isoflurane in medical grade 21% oxygen (flow of 2 liter/min). Apply ophthalmic ointment to the eyes of the mouse to prevent them from drying. Assess the depth of anesthesia by monitoring mice for toe pinch reflexes.
Keep a pad is over a water re-circulating heating pad (37°C) and place drapes (or towels) on the pad and/or nearby to assist in maintaining a clean field.
Wash hands with an antiseptic surgical scrub preparation and then aseptically put on sterile gloves for the procedure following general surgical guidelines. Also wear a clean lab coat and a surgical mask.
Place the mouse in dorsal recumbency and remove hair with clippers. An alternative method to remove fur is by plucking the fur because murine hair follicles are in resting phase, and hair can be removed without injury.
Dab the clipped or plucked area with a piece of adhesive tape or moistened gauze to remove loose fur that could otherwise migrate into the incision.
Perform 3 alternating betadine (or chlorhexidine) and 70% isopropyl alcohol (or sterile LRS, Saline or water) scrub. Repeat this scrub and rinse three times using a sterile sponge (or cotton tip swab for tiny animals). Apply antiseptic solution (povidone iodine solution) to the surgical site as a final prep and allow it to dry before the incision is made.
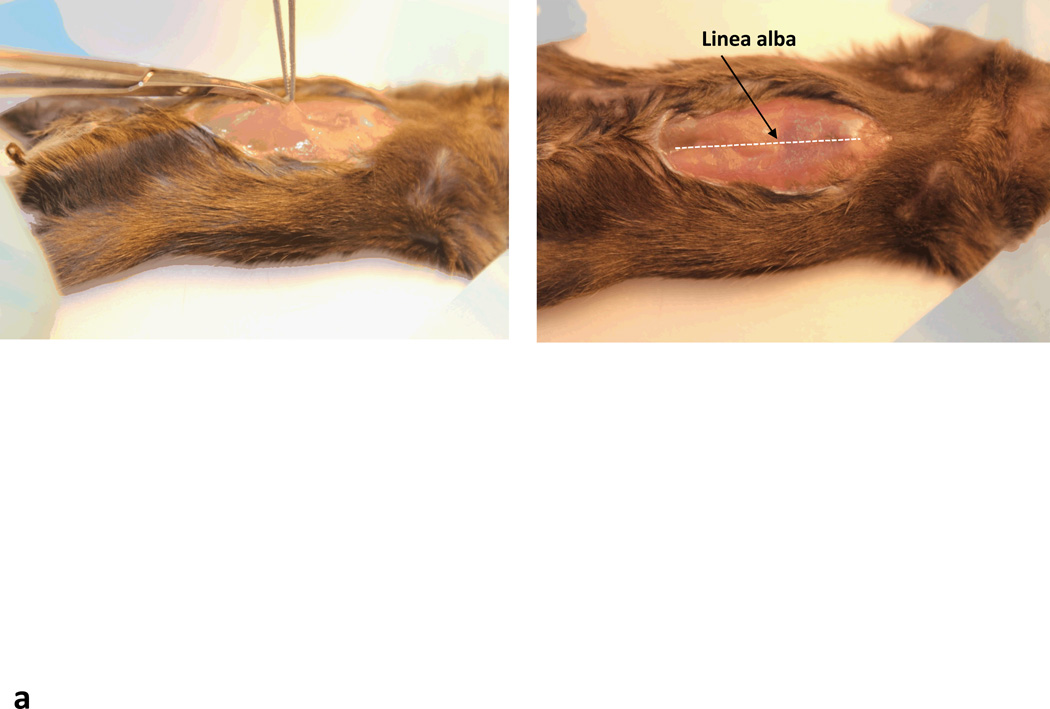
Make a 2 cm incision ventral midline incision starting just caudal to the xyphoid process and continue caudally. Enter the abdomen through the linea alba.(Fig. 3) Gently rotate the intestines in the right lower quadrant to the left and expose the superior mesenteric vein, which forms the portal vein with splenic vein. Cover the bowels with moist towels or gauze.
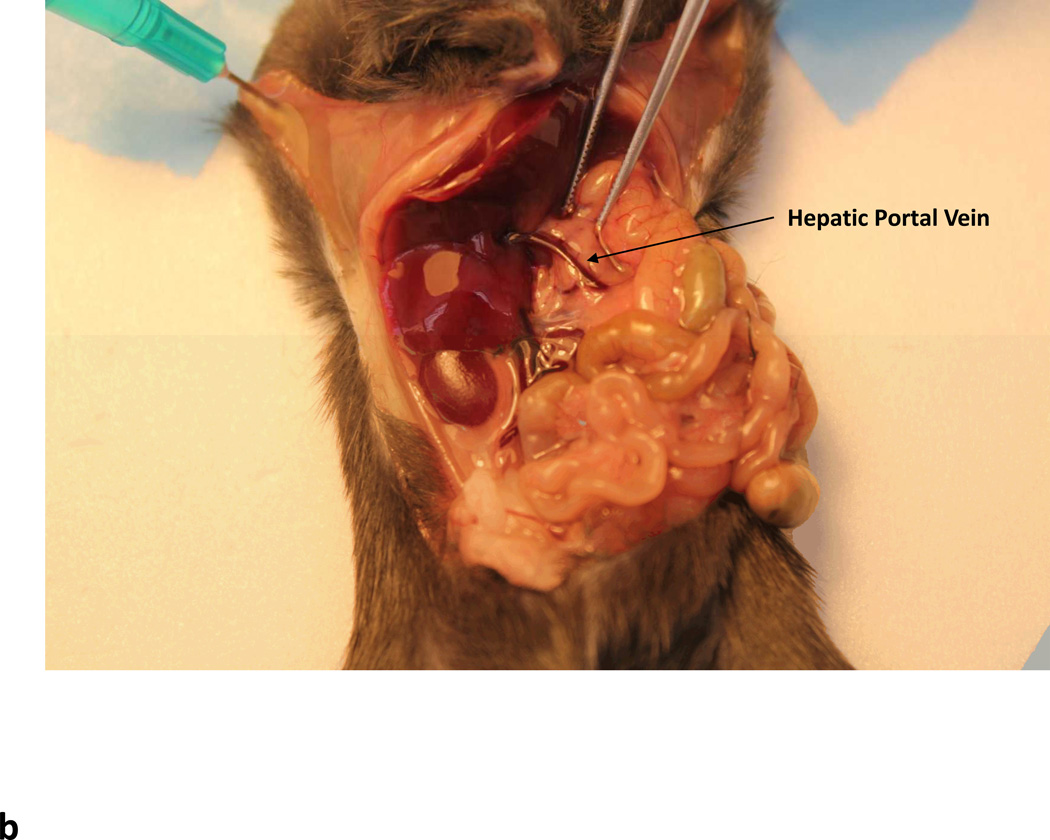
Using a cotton swab, retract the liver lobes cephalad and display the superior mesenteric and portal vein. Identify the portal vein by tracing its entry into the hepatic hilum and expose it.
Using a 1 ml syringe with 33G needle, inject up to 0.1 ml of the vector directly into the portal vein above the gastroduodenal tributary.
After injection, stop the bleeding from the puncture wound in the portal vein by compression (with a sterile cotton swab) or sterile oxycel (a hemostat) applied directly to the wound.
Put the intestines and liver lobes back to original postions. Following the operation, suture the abdominal wound using a 2 layer approach (Fig. 4) First, close the body wall with 4-0, 5-0 or 6-0 Polydioxanone (PDS) or Vicryl in a continuous suture pattern (Fig 4a). Use 3–4 interrupted sutures to reinforce with the suture security.
-
Close the skin in one of four ways:
Subcuticular continuous pattern with 4-0, 5-0 or 6-0 PDS or Vicryl.
Subcuticular interrupted pattern with 4-0, 5-0 or 6-0 PDS or Vicryl (Fig 4b). In this approach, place only a few (1–4) sutures to relieve tension and follow by tissue glue.
4-0, 5-0 or 6-0 Prolene or nylon in an interrupted pattern.
Wound clips. Wound clips should be removed 7–10 days post surgery.
Do not remove the mouse from the heat-controlled pad at 37°C immediately after the surgery. Once isoflurane is removed, mice are expected to recover within 10–15 min. Keep the animals under surveillance until they are fully awake and moving around. Monitor the animals for signs of post operative respiratory distress for approximately 1–2 hours until they are fully awake.
Monitor the animals hourly for the first 3 hours after recovery, and daily in the first week (including weekends and holidays) of the procedure for any signs of discomfort. Place food at the bottom of the cage to facilitate nutritional intake for some cases. If the animals appear to be in discomfort, follow an analgesic plan post-surgery.
Give the analgesic drug Buprenorphine at the time of surgery (0.05–0.1 mg/kg, subcutaneously, ≤20 ml/kg), and every 6–8 hours thereafter for 48 hours. Give Ketoprofen (5 mg subcutaneously) at 48 hours post-surgery, if the mice still seem to be in pain. Alternatively, Carprofen can be administered (5 mg/kg, subcutaneously, ≤20 ml/kg) on the same schedule as the Ketoprofen. If the pain continues past the 2nd day post-op, a veterinarian should be consulted.
Fig. 3.
Intraportal delivery of rAAV vectors. (a) Ventral incision through the linea alba. Dotted line shows position of linea alba (b) Arrow showing the location of the hepatic portal vein.
Fig. 4.
Sutures for closing the wound. (a) Continuous suture for closing the body cavity. (b) Interrupted suture for closing the skin.
ALTERNATE PROTOCOL 2: VECTOR DELIVERY BY INTRASPLENIC INJECTION
Although intraportal delivery is the most direct approach for liver gene transfer, intrasplenic injection is also used as a convenient alternative to deliver vectors to the liver via the portal circulatory system. Although this method also needs survival surgery in mice, it is much easier to inject vector to spleen compared to portal vein injection.
Materials
Same as the intraportal injection
Obtain IACUC approval for the protocol.
For the anesthesia and surgical procedures, please see the intraportal injection section.
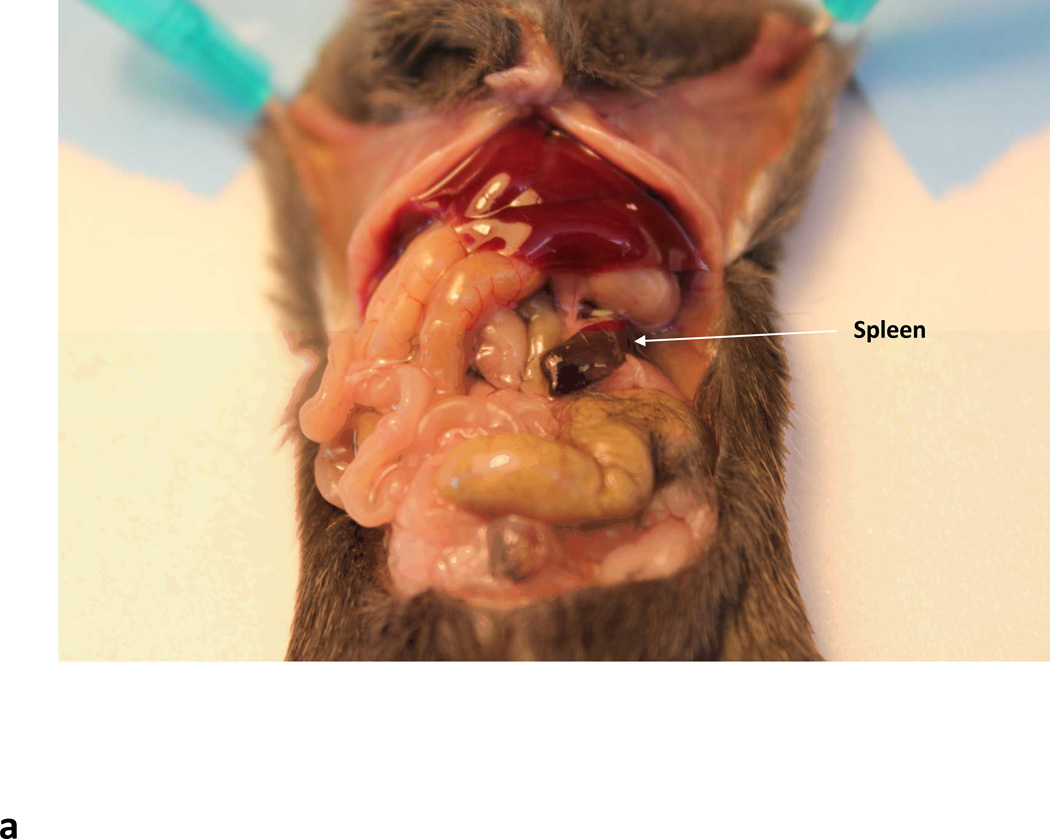
Following laparotomy, gently push the liver lobes to the left and move the stomach to reveal a slipper shaped red elongated organ- this is the spleen (Fig. 5A). Be careful not to rip the pancreas that is closely attached to the spleen.
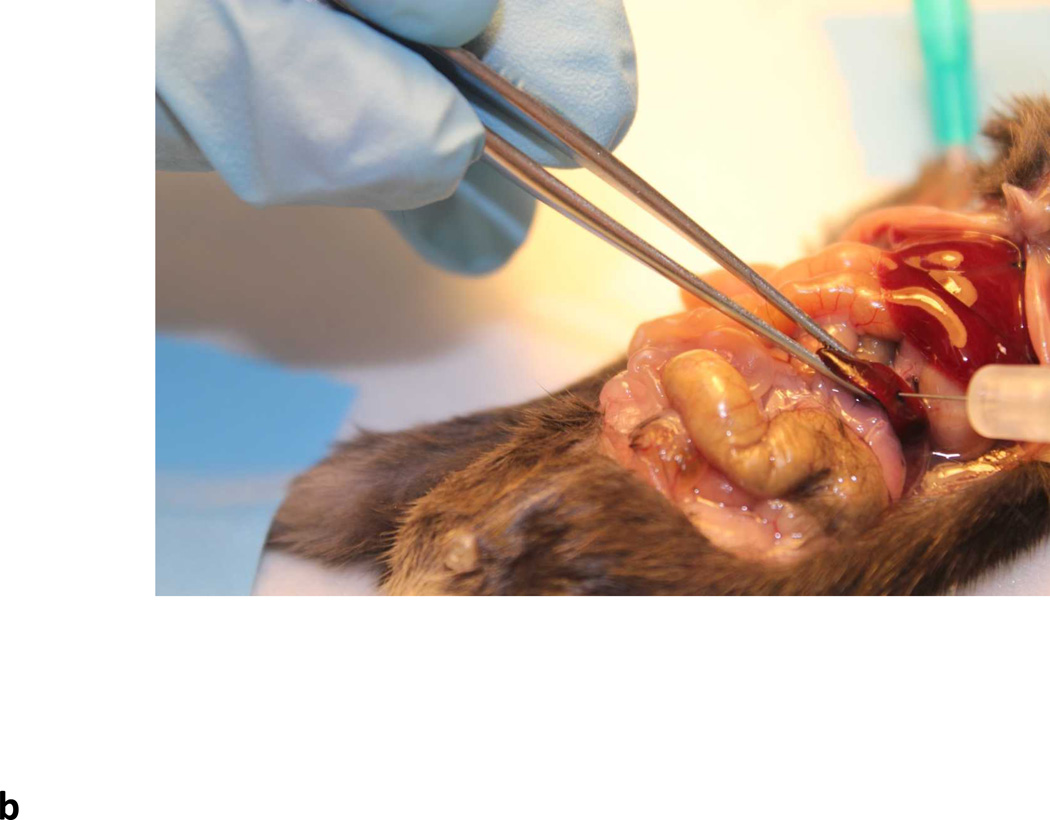
Inject up to 0.1 ml of viral vector directly into the splenic pulp using an syringe fitted with a 33 gauge needle (Fig. 5B).
Apply pressure to the stab wound (with a sterile cotton swab) until bleeding stops, at which time the incisional wound is closed in two layers. For post surgical care and analgesia, please see Basic Protocol 3: Vector Delivery By Intraportal Injection and CPI Unit 1.12
Fig. 5.
Intrasplenic delivery of rAAV vectors. (a) Arrow indicates the slipper like red spleen. (b) Needle entering the splenic pulp.
BASIC PROTOCOL 4: MONITORING TRANSDUCTION IN LIVE ANIMALS BY WHOLE BODY LIVE IMAGING
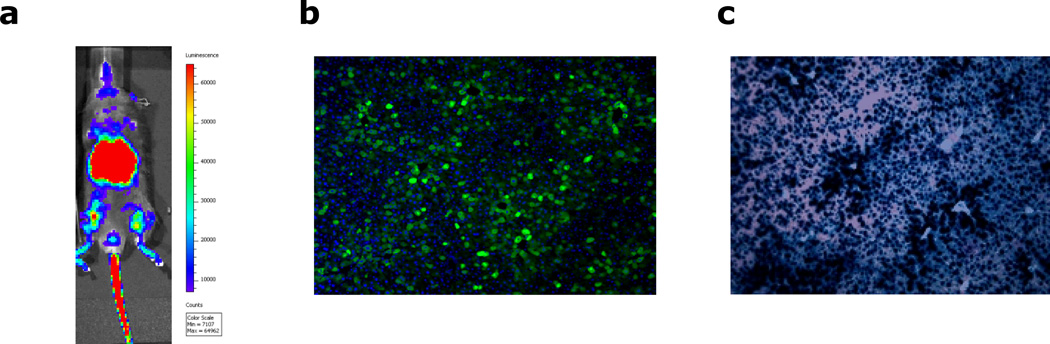
Whole body or in vivo imaging is a convenient non-invasive tool for obtaining information about physiological processes in living organisms that can augment in vitro microscopy data to provide a comprehensive overview of biological functions in integrated living systems. The method allows high spatial resolution in perceiving a variety of cellular processes by determining the biodistribution of bioluminescent or fluorescent reporters delivered by rAAVs and is widely used in preclinical studies. Liver, being a large organ, is a good target for evaluating such biodistribution; hence whole body imaging would be a useful tool for following up gene-transfer studies directed to the liver. Most of the current imaging systems are based on epi-illumination planar imaging and though the biological principle underlying fluorescent and bioluminescent imaging is essentially the same, they do have their own distinctive challenges. Fluorescent reporters require excitation light-sources that result in significant autofluorescence, leading to high background; in contrast, bioluminescent reporters are detected by systemic administration of a reporter-specific substrate, thus bypassing the auto-fluorescence problem. Differences in emission spectra between fluorescent and bioluminescent proteins favor luciferase-based reporters for detection of gene transfer deep within tissue, and the superior sensitivity and wider dynamic range of bioluminescent signals make such these ideal choice for use in monitoring rAAV-mediated hepatic gene transfer in vivo though the multitudes of new fluorescent probes with varying excitation spectra are also expanding equally. There are more than 700 luciferase containing luminous species (Widder, 2010) and the most commonly used luciferase enzymes include firefly luciferase (Fluc, from firefly photinus pyralis), Renilla luciferase (Rluc, from sea pansy Renilla reniformis) and Gaussia luciferase (Gluc, from copepod Gaussia princeps) (Close et al., 2011). Fluc is the most widely used luciferase and its long wavelength of emitted light (>600 nm) can penetrate mammalian tissues easily with much less absorbance and result in high sensitivity images in whole body live imaging studies (Close et al., 2011; Contag, 2007). However, its long coding sequence (1653 bp) limits its ability to be packaged by self-complementary (sc) rAAVs. Rluc and Gluc have shorter coding sequence of 936 and 558 bp, making them more suitable for gene transfer studies by scrAAVs. However, both these luciferases emit short wavelength light in the blue/green region, which is strongly absorbed and scattered by mammalian tissues and leads to poor sensitivity in imaging performance in whole body live imaging studies (de Almeida et al., 2011). Though the method currently poses a couple of problems, there is continuous research to address its shortcomings and consistently improve resolution and sensitivity for the experiments. Whole body live imaging for rAAV-mediated liver Fluc transduction in mice is described in this protocol because it has the best signal sensitivity (among all luciferases) from transduced animal tissues.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
Isoflurane (Webster Veterinary Supply, Inc.)
Anesthesia machine (Vetequip) (Colonial Medical Supply Co.,Inc.)
15 mg/ml Beetle luciferin substrate in D-PBS (Promega Co.)
Mice injected with rAAV-Fluc expression vector or D-PBS
1 ml syringe with a 28G needle (Fisher Sci)
Scale with 200-gram capacity (digital scales are ~700 ml tupperware (or similar) container with lid (for weighing animals)
Cooled CCD camera for in vivo bioluminescence imaging (Xenogen)
Obtain IACUC approval for the protocol.
Place Tupperware or similar container on scale and tare to zero. Place mouse in container, cover with lid, and record weight of the animal. Weighing animals is essential for accurate substrate dosing.
Inject the Beetle luciferin substrate at dosage of 150 mg/kg into test mice intraperitoneally.
After 5 minutes, anesthetize the mice with isoflurane using anesthesia machine. Details see Alternate Protocol 1.
Place the animal in a light-free warm chamber (30°C) and acquire photon counts using a cooled CCD camera at different exposure times.
-
Without moving the animal, take a light image using dim polychromatic illumination.
Refer to CCD camera for in vivo bioluminescence imaging vendor instructions for more details
Transfer animal to the pre-warmed, empty cage. Monitor animal until recovery and return to source cage.
Process CCD images in accordance with camera manufacturer’s instructions analysis software. Distinguish bioluminescence signals significantly greater than background by automatic intensity contour mapping. Quantify transgene reporter activity via analysis of mean photon count for regions of interest.
Visualize localization and intensity of reporter activity as a function of anatomical distribution by pseudocolor overlay of bioluminescence images with corresponding light images.
Analyze data by plotting photons versus time on the y- and x-axis, respectively.
SERUM REPORTER ASSAYS
Liver biopsy has been the gold standard for the assessment of liver diseases like fibrosis and monitoring the effect of liver directed gene therapy but the technique though useful is highly invasive. A useful non-invasive assessment would include estimation of markers in the serum. Serum reporters represent sensitive, non-invasive and convenient tools for use in ex vivo monitoring of rAAV-mediated liver gene transfer. Amongst the most sensitive and commonly used serum reporters are secreted family-members of the bioluminescent luciferase proteins. Secreted alkaline phosphatase (SEAP) derived from the placenta (Berger et al., 1988) is one of the most commonly used reporter since, in addition to being detectable in the serum, its expression can be directly correlated to the level of mRNA and the number of cells expressing the protein. Following gene transfer, SEAP has been used in several studies to monitor levels of gene expression (Abruzzese et al., 1999), study tumor growth and evaluate drug efficacy (Bao et al., 2000) and monitor microscopic inflammation (Meng et al., 2005). The assays to detect and A1AT and Gluc are discussed in Basic Protocols 5 and 6, respectively.
(Note: For serum collection from experimental animals, please see Support Protocol 3 biofluids collection and storage.)
BASIC PROTOCOL 5: Serum Reporter Assay via A1AT detection
An emerging serum reporter for monitoring gene expression for liver based gene therapy is Alpha 1 antitrypsin (A1AT), a protein that is synthesized in liver, secreted in the blood and functional in lungs. Since A1AT is an acute phase protein, systemic inflammation increases its synthesis and release from the hepatocytes (Fairbanks and Tavill, 2008), also making it an ideal reporter for estimating liver damage.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
Serum from mice injected with rAAV-A1AT expression vector and rAAV D-PBS (see Basic Protocols 2 and 3 and Alternate Protocols 1 and 2 for information regarding vector preparation and delivery)
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 15 ml conical tubes (Corning Corp.)
Sterile 50 ml conical tubes (Corning Corp.)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
96-well flat bottom plates high binding (Costar)
Heat resistant NUNC plates (Fisher Sci)
Multi-channel-12 pipette P200 (Rainin)
Plate sealers: Sealing tapes (Fisher Sci)
Squirt bottle, plate washer or dunking bucket (Fisher Sci)
Microcentrifuge (Thermo Sci)
Blocking Solution Concentrate BSA (KPL)
Wash Solution Concentrate (KPL)
ABTS Peroxidase Substrate (KPL)
Coating Solution Concentrate (KPL)
Tween 20 (Sigma-Aldrich)
10mg/ml stock A1AT Standard (Sigma-Aldrich)
Conjugate: Goat anti-α1-anti-trypsin-HRP antibody (Fitzgerald)
Anti-Human Alpha 1-Antitripsin (A1AT) developed in rabbit (Sigma-Aldrich)
Plate luminometer equipped with injector (BioTek instruments)
Dilute the A1AT coating antibody (1/1000) in coating buffer, vortex well and add to each well of the 96-well plate ensuring equal distribution. Seal each plate, using a plate sealer (coated plates can be stored at 4°C for up to a week)
Rinse each well with 300 µl of wash buffer; using a multi-channel, squirt bottle, plate washer, or a dunking bucket. Decant after each wash. Repeat three additional times.
After the 4th wash; tap the plate(s) on a dry clean paper towel to remove excess liquid (do not let the wells dry out)
Pour 30ml of Block Solution into a basin and add a total of 300 µl/well of block solution. Incubate at room temperature (22 ~ 25°C) for a minimum of 1 hour.
In a Nunc plate add sample buffer and serum for appropriate dilution of wells (e.g. add 190 µl/well of sample buffer and 10 µl of serum for 1/20 dilution and so on) keeping the final volume as 200 µl.
Next prepare serial dilutions for the assay (e.g. mix and transfer 20 µl from 1/20 dilution wells to the 1/200 well containing 180 µl). Repeat until desired dilutions are obtained.
-
Table summary of Serum dilutions:
1:20= 190 µl of sample buffer + 10 µl of serum
1:200= 180 µl of sample buffer + 20 µl of 1:20 dilution
1:2000= 180 µl of sample buffer + 20 µl of 1:200 dilution
1:20000= 180 µl of sample buffer + 20 µl of 1:2000 dilution
To prepare standard solution, thaw one vial of 10 mg/ml stock A1AT and add 10 µl to one 15 ml conical tube containing 10 ml sample buffer labeled 10 µg/ml and vortex well. Add 100 µl of diluted A1AT from this tube to the second tube labeled 100 ng/ml containing 10 ml sample buffer. Keep both tubes on ice.
Add 500 µl sample buffer to seven 1.5 ml Eppendorf® tubes labeled 50, 25, 12.5, 6.25, 3.125, 0 and Blank
Add 1 ml of the 100 ng/ml A1AT solution to an empty Eppendorf® tube labeled 100.
Prepare serial dilutions transferring 500 µl to each successive tube and vortex well each time (Note: 0 and Blank are 100 % sample buffer.)
Wash the blocked 96-well coated plates prepared earlier 4 times and tap on paper towel
Add 100 µl of sample dilutions and standards to this plate and incubate at 4°C overnight (~20 hours).
Equilibrate the ABTS KPL substrate bottle to 22 ~ 25°C before use.
Remove plates from 4°C and wash 4 times and tap on paper towel.
Prepare 1:5500 dilution of Conjugated Goat anti-A1AT-HRP antibody in sample buffer for a total volume of 11 ml/plate and vortex.
Add 100 µl of conjugate solution to each well and incubate for 2 hours room temperature in the dark.
Wash 6 times and tap on paper towel.
Add 100µl of room temperature ABTS KPL solution to each well; incubate until ΔOD (Read out at 405nm – read out at 600nm) reaches over 1.2 OD. Incubation times vary between 30 minutes −1 hour depending on quality/stability of antibodies.
Data analysis is based on a linear regression comparison and interpolation of the standards against unknown samples. The R2 value for the standard curve should be 0.95 or higher. The measured OD value of the unknowns should fall within the range of the standards for a particular dilution starting the analysis with highest dilution to the lowest dilution.
Manually analyze the raw OD data, circle all OD’s within the STD curve range of 100 ~ 3.125 ng/ml; anything outside the range of 100 ~ 3.125 ng/ml is beyond the limit of detection for this assay. Transfer the circled values to the calculated concentration section and report those values in ng/ml.
Report data in Excel table using SD and average for each of the animal groups.
BASIC PROTOCOL 6: Serum Reporter Assay via Gluc detection
Another secreted reporter was established by Wurdinger et al (Tannous, 2009) where they characterized a naturally occurring luciferase from the marine copepod Gaussia princeps called Gaussia luciferase (Gluc). Mammalian cells can secrete this luciferase into the blood and urine which can be easily quantified using its substrate coelenterazine. The levels of Gluc have a linear correlation with cell numbers. Secreted reporter proteins like Gluc, SEAP and A1AT allow for the rapid and reliable detection and quantitation of rAAV transgene expression in miniscule volumes of serum permitting frequent sample collection without risk of undue harm or stress to animals. Furthermore, the very short in vivo half-life (twenty minutes) of reporters like Gluc prevents their accumulation, thus providing a powerful means to monitor expression of the transgene of interest in real time.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (with Ca2+ and Mg2+) (Fisher Sci)
Concentrated HCl (Fisher Sci)
Methanol (Fisher Sci)
Coelenterazine-h or Coelenterazine-n substrate (Promega)
Serum from mice injected with rAAV-Gluc expression vector and D-PBS (see Basic Protocols 2 and 3 and Alternate Protocols 1 and 2 for information regarding vector preparation and delivery)
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile 1.5 ml Eppendorf® centrifuge tube (Fisher Sci)
Black or white opaque 96-well microtiter plates (Costar)
Plate luminometer equipped with injector (BioTek instruments)
-
Preparation of coelenterazine solution:
-
1.1.
Add one drop of concentrated HCl into 10 ml of methanol to make acidified methanol.
-
1.2.
Add 4.07 mg coelenterazine-h or 4.23 mg coelenterazine-n to 10 ml acidified methanol to make a 1 mM stock substrate solution. The stock solution can be aliquoted and stored at −80°C.
-
1.3.
Freshly add 30 µl stock solution to 20 ml D-PBS to make a 1.5µM concentration working solution. Allow working solution to reach room temperature.
-
1.1.
Collect blood from animals to get serum for testing (Please see Support Protocol 3 biofluids collection and storage and CPI Unit 1.7 for information on how to prepare serum). Pipette 5 µl of serum into the 96-well plate in triplicate. (3 wells/ sample for each sample).
Place plate in luminometer, add substrate to injector reservoirs, and set injector to administer 100 µl of coelenterazine working solution/well and acquisition time to 10 seconds in accordance with manufacturer’s instructions.
Run assay, and analyze triplicate RLU values obtained from empty vector control samples to obtain background luminescence values. Perform background subtraction from each experimental sample.
Analyze background-subtracted experimental samples in triplicate or as appropriate.
SAMPLE COLLECTION AND STORAGE
At the end-point of the study, the animals will be necropsied and liver and other organs will be collected from the mice for further studies to detect the biodistribution of viral vectors, transgene expression (RT-PCR, Western blot, histochemistry and fluorescence microcopy) and immune response to the viral vectors and transgene. The methods for tissue collection and storage are variable dependent on the study purpose. The following protocols describe the collection and storage of tissue samples for biodistribution, transgene expression, and histopathology studies, as well as various methods of blood collection.
SUPPORT PROTOCOL 1: TISSUE COLLECTION AND STORAGE FOR BIODISTRIBUTION STUDIES
The purpose of biodistribution study is to determine which organs contain vector copies, generally using quantitative polymerase chain reaction (qPCR) assays. Due to the high sensitivity of the assay, it is critical that cross-contamination must be prevented to the maximum extent possible. Tissue collection must be performed in an area which is not used for preparation, storage or handling of vector or reagents used in any of these or related processes. A Biological Safety Cabinet (bio-hood) is recommended for this purpose. If more than one animal is to be necropsied at the same time, separate stations must be used and personnel performing the necropsies should not travel between stations. Animals must not be necropsied in close proximity to one another and separate instruments must be used. Species appropriate Personal Protective Equipment (PPE) must be worn during all stages of animal handling and tissue collection.
Materials
10% Bleach solution (Clorox Professional Products Co.)
Sterile, disposable scissors and forceps (Kent Scientific Corp.)
1 ml sterile, disposable syringes (Fisher Sci)
Sterile, disposable 27 to 22G needles (Fisher Sci)
Microcentrifuge (Thermo Sci)
Liquid Nitrogen (Middlesex Gases & Technologies, Inc.)
Dry ice (Middlesex Gases & Technologies, Inc.)
Microcentrifuge tubes (Fisher Sci)
Mice injected with recombinant rAAV vector or D-PBS
Clear the surface of the table or counter top with 10% bleach solution.
Euthanize the mice in a manner approved by IACUC (carbon dioxide asphyxiation followed by cervical dislocation) and collect the liver (target organ) and other core organs, such as the gonads, lungs, heart, and kidney if required.
Collect the organs in a manner that minimizes the chances of cross contamination i.e. start with negative controls that have not had vector administered and then move to the vector-administered animals, progressing from low dose to high dose groups.
Use clean instruments and take care that separate sets of instruments are used for isolation of each organ from each animal.
Disposable forceps and razor blades may be used to harvest the tissues.
Organs should be collected sequentially with the organs harboring the least amount of vector collected first followed by ones that harbor more vectors. The target organ (liver) should be collected last as it has the highest concentration of vectors. To collect bone marrow, dissect the femurs, expose and remove the adipose tissue and large vasculature. Cut the ends of the bones with sharp surgical scissors, and flush the marrow plugs into 1–2 ml cold PBS using a sterile 21G needle attached to a 3-ml syringe.
Only instruments should touch the tissues and fingers should not be used. If the gloved hand becomes soiled, replace the gloves.
Put each collected organ and tissue in a container (e.g. Eppendorf® tube) and freeze it in liquid nitrogen and immediately transfer to dry ice for temporary storage.
Store the frozen organs and tissue in −80 °C freezer for future biodistribution studies.
After completing the necropsy, discard all absorbent pads in a biohazard bag. The remainder of the carcasses, if any, should be properly disposed and the surface of the table or counter top decontaminated with 10% bleach solution.
SUPPORT PROTOCOL 2: TISSUE COLLECTION AND STORAGE FOR TRANSGENE EXPRESSION, HISTOPATHOLOGY, AND IMMUNOHISTOCHEMISTRY STUDIES
Liver tissue for nucleic acid and protein isolation should be collected and frozen in liquid nitrogen immediately to detect the transgene expression after vector delivery. Liver tissue to be used for detection of direct fluorescence [Green Fluorescent protein (GFP) or Red Fluorescent protein (RFP), etc] by, immunohistochemistry (IHC) or immunofluorescence (IFL) microscopy may be collected and preserved in an appropriate fixative solution. For detection of transduced enzyme activity (β–galactosidase, and alkaline phosphatase etc.), fresh tissue should be embedded in Optimal Cutting Temperature (OCT) compound followed by freezing immediately. Although cross-contamination is not a critical issue in these situations, tissue collection must be performed in a clean area to prevent other contaminations.
Materials
10% Bleach solution (Clorox Professional Products Co.)
Sterile, disposable scissors and forceps (Kent Scientific Corp.)
4% paraformaldehyde (Sigma-Aldrich) or 10% neutral buffered formalin (Sigma-Aldrich)
30% sucrose (Fisher Sci)
Optimal Cutting Temperature (OCT) (Sakura Finetek USA, Inc.)
Embedding cup: made by aluminum foil by using small flat-bottomed bottle (about 1 cm in diameter) or embedding vinyl molds (Fisher Sci)
Ethanol (Decon Labs, Inc.) or isopropyl alcohol (Fisher Sci)
Mice injected by rAAV vectors or D-PBS
Liquid Nitrogen (Middlesex Gases & Technologies, Inc.)
Dry ice (Middlesex Gases & Technologies, Inc.)
Microcentrifuge tubes (Fisher Sci)
Clean the surface of the table or counter top with 10% bleach solution.
Euthanize the mice in a manner approved by IACUC (carbon dioxide asphyxiation followed by cervical dislocation).
For nucleic acid and protein isolation, collect the liver lobes and put in a microcentrifuge tube followed by freezing in liquid nitrogen immediately. Store the tissue in −80 °C freezer which can be later used for isolation of RNA and protein.
-
Fixed-frozen liver tissue sections will be used for direct fluorescence (GFP, RFP etc) microcopy detection.
-
4.1
Preserve the liver lobes in 4% paraformaldehyde or 10% neutral buffered formalin at 4 °C for fixation overnight.
-
4.2
Rinse the fixed tissue in D-PBS followed by cryoprotection (dehydration) in 30% sucrose until sample sinks at 4’C.
-
4.3
The tissue will be subjected to OCT embedding and sectioning (For details of OCT embedding and sectioning, please see Support Protocol 5).
-
4.1
Both fixed-frozen (as described in section 4) and fixed-paraffin liver tissue sections can be used for histopathology, IHC or IFL staining detection. For fixed-paraffin sections, preserve the liver lobes in 4% paraformaldehyde or 10% neutral buffered formalin at 4 °C for fixation overnight after which the fixed tissue will be dehydrated and subjected to paraffin embedding and sectioning. (For details of paraffin embedding and sectioning, please Basic Protocol 9.)
To detect transduced enzyme activity, such as β–galactosidase, and alkaline phosphatase, collect fresh liver lobes subject them to OCT embedding, which is described in Support Protocol 5.
SUPPORT PROTOCOL 3: BIOFLUIDS COLLECTION AND STORAGE
After vector delivery, collect the biofluids, mainly blood from the mice for detection of transgene expression, enzyme activity, neutralization antibody, and vector clearance. Based on the study purpose, serum collection tubes or anti-coagulant tubes may be used to collect mouse serum or whole blood. The blood may be collected by lateral tail vein puncture, facial (submandibular) vein puncture or retro-orbital bleeding. It is critical that hemolysis be prevented to the maximum extent possible, since it would interfere with the analysis.
Materials
Serum collection tube or anti-coagulant tube (Fisher Sci)
50 µl sterile Natelson tube (Fisher Sci)
Goldenrod animal bleeding lancet (Medipoint, Inc.)
19-, 21-, or 23- G needle (Fisher Sci)
Isoflurane (Webster Veterinary Supply, Inc.)
Anesthesia machine (Vetequip) (Colonial Medical Supply Co.,Inc.)
Blood collection (phlebotomy) by lateral tail vein puncture
Obtain IACUC approval for the protocol.
Collect blood by lateral tail vein puncture weekly or monthly after injection (Please see CPI Unit 1.7 for a detailed description). The procedure of animal preparation and tail vein identification is the same as tail vein injection (see Basic Protocol 2).
Aspirate approximately 0.1–0.25 ml or less (according the animal's body weight, less than 10% of total blood volume) into a syringe. Alternatively, puncture the tail vein with a small gauge needle and collect the free flowing drops of blood. Blood should not be collected more than 8 times in a period of 4 months.
After removing the needle, use gauze to stop the bleeding, if any, before returning the mice to their cages.
Separate the serum or plasma by spinning down the blood in a serum collection tube or anti-coagulant tube, respectively at 8,000rpm for 5 minutes using a microcentrifuge. Use for test immediately or store in −80 °C freezer for future use. Store whole blood in anti-coagulant tubes at 4 °C. Don’t put whole blood in freezer to avoid hemolysis except for experiments that require cell lysis.
Blood collection (phlebotomy) by retro-orbital bleeding
Obtain IACUC approval for the protocol.
In some experiments, peripheral blood is analyzed by retro-orbital bleeding. It is important to use retro-orbital bleeding because platelets tend to clump and give incorrect numbers when tail bleeding is used.
Anesthetize the mice and prepare them as described in retro-orbital sinus injection section.
After the animal’s eye bulges slightly, direct a 50 µl sterile Pasteur pipette into the medial canthus (junction of eyelids closest to the animal's nose) of the eye rotating slightly as the tube is directed to a point directly behind the globe. Sufficient pressure must be applied to cut through the fibrous layer that surrounds the sinus. Blood will flow through the tube and occasionally around the tube once the sinus has been penetrated.
After blood collection, remove the tube and close the eyelids. Apply a dry cotton pad over the eye with gentle pressure to prevent retro-orbital hemorrhage. Return the mice immediately to their cages. Blood should not be collected from the same eye more than 2 times, allowing at least 2 weeks between collections. Apply an antibiotic ophthalmic ointment to the eyes following bleeding.
The sample storage is described as previous section.
SAMPLE ANALYSIS METHODS
Following delivery of rAAV vector into the animal, the quantity of rAAV genome and efficiency of rAAV mediated transgene expression in liver as well as the potential rAAV-induced liver pathological outcome will be monitored as according to experimental requirements. The primary methods for analyses of gene expression post sample collection are quantification of vector genome by real time PCR and staining of fixed tissue for reporter genes like X-gal or for morphological studies by Hematoxylin and Eosin (H&E) staining. The methods for liver sample analysis for these purposes will be discussed in the following protocols.
BASIC PROTOCOL 7: VECTOR GENOME COPY QUANTIFICATION BY REAL-TIME PCR
The objective of this assay is to determine the number of viral genomes particles in liver and other tissue samples after vector delivery. This assay provides valuable information in both vector biology and liver gene therapy pre-clinical studies. DNase I is used to eliminate un-encapsidated rAAV genome DNA and/or contaminating plasmid DNA in the evaluation of virus uncoating. The DNA slot-blot has been used to quantify the genome copy in mouse tissues (Kube and Srivastava, 1997). However, the real-time qPCR can provide more accurate information and decrease the usage of radiation, which is discussed in this section, Both SYBR green and Taqman real-time qPCR can be used for this assay. The primer and probe sets used in rAAV qPCR titration, which target common elements present in most AAV vector genomes, such as promoters and poly A signals, usually work well for this purpose. Linearized pCIS plasmid used for vector production of known copy numbers, can be used as standard for qPCR. Same amounts of mouse liver genomic DNA as test samples should be spiked in the standards to eliminate the interference effect by genomic DNA in PCR reaction. A blank control containing nuclease-free water only should be used to control potential cross-contamination between samples during the preparation process. A validation vector sample with a known titer may be included in assay to monitor inter-assay variability.
Materials
DNA as prepared in Support Protocol 4
Sterile disposable tips with filter and pipettes (Fisher Sci)
96-well PCR plate and appropriate adhesive plate cover (Fisher Sci)
96-well plate holder (Fisher Sci)
PCR Standards (linearized plasmid DNA containing the vector genome)
DNase-free water (Qiagen)
GoTaq® qPCR Master Mix (Applied Biosystems)
Perfecta Sybr Green FastMix (Applied Biosystems)
GoTaq® Flexi DNA polymerase (Applied Biosystems)
dNTP mix (Applied Biosystems)
Primers (Eurofins MWG Operon)
Probes (Applied Biosystems)
Spike mouse liver genomic DNA (UMass Gene Therapy Center)
Microcentrifuge (Thermo Sci)
Real-time PCR system (Applied Biosystems)
Prepare work space on bench: swab area with 70% ethanol and cover bench with absorbent bench cover.
Wear disposable gown, surgical cap, and surgical mask.
Set up PCR plate: No Amplification Control (NAC) - triplicates; No Template Control (NTC) - triplicates; Flow through control (FTC) - duplicate; Validation vector control – duplicate; Standards (Std) – duplicates or triplicates, 0.1 µg mouse gDNA spiked, 101 copies through 108 copies; Samples (Spl) –duplicates or triplicates, 0.1 µg DNA per PCR reaction. The DNA was prepared in accordance with Support Protocol 4.
Prepare master mix solutions using GoTaq® qPCR Master Mix, primer sets, probe and dNTP for Taqman qPCR) or Perfecta Sybr Green FastMix, primer sets and dNTP for Sybr Green qPCR. The total master mix volume per reaction may be 18 µl and 0.1µg mouse gDNA is spiked in the standards.
Aliquot master mix to labeled 0.5ml microfuge tubes and add 2 µl of samples to master mix.
Vortex and transfer 20 µl reaction mix into the appropriate wells of the PCR plate and cover the PCR plate with an appropriate adhesive plate cover.
Perform PCR reactions in the Real-time PCR Machine following manufacturer directions: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute.
Analyze data and calculate vector genome copy number.
SUPPORT PROTOCOL 4: DNA EXTRACTION FROM LIVER TISSUE
Since qPCR is highly sensitive, it is critical to prevent cross-contamination to the maximum extent possible when the DNA extraction is performed. Caution and requirement is same as the protocol 4.1.1. Homogenize frozen liver tissue (See Support Protocol 5 for extraction of fresh liver tissue) in lysis buffer using TissueLyser II (Qiagen, Valencia, CA) and extract total cellular DNA by the Qiagen QIAamp DNA Mini kit.
Materials
Sterile disposable tips with filter and pipettes (Fisher Sci)
Sterile Microfuge tubes (Fisher Sci)
Stainless steel beads, 5 mm (Qiagen)
TissueLyser II (Qiagen)
Qiagen QIAamp DNA Mini kit (Qiagen)
Proteinase K (Qiagen)
RNaseA (Qiagen)
Ethanol (Decon Labs, Inc.)
Microcentrifuge (Thermo Sci)
Prepare work space in Tissue Culture Hood. Close shield of hood and UV irradiate all surfaces for 10 to 15 minutes. Wipe all surfaces with Lysol followed by 70% ethanol.
Label one 2 ml microfuge tube for each sample to be extracted and one flow-through control (FTC) and place one 5mm steel bead in each of the labeled tubes.
Thaw the liver tissues, quickly cut about 25 mg of each tissue and place in the labeled tubes.
Add 180 µl Buffer A to each sample and homogenize tissues in Qiagen TissueLyser II (20 seconds at frequency 30) followed by pulse spin (30 seconds, max speed).
Add 20 µl Proteinase K and digest tissue in 56°C water bath 3 hours to overnight. If necessary, digested tissue can be stored at 4°C.
Pre-warm elution buffer AE to 70°C and warm 100 µl buffer for each sample being extracted plus excess volume.
Spray tubes of digested tissue with 70% ethanol and pulse spin (30 seconds, max speed) all samples.
Add 5 µl RNaseA to each digested tissue and incubate at 37°C heat for 15 minutes.
Add 200 µl Buffer AL and vortex to mix to each digested tissue followed by incubation at 70°C for at least 10 minutes.
Add 200 µl 100% ethanol (room temperature) to each sample and vortex vigorously for 10 seconds and apply entire mixture to QIAamp column.
Spin column at 8000rpm for 1 minute and transfer column to new 2 ml collection tubes.
Add 500µl Buffer AW1 to each column and spin at 8000rpm for 1 minute and transfer column to new 2 ml collection tubes.
Add 500µl Buffer AW2 to each column and spin at 14000rpm for 3 minutes and transfer column to new 1.5 ml elution tube
Label tubes with tissue type, animal ID, and study ID.
Elute DNA in 2 × 50 µl Buffer AE and store DNA at 4°C for concentration measurement and qPCR.
BASIC PROTOCOL 8: DETECTION OF TRANSGENE EXPRESSION IN rAAV-TRANSDUCED LIVER BY X-GAL STAINING
Liver transgene expression after rAAV vector delivery can be detected at 4-week post-injection or other time points upon experimental design. Protein and mRNA expression can be detected by Western Blotting and RT-PCR. However, the most common used method is to detect the transgene expression in the tissue section in situ under microscopy or fluorescence microcopy. For example, reporter gene β–galactosidase expression can be detected under microcopy after X-gal staining. Green Florescent protein (GFP) or Red Florescent protein (RFP) can be detected by fluorescence microcopy directly. Ttherapeutic gene expression can also be detected by immunohistochemistry or immunofluorescence staining. The quantification of transgene expression can be assessed as total area of staining or fluorescence particles using image softwares such as NIH ImageJ. X-gal staining using fresh frozen liver tissue sections is described in this section as an example for quantifying rAAV-mediated liver transgene expression. Liver tissue section preparation for direct fluorescence (GFP, RFP etc) detection was described in protocol 4.1.2.
Materials
Sections as prepared in Support Protocol 5
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
Fixing solution: 0.5% Glutaraldehyde (Sigma-Aldrich) in D-PBS
Wash solution: 2 mM MgCl2 (Sigma-Aldrich) in D-PBS
50mg/ml of X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside) (Sigma-Aldrich MO) stock in dimethylformamide (DMF) (Sigma-Aldrich)
50mM potassium ferrocyanide (K4Fe(CN)6-3H2O) (Sigma-Aldrich) stock
50mM potassium ferricyanide (K3Fe(CN)6) ((Sigma-Aldrich) stock
X-gal staining solution: D-PBS supplemented with 2mM MgCl2, 3mM potassium ferrocyanide and 3mM potassium ferricyanide. Add X-gal to a final concentration of 1 mg/ml before use.
Ethanol (Decon Labs, Inc.)
Xylene (Fisher Sci)
Nuclear Fast Red (Vector Laboratories, Inc.)
Permount® (Vector Laboratories, Inc.)
Coverslips (Fisher Sci)
Glass or metal slide holder (Fisher Sci)
Staining containers (Fisher Sci)
Light microscope with digital camera (Olympus)
NIH ImageJ (NIH) or other image analysis software
Prepare fixation and staining solutions as described in Materials and store in different staining containers. Use glass or metal slide holder to hold the slides.
-
Serially put the slides in the staining containers containing different solution as following.
-
2.1
Fix in fixing solution at room temperature for 5 minutes.
-
2.2
Wash with washing solution twice, 5 minutes per wash.
-
2.3
Add X-gal stock into X-gal staining solution while washing and immerse slides in complete X-gal staining solution at 37°C for overnight.
-
2.4
Wash slides with D-PBS. Store at 4 °C or go to next step.
-
2.1
Rinse slides in tap water.
-
Counterstain slides with Nuclear Fast Red.
-
4.1
Immerse slides in vector Nuclear fast red or apply counterstain directly to slide, completely covering the tissue section.
-
4.2
Incubate sections for 1–10 minutes to obtain expected stain intensity.
-
4.3
Wash slides in tap water for 10 minutes. (Note: washing for longer than 10 minutes may lead to decreased counterstain intensity)
-
4.1
-
Serially dehydrate, and clear the slides as following.
-
5.1
70% Ethanol, 1 minute.
-
5.2
95% Ethanol, 2 minutes.
-
5.3
100% Ethanol, 2 minutes.
-
5.4
100% Ethanol, 2 minutes.
-
5.5
Xylene, 2 minutes.
-
5.6
Xylene, 2 minutes.
-
5.1
Mount the tissue sections in the slide: add a few drops of Permount® to one side of the slide and cover the sections with a thin and long cover glass.
Place the slides in hood till dry.
Observe the stained slide under light microscopy. The β–galactosidase expression corresponds to blue stain. Take pictures for at least 5 visual fields and save the pictures in the computer.
-
Analyze the pictures with NIH ImageJ or other appropriate image analysis software using the following instructions.
-
9.1
Open image files with NIH ImageJ or other appropriate image analyze software.
-
9.2
Change the image type to 8-bit black/white.
-
9.3
Adjust brightness and contrast to diminish the background.
-
9.4
Adjust the threshold with Black/white (B&W).
-
9.5
Select “Analyze Particles” under the Analyze tab.
-
9.1
The quantification of transgene expression will be assessed as total area of particles (pixel2) per visual field. If the picture has a scale bar, go to the line tool trace the scale bar and open the Analyze tab and select the “Set scale” option and fill in the appropriate values to get total area of particles in appropriate units.
SUPPORT PROTOCOL 5: PREPARATION OF FRESH FROZEN LIVER SECTIONS
The fresh frozen liver tissue sections can be used for a number of analyses most importantly for estimating in situ enzyme assays like x-gal staining.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
Embedding cup: made by aluminum foil by using small flat-bottomed bottle (about 1 cm in diameter) or embedding vinyl molds (Fisher Sci)
Sterile, disposable scissors and forceps (Kent Scientific Corp.)
Dry ice (Middlesex Gases & Technologies, Inc.)
Ethanol (Decon Labs, Inc., King of Prussia, PA) or isopropyl alcohol (Fisher Sci)
Optimal Cutting Temperature (OCT) (Sakura Finetek USA, Inc.)
Slides (Fisher Sci)
Mice injected by rAAV vectors and D-PBS.
Make a freezing bath using a mixture of dry ice and 100% ethanol or isopropyl alcohol.
Euthanize the mice in a manner approved by IACUC.
Collect the liver tissue, cut a small piece of liver lobe and wash away the attached blood by cold D-PBS. Absorb the extra D-PBS on the liver lobe using a paper towel.
Prepare an embedding cup (aluminum foil or vinyl mold) with a shallow layer of OCT place the liver lobe in desired orientation and put more OCT to cover the tissue. Place a label in the cup for identification.
Lower the bottom of the cup into freezing bath and gradually freeze the whole block. Retrieve the cup from bath before the block freeze completely, wrap it in foil, and place on dry ice immediately. Store the block in −80°C or move it to cryostat. For block sectioning, let the block equilibrate for 5 minutes in a cryostat (~ −20°C). Liver sections are usually obtained at the temperature of ~ −15°C.
Fix the block to the cryostat sectioning holder with OCT and cut 6–8°m sections.
Mount the sections on slides and let sections dry for 30 min at room temperature, and then stain for X-gal.
The slides can be stored at 4°C for a few days. If long-term storage is required, place in −80°C.
BASIC PROTOCOL 9: HISTOLOGY STUDY OF LIVER SAMPLES USING H&E STAINING
Liver biopsies are an important tool for pathologists being a gold standard for diagnosis for hepatic diseases and are commonly used prior to initiation of antiviral therapies and following that to monitor the progression of fibrosis. The major advantages of histopathology include its inexpensiveness, rapidity and the ability to provide a probable idea of infective or foreign organisms. Most common stains used for studying liver histology are the Hematoxylin and Eosin stain (H&E), Masson’s trichrome stain and Reticulin stain for assessing morphology; Periodic Acid Schiff (PAS) for storage of abnormal metabolites and A1AT and Oil red O to study accumulation of fat in the liver tissue. Other stains like Perl’s and rhodanine stains detect intracellular deposition of iron and copper respectively. H&E stained samples can be used for a wide variety of diagnoses hence the stain is termed as a routine stain. Stained liver biopsies are analyzed according to the Metavir scoring system to assess progression of fibrosis from F0-F4. A score between F2-F4 indicates significant fibrosis whereas F3-F4 indicates advanced fibrosis. The system also scores for inflammation from A0-A3 where A0= no inflammation, A1 = minimal inflammation, A2 = moderate inflammation, A3 = severe inflammation. Limitations to the histology approach include the invasive nature of sample collection, the fact that a small section of the liver is included which may exclude affected areas and that diagnosis is graded subjectively.
Materials
Dulbecco’s Phosphate Buffered Saline Solution (D-PBS) (Fisher Sci)
4% paraformaldehyde (Sigma-Aldrich)
Embedding cup: made by aluminum foil by using small flat-bottomed bottle (about 1 cm in diameter) or embedding vinyl molds (Fisher Sci)
Sterile, disposable scissors and forceps (Kent Scientific Corp.)
Ethanol (Decon Labs, Inc., King of Prussia, PA) or isopropyl alcohol (Fisher Sci)
Optimal Cutting Temperature (O.C.T.) (Sakura Finetek USA, Inc.)
Slides and coverslips (Fisher Sci)
Paraffin wax (Fisher Sci)
Permount® (Vector Laboratories, Inc.)
Glass or metal slide holder (Fisher Sci)
Staining containers (Fisher Sci)
Ethanol (Decon Labs, Inc.)
Xylene (Fisher Sci)
Hematoxylin solution (Sigma-Aldrich)
Eosin solution (Sigma-Aldrich)
Microtome (Triangle Biomedical Sciences)
Light microscope with digital camera (Olympus)
Mice injected by rAAV vectors or D-PBS
Preparation of slides
Euthanize the animal according to IACUC guidelines (carbon dioxide asphyxiation followed by cervical dislocation) and place in dorsal recumbency. Open the abdomen with an incision to expose the liver.
Extract the liver and cut a small block approx 1×1x4 cm3 and place in 4% paraformaldehyde overnight.
Serially dehydrate tissue by immersing sample 2 times for 1 hour each in 70%, 80% and 95% ethanol and 3 times 1 hour each in 100% ethanol and xylene.
Place dehydrated tissue in paraffin wax (56 ~ 58°C), 2 changes for 1.5 hour each and then place the tissue in embedding cups.
Transfer paraffin wax block in cups to a cold block at −3°C
Trim paraffin blocks to an optimal cutting surface including the sample with a small paraffin frame.
Section paraffin blocks continuously at 4–6 µm thickness using a microtome and float paraffin ribbon on a 40–45°C water bath containing deionized water.
Fish out swimming paraffin section using glass slides and a brush; position the section to the slide and remove wrinkles.
Dry sections overnight at 37°C (lower baking temperatures are better for subsequent antibody detection).
Place in drying oven at 60°C for 45 minutes prior to deparaffinization.
Staining
Place slides containing paraffin sections in a slide holder (glass or metal) to be used for all further steps.
Deparaffinize the sections 3 times for 3 minutes in Xylene. Blot excess xylene before going into ethanol.
Rehydrate sections by serially immersing the slides 3 times for 3 minutes in 100% ethanol, followed by 3 minutes immersion 1 time each in 95%, 80%, 70%, 50%, 30% ethanol and for 5 minutes 1 time in deionized water.
Skim surface of hematoxylin solution with a Kimwipe to remove oxidized particles.
Blot excess water from slide holder and immerse in hematoxylin 1X for 3mins
Rinse in deionized water and then 1 time for 5 minutes in tap water to allow stain to develop
Dip the slide holder 10–12 times very fast in acid ethanol to destain and rinse 2 times for 1 minute in tap water.
Rinse 1 time for 2 minutes in deionized water. Slides can be left overnight at this stage.
Blot excess water from slide holder and immerse for 30 ~ 5 seconds in Eosin solution.
Serially dehydrate by successive immersions for 3 minutes each in 30%, 50%, 70%, 80% ethanol, for 3 times for 5 minutes each in 95% and 100% ethanol. Blot excess ethanol before next step.
Immerse 3 times for 15 minutes each in xylene (longer is preferred to completely remove water)
Using a glass rod, place a drop of Permount® on the slide, angle the coverslip and slowly lower it taking care to leave no bubbles and to ensure that all the sections are covered
Dry the slides overnight in the hood.
Observe liver sections under light microscopy and take pictures for analyses.
COMMENTARY
Background Information
The liver is the site of many important metabolic functions; hence many inherited metabolic disorders such as hemophilia and hypercholesterolemia have their roots in liver dysfunction. Other inherited inborn errors of metabolism that are hepatic in origin include urea cycle disorders, lysosomal storage diseases and phenylketonuria. Since orthotropic liver transplants are risky, invasive, associated with high mortality and require lifelong immunosuppression, a better alternative is viral vector-based gene therapy. An ideal therapeutic vector for liver directed gene therapy should specifically target the liver, be capable of transducing quiescent hepatocytes, not be toxic or immunogenic, and should express the transgene for extended periods of time. Most published studies on liver-directed gene therapy have utilized adenoviruses as vectors (Ali et al., 1994; Jolly, 1994) but gradually rAAV-mediated gene therapy is gaining popularity because of its low immune activation, easy manipulation and episomal persistence.
Use of rAAV-based vectors could circumvent the requirement of high cellular proliferation since rAAVs can persist episomally for extended periods of time in non-dividing cells. Recent reports describing the systemic delivery of rAAVs have shown a liver tropism for some rAAV serotypes making them ideal choices for liver directed gene therapy. The scrAAV vectors can achieve much higher transduction efficiency in murine hepatocytes in vivo compared to the conventional rAAV vector (McCarty and others 2003; Wang and others 2003). Recently, a combination of rAAV serotype 8 and sc vector genome led to a successful hemophilia B gene therapy trial (Nathwani et al., 2011). The key to obtaining liver specific expression of genes lies on the use of liver specific promoter-enhancer combinations. In addition to the gene replacement, editing of the human genome by rAAV-mediated gene targeting to correct disease-causing mutations provides an alternative approach for the treatment of genetic disorders. In a recent study (Li et al., 2011), rAAV-mediated zinc finger nucleases (ZFNs)-driven in vivo gene targeting in liver has successfully used to restore hemostasis in a mouse model of hemophilia B, raising the possibility of this new strategy for liver gene therapy.
Possible limitations of using rAAVs for liver gene transfer include the presence of neutralizing antibodies (Li et al., 2012; Monteilhet et al., 2011) and CD8+ T-cell responses against rAAV capsids (Manno et al., 2006; Mingozzi et al., 2007) in human, which may interfere with gene transfer and also preclude repeated gene transfer with these vectors. Another drawback associated with rAAVs is the limited packaging space (4.7 kb) that precludes the use of larger genes like the coagulation factor VIII.
In addition to the various therapeutic benefits of rAAV-mediated gene transfer, gene delivery to the liver can also be used as a tool to understand basic biology. rAAV-mediated overexpression or knockdown of genes or miRNAs as well as rAAV-mediated gene targeting can provide an insight into gene and small RNA functions in liver. Recently, rAAV vectors were used to delivery miRNA 'tough decoys' (TuDs) into mouse liver to inhibit specific miRNAs (miR122 and let-7), and revealed that TuDs could induce miRNA tailing and trimming in vivo (Xie et al., 2012). This study provides a simple way to study miRNA function and accelerate analysis of miRNA biology in mammals in vivo. Russell et al used rAAV for in vivo gene targeting to insert an enhancer-promoter element at an imprinted chromosome 12 locus in mice, resulting in activation of multiple mRNAs, snoRNAs, and microRNAs in the 300-kb chromosomal domain and thereby converting normal hepatocytes into hepatocellular carcinoma (HCC) (Wang et al., 2012). This study demonstrates that rAAV-mediated gene targeting can also become a power tool in studying tumor biology.
Critical Parameters and Troubleshooting
Lateral tail vein injection
Mice lateral tail vein injection is a technically challenging procedure, which takes time and practice to perform well. One of the keys to successful injection is to identify the lateral vein in the mice tail. As shown in Fig 1A, there are two lateral veins, a single dorsal vein (especially visible in white mice, such as Balb/c strain) and a single ventral artery in mice tail. Both lateral veins are very superficial, and are generally visible as dark lines running along either side of the tail. These veins are easily accessible and generally used for IV injection. Sometimes, the injection fails because the wrong vessel is used for delivery. For example, in white mouse strains, the dorsal vein is also visible, but it’s more difficult to access and the injection to this vein always leads to failure. Thus, it is very important to distinguish the lateral veins and the dorsal vein in certain mouse strains. Sometimes the dorsal vein needs to be labeled with a marker pen when the mouse is very active and moves its tail frequently.
The second key is to warm the mice, particularly the tail, to dilate the vein. Mice cages can be warmed using a 60W lamp above the cage. Placing the tail in a small plastic tube of warm water can also do the trick. However, take care to avoid any over-heating and dehydrating the mice. Another key point is to have the tail bent slightly so the approach angle of the needle can be almost parallel to the vein. If the needle is not inserted into the vein, the solution will be diffuse into the surrounding tissue, resulting in a blanching and outward bulging of the skin. In this case, the plunger of the syringe offers a lot of resistance, which indicate the injection failed. In this event, carefully withdraw the needle and re-do the injection in a more proximal location. Sometimes, if multiple attempts fail, put the mice back in the cage and try again after some time has elapsed. The syringe should also be inspected to make sure the injection material is free of bubbles, which may lead to sudden death of the animal. It may also be useful to temporarily occlude the vein at the base of the tail, to block the flow of blood back to the body and thereby swell the tail vein, which will facilitate the needle access.
Overall, the vectors should be easily and smoothly delivered into the lateral tail vein by accurate injection.
Retro-orbital injection and blood collection
Usually retro-orbital injection is an easy and reliable method for intravascular delivery of rAAV vector into both adult and neonatal or young age mice for liver transduction. Retro-orbital bleeding is commonly used for blood collection in adult mice. However, sufficient training on cadavers or terminally anesthetized mice is necessary for a beginner. Anesthesia is required for these procedures. There are some concerns for these techniques: Only one injection per eye per day and at least a one day interval between injections; one bleed is allowed once per week from one eye and subsequent bleeds should use alternate eyes; the maximum number of bleeds for each animal is two bleeds per eye; the injection volume is not to exceed 100 µl per eye; the maximum amount of blood that may be collected at one time from one location is 1% of the animal's body weight (e.g., 0.25 ml from a 25 g adult mouse)
One potential adverse effect of retro-orbital injection and bleeding is swelling around injection or insert sites or eye trauma, which is usually caused by incorrect placement of the needle or capillary tubes in the soft tissue or eyeball. Also, the needle or tubes must have minimal movement once inserted into the retro-orbital sinus to prevent the vessels from rupturing, which will cause bleeding and loss of the vectors into the tissues behind the eye. If the needle is placed too deeply or too aggressively, the thin bone of the skull behind the eye can be pierced and the vector will be injected into the skull. If the mice experience swelling, conjunctivitis, uncontrolled bleeding or other sequelae related to injection or insert trauma, a veterinarian must be contacted.
Intraportal and intrasplenic injections
These two procedures require animal surgery, and the mice should be treated and monitored extremely carefully at pre and post-operative stage as well as during the operation.
Whole body live imaging
In vivo imaging requires expensive instruments including but not limited to cooled CCD cameras. Using whole animals for fluorescence imaging is wrought with additional challenges being limited by tissue based photon absorption. Photons that contribute to the detected signal are scattered multiple times during excitation and emission; in addition cellular and sub-cellular components of tissues collectively absorb light in the ultraviolet and visible spectra thus limiting the light penetration in the whole body to a couple of microns. Therefore, currently this technique is not practical for large animal model since the imaging performance is poor. Use of the far-red or near-infrared (NIR) wavelength range for excitation would allow signals to penetrate to several centimeters of tissue since only deoxyhemoglobin, oxyhemoglobin, water and lipids absorb the light signal. Therefore, it is critical to choose the optimal reporter gene for liver gene transfer study in case of whole body live imaging. For example, though Gluc has short coding sequence, it is not a very good choice since it emits short wavelength light in the blue/green region, which is strongly absorbed and scattered by mammalian tissues and lead to poor sensitivity of imaging performance (de Almeida et al., 2011). If the Gluc is used for the whole body live imaging, it is extremely important to image animals immediately after coelenterazine injections because the Gluc reaction is very fast and to keep the time between injection and imaging constant for all animals (Close et al., 2011). Incubation of coelenterazine diluted in PBS for long might lead to accumulation of a big cloudy precipitate that may clot the blood and result in the death of animals. A small precipitate/cloudy solution might form during injection which normally does not interfere with imaging. However, if coelenterazine diluted in PBS is incubated for a long period of time, a larger precipitate will form which might lead to blood-clots and death of the animal.
This technique is also time-consuming, involves frequent anesthesia and repeated systemic substrate injections which make it difficult to monitor relatively large cohorts of animals at small time intervals.
Tissue collection and vector genome copy quantification by real-time PCR
The most common concern for this procedure is to prevent DNA contamination from environment or cross-contamination from mouse tissues and organs, since the qPCR technique is very high sensitive; if tissues or organs other than the liver are to be collected in the experiment, the liver is almost always collected last, since it is always the most efficiently transduced organ in mice intravenously injected with rAAV vectors. A Biological Safety Cabinet (bio-hood) is recommended for this purpose. Species appropriate Personal Protective Equipment (PPE) must be worn during all stages of animal handling and tissue collection.
Anticipated Results examples
Vectors such as rAAV8 and rAAV9 can transduce adult mouse liver with high efficiency after IV injection. Different procedures can be used to monitor or detect the transgene expression upon different report genes are used. The live image of luciferase-based bioluminescent signals can be used to monitor the luciferase expression (Fig 6A), fluorescence microscopy can be adapted to detect the EGFP expression in liver section (Fig 6B) and X-gal staining followed with microscopy was used to observe the nuclear targeted LacZ (nLacZ) expression in liver section (Fig 6C). The ELISA is used to detect the A1AT expression in the sera collected from the mice and Gaussia luciferase activity is measured using a luminometer. Both A1AT and Gaussia luciferase activities are good readout parameters to monitor the rAAV-mediated transgene expression in liver animal at different time points.
Fig. 6.
rAAV-mediated liver transduction after IV injection. AAV9 vectors containing different report genes were injected to C57Bl6 mice via lateral tail vein at dosage of 1×1011 GC/mouse. Transgene expression was detected at 28 days post-injection. (A) Luciferase expression was monitored by live imaging of luciferase-based bioluminescent signals. (B) EGFP expression in liver section was detected by fluorescence microscopy (C) Nuclear LacZ (nLacZ) expression in liver section was observed under microscopy followed by X-gal staining.
Time Considerations
rAAV vector preparation takes about 1 hour and animal injection takes about couple of hours depending upon animal numbers and the skill of the injector. The rAAV-mediated liver transduction peaks at about 4 weeks post-injection. Therefore, animals usually are terminated at this time point for readout. For monitoring transgene expression in live animal, the time points are designed depending on different experimental requirements.
Acknowledgment
This work was supported by Public Health Service grant P01, UL1RR031982, 2P01 HL059407, 1P01AI100263-01, and 2R01NS076991-01 from the National Institutes of Health (to GG), and in part by a UMSS internal grant and a National High Technology Research and Development Program ("863"Program) of China (2012AA020810) (to GG) as well as a research grant from the Fanconi Anemia Research Fund, Inc (to LZ). We thank Mengxin Li and Jia Li for technical support and data preparation. Fig2A and Fig2B were adapted from Lab Anim (NY). 2011 May; 40(5): 155–160 (government works and no requirement for reuse permission).
LITERATURE CITED
- Abruzzese RV, Godin D, Burcin M, Mehta V, French M, Li Y, O’Malley BW, Nordstrom JL. Ligand-dependent regulation of plasmid-based transgene expression in vivo. Hum Gene Ther. 1999;10:1499–1507. doi: 10.1089/10430349950017833. [DOI] [PubMed] [Google Scholar]
- Ali M, Lemoine NR, Ring CJ. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- Bao R, Selvakumaran M, Hamilton TC. Use of a surrogate marker (human secreted alkaline phosphatase) to monitor in vivo tumor growth and anticancer drug efficacy in ovarian cancer xenografts. Gynecol Oncol. 2000;78:373–379. doi: 10.1006/gyno.2000.5925. [DOI] [PubMed] [Google Scholar]
- Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Close DM, Xu T, Sayler GS, Ripp S. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel) 2011;11:180–206. doi: 10.3390/s110100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contag CH. In vivo pathology: seeing with molecular specificity and cellular resolution in the living body. Annu Rev Pathol. 2007;2:277–305. doi: 10.1146/annurev.pathol.2.010506.091930. [DOI] [PubMed] [Google Scholar]
- de Almeida PE, van Rappard JR, Wu JC. In vivo bioluminescence for tracking cell fate and function. Am J Physiol Heart Circ Physiol. 2011;301:H663–H671. doi: 10.1152/ajpheart.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher KJ, Engelhardt JF. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks KD, Tavill AS. Liver disease in alpha 1-antitrypsin deficiency: a review. Am J Gastroenterol. 2008;103:2136–2141. doi: 10.1111/j.1572-0241.2008.01955.x. quiz 2142. [DOI] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, Calcedo R, Sanmiguel J, Abbas Z, Wilson JM. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B, Yu QC, Marsh JA, Conceicao CM, Wilson JM. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. Viral vector systems for gene therapy. Cancer Gene Ther. 1994;1:51–64. [PubMed] [Google Scholar]
- Kube DM, Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic Acids Res. 1997;25:3375–3376. doi: 10.1093/nar/25.16.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, Manco-Johnson MJ, Monahan PE. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Young SM, Jr., Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Yamauchi K, Takeda M, Kawachi H, Shimizu F, Yao J, Kitamura M. Continuous, noninvasive monitoring of local microscopic inflammation using a genetically engineered cell-based biosensor. Lab Invest. 2005;85:1429–1439. doi: 10.1038/labinvest.3700341. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lu Y, Bish LT, Calcedo R, Wilson JM, Gao G. Molecular analysis of vector genome structures after liver transduction by conventional and self-complementary adeno-associated viral serotype vectors in murine and nonhuman primate models. Hum Gene Ther. 2010;21:750–761. doi: 10.1089/hum.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wilson JM, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- Wang PR, Xu M, Toffanin S, Li Y, Llovet JM, Russell DW. Induction of hepatocellular carcinoma by in vivo gene targeting. Proc Natl Acad Sci U S A. 2012;109:11264–11269. doi: 10.1073/pnas.1117032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder EA. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science. 2010;328:704–708. doi: 10.1126/science.1174269. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Ameres SL, Friedline R, Hung JH, Zhang Y, Xie Q, Zhong L, Su Q, He R, Li M, Li H, Mu X, Zhang H, Broderick JA, Kim JK, Weng Z, Flotte TR, Zamore PD, Gao G. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods. 2012;9:403–409. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY) 2011;40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Muzyczka N. In vitro packaging of adeno-associated virus DNA. J Virol. 1998;72:3241–3247. doi: 10.1128/jvi.72.4.3241-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]