Abstract
The widespread occurrence of multiple infections and the often vast range of nutritional resources for their hosts allow that interspecific parasite interactions in natural host populations might be determined by host diet quality. Nevertheless, the role of diet quality with respect to multispecies parasite interactions on host population level is not clear. We here tested the effect of host population diet quality on the parasite community in an experimental study using Daphnia populations. We studied the effect of diet quality on Daphnia population demography and the interactions in multispecies parasite infections of this freshwater crustacean host. The results of our experiment show that the fitness of a low-virulent microsporidian parasite decreased in low, but not in high-host-diet quality conditions. Interestingly, infections with the microsporidium protected Daphnia populations against a more virulent bacterial parasite. The observed interspecific parasite interactions are discussed with respect to the role of diet quality-dependent changes in host fecundity. This study reflects that exploitation competition in multispecies parasite infections is environmentally dependent, more in particular it shows that diet quality affects interspecific parasite competition within a single host and that this can be mediated by host population-level effects.
Keywords: Daphnia magna, disease dynamics, food quality, host population demography, interspecific parasite competition, multispecies infections, parasite ecology, unicellular gut parasite, white bacterial disease
Introduction
Parasites play a key role as consumers in our environment (Dobson et al. 2008), for example in food webs (Lafferty et al. 2006) and during species invasions (Johnson and Thieltges 2010; Dunn et al. 2012). Their commonness and significance for ecosystems is gaining more and more attention (Hudson et al. 2006; Holdo et al. 2009; Sato et al. 2012). It is frequently observed that hosts are infected by multiple parasite species (Ebert 2005; Poulin 2007; Schmid-Hempel 2011). Multispecies infections co-infecting the same host individual may be independent, but parasites can also interact in a synergistic or antagonistic way, modifying each other's effect on the host (Hughes and Boomsma 2004; Pedersen and Fenton 2007; Graham 2008; Thumbi et al. 2013). Beyond interactions within individual hosts, synergistic and antagonistic parasite interactions can also be observed within host populations (Dobson 1985; Lello et al. 2004; Bell et al. 2006; Graham 2008; Telfer et al. 2008, 2010; Mideo 2009).
Interacting parasites can affect each other's survival (de Roode et al. 2004; Alizon et al. 2013) and virulence (Balmer et al. 2009). The strength of the interactions depends on many different factors, such as host behavior (Bush and Malenke 2008), the infection dose to which the host is exposed (Fellous and Koella 2009), or the order in which multiple infections occur (Lohr et al. 2010; Hoverman et al. 2013). Besides these intrinsic host properties, parasites can be largely affected by the environmental factors to which their hosts are exposed (Wolinska and King 2009; Karvonen et al. 2010; Pech et al. 2010; Vale et al. 2011). Evidence for the relevance of these factors, with respect to single species infections, is amply present in a number of different systems: bacteria–phage interactions (Forde et al. 2004), plant–pathogen interactions (Laine and Tellier 2008; Burdon and Thrall 2009), and invertebrate–pathogen interactions (McKenzie and Townsend 2007; Garbutt et al. 2014; Hall et al. 2013; for an overview see Schmid-Hempel 2011). Nevertheless, with the exception of temperature and humidity (see Thomas et al. 2003; Malenke et al. 2011), the effects from environmental factors on multispecies parasite interactions have been largely neglected, even though ecological constraints of host resources are considered to play an important role (Pedersen and Fenton 2007; Bukovinszky et al. 2009; Sarfraz et al. 2009).
Contrary to food quantity (amount in mg Carbon/L), food quality is usually defined by elemental ratios, such as low carbon-to-nitrogen (associated with high protein quality, Anderson et al. 2004) or low carbon-to-phosphor ratios (largely associated with P-rich ribosomal RNA production, Elser et al. 2000) or by particular lipids, such as PUFAs or sterols (Martin-Creuzburg and Von Elert 2009). In vertebrates, parasites mostly suffer from both a higher quantity or a better quality diet of the host, based on increased host immune system activity (Wiehn and Korpimäki 1998; Lochmiller and Deerenberg 2000). Reversely, monospecies parasite infections of invertebrate hosts often benefit from an increase of host food quantity (McKenzie and Townsend 2007; Seppälä et al. 2008; Civitello et al. 2013), although quantity levels that are too high can also terminate parasite epidemics (Dallas and Drake 2014). To date, however, possible effects of host diet on multispecies parasite infections remain elusive. We here focus on this research question, evaluating the environmental factor of host diet quality, as it is a determining and ubiquitous factor for heterotrophic animal hosts, which has shown to affect growth and virulence of individual parasite species (Hall et al. 2009; Choisy and de Roode 2010). We consider diet quality as a difference in the presence of particular nutrients that are important for the growth and reproduction of a heterotrophic animal and observed the difference in host population density as well as parasite fitness in different food quality environments.
In natural ecosystems, parasites live in host populations and transmission between different host individuals is usually an essential fitness parameter that determines parasite epidemiology and virulence (Dieckmann 2002; Poulin 2007). Despite the importance of host to host transmission, most studies investigating infected host populations are based on within-host model systems, and from these individual host studies, one extracts expectations for host population-level effects of parasitism (Choisy and de Roode 2010). In particular, parasite transmission between host individuals in a population may be relevant for understanding multispecies parasite interactions. One of the few studies, where a host population model was used, found fundamental differences in host population dynamics in the monoparasitic Plodia interpunctella granulovirus system, due to changes in food quality (McVean et al. 2002). While a range of studies confirmed that even parasite communities can differ depending on environmental conditions (Rohde and Heap 1998; Nunn et al. 2005), these descriptive analyses could not uncover interactions between specific parasites (Rigaud et al. 2010; Johnson and Buller 2011). For these reasons, we have chosen to study host diet quality effects on multispecies parasites interactions experimentally, explicitly allowing host population-level-mediated effects, while controlling the identity of parasite species.
Here, we use the established experimental invertebrate model organism Daphnia magna that is infected by a multitude of microparasites, most of them transmitting horizontally between individuals in the host population, and many of which are known and their life cycle described in the literature (Decaestecker et al. 2005; Ebert 2005; Wolinska et al. 2009; Duffy et al. 2010; Jansen et al. 2010). Earlier experimental studies, investigating uninfected D. magna individuals, suggested a possibly strong effect of food quality on Daphnia population dynamics, because somatic growth depends on nutrient and cholesterol availability, while reproduction depends on PUFA availability (Wacker and Martin-Creuzburg 2007; Martin-Creuzburg and Von Elert 2009). In Daphnia, food quality has also been shown to affect parasitism. In particular, the relative availability of nutrients and the presence of PUFAs can affect Daphnia–parasite interactions (Frost et al. 2008; Hall et al. 2009; Schlotz et al. 2013), but effects on population level have rarely been studied and are not straightforward (Aalto et al. 2014; Dallas and Drake 2014). These findings allow us to target the availability of sterols and PUFAs, by using different food choices with variable sterol and PUFA contents, and here we measure its direct effect on population density rather than on host individuals. We chose two parasites for this study, the unicellular gut parasite (UGP) and the parasite that causes white bacterial disease (WBD). These two parasites differ strongly in their effect on D. magna mortality and fecundity (Ebert et al. 2000; Ebert 2005) and co-occur in natural host populations (Decaestecker et al. 2005; Ebert 2005). The aim of this work was to investigate the role of host diet quality in parasitized D. magna populations, comparing monospecies- versus multispecies-infected populations. More specifically, the objectives of the study were to determine the effect of diet quality on: (1) competition between two co-infecting parasite species; (2) single- and multiple-infected host population densities; and (3) host population demography, particularly the density of adult/juvenile hosts.
Materials and Methods
Host-parasite system
To study food quality effects on parasite interactions, we used the water flea D. magna STRAUSS and two of its (endo-)parasites. Monoclonal offspring of a female Daphnia were used in the experiments. The female was collected from a Belgian pond (situated in the coastal zone, 51°21′25″N, 3°20′34″E) in May 2010. Two Daphnia parasites were isolated from the same pond: White bacterial disease (WBD), also known as white fat cell disease, is a highly virulent horizontally transmitting parasite that infests the fat tissue of its host (Ebert 2005; Coopman et al. 2014). During early infection, stages of the parasite are not yet visible to the naked eye. After several days, the host's fat cells start to have a whitish-green shine and the infection is clearly visible. In earlier studies, infected animals died within 3 weeks postinfection (Ebert et al. 2000; Van De Bund and Van Donk 2002; Decaestecker et al. 2003). The second parasite used here is the unicellular gut parasite (UGP), also described before as Micro1 in Decaestecker et al. (2003, 2005). It is a horizontally transmitting microsporidian parasite that can be found primarily at the end of the host gut and is characterized by low virulence, a very small parasite-induced increase in mortality (Refardt and Ebert 2012).
Culture conditions
For our experiments, food treatments differing in cholesterol and PUFA availability were chosen. Strains of the algae Scenedesmus obliquus (SAG 276-3a; culture collection of algae, University of Göttingen in Germany), Chlamydomonas reinhardtii (SAG 77.81), and Cryptomonas sp. (SAG 26.80) were grown in modified WC medium with vitamins at 20 ± 1°C (Guillard 1975). In particular, with respect to PUFAs, S. obliquus and C. reinhardtii are described as low food quality, while Cryptomonas sp. is considered to be high food quality (Weers and Gulati 1997; Martin-Creuzburg and Von Elert 2009; Basen et al. 2011). Prior to all experiments, the animals were fed with the green alga S. obliquus for several months to standardize food conditions for all host populations (Taipale et al. 2011). For the experiments, food quality was standardized for organic Carbon according to Bird et al. (2011) so that there is no quantitative but only a qualitative difference between food treatments. Experiments were conducted under a constant light/dark cycle (16:8 h) at a temperature of 20 ± 1°C. The artificial Daphnia medium ADaM (modified by using only 5% of the recommended SeO2 concentration) was used for all cultures (Klüttgen et al. 1994).
Experimental setup
To test the influence of food quality on parasite interactions, a host population experiment was conducted using ten replicated populations per treatment. Infected or uninfected (control) populations of ten hosts (five adults, five juveniles) were set up in beakers containing 300 mL of medium. The populations were either fed high or low food quality three times per week (2 mg Carbon/L). After 5 weeks, the treatment was increased to 4 mg Carbon/L. Medium was changed in weekly intervals, and adult and juvenile Daphnia were counted. Dead animals were not transferred to the new medium because spore transmission and bacterial growth might influence the host–parasite dynamics (Ebert et al. 2000). The experiment lasted 10 weeks, which equals about five host generations. During this time, WBD-exposed populations were checked visually once per week for WBD infections. After 10 weeks, five adult host individuals were randomly chosen from each surviving host population. Then, Daphnia body length (as in Ranta et al. 1993) and amount of UGP spores (as in Decaestecker et al. 2005) were assessed for each individual in the UGP treatments. All animals surviving the other treatments were checked for UGP infection at the end of the experiment. Absence of WBD infections, in the controls and solely UGP-exposed populations, was verified by weekly observations during the experiment.
Data analysis
In the parasite fitness part of this study, we were primarily interested in the effects of co-infection and food quality. We analyzed our experimental data on parasite fitness using an analysis of variance (ANOVA). The full model contained the following main explanatory variables: (1) fixed effects of ‘host body size’ (continuous), (2) ‘food’ (nominal) quality, or (3) ‘WBD’ infection (nominal: singly infected population versus co-infected population with WBD & UGP), and the interactions between these explanatory variables. The response variable was the average number of UGP spore clusters for each host population. We followed backward elimination of nonsignificant terms, and thus removed the interactions between host body size and food as well as host body size and WBD.
In the host population part of this study, the mean host density of weeks eight to ten (equilibrium density) was used for analysis. To that purpose, the arithmetic mean of population density of adults, juveniles, or both together were compared with the mean population density of uninfected populations within the same food and parasite treatment combination, using a Wilcoxon two-sample test. Because several populations became extinct after 10 weeks of WBD treatment, a chi-square test for host extinctions comparing parasite to uninfected treatment was used instead of the Wilcoxon two-sample test. Generally, statistical significance was accepted at the α < 0.05 level. To counteract type I errors, the results of the Wilcoxon two-sample test were Bonferroni corrected and thus only accepted at the α < 0.003 level. All analyses were performed in R (R Development Core Team 2009).
Results
Effects of host diet and co-infection on UGP fitness
Parasite fitness was measured by assessing the number of UGP spore clusters in Daphnia. The number of UGP clusters was affected by interactions of UGP with WBD and food quality (Table 1; Fig. 1). The mean number of UGP clusters was higher in hosts of single-UGP-infected populations than in hosts of co-infected populations in the low food quality treatment (single infected: 66.38 ± 13.42 (mean ± SD); co-infected: 26.11 ± 13.65; Welch's test, t16.00 = 6.31, P < 0.0001). In contrast, no difference in UGP cluster number between the single and co-infected hosts was observed in the high food quality treatment (single infected: 55.70 ± 39.43; co-infected: 72.28 ± 34.82; Welch's test, t15.80 = −0.95, P < 0.36).
Table 1.
ANOVA results of population experiment for the effects of host body size, food quality treatment, and WBD (white bacterial disease) infection on the number of clusters of UGP (unicellular gut parasite) after 10 weeks. Five host individuals per population were measured.
| Explanatory variable(s) | df | F | P |
|---|---|---|---|
| Size | 1, 31 | 9.11 | 0.006 |
| Food | 1, 31 | 4.12 | 0.051 |
| WBD | 1, 31 | 3.52 | 0.071 |
| Food × WBD | 1, 31 | 4.47 | 0.043 |
Figure 1.
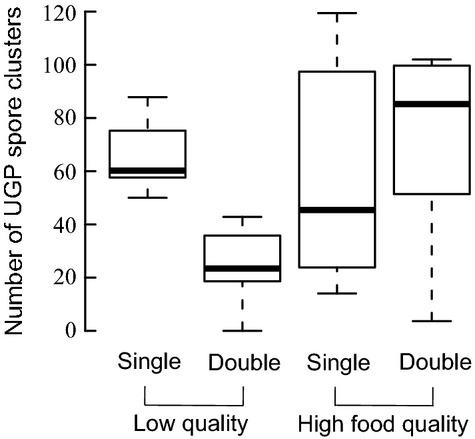
Box-Whiskers plot showing the mean number of UGP (unicellular gut parasite) spore clusters after 10 weeks in surviving host populations in UGP infected (single) or co-infected (UGP & WBD (White bacterial disease), double) populations. The outer x-axis shows the feeding regime of the host populations (low or high food quality). Mean UGP spore cluster numbers were calculated from randomly selected adult individuals for each host population [n = 5].
Effects of host diet quality and co-infection on host population dynamics
Infected host populations differed in the rate of population extinction. None of the control populations became extinct. Most singly WBD-infected populations went extinct, while all other parasite treatments showed intermediate host population extinction rates (Table 2). Higher food quality led to a higher host equilibrium population density (Fig. 2). The equilibrium density of the UGP-infected populations did not differ from the density of the control populations (neither at high, nor at the low food quality treatment). However, the age structure in UGP-infected populations showed significantly more juveniles than the control populations at high, but not at the low food quality (Table 2). Daphnia equilibrium population densities in co-infected and WBD-infected populations were half the density of UGP-infected populations under the high food quality treatment (Fig. 2). The equilibrium density of co-infected populations was significantly higher than that of WBD-infected populations at low (co-infected: 12.93 ± 0.18; WBD-infected: 0.27 ± 0.18; Welch's test, t9.09 = 4.84, P < 0.001), but not at high food quality (co- infected: 48.50 ± 8.67; WBD-infected: 43.00 ± 9.11; Welch's test, t17.69 = 0.44, P < 0.67). Daphnia equilibrium density in co-infected populations was also intermediate compared to the density of the UGP-infected populations under the low food quality treatment (Fig. 2).
Table 2.
Summary of population experiment results with two different food quality and two parasite species treatments. Shown are relative densities of infected host populations statistically compared with uninfected populations in the same food and parasite treatment combination.
| Food treatment | Parasite treatment | Host extinctions after 5/10 weeks | Equilibrium density of adult and juvenile hosts (±SE) | Equilibrium density of adult hosts (±SE) | Equilibrium density of juvenile hosts (±SE) |
|---|---|---|---|---|---|
| Low quality food | UGP | 0/1 | 0.82 ± 0.11 | 0.82 ± 0.11 | 0.82 ± 0.12 |
| WBD | 6/10*** | NA | NA | NA | |
| UGP & WBD | 0/1 | 0.47 ± 0.09*** | 0.36 ± 0.08*** | 0.52 ± 0.11 | |
| High quality food | UGP | 0/0 | 1.22 ± 0.03 | 0.62 ± 0.04*** | 1.77 ± 0.05*** |
| WBD | 0/4*** | 0.59 ± 0.12 | 0.47 ± 0.11 | 0.70 ± 0.16 | |
| UGP & WBD | 0/2 | 0.66 ± 0.12 | 0.47 ± 0.07*** | 0.84 ± 0.19 |
WBD, white bacterial disease; UGP, unicellular gut parasite.
P < 0.001.
Figure 2.
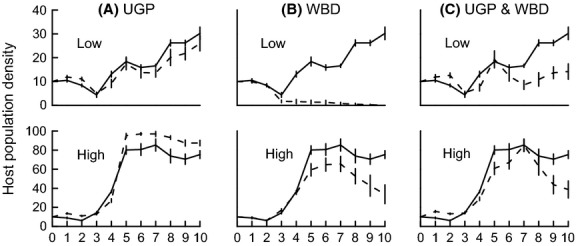
Population dynamics of uninfected Daphnia populations (continuous line, means of 10 replicate populations with SE) and infected Daphnia populations (dashed line, means of ten replicate populations with SE). Infected populations carried (A) UGP (unicellular gut parasite), (B) WBD (white bacterial disease), or (C) UGP and WBD together. The populations were kept under a low food quality (Low) or high food quality (High) treatment. For each food quality, the same uninfected control populations were plotted for all parasite treatments.
Discussion
We investigated the effect of host resources, mediated by diet quality on the interspecific association between two parasites infecting the freshwater crustacean D. magna. In particular, infected or uninfected experimental host populations, instead of host individuals, were treated with high or low food quality and resulting population dynamics were analyzed. Our results reflect interspecific parasite interactions and showed that a low-virulent microsporidian species (UGP) suffered from co-infection with a high-virulent bacterial (WBD) parasite at low, but not at high food quality. Similarly, results of earlier studies on within-host competition showed fitness disadvantages for lesser virulent parasites (Gower and Webster 2005; Bell et al. 2006). The disadvantage is explained by competition favouring increased host exploitation and thus increased virulence (Choisy and de Roode 2010). The result could also be attributed to increased host defenses (top-down control; Pedersen and Fenton 2007; Graham 2008; Johnson and Buller 2011), due to the possible presence of co-infecting high-virulence parasites which would strongly activate the host's immune system.
Here, we chose to use a host population-level approach, rather than a host individual approach, as individual-based infection trials exclude host–host interactions and parasite transmission between hosts. These interactions influence the fitness and virulence of parasites in natural populations (Ebert 1998; Schmid-Hempel and Ebert 2003; Woolhouse and Gowtage-Sequeria 2005; Refardt and Ebert 2007). Consequently, competition, which could not be observed in individual host infection trials, might also emerge between parasites as a result of more complicated mechanisms at the host population level (Pedersen and Fenton 2007; Johnson and Buller 2011). In particular, competition may arise due to a change in the host population's demographic structure, which is what we observed here. In our study, higher food quality increased the host fecundity (reflected in a higher juvenile compared to adult host population density), both in the absence and presence of parasitism. This outcome tallies with previous studies, which showed that food quality, more precisely PUFA and sterol availability in general, leads to a higher fecundity of copepods and cladocerans (Arendt et al. 2005; Wacker and Martin-Creuzburg 2007; Martin-Creuzburg and Von Elert 2009). Although parasites can shape host population dynamics (Ebert et al. 2000), the fecundity increase caused by the higher food quality overruled the negative effect of the parasites. Higher food quality led to a higher host offspring production in a straightforward way, but the parasites’ effect on host reproduction is more complex. One effect that may have occurred is that parasite infections lead to fecundity compensation, which is a nonimmunological mechanism that contributes to host defense (Parker et al. 2011; Schmid-Hempel 2011). Fecundity compensation can be realized by inducing earlier host reproduction in infected hosts as a mean to increase its fitness (reproduction shift), and as such may affect host population dynamics (as shown for Daphnia parasitism in Chadwick and Little 2005). As increased fecundity can be affected by both food quality and the presence of parasites, it is likely the cause of the interaction between food quality and parasites found in this study. More specifically, the increased fecundity is likely to affect the higher equilibrium population density for the UGP-infected populations compared to the other infected populations at high food quality.
Our results suggest that the host populations were protected against the more virulent WBD by the less virulent UGP under the low food quality treatment. Under low food quality, WBD-infected host populations were less viable than co-infected populations, showing that competition between parasites can be beneficial to the host. It is also at low food quality that we detected a lower UGP growth rate in the co-infected populations. Thus apparently the growth of UGP is more constrained, but at the same time the host is more protected against WBD in the co-infected condition. Similarly, but then at the within-species level, co-infections with different strains of the protozoan parasite Trypanosoma brucei have been shown to result in higher host survival (Balmer et al. 2009). The consequences of co-infections with different strains have also been investigated for the parasitic genus Plasmodium (Read and Taylor 2001; de Roode et al. 2005; Wargo et al. 2007). Observations of Plasmodium falciparum implied competition between strains, leading possibly to protection against genetically distinct Plasmodium superinfections (Mercereau-Puijalon 1996; Smith et al. 1999). Between parasite species, especially bacteria, competition and mechanisms preventing superinfections have been shown (Harrison et al. 2008; Hibbing et al. 2010). The results presented here are to our knowledge the first results that show that food quality determines competition between different parasite species and that this may have a beneficial, protective effect for the host population. This result shows that interactions between parasites are not only influenced by host and parasite genotypes (Thomas et al. 2003; de Roode et al. 2004), but also by food quality provided to the host.
There might be a mechanistic explanation for the food quality-dependent interactions between the two parasites we tested here based on their different life cycles and shared resources (Griffiths et al. 2014). UGP causes chronic infection with increased parasite spore production when the host gets older. Thus, UGP depends on long-living host individuals that, once infected, spread an increasing amount of spores until their death (Ebert 2005). In contrast, WBD kills its host relatively fast and is not dependent on long host lifespan (Decaestecker et al. 2003; Coopman et al. 2014). However, a host that is already infected with UGP might be less susceptible to infection or at least less viable as a host for WBD. While in high food quality levels, the negative effects of these parasites on each other might be reduced, in low food quality environments, UGP cannot compensate the WBD-induced death of UGP spreaders anymore, as a result of which WBD has a stronger impact on the host population. Accordingly, in our experiment host population density of co-infected populations increased, compared to singly WBD-infected populations, in low but not in high host diet quality. Because the life history of both parasites depending on the same host populations collide and fitness of UGP can be lowered by presence of WBD, the association between these two parasites can be described as exploitation competition (Dunn 2005; Dunn et al. 2012).
In conclusion, the host population design of this study allowed us to omit the limitations of individual-based infection trials and increased the robustness of the results. We infer that food quality in the environment modifies interspecific competition between parasite species and that this outcome is likely associated with population demographic effects. From these insights, we believe that understanding resource-dependent co-occurrence of different parasite species will help us to better grasp the make-up of natural parasite communities and to gather further insights into disease dynamics. Furthermore, environmental factors, such as changing temperatures, may influence the effect of Daphnia nutrition on its reproduction (Pajk et al. 2012) and in consequence Daphnia–parasite interactions (Mitchell et al. 2005) and would therefore be interesting additions to any future investigation.
Acknowledgments
B. Lange is supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen, grant number: 101476). This research was also financially supported by Fund for Scientific Research (FWO-Flanders) project G.0506.07 and KU Leuven Research Fund projects STRT/1/08/019, the Excellence Centre Financing PF/2010/007 and Beslpo IAP/SPEEDY P7/4. We thank two anonymous reviewers for comments on the manuscript and Veronica Comper for linguistic advice.
Data Accessibility
Original experimental data will be stored at Dryad.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Daphnia magna with white bacterial disease. Picture by Joachim Mergeay, Leuven.
References
- Aalto SL, Ketola T, Pulkkinen K. No uniform associations between parasite prevalence and environmental nutrients. Ecology. 2014 http://www.esajournals.org/doi/abs/10.1890/13-2007.1) [Google Scholar]
- Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013;16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Boersma M, Raubenheimer D. Stoichiometry: linking elements to biochemicals. Ecology. 2004;85:1193–1202. [Google Scholar]
- Arendt K, Jónasdóttir S, Hansen P, Gärtner S. Effects of dietary fatty acids on the reproductive success of the calanoid copepod Temora longicornis. Mar. Biol. 2005;146:513–530. [Google Scholar]
- Balmer O, Stearns SC, Schotzau A, Brun R. Intraspecific competition between co-infecting parasite strains enhances host survival in African trypanosomes. Ecology. 2009;90:3367–3378. doi: 10.1890/08-2291.1. [DOI] [PubMed] [Google Scholar]
- Basen T, Martin-Creuzburg D, Rothhaupt K-O. Role of essential lipids in determining food quality for the invasive freshwater clam Corbicula fluminea. J. North. Am. Benthol. Soc. 2011;30:653–664. [Google Scholar]
- Bell AS, De Roode JC, Sim D, Read AF. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution. 2006;60:1358–1371. [PubMed] [Google Scholar]
- Bird MI, Wurster CM, de Paula Silva PH, Bass AM, de Nys R. Algal biochar – production and properties. Bioresour. Technol. 2011;102:1886–1891. doi: 10.1016/j.biortech.2010.07.106. [DOI] [PubMed] [Google Scholar]
- Bukovinszky T, Poelman EH, Gols R, Prekatsakis G, Vet LEM, Harvey JA, et al. Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia. 2009;160:299–308. doi: 10.1007/s00442-009-1308-y. [DOI] [PubMed] [Google Scholar]
- Burdon JJ, Thrall PH. Co-evolution of plants and their pathogens in natural habitats. Science. 2009;324:755–756. doi: 10.1126/science.1171663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush SE, Malenke JR. Host defence mediates interspecific competition in ectoparasites. J. Anim. Ecol. 2008;77:558–564. doi: 10.1111/j.1365-2656.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- Chadwick W, Little TJ. A parasite-mediated life-history shift in Daphnia magna. Proc. Biol. Sci. 2005;272:505–509. doi: 10.1098/rspb.2004.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choisy M, de Roode JC. Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 2010;175:E105–E118. doi: 10.1086/651587. [DOI] [PubMed] [Google Scholar]
- Civitello DJ, Penczykowski RM, Hite JL, Duffy MA, Hall SR. Potassium stimulates fungal epidemics in Daphnia by increasing host and parasite reproduction. Ecology. 2013;94:380–388. doi: 10.1890/12-0883.1. [DOI] [PubMed] [Google Scholar]
- Coopman M, Muylaert K, Lange B, Reyserhove L, Decaestecker E. Context dependency of infectious disease: the cyanobacterium Microcystis aeruginosa decreases white bacterial disease in Daphnia magna. Freshw. Biol. 2014;59:714–723. [Google Scholar]
- Dallas T, Drake JM. Nitrate enrichment alters a Daphnia-microparasite interaction through multiple pathways. Ecol. Evol. 2014;4:243–250. doi: 10.1002/ece3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker E, Vergote A, Ebert D, De Meester L. Evidence for strong host clone-parasite species interactions in the Daphnia microparasite system. Evolution. 2003;57:784–792. doi: 10.1111/j.0014-3820.2003.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, Declerck S, De Meester L, Ebert D. Ecological implications of parasites in natural Daphnia populations. Oecologia. 2005;144:382–390. doi: 10.1007/s00442-005-0083-7. [DOI] [PubMed] [Google Scholar]
- Dieckmann U. Adaptive dynamics of pathogen–host interactions. Adaptive dynamics of infectious diseases. In: Dieckmann U, Metz JAJ, Sabelis MW, Sigmund K, editors. Pursuit of virulence management. 1st ed. Cambridge, U.K.: Cambridge Univ. Press; 2002. pp. 39–59. [Google Scholar]
- Dobson AP. The population dynamics of competition between parasites. Parasitology. 1985;91:317–347. doi: 10.1017/s0031182000057401. [DOI] [PubMed] [Google Scholar]
- Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl Acad. Sci. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MA, Cáceres CE, Hall SR, Tessier AJ, Ives AR. Temporal, spatial, and between-host comparisons of patterns of parasitism in lake zooplankton. Ecology. 2010;91:3322–3331. doi: 10.1890/09-1611.1. [DOI] [PubMed] [Google Scholar]
- Dunn RR. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005;19:1030–1036. [Google Scholar]
- Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM, Byers JE, et al. Indirect effects of parasites in invasions. Funct. Ecol. 2012;26:1262–1274. [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. [DOI] [PubMed] [Google Scholar]
- Ebert D. Ecology, epidemiology, and evolution of parasitism in Daphnia [Internet] Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2005. See http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books. [Google Scholar]
- Ebert D, Lipsitch M, Mangin KL. The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am. Nat. 2000;156:459–477. doi: 10.1086/303404. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, et al. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000;3:540–550. [Google Scholar]
- Fellous S, Koella JC. Infectious dose affects the outcome of the within-host competition between parasites. Am. Nat. 2009;173:E177–E184. doi: 10.1086/598490. [DOI] [PubMed] [Google Scholar]
- Forde SE, Thompson JN, Bohannan BJM. Adaptation varies through space and time in a coevolving host-parasitoid interaction. Nature. 2004;431:841–844. doi: 10.1038/nature02906. [DOI] [PubMed] [Google Scholar]
- Frost PC, Ebert D, Smith VH. Responses of a bacterial pathogen to phosphorus limitation of its aquatic invertebrate host. Ecology. 2008;89:313–318. doi: 10.1890/07-0389.1. [DOI] [PubMed] [Google Scholar]
- Garbutt JS, Scholefied JA, Vale PF, Little TJ. Funct. Ecol. 2014;28:424–431. [Google Scholar]
- Gower CM, Webster JP. Intraspecific competition and the evolution of virulence in a parasitic trematode. Evolution. 2005;59:544–553. [PubMed] [Google Scholar]
- Graham AL. Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA. 2008;105:566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EC, Pedersen AB, Fenton A, Petchey OL. Analysis of a summary network of co-infection in humans reveals that parasites interact most via shared resources. Proc. Biol. Sci. 2014;281:20132286. doi: 10.1098/rspb.2013.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL. Culture of marine invertebrate animals. New York, NY: Plenum Press; 1975. [Google Scholar]
- Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Cáceres CE. Quality matters: resource quality for hosts and the timing of epidemics. Ecol. Lett. 2009;12:118–128. doi: 10.1111/j.1461-0248.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- Hall MD, Vettiger A, Ebert D. Interactions between environmental stressors: the influence of salinity on host-parasite interactions between Daphnia magna and Pasteuria ramosa. Oecologia. 2013;171:789–796. doi: 10.1007/s00442-012-2452-3. [DOI] [PubMed] [Google Scholar]
- Harrison F, Paul J, Massey RC, Buckling A. Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J. 2008;2:49–55. doi: 10.1038/ismej.2007.96. [DOI] [PubMed] [Google Scholar]
- Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdo RM, Sinclair ARE, Dobson AP, Metzger KL, Bolker BM, Ritchie ME, et al. A Disease-mediated trophic cascade in the serengeti and its implications for ecosystem C. PLoS Biol. 2009;7:e1000210. doi: 10.1371/journal.pbio.1000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoverman JT, Hoye BJ, Johnson PTJ. Does timing matter? How priority effects influence the outcome of parasite interactions within hosts. Oecologia. 2013;4:1471–1480. doi: 10.1007/s00442-013-2692-x. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Dobson AP, Lafferty KD. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hughes WOH, Boomsma JJ. Let your enemy do the work: within-host interactions between two fungal parasites of leaf-cutting ants. Proc. Biol. Sci. 2004;271:S104–S106. doi: 10.1098/rsbl.2003.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Stoks R, Decaestecker E, Coors A, Van De Meutter F, De Meester L. Local exposure shapes spatial patterns in infectivity and community structure of Daphnia parasites. J. Anim. Ecol. 2010;79:1023–1033. doi: 10.1111/j.1365-2656.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Buller ID. Parasite competition hidden by correlated coinfection: using surveys and experiments to understand parasite interactions. Ecology. 2011;92:535–541. doi: 10.1890/10-0570.1. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Thieltges DW. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J. Exp. Biol. 2010;213:961–970. doi: 10.1242/jeb.037721. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Rintamäki P, Jokela J, Valtonen ET. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010;40:1483–1488. doi: 10.1016/j.ijpara.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Klüttgen B, Dülmer U, Engels M, Ratte HT. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. [Google Scholar]
- Lafferty KD, Dobson AP, Kuris AM. Parasites dominate food web links. Proc. Natl Acad. Sci. USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine A-L, Tellier A. Heterogeneous selection promotes maintenance of polymorphism in host–parasite interactions. Oikos. 2008;117:1281–1288. [Google Scholar]
- Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. Competition and mutualism among the gut helminths of a mammalian host. Nature. 2004;428:840–844. doi: 10.1038/nature02490. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Lohr JN, Yin M, Wolinska J. Prior residency does not always pay off co-infections in Daphnia. Parasitology. 2010;137:1493–1500. doi: 10.1017/S0031182010000296. [DOI] [PubMed] [Google Scholar]
- Malenke JR, Newbold N, Clayton DH. Condition-specific competition governs the geographic distribution and diversity of ectoparasites. Am. Nat. 2011;177:522–534. doi: 10.1086/658176. [DOI] [PubMed] [Google Scholar]
- Martin-Creuzburg D, Von Elert E. Good food versus bad food: the role of sterols and polyunsaturated fatty acids in determining growth and reproduction of Daphnia magna. Aquat. Ecol. 2009;43:943–950. [Google Scholar]
- McKenzie VJ, Townsend AR. Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth. 2007;4:384–396. [Google Scholar]
- McVean RIK, Sait SM, Thompson DJ, Begon M. Effects of resource quality on the population dynamics of the Indian meal moth Plodia interpunctella and its granulovirus. Oecologia. 2002;131:71–78. doi: 10.1007/s00442-001-0862-8. [DOI] [PubMed] [Google Scholar]
- Mercereau-Puijalon O. Revisiting host/parasite interactions: molecular analysis of parasites collected during longitudinal and cross-sectional surveys in humans. Parasite Immunol. 1996;18:173–180. doi: 10.1046/j.1365-3024.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- Mideo N. Parasite adaptations to within-host competition. Trends Parasitol. 2009;25:261–268. doi: 10.1016/j.pt.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Mitchell SE, Rogers ES, Little TJ, Read AF. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- Nunn CL, Altizer SM, Sechrest W, Cunningham AA. Latitudinal gradients of parasite species richness in primates. Divers. Distrib. 2005;11:249–256. [Google Scholar]
- Pajk F, Von Elert E, Fink P. Interaction of changes in food quality and temperature reveals maternal effects on fitness parameters of a keystone aquatic herbivore. Limnol. Oceanogr. 2012;57:281–292. [Google Scholar]
- Parker BJ, Barribeau SM, Laughton AM, de Roode JC, Gerardo NM. Non-immunological defense in an evolutionary framework. Trends Ecol. Evol. 2011;26:242–248. doi: 10.1016/j.tree.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Pech D, Aguirre-Macedo ML, Lewis JW, Vidal-Martínez VM. Rainfall induces time-lagged changes in the proportion of tropical aquatic hosts infected with metazoan parasites. Int. J. Parasitol. 2010;40:937–944. doi: 10.1016/j.ijpara.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Poulin R. Evolutionary ecology of parasites. 2nd ed. Princeton, NJ: Princeton Univ. Press; 2007. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Ranta E, Bengtsson J, McManus J. Growth, size and shape of Daphnia longispina Daphnia magna and Daphnia pulex. Ann. Zool. Fenn. 1993;30:299–311. [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Refardt D, Ebert D. Inference of parasite local adaptation using two different fitness components. J. Evol. Biol. 2007;20:921–929. doi: 10.1111/j.1420-9101.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- Refardt D, Ebert D. The impact of infection on host competition and its relationship to parasite persistence in a Daphnia microparasite system. Evol. Ecol. 2012;26:95–107. [Google Scholar]
- Rigaud T, Perrot-Minnot M-J, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. Biol. Sci. 2010;277:3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde K, Heap M. Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. Int. J. Parasitol. 1998;28:461–474. doi: 10.1016/s0020-7519(97)00209-9. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Culleton R, Cheesman SJ, Carter R, Read AF. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc. Biol. Sci. 2004;271:1073–1080. doi: 10.1098/rspb.2004.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode JC, Helinski MEH, Anwar MA, Read AF. Dynamics of multiple infection and within-host competition in genetically diverse malaria infections. Am. Nat. 2005;166:531–542. doi: 10.1086/491659. [DOI] [PubMed] [Google Scholar]
- Sarfraz M, Dosdall LM, Keddie BA. Host plant nutritional quality affects the performance of the parasitoid Diadegma insulare. Biol. Control. 2009;51:34–41. [Google Scholar]
- Sato T, Egusa T, Fukushima K, Oda T, Ohte N, Tokuchi N, et al. Nematomorph parasites indirectly alter the food web and ecosystem function of streams through behavioural manipulation of their cricket hosts. Ecol. Lett. 2012;15:786–793. doi: 10.1111/j.1461-0248.2012.01798.x. [DOI] [PubMed] [Google Scholar]
- Schlotz N, Ebert D, Martin-Creuzburg D. Dietary supply with polyunsaturated fatty acids and resulting maternal effects influence host-parasite interactions. BMC Ecol. 2013;13:41. doi: 10.1186/1472-6785-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford, U.K.: Oxford Univ. Press; 2011. [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. [Google Scholar]
- Seppälä O, Liljeroos K, Karvonen A, Jokela J. Host condition as a constraint for parasite reproduction. Oikos. 2008;117:749–753. [Google Scholar]
- Smith T, Felger I, Tanner M, Beck H-P. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans. R. Soc. Trop. Med. Hyg. 1999;93:S1/59–S1/64. doi: 10.1016/s0035-9203(99)90329-2. [DOI] [PubMed] [Google Scholar]
- Taipale SJ, Kainz MJ, Brett MT. Diet-switching experiments show rapid accumulation and preferential retention of highly unsaturated fatty acids in Daphnia. Oikos. 2011;120:1674–1682. [Google Scholar]
- Telfer S, Birtles R, Bennett M, Lambin X, Paterson S, Begon M. Parasite interactions in natural populations: insights from longitudinal data. Parasitology. 2008;135:767–781. [Google Scholar]
- Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MB, Watson EL, Valverde-Garcia P. Mixed infections and insect-pathogen interactions. Ecol. Lett. 2003;6:183–188. [Google Scholar]
- Thumbi SM, de C Bronsvoort BM, Poole EJ, Kiara H, Toye P, Ndila M, et al. Parasite co-infections show synergistic and antagonistic interactions on growth performance of East African zebu cattle under one year. Parasitology. 2013;140:1789–1798. doi: 10.1017/S0031182013001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale PF, Wilson AJ, Best A, Boots M, Little TJ. Epidemiological, evolutionary and co-evolutionary implications of context-dependent parasitism. Am. Nat. 2011;177:510–521. doi: 10.1086/659002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Bund WJ, Van Donk E. Impact of ‘white bacterial disease’ on a Daphnia magna population. Verhandlungen der Internationalen Vereinigung Limnologie. 2002;28:303–306. [Google Scholar]
- Wacker A, Martin-Creuzburg D. Allocation of essential lipids in Daphnia magna during exposure to poor food quality. Funct. Ecol. 2007;21:738–747. [Google Scholar]
- Wargo AR, de Roode JC, Huijben S, Drew DR, Read AF. Transmission stage investment of malaria parasites in response to in-host competition. Proc. Biol. Sci. 2007;274:2629–2638. doi: 10.1098/rspb.2007.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weers PMM, Gulati RD. Effect of the addition of polyunsaturated fatty acids to the diet on the growth and fecundity of Daphnia galeata. Freshw. Biol. 1997;38:721–729. [Google Scholar]
- Wiehn J, Korpimäki E. Resource levels, reproduction and resistance to haematozoan infections. Proc. Biol. Sci. 1998;265:1197–1201. [Google Scholar]
- Wolinska J, King KC. Environment can alter selection in host-parasite interactions. Trends Parasitol. 2009;25:236–244. doi: 10.1016/j.pt.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Wolinska J, Giessler S, Koerner H. Molecular identification and hidden diversity of novel Daphnia parasites from European lakes. Appl. Environ. Microbiol. 2009;75:7051–7059. doi: 10.1128/AEM.01306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Daphnia magna with white bacterial disease. Picture by Joachim Mergeay, Leuven.
Data Availability Statement
Original experimental data will be stored at Dryad.


