Abstract
Human and canine periodontium are often affected by an inflammatory pathology called periodontitis, which is associated with severe damages across tissues, namely, in the periodontal ligament, cementum, and alveolar bone. However, the therapies used in the routine dental practice, often consisting in a combination of different techniques, do not allow to fully restore the functionality of the periodontium. Tissue Engineering (TE) appears as a valuable alternative approach to regenerate periodontal defects, but for this purpose, it is essential to develop supportive biomaterial and stem cell sourcing/culturing methodologies that address the complexity of the various tissues affected by this condition. The main aim of this work was to study the in vitro functionality of a newly developed double-layer scaffold for periodontal TE. The scaffold design was based on a combination of a three-dimensional (3D) fiber mesh functionalized with silanol groups and a membrane, both made of a blend of starch and poly-ɛ-(caprolactone). Adipose-derived stem cells (canine adipose stem cells [cASCs]) were seeded and cultured onto such scaffolds, and the obtained constructs were evaluated in terms of cellular morphology, metabolic activity, and proliferation. The osteogenic potential of the fiber mesh layer functionalized with silanol groups was further assessed concerning the osteogenic differentiation of the seeded and cultured ASCs. The obtained results showed that the proposed double-layer scaffold supports the proliferation and selectively promotes the osteogenic differentiation of cASCs seeded onto the functionalized mesh. These findings suggest that the 3D structure and asymmetric composition of the scaffold in combination with stem cells may provide the basis for developing alternative therapies to treat periodontal defects more efficiently.
Introduction
The tooth is involved by a complex organ, called the periodontium, which comprises different tissues, namely, the alveolar bone, maxilla and mandible, the cementum (which is part of the tooth root), the periodontal ligament (PDL), and the gingiva.1 The above-mentioned structures have an important role in the chewing sensibility and, above all, act as a dynamic support apparatus for the tooth structure.2 Periodontitis is the most important periodontal pathology, known to cause tissue destruction that leads to tooth exfoliation, when not treated in due time.3,4 The main goals of current periodontal therapies are to preserve the natural dentition, as well as the esthetics and function of the periodontium. However, none of the currently available treatments for treating periodontal defects has shown the ability to restore completely the functionality of all the damaged tissues. A complete regeneration is only achieved when the Sharpey's fibers of the PDL between the bone and the radicular cementum are formed with oblique orientation, and when it is possible to avoid the expansion of the gingival epithelium and connective tissue into the lesion, as well as the occurrence of the ankylosis and radicular resorption.1,4–6
In the clinical routine, the periodontologists are forced to combine different techniques to achieve better results. However, the outcomes are quite variable and difficult to predict. Tissue Engineering (TE), using supportive biomaterials and cells, might provide new approaches for periodontal regeneration as suggested in recent scientific reports.7–12 Different strategies have been proposed, including the use of adipose-derived stem cells (ASCs) combined with platelet-rich plasma,7 collagen sponges seeded with autologous PDL cells,11 autologous bone marrow stem cells laden in a collagen gel,10 and a combination of multilayered PDL cell sheets with a polyglycolic acid membrane and with a tricalcium phosphate.9 However, these strategies have not shown a clear potential to enhance, synchronically, the alveolar bone regeneration and the formation of a new functional PDL.
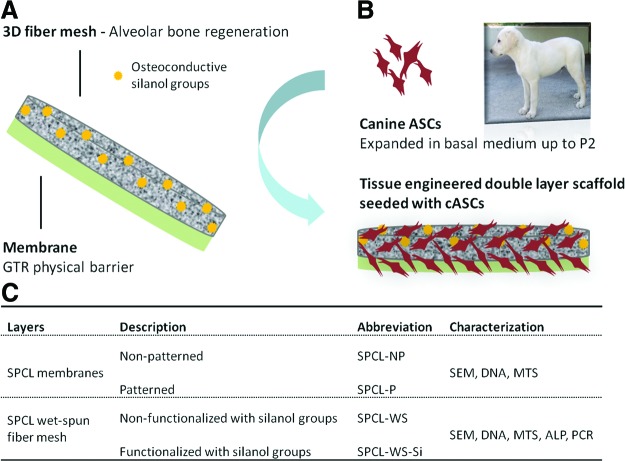
In previous studies, we have developed a scaffold specifically designed for periodontal regeneration, which is composed by two layers combined in a single matrix: a starch+poly (ɛ-caprolactone) (SPCL) membrane aiming at acting as a physical barrier between the gingival epithelium and the bone and ligament compartment of the periodontium, creating a selective space for bone and PDL regeneration; and a SPCL fiber mesh functionalized with osteoconductive silanol groups, which is aimed at promoting new bone formation.13–15 The characterization of these constructs performed so far, namely, regarding the morphology/structure, surface chemical composition, mechanical properties, and in vitro degradability, suggests their suitability for the envisioned application.16
The present work focused on studying the in vitro behavior of ASCs seeded and cultured onto both layers of the described scaffold, to further assess the potential of this TE scaffold for the proposed application. The potential of such scaffolds for the envisioned application can only be fully assessed upon performing in vivo studies in relevant animal models. Nevertheless, the in vitro studies are expected to demonstrate the ability of the proposed double-layer scaffold to promote either cellular proliferation or osteogenic differentiation, onto the membrane and fiber mesh layers, respectively. ASCs have been extensively described in terms of stemness and osteogenic differentiation capacity and proposed for the treatment of distinct pathological conditions, including tissue-engineered approaches for periodontal regeneration.7,17,18 ASCs seeded/cultured onto the membrane layer were characterized in terms of adhesion, proliferation, and metabolic activity and ASCs seeded/cultured onto the fiber mesh layer were further evaluated concerning their osteogenic differentiation by real-time reverse transcription-polymerase chain reaction (RT-PCR) and alkaline phosphatase (ALP) activity analysis.
This versatile double-layer material was shown to enhance osteogenic differentiation of ASCs cultured onto the fiber mesh layer functionalized with silanol groups. Additionally, it was observed that the membrane has the potential to allow the adhesion and proliferation of the undifferentiated ASCs.
Materials and Methods
Preparation of the materials
The double-layer scaffolds were prepared from a blend of starch and poly(ɛ-caprolactone) (30:70% w/w, SPCL; Novamont) by combining two different components/layers: a wet-spun fiber mesh and a solvent casting membrane (Fig. 1A) as described previously.16 Briefly, to prepare the fiber meshes, the SPCL was dissolved in chloroform (Sigma-Aldrich) at a concentration of 20% (w/v). This solution was loaded into a 5-mL plastic syringe with a metallic needle (21 G×1.5′′), which was connected to a programmable syringe pump (KD Scientific, World Precision Instruments). The solution was injected at a controlled pumping rate of 5 mL/h into the coagulation bath that was randomly moved to allow the formation of the fiber mesh structure. Two different coagulation baths were used: methanol (Vaz Pereira) to produce the SPCL wet-spun fiber meshes (SPCL-WS) and a calcium silicate solution to produce SPCL-WS fiber meshes functionalized with silanol groups (SPCL-WS-Si) as described previously.13,14,16 SPCL-WS were then dried at room temperature overnight to remove any remaining solvents and the SPCL-WS-Si were dried at 60°C for 24 h.
FIG. 1.
Schematic representation of the envisioned tissue engineering strategy based on the use of a double-layer scaffold with two different target functionalities (A) to culture the harvested/isolated cASCs (B). Description of the different components of the double-layer scaffolds studied in the present work and respective abbreviation (C). cASCs, canine adipose stem cells. Color images available online at www.liebertpub.com/tea
The membrane was obtained by casting 3 mL of the same polymeric solution onto a 5-cm-diameter either patterned/nonpatterned Teflon mold, to obtain patterned (SPCL-P) or smooth/nonpatterned (SPCL-NP) membranes, respectively. The objective was to analyze the effect of topography on cellular attachment and proliferation. Membranes were dried in a hood and then cut into disc samples of 6 mm of diameter.
Both membranes and fiber meshes were cut into 6-mm-diameter samples and sterilized by ethylene oxide before the biological assays described below.
Seeding/culturing of the canine adipose stem cells onto materials
Canine adipose stem cells (cASCs) were isolated from subcutaneous abdominal tissue of adult healthy dogs and expanded in a basal medium composed of the Dulbecco's Modified Eagle's Medium (Sigma Aldrich) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% antibiotic/antimicotic (Sigma) until passage 2 (P2), as described in a previous work18 (Fig. 1B). The rationale behind the use of cASCs resides in the fact that dog is the most suitable animal model used in periodontology, and thus, this could facilitate the future autologous assessment of the proposed TE approach.
The two components of the double-layer scaffolds were assessed separately by seeding/culturing them with ASCs, as we intent to analyze their distinct functionalities.
The SPCL-P and SPCL-NP membranes were seeded with 5.0×104 cASCs/samples and then maintained in the basal medium for 2 h to allow cell attachment. Afterward, 1 mL of medium was added to each culture well and cell-seeded membranes were further cultured for 1, 3, 7, and 14 days.
The SPCL-WS and SPCL-WS-Si fiber meshes were seeded with cASCs at a concentration of 1.0×105 cells/samples and then cultured in either a basal or osteogenic medium, containing the alpha Modification Eagle's Medium (Sigma Aldrich), 10% FBS, 1% antibiotic/antimicotic supplemented with 50 μg/mL ascorbic acid (Sigma), 10−8 M dexamethasone (Sigma), and 10 mM β-glycerophosphate (Sigma) for 7, 14, 21, and 28 days.
Characterization of the membranes/fiber meshes cultured with cASCs
After each selected culturing period of time, the culture medium was aspirated and the cell membrane/fiber mesh samples were carefully washed with sterile phosphate-buffered saline (PBS) and then, characterized using different techniques, namely, scanning electron microscopy (SEM), histology, DNA quantification, 3-(4,5-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl)-2- (4-sulfophenyl)-2H-tetrazolium (MTS) assay, ALP activity analysis, and PCR analysis, as described in detail below.
Cellular morphology
Scanning electron microscopy: For SEM evaluation, samples were fixed in 2.5% glutaraldehyde (Merck Millipore) solution in PBS for 1 h at 4°C and then dehydrated by immersion in a series of ethanol–water solutions with increasing concentrations (30%, 50%, 70%, 90%, and 100%, v/v). Afterward, the samples were dried at room temperature overnight.
SEM was performed using the Hitachi SU-70 UHR Schottky (Hitachi) equipment. Before any SEM observations, all the scaffolds were sputter coated with gold (Polaron SC502; Fisons Instruments). Three samples of each material/condition under study were analyzed.
Histology–Donath technique: The SPCL-WS and SPCL-WS-Si, cultured for 28 days with cASCs in both culturing conditions, were fixed in 4% phosphate-buffered formalin for 1 h at 4°C for further histological analysis by the Donath technique.
Briefly, the fiber mesh–cell constructs were dehydrated in alcohol solutions of increased concentrations, embedded in glycol methacrylate (Technovit 7200 VLC; Heraeus Kulzer), and processed for ground sectioning according to the method described by Donath and Breuner.19 The slides with a thickness of approximately 30 μm were prepared by microcutting and grinding (Exakt), and stained using a modified Lévai Laczkó stain.20
Cellular metabolic activity
The cell metabolic activity was assessed using the MTS test. For this purpose, the cultured constructs (membranes and fiber meshes seeded/cultured with cASCs), retrieved at different time points, were rinsed with PBS, immersed in a mixture consisting of serum-free cell culture medium and MTS reagent (VWR) in a 5:1 ratio, and incubated for 3 h at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, 200 μL of this mixture were transferred to new 96-well plates, and finally, the optical density was measured on a microplate reader (BioTek) using an absorbance of 490 nm.
Cellular proliferation
Double-strain deoxyribonucleic acid (dsDNA) quantification was performed to assess the proliferation of the cASCs seeded/cultured onto the membranes and fiber meshes under study. For this purpose, samples removed from culture were rinsed twice in a PBS solution and transferred into 1.5-mL microtubes containing 1 mL of ultrapure water. Afterward, the samples were incubated for 1 h at 37°C in a water bath and stored in a −80°C freezer, promoting a thermal shock variation and thus inducing cell lysis. Before assessing DNA levels, constructs were thawed and sonicated for 15 min.
A fluorimetric dsDNA quantification kit (PicoGreen; Molecular Probes) was used to determine the cellular proliferation. Samples and standards (0–2 μg/mL) were prepared in triplicate. Briefly, in each well of a 96-well white plate (Costar; Becton-Dickinson), were deposited 28.7 μL of sample or of each standard, 71.3 μL of PicoGreen solution, and 100 μL TE 1× (Life Technologies), and then the plate was incubated for 10 min in the dark and the fluorescence was read using a microplate reader (BioTek) at an excitation of 485/20 nm and an emission of 528/20 nm. A standard curve was developed to read DNA values of samples from the standard graph.
Osteogenic differentiation of the cASCs on the wet-spun fiber meshes
ALP activity: ALP activity assay was used to assess the osteogenic differentiation of the cASCs cultured onto the wet-spun fiber mesh layers, SPCL-WS and SPCL-WS-Si. For this purpose, in each well of a 96-well plate (Costar; Becton-Dickinson) were added 20 μL of each sample (the same used for DNA assay) plus 60 μL of a substrate solution consisting of 0.2% (w/v) p-nytrophenyl phosphate (Sigma) in a substrate buffer with 1 M diethanolamine HCl (Merck), pH 9.8. The plate was then incubated in the dark for 45 min at 37°C. After this incubation period, 80 μL of stop solution (2 M NaOH [Panreac] plus 0.2 mM ethylenediaminetetraaceticacid [Sigma]) was added to each well. Standards were prepared with p-nytrophenol (10 μM/mL; Sigma) to achieve final concentrations in the range 0–0.3 μM/mL. Samples and standards were prepared in triplicates. Absorbance was read at 405 nm and sample concentrations were read from the standard.
Gene expression of specific osteogenic markers: Real-time RT-PCR analysis was performed to analyze the relative gene expression of specific osteogenic markers, namely, collagen type I α1 (COLIA1), runt-related transcription factor 2 (RUNX2), and osteocalcin on the SPCL-WS and SPCL-WS-Si cultured with cASCs. For this purpose, the samples retrieved at each time point were kept in 800 μL of TRIzol reagent (Invitrogen). The mRNA was extracted with TRIzol following the procedure provided by the supplier. Briefly, after an incubation of 5 min at 4°C, additional 160 μL of chloroform (Sigma Aldrich) was added; the samples were incubated for 15 min at 4°C and then centrifuged at 13,000 rpm for 15 min at the same temperature. Subsequently, the aqueous part was collected and an equal part of isopropanol (VWR) was added. The samples were further incubated for 2 h at −20°C, and afterward, washed in ethanol, centrifuged at 4°C and 9000 rpm for 5 min, and resuspended in 12 μL of RNase/DNase free water (Gibco). The samples were quantified using the NanoDrop ND1000 Spectrophotometer (NanoDrop Technologies). For the cDNA synthesis, the samples with a 260/280 ratio between 1.7 and 2.0 were used. The cDNA synthesis was performed in the Mastercycler real-time PCR equipment (Eppendorf) using the iScript cDNA Synthesis Kit (Quanta Biosciences) with an initial amount of mRNA of 2 μg in a total volume of 20 μL. RNAse free water (Gibco) was used as a negative control.
After the synthesis of the cDNA, real-time PCR analysis was performed using the Mastercycler real-time PCR equipment (Eppendorf) using the PerfeCta Sybr-Green FastMix (Quanta Biosciences) to analyze the relative expression of the three studied markers in each sample, using GAPDH as the housekeeping gene. The primers were previously designed using the Primer 3 Plus v0.4.0 (MWG Biotech) (Table 1). The Delta Delta Ct method, according to Livak and Schmittgen (2001),21 was performed, using the data obtained for the cell–fiber mesh constructs upon seeding as a calibrator.
Table 1.
Primer Sequences for Targeted cDNAs
| Primer | RefSeqID | Product length (bp) | 5′-3′ sequence (F: forward; R: reverse) |
|---|---|---|---|
| COL1A1 | ENSCAFT00000026953 | 170 | F: ATGCCATCAAGGTTTTCTGC |
| R: CTGGCCACCATACTCGAACT | |||
| RUNX2 | ENSCAFT00000020482 | 148 | F: CAGACCAGCAGCACTCCATA |
| R: CAGCGTCAACACCATCATTC | |||
| Osteocalcin | ENSCAFT00000026668 | 166 | F: GATCGTGGAAGAAGGCAAAG |
| R: AGCCTCTGCCAGTTGTCTGT | |||
| GAPDH | NM_001003142.1 | 238 | F: CCAGAACATCATCCCTGCTT |
| R: GACCACCTGGTCCTCAGTGT |
COLIA1, collagen type I α1; RUNX2, runt-related transcription factor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
The obtained results were elaborated using the software JMP v9.0.1 (SAS Institute, Inc.). The statistical significance was assessed by the analysis of variance single factor method. Data are presented as mean±standard error of the mean and differences between data groups were considered to be statistically significant for p<0.05 (Student's t-test).
Results
Cellular morphology
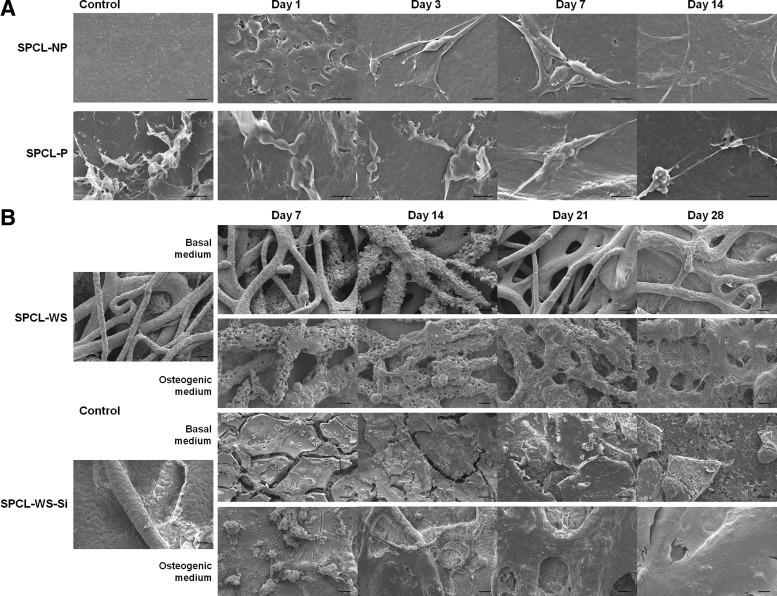
SEM micrographs of the membrane layer (Fig. 2A) upon 1, 7, 14, and 21 days on culture with cASCs revealed a good attachment of the cells onto both surface types (patterned and nonpatterned), exhibiting an elongated shape, typical of the mesenchymal stem cells. It was not possible to observe differences in the attachment or morphology of the cells seeded onto the two different membranes.
FIG. 2.
Scanning electron microscopy micrographs of (A) patterned (SPCL-P) and nonpatterned (SPCL-NP) membranes cultured with cASCs for 1, 3, 7, and 14 days in the basal medium (scale bar=20 μm). (B) SPCL-WS and SPCL-WS-Si cultured with cASCs for 7, 14, 21, and 28 days in both basal and osteogenic media (scale bar=100 μm). SPCL, starch+poly (ɛ-caprolactone); SPCL-WS, SPCL wet spun; SPCL-WS-Si; SPCL-WS fiber meshes functionalized with silanol groups.
Regarding the wet-spun fiber meshes (Fig. 2B), SEM observation suggests that both functionalized and nonfunctionalized materials allowed attachment and uniform distribution of the seeded cells, independently of the culturing conditions, either basal or osteogenic medium. The images show a clear increase in the amount of cells along the culturing time and suggest the formation of an extracellular matrix that in some cases covered almost the fiber mesh at the latest time point studied.
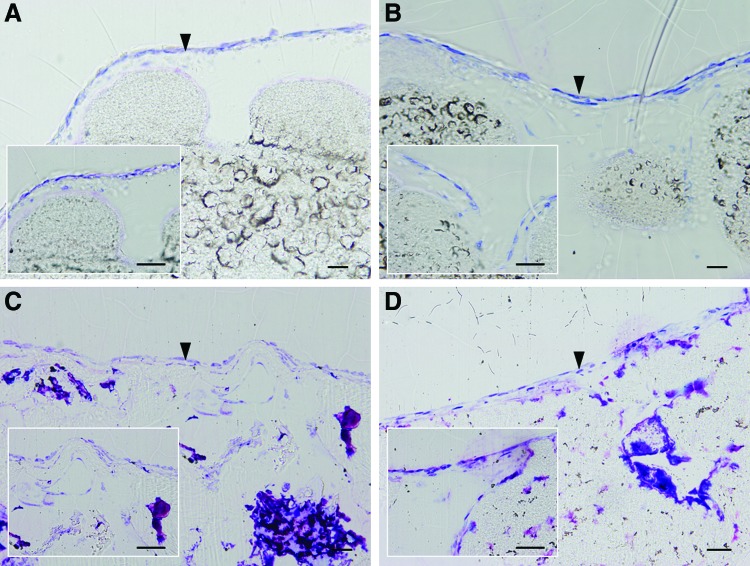
The histological sections of the SPCL-WS and SPCL-WS-Si stained with Lévai Laczkó (Fig. 3) shown that a monolayer of cells was formed along the surface of the fiber meshes. Moreover, these cells did not migrate throughout the material to the opposite layer of the material.
FIG. 3.
Optical microscopy images of cASCs (indicated by the arrowheads) cultured on the SPCL-WS (A, B) and SPCL-WS-Si (C, D) for 28 days both in basal (A, C) and osteogenic media (B, D) (Lévai Laczkó staining, scale bar=50 μm). Color images available online at www.liebertpub.com/tea
Cellular metabolic activity—MTS assay
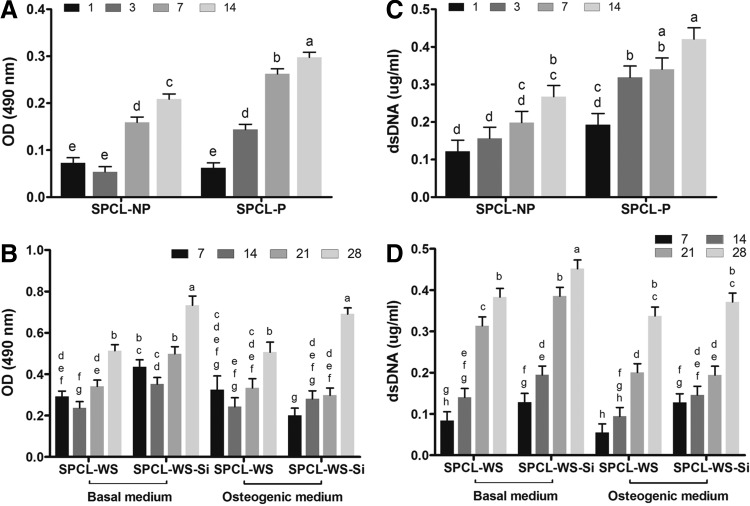
In terms of cell metabolic activity, the values were found higher for the cASCs cultured on the patterned (SPCL-P) than nonpatterned (SPCL-NP) membranes (Fig. 4A), particularly, in the later culturing times. The metabolic activity of the cells cultured onto the SPCL-WS and SPCL-WS-Si layer (Fig. 4B) was generally found higher in the functionalized fiber meshes (SPCL-WS-Si), particularly, in the last day of culture. It was also possible to observe that the different culturing media used did not induce any significant effect in the metabolic activity of cells cultured in the different fiber meshes.
FIG. 4.
cASCs metabolic activity (A, B) and proliferation (C, D) upon culturing onto the SPCL-NP and SPCL-P membranes for 1, 3, 7, and 14 days in basal medium (A, C) and culturing onto the SPCL-WS and SPCL-WS-Si for 7, 14, 21, and 28 days in basal and osteogenic media (B, D). Levels connected by different letters are significantly different (p<0.05, Student's t-test).
Cellular proliferation—dsDNA assay
In general, the results obtained from the dsDNA assay (Fig. 4C) showed increased values along the culturing time for SPCL-P and SPCL-NP, demonstrating that both membranes supported the adhesion and further proliferation of the seeded cASCs. Nevertheless, the cellular content was significantly higher in the SPCL-P as compared with the SPCL-NP at all the time points.
Similarly, for the fiber meshes (Fig. 4D), an increase in the cell content with increasing culturing times was also observed, indicating that cASCs were able to proliferate in both functionalized and nonfunctionalized scaffolds cultured either in the basal medium or in the osteogenic medium. However, the first evidence is that, in general, the basal medium promoted a higher cellular proliferation, mainly in the later time points. It is also possible to observe that the functionalization with silanol groups had a positive effect in the cellular proliferation, which is more significant on the cells cultured in the basal medium.
Osteogenic differentiation assessment
The osteogenic differentiation of the cASCs cultured onto the fiber mesh layer was evaluated by ALP activity measurement and by real-time RT-PCR analysis of the expression profile of three different osteogenic markers, namely, COLIA1, RUNX2, and Osteocalcin.
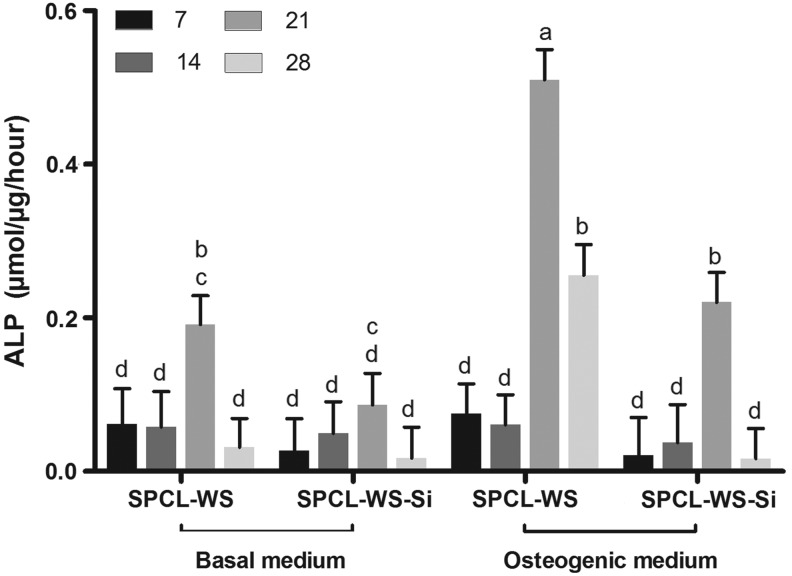
ALP quantification revealed, in general, a significant increase until the 21st day of culturing followed by a decrease in the last experimental time point (Fig. 5). The ALP activity was significantly higher in the cells cultured under the osteogenic medium, particularly, in the latest time points and for the nonfunctionalized samples (SPCL-WS).
FIG. 5.
Alkaline phosphatase activity of the cASCs cultured onto the SPCL-WS and SPCL-WS-Si for 7, 14, 21, and 28 days in basal and osteogenic media. Levels connected by different letters are significantly different (p<0.05, Student's t-test).
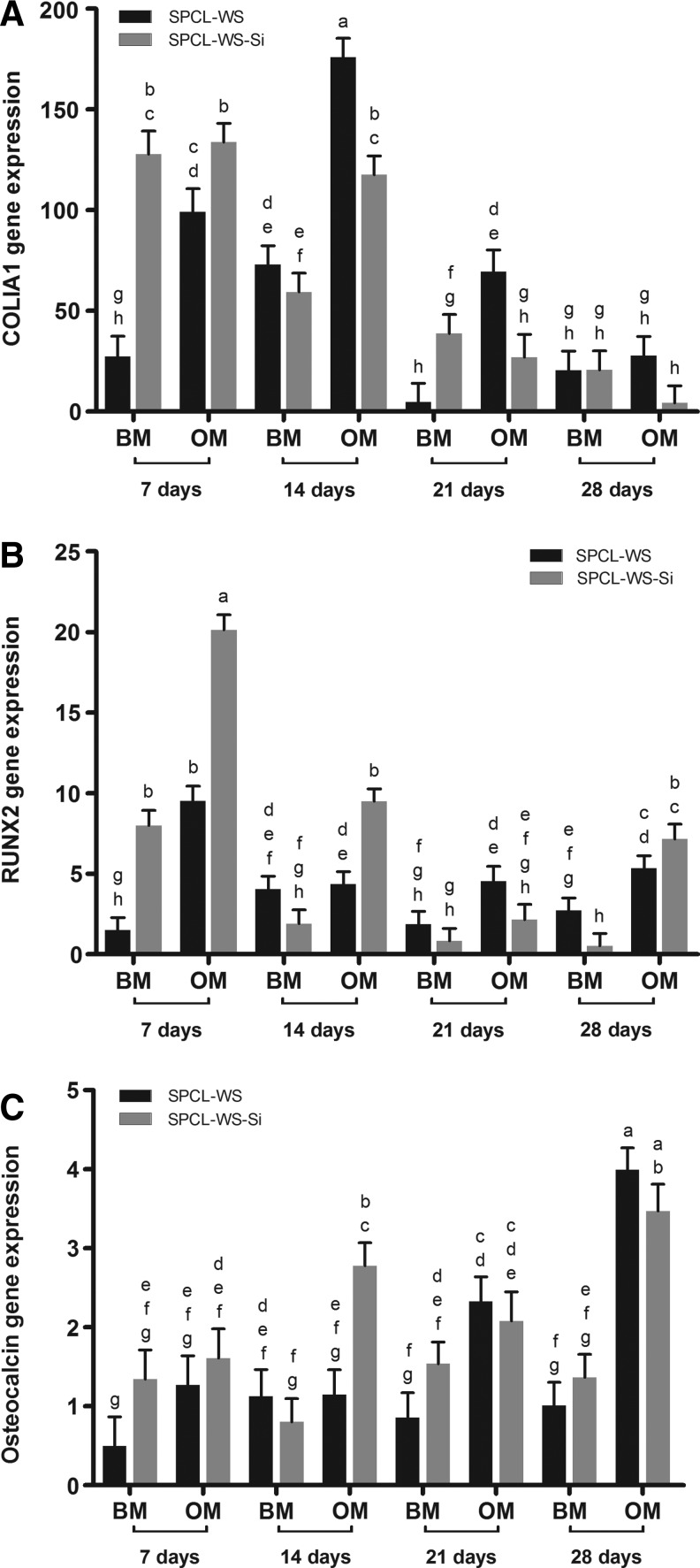
In Figure 6, it is possible to observe the different expression profiles of the analyzed osteogenic markers. The expression of COLIA1 was higher in the beginning of the studied period, followed by a decrease between the 14th and 21st culturing days in all conditions. In the 7th day, the expression of this marker was significantly higher in the SPCL-WS-Si samples in both the culturing media, and for the later time points the expression levels were variable being, in general, higher in the SPCL samples cultured in the osteogenic medium. Regarding the expression of the RUNX2 gene, an early marker for osteogenic differentiation, a significant upregulation was found in the initial culturing times, particularly in the SPCL-WS-Si scaffolds. This marker was expressed along all the experiment, particularly on the cASCs cultured in the osteogenic medium. Concerning the expression of Osteocalcin, a late marker, increased levels of expression along the time were registered, significantly higher in the samples cultured in the osteogenic medium. The SPCL-WS-Si samples promoted, in general, a higher expression of this marker in almost all conditions.
FIG. 6.
Real-time reverse transcription-polymerase chain reaction analysis of osteoblastic genes: COLIA1 (A), RUNX2 (B), and Osteocalcin (C) analyzed in cASCs cultured on the SPCL-WS and SPCL-WS-Si for 7, 14, 21, and 28 days in BM and OM (mean±standard error of the mean). Considering each marker independently, levels connected by different letters are significantly different (p<0.05, Student's t-test). COLIA1, collagen type I α1; RUNX2, runt-related transcription factor 2; BM, basal medium; OM, osteogenic medium.
Discussion
SPCL has been used in the development of several scaffold architectures with different physicochemical characteristics aimed at being used in TE applications, having been extensively tested both in vitro14,22–24 and in vivo.25–27 The feasibility of producing an SPCL double-layer scaffold specifically designed for periodontal regeneration was demonstrated in a previous work.16
Good elasticity and mechanical strength, as well as its degradability, allow to hypothesize that, once implanted in a periodontal defect, this scaffold will enable to maintain an occlusive space for some months while tissue regeneration is accomplished.
The cASCs used in this wok were previously characterized in terms of stemness and osteogenic potential along culture passages.18 These studies have shown that the cASCs lose their stemness and osteogenic differentiation potential along the passages and, for this reason, in the present work, they were used at passage 2.
As described above, the proposed scaffold is composed by two layers, an osteoconductive wet-spun fiber mesh and a solvent casting membrane that can be further enriched with stem cells, envisioning to achieve two main functions upon implantation in a periodontal defect, namely, to induce the formation of a new alveolar bone and act as a guided tissue regeneration barrier, respectively. Specifically, to evaluate the osteogenic potential of the bioactive fiber mesh layer, we have in this study assessed the ability of such a scaffold to promote the differentiation of cASCs into the osteogenic lineage. As for the membrane, it is thought to avoid the migration of epithelial cell ingrowth to the periodontal defect hindering the anchorage of other cell types essential for regeneration and also to simultaneously promote an effective integration with the surrounding smooth gingival tissue. Therefore, it was evaluated the effect of the surface topography (patterned and nonpatterned) of the solvent casting membrane layer, to select the most adequate to allow cellular adhesion and proliferation.
The SEM images of the membrane and fiber mesh layers revealed that both aspects of the double-layer scaffold allowed the adhesion of cells displaying the typical filopodia morphology of the ASCs, independently of the surface topography and presence/absence of silanol groups. The fact that the cASCs grow preferentially on the surface of the fiber meshes has been typically reported in static cultures that hinder the distribution of cells toward the interior of the scaffolds and prevent their viability within the scaffold due to diffusion limitations28,29 and might be overcome by culturing constructs in adequate bioreactors.
In all the studied materials, it was observed an increase of cellular metabolic activity along the culturing period, independently of the conditions or the composition/topography of the scaffolds, further demonstrating the biocompatibility of the materials selected.
Concerning the outcomes of the DNA assays performed on the membrane layer, it was shown a positive effect of the patterning on the membrane layer. This shows that a simple methodology can not only be used to promote cell proliferation but also paves the way for the development of other topographies specifically designed to guide the cellular migration along the surface, either restricting some cellular populations as the epithelial cells or promoting the migration of others, such as the PDL cells. Regarding the DNA assay results registered for the fiber mesh layers, it was found that the functionalization with silanol groups increased the proliferation of the cASCs on the fiber meshes, which is in good agreement with previous results.16
The incorporation of a three-dimensional functionalized fiber mesh in the design of the proposed double-layer scaffold was aimed at enhancing the osteogenic differentiation of seeded stem cells and/or to promote the ingrowth of alveolar bone tissue into the porous structure of the mesh layer upon implantation in a periodontal defect. This osteogenic potential was evaluated in vitro by quantifying the ALP activity, and by assessing the gene expression of specific osteogenic markers along the culturing period in the basal and osteogenic media.
The ALP is an enzyme anchored to the membrane inositol-phosphate on the outer surface of osteoblasts30 that reduces phosphate-containing substances and hydrolyzes pyrophosphate, which is known to inhibit hydroxylapatite formation,31 and is considered an early osteogenic differentiation marker.32 In this work, it was revealed that the SPCL-WS induced higher levels of ALP activity than the SPCL-WS-Si, as reported in a previous study using human ASCs cultured onto similar materials14 as well as other studies using various cell types and materials.33 In fact, some osteoconductive calcium- and silicon-rich materials have shown a negative effect in ALP activity, such as a bioactive glass-based scaffolds cultured with human ASCs when compared with nonbioactive scaffolds.33 The same negative effect of calcium in the ALP activity has also been reported on human osteosarcoma cells (SaOs-2) cultured with the Ca-enriched culture medium.30
Gene expression analysis showed that all the studied osteogenic markers were expressed along the culturing time of cASCs onto the SPCL-WS-Si and SPCL-WS. The highest levels of Osteocalcin expression, a late marker of differentiation34 were registered at the 21st day of culturing, concomitantly with higher activity of the ALP, and after the expression peaks of RUNX2 and COLIA1 at the 7th and 14th culturing days, respectively. This result was particularly evident for the samples cultured in the osteogenic medium. The expression of these markers has been also reported in cASCs cultured in two-dimensional cultures under the osteogenic medium, where it was observed a later peak of COLIA1 expression at the 21st day, also followed by the Osteocalcin,18 which suggests that the functionalized scaffolds might be accelerating the onset of the cASC osteogenic differentiation. Moreover, the expression profile of COLIA1 in the cASCs fiber meshes was similar to that observed during the natural healing of the canine mandibular alveolar bone.35
The positive effect of silicon has been also reported in cells attached to silicon-rich calcium phosphate nanocomposite, which showed higher expression of COLIA1 and osteocalcin compared with cells cultured on tissue culture plates,36 as well as reported by Botelho et al., who observed that Si-doped hydroxyapatite (HA) has improved performance compared with pure HA on human osteoblast differentiation.37 Silicon and calcium were shown to upregulate osteoblastic markers and accelerate osteogenic differentiation of cultured stem cells.38,39 The expression of the studied genes on the cultured cASCs on the SPCL-WS-Si, lead us to conclude that silanol groups promote the osteogenic differentiation of the cASCs, despite the lower ALP activity registered for the functionalized fiber meshes. Nevertheless, it is recognized the importance of further investigating the role of silicon in the mechanism of osteogenesis.
Overall, the proposed asymmetric double-layer scaffold supported the attachment and proliferation of cASCs in both layers, enhancing the osteogenic differentiation of the cASCs cultured on the osteoconductive fiber mesh layer. These findings, together with physicochemical characteristics previously reported, clearly demonstrate that the proposed scaffold might be used as a matrix for cell seeding/culturing before implantation in a tissue defect. Nevertheless, to address its suitability to promote the required functionalities, supporting the simultaneous regeneration of different tissue in a periodontal defect, either with or without the preculture of stem cells, in vivo studies must be performed. These studies should start by assessing the bone regeneration ability in small animal models, but ultimately, it should be used the canine circumferential periodontal defect models, which are considered the most adequate models for research in periodontology.
Conclusion
The present study described a new TE approach aimed at being used in periodontal regeneration, based on a recently developed construct composed of a biodegradable double-layer scaffold and mesenchymal stem cells obtained from adipose tissue.
The results obtained in the present work demonstrated that the wet-spun fiber mesh layer functionalized with silanol groups stimulated the osteogenic differentiation of cASCs, while the membrane layers enabled a good cell attachment and proliferation. Considering these results together with those obtained previously suggested the potential of these double-layer scaffolds to be used in periodontal TE approaches. In such strategies, the double-layer scaffold, with or without precultured stem cells, is envisioned to enhance osteogenesis, which is essential for the regeneration of the alveolar bone compartment of the periodontium, while simultaneously promoting colonization with a distinct cellular population of the periodontium and to avoid the migration of epithelial cells into the defect, through the specific features of the fiber mesh and the membrane layers, respectively. This approach should be assessed in a suitable animal model to be proposed as a viable alternative for treating periodontal defects.
Acknowledgments
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° REGPOT-CT2012-316331-POLARIS. J.F.R. acknowledges the Portuguese Foundation for Science and Technology (FCT) for his PhD scholarship (SFRH/BD/44143/2008).
Disclosure Statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the article.
References
- 1.Polimeni G., Xiropaidis A.V., and Wikesjo U.M.E.Biology and principles of periodontal wound healing/regeneration. Periodontology 200041,30, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chen F.M., and Jin Y.Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev 16,219, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Kuo L.-C., Polson A.M., and Kang T.Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health 122,417, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bosshardt D.D., and Sculean A.Does periodontal tissue regeneration really work? Periodontology 200051,208, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Wikesjo U.M.E., Lim W.H., Thomson R.C., and Hardwick W.R.Periodontal repair in dogs: gingival tissue occlusion, a critical requirement for GTR? J Clin Periodontol 30,655, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bartold P.M., McCulloch C.A.G., Narayanan A.S., and Pitaru S.Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 200024,253, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Tobita M., Uysal A.C., Ogawa R., Hyakusoku H., and Mizuno H.Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A 14,945, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Dogan A., Ozdemir A., Kubar A., and Oygur T.Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue Eng 9,1189, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Iwata T., Yamato M., Tsuchioka H., Takagi R., Mukobata S., Washio K., Okano T., and Ishikawa I.Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 30,2716, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi H., Hirachi A., Hasegawa N., Iwata T., Hamaguchi H., Shiba H., Takata T., Kato Y., and Kurihara H.Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 75,1281, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Nakahara T., Nakamura T., Kobayashi E., Kuremoto K.I., Matsuno T., Tabata Y., Eto K., and Shimizu Y.In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng 10,537, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Requicha J.F., Viegas C.A., Muñoz F., Reis R.L., and Gomes M.E.Periodontal tissue engineering strategies based on non-oral stem cells. Anat Rec 297,6, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Leonor I.B., Rodrigues M.T., Gomes M.E., and Reis R.L.In situ functionalization of wet-spun fibre meshes for bone tissue engineering. J Tissue Eng Regen Med 5,104, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues A.I., Gomes M.E., Leonor I.B., and Reis R.L.Bioactive starch based scaffolds and human adipose stem cells are a good combination for bone tissue engineering. Acta Biomater 8,3765, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Ni S., Chang J., Chou L., and Zhai W.Comparison of osteoblast-like cell responses to calcium silicate and tricalcium phosphate ceramics in vitro. J Biomed Mater Res B Appl Biomater 80,174, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Requicha J.F., Viegas C.A., Hede S., Leonor I.B., Reis R.L., and Gomes M.E.Design and characterization of a biodegradable double-layer scaffold aimed at periodontal tissue-engineering applications. J Tissue Eng Regen Med [Epub ahead of print]; DOI: 10.1002/term.1816,2013 [DOI] [PubMed] [Google Scholar]
- 17.Vieira N., Brandalise V., Zucconi E., Secco M., Strauss B., and Zatz M.Isolation, characterization and differentiation potential of canine adipose-derived stem cells. Cell Transplant 19,279, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Requicha J., Viegas C., Albuquerque C., Azevedo J., Reis R., and Gomes M.Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev 8,1211, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Donath K., and Breuner G.A method for the study of undecalcified bones and teeth with attached soft tissues. J Oral Pathol 11,318, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Laczkó J., and Lévai G.A simple differential staining method for semi-thin sectionsof ossifying cartilage and bone tissues emhedded in epoxy resin. Mikroskopie 31,1, 1975 [PubMed] [Google Scholar]
- 21.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2 (T) (-Delta Delta C) method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Gomes M.E., Bossano C.M., Johnston C.M., Reis R.L., and Mikos A.G.In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng 12,177, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Puppi D., Piras A.M., Chiellini F., Chiellini E., Martins A., Leonor I.B., Neves N., and Reis R.Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J Tissue Eng Regen Med 5,253, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Tuzlakoglu K., Pashkuleva I., Rodrigues M.T., Gomes M.E., van Lenthe G.H., Muller R., and Reis R.L.A new route to produce starch-based fiber mesh scaffolds by wet spinning and subsequent surface modification as a way to improve cell attachment and proliferation. J Biomed Mater Res Part A 92A,369, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues M.T., Gomes M.E., Viegas C.A., Azevedo J.T., Dias I.R., Guzon F.M., and Reis R.L.Tissue-engineered constructs based on SPCL scaffolds cultured with goat marrow cells: functionality in femoral defects. J Tissue Eng Regen Med 5,41, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Santos T.C., Marques A.P., Horing B., Martins A.R., Tuzlakoglu K., Castro A.G., van Griensven M., and Reis R.L.In vivo short-term and long-term host reaction to starch-based scaffolds. Acta Biomater 6,4314, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Link D., Gardel L., Correlo V., Reis R., and Gomes M.Osteogenic properties of starch poly(ɛ-caprolactone) (SPCL) fiber meshes loaded with osteoblast-like cells in a rat critical-sized cranial defect. J Biomed Mater Res Part A 101,3059, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Gomes M.E., Sikavitsas V.I., Behravesh E., Reis R.L., and Mikos A.G.Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J Biomed Mater Res Part A 67A,87, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Goncalves A., Costa P., Rodrigues M.T., Dias I.R., Reis R.L., and Gomes M.E.Effect of flow perfusion conditions in the chondrogenic differentiation of bone marrow stromal cells cultured onto starch based biodegradable scaffolds. Acta Biomater 7,1644, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Anh D.J., Dimai H.P., Hall S.L., and Farley J.R.Skeletal alkaline phosphatase activity is primarily released from human osteoblasts in an insoluble form, and the net release is inhibited by calcium and skeletal growth factors. Calcified Tissue Int 62,332, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Balcerzak M., Hamade E., Zhang L., Pikula S., Azzar G., Radisson J., Bandorowicz-Pikula J., and Buchet R.The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol 50,1019, 2003 [PubMed] [Google Scholar]
- 32.Nandakumar A., Yang L., Habibovic P., and van Blitterswijk C.Calcium phosphate coated electrospun fiber matrices as scaffolds for bone tissue engineering. Langmuir 26,7380, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Haimi S., Suuriniemi N., Haaparanta A.M., Ella V., Lindroos B., Huhtala H., Raty S., Kuokkanen H., Sandor G.K., Kellomaki M., Miettinen S., and Suuronen R.Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A 15,1473, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Rodan G.A., and Noda M.Gene expression in osteoblastic cells. Crit Rev Eukaryot Gene Expr 1,85, 1991 [PubMed] [Google Scholar]
- 35.Sun L.Y., Wu L., Bao C.Y., Fu C.H., Wang X.L., Yao J.F., Zhang X.D., and van Blitterswijk C.A.Gene expressions of collagen type I, ALP and BMP-4 in osteo-inductive BCP implants show similar pattern to that of natural healing bones. Mater Sci Eng C 29,1829, 2009 [Google Scholar]
- 36.Gupta G., Kirakodu S., and El-Ghannam A.Dissolution kinetics of a Si-rich nanocomposite and its effect on osteoblast gene expression. J Biomed Mater Res A 80,486, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Botelho C.M., Brooks R.A., Best S.M., Lopes M.A., Santos J.D., Rushton N., and Bonfield W.Human osteoblast response to silicon-substituted hydroxyapatite. J Biomed Mater Res Part A 79A,723, 2006 [DOI] [PubMed] [Google Scholar]
- 38.El-Ghannam A., and Ning C.Q.Effect of bioactive ceramic dissolution on the mechanism of bone mineralization and guided tissue growth in vitro. J Biomed Mater Res Part A 76A,386, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Beck G.R., Jr., Moran E., and Knecht N.Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res 288,288, 2003 [DOI] [PubMed] [Google Scholar]