Abstract
Mesenchymal stem cells (MSCs) hold tremendous potential for vascular tissue regeneration. Research has demonstrated that individual factors in the cell microenvironment such as matrix elasticity and growth factors regulate MSC differentiation to vascular lineage. However, it is not well understood how matrix elasticity and growth factors combine to direct the MSC fate. This study examines the combined effects of matrix elasticity and vascular endothelial growth factor (VEGF) on both MSC differentiation into endothelial lineage and MSC paracrine signaling. MSCs were seeded in soft nanofibrous matrices with or without VEGF, and in Petri dishes with or without VEGF. Only MSCs seeded in three-dimensional soft matrices with VEGF showed significant increases in the expression of endothelial markers (vWF, eNOS, Flt-1, and Flk-1), while eliminating the expression of smooth muscle marker (SM-α-actin). MSCs cultured in VEGF alone on two-dimensional dishes showed increased expression of both early-stage endothelial and smooth muscle markers, indicating immature vascular differentiation. Furthermore, MSCs cultured in soft matrices with VEGF showed faster upregulation of endothelial markers compared with MSCs cultured in VEGF alone. Paracrine signaling studies found that endothelial cells cultured in the conditioned media from MSCs differentiated in the soft matrix and VEGF condition exhibited increased migration and formation of capillary-like structures. These results demonstrate that VEGF and soft matrix elasticity act synergistically to guide MSC differentiation into mature endothelial phenotype while enhancing paracrine signaling. Therefore, it is critical to control both mechanical and biochemical factors to safely regenerate vascular tissues with MSCs.
Introduction
In 2008, cardiovascular diseases accounted for one in three deaths in the United States.1 Regeneration of functional vascular tissues, including capillary network and small arteries, remains a critical barrier to the successful treatment of these diseases. Vascular endothelial cells (ECs) are key building blocks for vascular tissue repair, as they perform vital anti-thrombogenic functions and participate in angiogenesis.2 However, attempts to produce functional vascular tissue with autogenous ECs have limited success due to the need for invasive surgery, poor cell expansion in vitro, and inadequate cell proliferation or migration in vivo.3 Mesenchymal stem cells (MSCs) are a powerful cellular alternative for vascular tissue regeneration, as they are easily obtainable, multipotent, thrombo-resistant, and low in immunogenicity.4,5
Previous studies have shown MSCs are capable of regenerating vascular tissues by transdifferentiation or paracrine signaling.2,4,6,7 Transdifferentiation is the direct differentiation of MSCs into a specific vascular phenotype.8,9 Paracrine signaling is the release of molecular mediators from MSC that aid in the migration and proliferation of surrounding vascular cells, leading to increased capillary formation.10 Direct injection of MSCs into the heart increased vascular density, and resulted in transdifferentiation of MSCs into ECs along the vessel lumen.6 Hashi et al. cultured MSCs on aligned electrospun vascular grafts made of poly-l-lactide acid, and found that distinct EC and smooth muscle cell (SMC) layers formed on the grafts.11 However, other studies have led to concerns about the safety and efficacy of utilizing MSCs for vascular regeneration. O'Shea et al. recently found that bolus delivery of MSCs to injured vasculature produced a dysfunctional endothelium, leading to a higher rate of vessel occlusion.12 Research indicates a poorly defined vascular microenvironment for MSC differentiation can lead to highly heterogeneous cell populations with low vascular lineage commitment and functionality.13 In turn, these MSC-derived progenitors may be involved in diseased vascular remodeling.14 The microenviromental factors that guide MSC differentiation into healthy or diseased vascular phenotypes are not well studied. To improve the safety and efficacy of MSCs in cardiovascular therapies, it is critical to define the factors in local vascular microenvironments that regulate MSC differentiation.
In vivo, factors in the cellular microenviroments such as soluble biochemical factors and matrix elasticity guide the paracrine signaling and transdifferentiation of MSCs. Studies in the last few years have demonstrated the importance of local matrix elasticity in directing stem cell differentiation. Engler et al. demonstrated that gels that replicated the modulus of neural, muscle, and bone tissue directed the differentiation of MSCs toward neural, myogenic, and osteogenic cells, respectively.15 In a healthy vascular microenvironment, ECs reside in the intima tissue, composed of a fibrous matrix with soft elasticity in the low kilopascal (kPa) range.16,17 We previously showed that three-dimensional (3D) fibrous matrices that replicated the intima elasticity directed MSCs toward the endothelial lineage expressing an early endothelial marker (Flk-1).18 The substrate elasticity can also play a role in modulating paracrine signaling of MSCs. Seib et al. found MSCs cultured on a soft substrate decreased the release of pro-inflammatory factors, compared with those grown on a stiffer substrate.19 In addition to mechanical properties of matrices, vascular endothelial growth factor (VEGF) is known as one of the most important factors for endothelial differentiation of MSCs.9,20,21 VEGF also plays a significant role in paracrine signaling of MSCs to promote angiogenesis and myocardial repair.20,22 Though previous studies have examined the individual effects of biochemical and mechanical factors on vascular differentiation or paracrine signaling of MSCs, the combined effect of these factors as MSCs experience in vivo remains unknown. This study aims to understand how factors in the local microenvironment interact to modulate both the paracrine signaling and transdifferentiation capabilities of MSCs. We demonstrate that the combined effects of VEGF and soft matrix elasticity result in MSC differentiation into a more mature endothelial lineage and increased MSC paracrine signaling abilities, compared to the separate use of VEGF and matrix.
Materials and Methods
Fabrication of 3D nanofibrous graft scaffolds
Polyethylene glycol dimethacrylate (PEGDM) with a molecular weight of 3000 was synthesized as we previously described.18 Approximately 90% of the end groups were modified with methacrylates as determined by 1H NMR analysis. An electrospinning solution composed of 3.2% wt PEGDM 3000, 3.4% wt PEO (MW 40,000; Sigma-Aldrich, Inc., St Louis, MO), 0.4% wt of Irgacure 2959 (I2959, 0.6 mg/mL in deionzied [DI] H2O; Ciba, Tarrytown, NY), and 93% DI H2O was mixed for 1 h with magnetic stir bar. PEGDM 3000 photo-polymerizable nanofibrous grafts (NFGs) were fabricated by electrospinning on a custom system composed of a high voltage power supply (Gamma High Voltage Research, Ormond Beach, FL), grounded collecting surface, motorized syringe pump, and a 14 mm syringe. The solution (2 mL) was spun at a distance of 26 cm from the stationary collecting surface, a voltage of 22 kV, and a flow rate of 1.10 mL/h. NFGs with a thickness of 0.3 mm were cut into 2 inch diameter disks and placed in glass vials. Vials were then vacuum-sealed and NFGs were photopolymerized under 365 nm light with an average intensity of 15 mW/cm2 for 5 min. NFGs were submerged in DI H2O for 24 h and sterilized with 70% ethanol prior to cell seeding. With random sampling of NFGs, the compressive modulus of ∼2 kPa was verified using the protocol we previously described.18
Cell culture and cell seeding
Rat bone marrow-derived MSCs from Lonza Group Ltd (Basel, Switzerland) with passages 3–8 were cultured in Dulbecco's modified Eagles media (DMEM; Sigma-Aldrich, Inc.), with 10% stem cell qualified fetal bovine serum for MSCs (Atlanta Biologicals, Lawrencdeville, GA) and 1% penicillin/streptomycin (Invitrogen, Inc., Carlsbad, CA). This media was the standard utilized for all MSC experiments unless otherwise noted. Cells were maintained at 37°C/5% CO2 and the cell culture medium was changed every second day. To examine the effect of soluble chemical factors on vascular differentiation of MSCs, VEGF-A (Shenandoah Biotechnology, Warwick, PA) was added to the media. For transdifferentiation experiments, MSCs were seeded in the following four experimental conditions: (1) PS, a standard polystyrene cell culture plate; (2) VEGF, a standard PS plate with 10 ng/mL of VEGF; (3) 2 kPa, a 3D NFG with compressive elasticity of ∼2–3 kPa; and (4) 2 kPa+VEGF, a 3D NFG with compressive elasticity of ∼2–3 kPa and 10 ng/mL of VEGF. Prior to cell seeding, all substrates were coated with 0.3% type I rat tail collagen (BD Biosciences, Bedford, MA). NFGs were seeded in ultra-low attachment plates (Sigma-Aldrich, Inc.). Our previous studies have shown that MSCs are able to penetrate the NFG scaffolds, which provide cells with 3D adhesion and culture.18
Immunofluorescent staining
Immunofluorescent staining of cells to examine VE-cadherin (VECAD), platelet endothelial cell adhesion molecule (PECAM), Flk-1, smooth muscle α-actin (SMA), or F-actin was performed to characterize vascular differentiation or MSC morphology. For VECAD and Flk-1 staining, cells were seeded for 168 h in media with 0, 10, and 50 ng/mL VEGF. For F-actin and SMA staining, 1×105 MSCs were seeded for 168 h on all the experimental conditions. For PECAM staining, 1×106 MSCs were seeded for 24 h on the two NFG conditions. For F-actin staining, samples were fixed with 3.7% formaldehyde at room temperature, permeated with 0.1% Triton, and blocked with 3% bovine serum albumin (BSA). Then, samples were incubated in Alexa488-phalloidin (Invitrogen, Inc., Eugene, OR) in 1% BSA for 1 h. For immunostaining of vascular biomarkers, samples were first incubated with a primary antibody in 1% BSA for 2 h at room temperature. Following primary antibody coupling, samples were washed in phosphate-buffered saline (PBS) and incubated with secondary antibodies. All samples were finally mounted with DAPI SlowFade (Invitrogen, Inc.) and imaged using an epifluorescence microscope (Zeiss, Peabody, MA). Images from each fluorescence channel were merged using ImageJ software (NIH, Bethesda, MA). Primary antibodies used were as follows: rabbit polyclonal anti-SMA (Sigma-Aldrich, Inc.), mouse monoclonal anti-Flk1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PECAM (Novus Biologicals, Littleton, CO), and rabbit polyclonal anti-VECAD (Alexis Biochemicals, San Diego, CA). Secondary antibodies were as follows: anti-mouse IgG antibody conjugated with Alexa 488 and anti-rabbit IgG antibody conjugated with Alexa 594 (Invitrogen, Inc.).
MSC formation of capillary-like tube structures on NFGs
Bone marrow-derived MSCs (2×105) were seeded in the PS, VEGF, 2 kPa, and 2 kPa+VEGF conditions, and reduced growth factor matrigel (BD, Franklin Lakes, NJ). MSCs were seeded on matrigel per procedure outlined in Analysis of MSC Paracrine Signaling: Capillary-Like Tube Formation of EC section. Cells were incubated for 24 h before they were fixed, stained with F-actin, and imaged. To quantify the formation of capillary-like tube structures, the total tube length was measured on each sample using ImageJ software. The total tube length per condition was averaged across the three samples used.
Real-time PCR
Total cellular mRNA from each sample was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized from 1 ng of total cellular RNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Primer sequences for amplification are shown in Table 1. The SYBR Green I assay and the iCycler iQ real-time PCR detection system (MyiQ Real-Time PCR System; Bio-Rad) were used for detecting real-time quantitative PCR products from 2 ng of reverse-transcribed cDNA. PCR thermal profile consisted of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 95°C for 1 min. Genes were normalized to the housekeeping gene GADPH, and the fold change relative to the PS condition was calculated using the comparative Ct method. All PCR graphs show ΔΔCt fold changes and standard deviations are calculated using the method described previously.23
Table 1.
Rat Primer Sequences
| Gene | Order | Primer |
|---|---|---|
| Flk-1 | Forward | 5′-AGCTCAGGTTTTGTGGAGGA-3′ |
| Reverse | 5′-CCAAGAACTCCATGCCCTTA-3′ | |
| Flt-1 | Forward | 5′-CTTTCTCAAGTGCAGAGGGG-3′ |
| Reverse | 5′-AGGATTGTATTGGTCTGCCG-3′ | |
| VWF | Forward | 5′-CACAGGTAGCACACATCACT-3′ |
| Reverse | 5′-CTCAAAGT-CTTGGATGAAGA-3′ | |
| eNOS | Forward | 5′-CCACAATCCTGGTGCGTC-3′ |
| Reverse | 5′-GCCTTTTTCCAGTTGTTCCA-3′ | |
| GAPDH | Forward | 5′-GCCTCGTCTCATAGACAAGATGGT-3′ |
| Reverse | 5′-GAAGGCAGCCCTGGTAACC-3′ |
Immunoblotting
Immunoblotting or western blotting was used to analyze eNOS protein expressions in MSCs seeded for 168 h in all four experimental conditions. Samples were prepared by first lysing cells in a lysis buffer containing homogenate buffer containing PBS (pH=7.5), 0.1% Triton X-100, and a protease inhibitor cocktail. Cells were then centrifuged at 1000 g, 4°C, for 15 min with an Eppendorf centrifuge (model 5417R; Brinkmann Instruments, Westbury, NY). The supernatant was collected, and protein concentrations were analyzed. The supernatant was mixed with an equal volume of sample buffer (100 mM Tris HCl, pH 6.8, 2% sodium dodecyl sulfate, 0.02% bromophenol blue, and 10% glycerol). Subsequently, protein samples were run on gradient (4–20%) minigels (Invitrogen, Inc.) at 100 V for 2 h. After transfer, membranes were rinsed with TPBS (PBS containing 0.05% Tween 20) and blocked with 5% nonfat dry milk for 1 h at room temperature. The blocked membranes were incubated in primary antibodies (diluted to 1:1000–1:3000 with TPBS and 5% BSA) at room temperature for 2 h. After washing with TPBS twice for 5 min each time, the membrane was incubated with peroxidase-linked secondary antibodies (diluted to 1:5000 with TPBS and 5% dry milk) at room temperature for 2 h. Following further washes, ECL solution was added for 5 min at room temperature, and then the membrane was exposed on X-ray film. ImageJ software was used to measure the band density. All eNOS bands were normalized to corresponding GAPDH (housekeeping molecule) bands.
Analysis of MSC paracrine signaling: capillary-like tube formation of ECs
MSCs (5×105) were seeded on at least three samples for each of the four experimental conditions. MSCs were cultured for 24 h in the experimental culture media, which were then removed. This was followed by washing the cells in PBS and then adding 1 mL of serum-free DMEM media to each cell sample. After 24 h, the conditioned serum-free media from MSCs were extracted from the samples and stored at −80°C for future use of EC culture. Following five serum-free medium conditions were used to culture ECs: (1) “PS-CM” is the conditioned media from MSCs seeded in the control “PS” condition as described in Cell Culture and Cell Seeding section; (2) “VEGF-CM” is the conditioned media from MSCs seeded in the “VEGF” condition; (3) “2 kPa-CM” the conditioned media from MSCs seeded in the “2 kPa” condition; (4) “2 kPa+VEGF-CM” the conditioned media from MSCs seeded in the “2 kPa+VEGF” condition; and (5) “SFM” is serum-free DMEM used as a negative control. Primary ECs from the vasa vasorum of bovine pulmonary artery (gift from Dr Evgenia Gerasimovskaya at the University of Colorado at Denver) were used at the passage 4. To analyze MSC paracrine signaling to EC tube formation, 10 μL of reduced growth factor matrigel (BD) was placed in each well of a μ-slide angiogenesis (ibidi, Martinsried, Germany) and incubated for 1 h at 37°C to allow gelation. For each of the five medium conditions, 50 μL of conditioned media was placed in the wells (n=3, from three separate samples), and ∼17,000 of ECs were then seeded on top of matrigel. Samples were incubated at 37°C for 24 h, before pictures of tube structure formation were taken on a phase contrast microscope (Nikon, Garden City, NY).
Analysis of MSC paracrine signaling: EC migration assay
The conditioned media from MSCs cultured in various experimental conditions were obtained with a similar method as described above, but MSCs were cultured for 168 h before the standard culture media were replaced with the conditioned media for additional 24-h culture. Transwell plates with 6.5 mm-diameter wells and polycarbonate membrane inserts having 8.0 μm pores (Sigma-Aldrich, Inc.) were used for the migration study. For each of the five medium conditions, 750 μL of conditioned media was placed in the wells (n=3, from three separate samples). ECs (∼1×105) in 200 μL of serum-free DMEM were seeded on top of the porous polycarbonate membrane inserts before the inserts were placed in the wells. Cells were then incubated for 24 h. To remove the ECs that did not migrate through the pores, the top side of the polycarbonate membrane inserts were gently cleaned with a cotton swab. Migrated cells on the bottom of the inserts were fixed in methanol and stained with crystal violet. Inserts were imaged using an upright microscope Axiovert S100 (Carl Zeiss, Oberkochen, Germany) at a 20×magnification. For each image, the area fraction was calculated as follows with the areas determined by ImageJ.
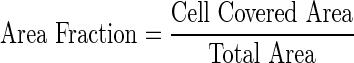 |
Statistical analysis
All data are shown as mean±standard deviation. Statistical analysis was performed using MVPstats software (MVP Programs, Vancouver, WA) or SPSS software (IBM, Chicago, IL). For comparing two groups with equal variances, a Student's t-test was used. For comparing two groups with unequal variances and unequal sample sizes, Welch's t-test was used. For multiple parametric group comparisons, a one-way ANOVA test was run on both the groups' means and variances. If the groups had equivalent variances, a Tukey post hoc was further performed. If the groups had unequivalent variances, a Games-Howell post hoc was further performed. For nonparametric groups, a Bonferroni or Kruskal–Wallis analysis was used. For PCR, statistics were calculated using the method described previously.23
Results
Effects of VEGF concentration on MSC differentiation toward endothelial lineage
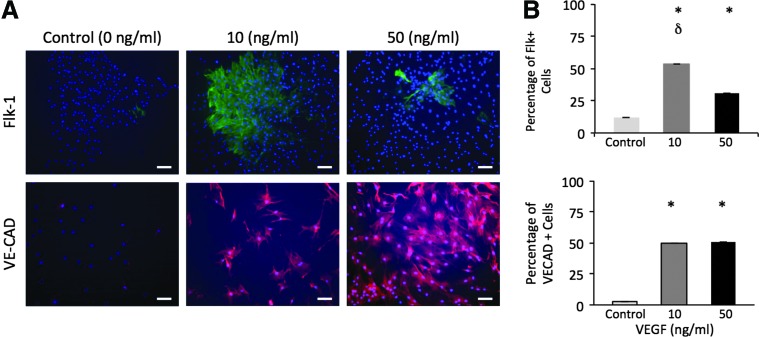
To determine an appropriate VEGF concentration for this study, MSCs were seeded in media containing 0, 10, or 50 ng/mL of VEGF, and the percentage of MSCs that differentiate toward endothelial lineage was quantified by immunostaining for early endothelial markers (Flk-1 and VECAD). Independent studies have utilized 10 or 50 ng/mL of VEGF to direct MSC differentiation toward endothelial lineage.9,24 However, the effects of VEGF concentration on the endothelial differentiation of MSCs remain unclear. Therefore, we compared the effectiveness of these VEGF concentrations in terms of differentiating MSCs toward endothelial lineage. Our results showed that both 10 and 50 ng/mL VEGF concentrations resulted in roughly 50% of MSCs with VECAD marker, a 500-fold increase compared with the PS condition (Fig. 1). MSCs seeded in 50 and 10 ng/mL of VEGF resulted in increased percentages of Flk-1+ cells by 1.8- and 3-fold respectively, when compared with the PS condition. As 10 ng/mL of VEGF was highly effective in driving MSCs to endothelial lineage, we continued using this concentration to explore the combined effects of matrix elasticity and VEGF on endothelial differentiation.
FIG. 1.
Effect of the vascular endothelial growth factor (VEGF) concentration on the mesenchymal stem cell (MSC) expression of early endothelial markers. (A) Representative images of MSC stained with endothelial markers (Flk-1 and VECAD) after 168-h incubation in the media supplied with 0, 10, or 50 ng/mL of VEGF. Scale bar is 10 μm. (B) Percentage of MSCs displaying Flk-1 marker and VE-CAD marker in 0, 10, or 50 ng/mL VEGF. *p<0.01 versus 0 ng/mL.δp<0.05 versus 50 ng/mL. Color images available online at www.liebertpub.com/tea
The combination of soft matrix and VEGF induces MSCs to upregulate gene expression of matured endothelial markers and to expedite endothelial differentiation
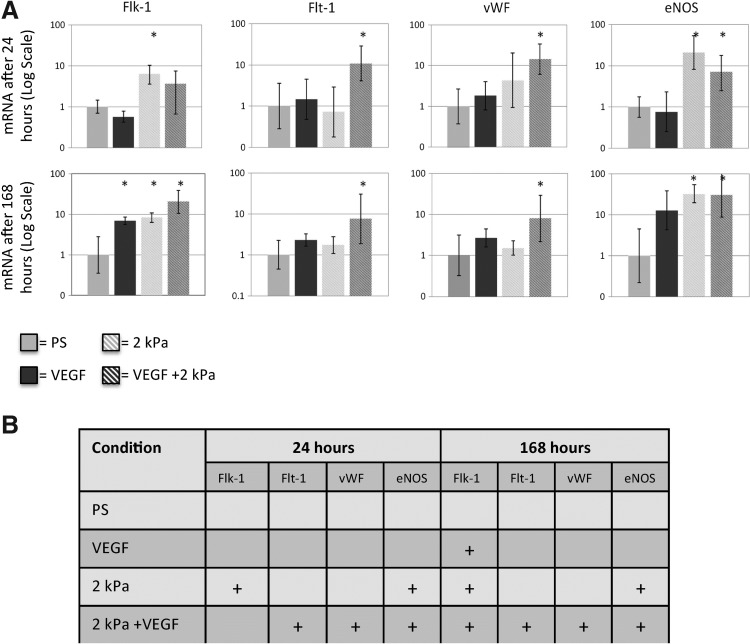
To evaluate the combined effects of soft matrix elasticity and VEGF on the differentiation of MSC to endothelial lineage, the gene expression of MSCs in the PS, VEGF, 2 kPa, and 2 kPa+VEGF conditions was quantitatively evaluated after culture for 24 and 168 h. Recent studies in developmental biology have identified a series of vascular biomarkers characterizing different differentiation stages of stem cells, including lineage commitment, differentiation, and maturation.25,26 To assess the maturity of differentiated MSCs, we examined genes of endothelial markers including Flk-1 (an early endothelial or progenitor marker), vWF, and Flt-1 (more mature endothelial differentiation markers), and eNOS (an endothelial-specific functional marker). Within 24 h, MSCs in the 2 kPa+VEGF condition showed an 8-fold increase of Flt-1, vWF, and eNOS genes compared with the PS condition (Fig. 2). After 168 h, MSCs in the 2 kPa+VEGF condition maintained the high increase in Flt-1, vWF, and eNOS gene expression, and Flk-1 gene expression increased by roughly 20-fold compared with the PS condition. For MSCs in the 2 kPa condition, Flk-1 and eNOS gene expression significantly increased by 8-fold compared with the PS condition at 24 h, and this increase was maintained at 168 h. Finally, MSCs seeded in VEGF showed an 8-fold increase in Flk-1 gene expression at 168 h compared with the PS condition. Therefore, while the use of the biochemical factor (VEGF) or the soft matrix (2 kPa) induced upregulation of certain endothelial genes, only MSCs in the VEGF+2 kPa condition had all four endothelial genes highly upregulated within 168 h. Furthermore, the combination of VEGF and 2 kPa matrix elasticity expedited the endothelial differentiation of MSCs.
FIG. 2.
The combination of VEGF and 2 kPa matrix elasticity upregulates genes showing early and mature endothelial phenotype in MSCs. (A) MSC gene expression of Flk-1, Flt-1, vWF, and eNOS after 24 and 168 h in seeding conditions. *p<0.05 versus PS, n>3 for all conditions. (B) Table summary on MSC expression of endothelial markers after 24 and 168 h of incubation. “+” indicates >8-fold increase (p<0.05) compared with the PS condition. PS, a standard polystyrene cell culture plate.
The combination of soft matrix and VEGF enhances MSC expression of endothelial-specific functional protein and inhibits expression of smooth muscle phenotype
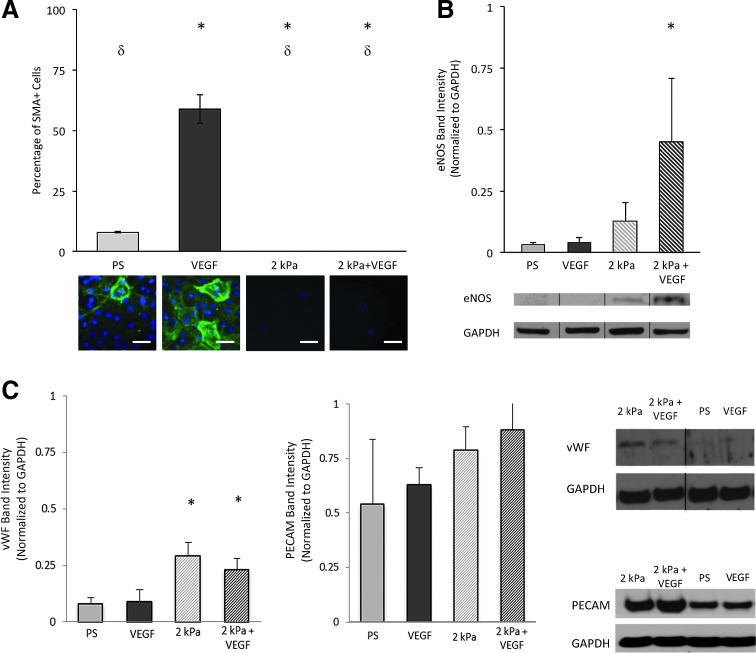
When utilizing MSCs for vascular regeneration, it is critical to direct cell differentiation to a specific, mature vascular phenotype, as opposed to vascular cell progenitor or diseased vascular phenotypes. Though VEGF is an exogenous growth factor present in the arterial microenvironment that is important for directing endothelial differentiation, increased VEGF was also found at the sites of neo-intima hyperplasia where diseased cells simultaneously express both endothelial and SMC markers (e.g., PECAM and SMA).27,28 To determine whether these vascular tissue biochemical and mechanical factors guided MSC differentiation into a highly specific endothelial phenotype, MSC protein expression was evaluated in all seeding conditions by immunostaining or immunoblotting. The protein expression of the functional endothelial marker (eNOS) and a smooth muscle biomarker (SMA) characterizing early smooth muscle differentiation was quantified. Results showed that MSCs in the 2 kPa+VEGF condition did not express SMA protein (Fig. 3A). Additionally, MSCs seeded in the 2 kPa+VEGF condition displayed an 8-fold increase in eNOS protein expression compared with the PS condition (Fig. 3B). For MSCs in the 2 kPa condition, the eNOS protein expression was not significantly increased compared to the PS condition, nor was any SMA protein expression found. For MSCs in the VEGF condition, eNOS protein was not significantly increased compared to the PS condition. Interestingly, the VEGF condition resulted in roughly 60% of MSCs showing the SMA marker, ∼6-fold increase compared with the PS condition. MSCs seeded in both PS and VEGF conditions had significantly greater SMA protein compared with MSCs cultured on 2 kPa or 2 kPa+VEGF conditions, suggesting that soft matrix elasticity may inhibit SMA protein expression. The VEGF condition increased both SMA and Flk-1 expression, suggesting that VEGF upregulated both endothelial and smooth muscle markers, promoting MSCs into vascular progenitors or pathological vascular phenotypes, which are often more proliferative than more matured vascular phenotypes. Cell proliferation assay (data not shown) showed that the VEGF condition significantly increased MSC proliferation by twofolds within 24 h, when compared with the PS condition. To further confirm the findings with eNOS expression results and to characterize early effects of soft matrix and VEGF conditions, two additional endothelial phenotypic markers (vWF and PECAM) were analyzed on the MSCs cultured in all the experimental conditions (Fig. 3C). Western blotting results showed that MSCs seeded in the 2 kPa and 2 kPa+VEGF conditions displayed 3-fold increases in vWF protein expression compared with the PS condition, demonstrating the role of the soft matrix in inducing rapid commitment of MSCs to endothelial lineage early in the differentiation process. MSC expression of PECAM in different experiment conditions also agreed with these findings. Therefore, these protein analysis results have demonstrated that only MSC cultured in the 2 kPa+VEGF condition highly upregulated endothelial-specific functional protein while lacking smooth muscle protein, demonstrating that both mechanical and chemical stimuli are required to direct MSC differentiation into specific vascular endothelial lineage.
FIG. 3.
The combination of VEGF and 2 kPa matrix elasticity upregulates MSC expression of endothelial-specific functional proteins while downregulating SMA expression. (A) Representative immunostaining images and quantitative measures showing SMA+ MSCs after cells were cultured in experimental conditions for 168 h. δp<0.05 VEGF. *p<0.05 with PS. Scale bar is 5 μm. (B) Western blotting results showing eNOS protein expression in MSCs after cells were cultured in experimental conditions for 24 h. *p<0.05 with PS. Representative eNOS and GAPDH protein bands were shown below the bar graph. (C) Western blotting results showing vWF and PECAM protein expression in MSCs after cells were cultured in experimental conditions for 24 h. *p<0.05 with PS. Representative protein bands were shown on the rightmost images. The black line in the blot images shows separated lanes obtained on the gel. PECAM, platelet endothelial cell adhesion molecule. Color images available online at www.liebertpub.com/tea
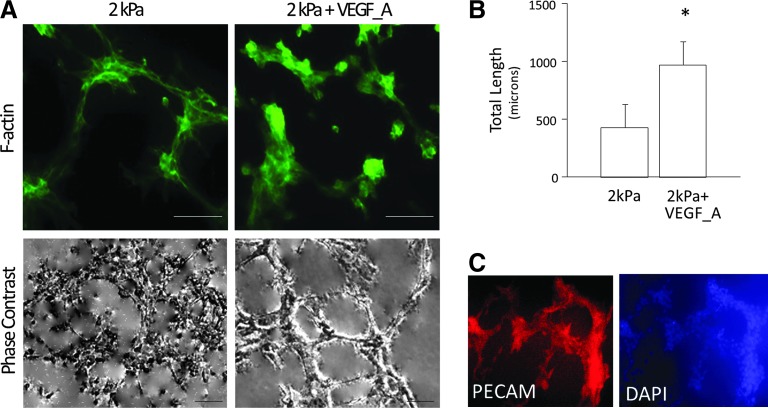
The combination of soft matrix and VEGF enhances capabilities of MSCs to form capillary-like structures
It is known that functional ECs are capable of undergoing angiogenic process to form capillary-like structures. To determine whether MSC differentiation under biomechanical and/or biochemical stimuli exhibited similar angiogenic capability, MSCs were cultured in the PS, VEGF, 2 kPa, and 2 kPa+VEGF conditions and cell organization was examined after 24 h. MSCs cultured in 2 kPa and 2 kPa+VEGF conditions formed capillary-like structures (Fig. 4A), whereas MSCs cultured on PS, VEGF, or matrigel with reduced growth factors did not form such structures (data not shown). To determine whether the addition of VEGF to 3D 2 kPa matrices improved the angiogenic capacity of differentiated MSCs, the total length of all capillary-like tube structures was determined. Results showed that the tubes formed by MSCs on 2 kPa+VEGF matrices exhibited increased total tube length by twofolds over those formed by MSCs on 2 kPa matrices (Fig. 4B), which indicates enhanced angiogenic capabilities of the differentiated cells. To further confirm whether differentiated MSCs in capillary-like structures expressed endothelial markers, MSCs seeded on 2 kPa and 2 kPa+VEGF conditions were stained with DAPI and PECAM. As illustrated in Figure 4C, MSCs in the soft matrix conditions expressed PECAM around capillary-like structures.
FIG. 4.
MSCs formed capillary-like tube structures in 2 kPa and 2 kPa+VEGF seeding conditions. (A) Representative fluorescent (F-actin) and phase contrast images of capillary-like structures formed by MSCs in 2 kPa and 2 kPA+VEGF conditions, after 24-h culture. Scale bar is 10 μm. (B) Quantitative analysis of the total tube length in capillary-like structures formed on 2 kPa and 2 kPa+VEGF conditions. * p<0.05 versus 2 kPa, n=3. (C) PECAM and DAPI immunostaining of capillary-like structures formed by MSCs in the 2 kPa+VEGF condition. Color images available online at www.liebertpub.com/tea
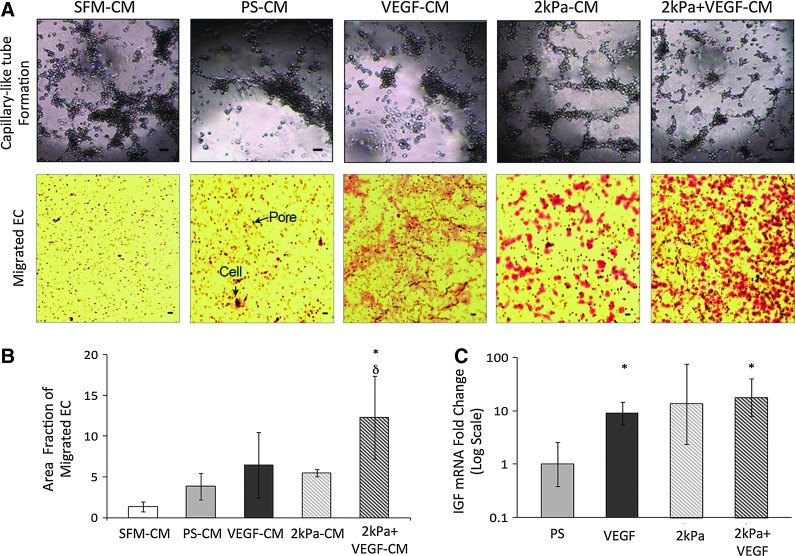
Matrix elasticity and VEGF synergistically enhanced MSC paracrine signaling to promote EC migration and formation of capillary-like structures
A number of recent in vivo studies have found that MSCs improve cardiovascular function by secreting a large reservoir of paracrine signals, which improve the angiogenenic capabilities of ECs.22,29 We thus examined how the combined use of chemical and mechanical factors influenced the paracrine signaling capabilities of MSCs. Since both EC migration and formation of capillary-like structures are critical steps in EC angiogenesis, we have performed these assays by culturing ECs in the conditioned media (CM) extracted from the four MSC culture conditions: PS (PS-CM), VEGF (VEGF-CM), 2 kPa (2 kPa-CM), and 2 kPa+VEGF (2 kPa+VEGF-CM). SFM was used as a negative control. Results showed that ECs cultured in 2 kPa+VEGF-CM exhibited significant increases in cell migration, compared with ECs cultured in the SFM and PS-CM conditions by fivefold and threefold, respectively (Fig. 5A, B). Additionally, we found that ECs cultured in 2 kPa-CM and 2 kPa+VEGF-CM conditions formed capillary-like structures on matrigel with reduced growth factors (Fig. 5A). These results demonstrate that matrix elasticity can independently enhance MSC paracrine signaling, which in turn increases EC capillary-like tube formation. However, it appears that both matrix elasticity and chemical growth factor are necessary to augment EC migration capabilities via MSC paracrine signaling. To further link increased EC migration and EC formation of capillary-like structures to the paracrine signaling factors secreted by MSCs, MSC expression of insulin-like growth factor (IGF) was studied in the PS, VEGF, 2 kPa, and 2 kPa+VEGF conditions. IGF-1 is one of the most potent natural activators of the AKT signaling, a stimulator of cell growth and proliferation, and recently was recognized as an important factor to improve endothelial function of progenitor cells.30 MSCs cultured in both VEGF and 2 kPa+VEGF conditions showed a 10-fold increase in IGF mRNA expression when compared with the PS condition (Fig. 5C). This suggests that VEGF increases IGF expression in MSCs, partly contributing to enhanced endothelial functions. Other signaling factors we examined did not show significant differences among different conditions. Future work is needed to elucidate the paracrine factors that are released by MSCs co-regulated by the matrix elasticity and VEGF.
FIG. 5.
MSC-conditioned media (CM) from cells differentiated in the 2 kPa+VEGF condition improves paracrine signaling capabilities of MSCs. (A) Top panel: Representative images showing endothelial cell (EC) formation of capillary-like structures on the matrigel with reduced growth factors in various CM. Bottom panel: Representative images of migrated ECs on filters. Arrows show pores in a membrane and ECs stained with crystal violet. For both panels, the scale bar is 10 μm. (B) Quantitative measure of the area fraction of filter covered by migrated ECs after 24 h of exposure to MSC-CM. δp<0.05 versus SFM. *p<0.05 versus PS-CM. n≥3. (C) Fold change of insulin-like growth factor (IGF) mRNA in MSCs after 168 h of seeding on PS, VEGF, 2 kPa, and 2 kPa+VEGF conditions. *p<0.05 with PS n=3. Color images available online at www.liebertpub.com/tea
Discussion
The present study has demonstrated that the combined use of VEGF and a soft (∼2 kPa) matrix synergistically enhances the capability of MSCs to regenerate the vascular endothelium. The synergism occurs through two mechanisms: MSC paracrine signaling to ECs, and MSC transdifferentiation into cells exhibiting matured endothelial phenotype. The paracrine signaling capabilities of MSCs were evaluated by EC migration and formation of capillary-like structures, both of which were increased by conditioned media from MSCs cultured on soft matrices supplied with VEGF. Additionally, the synergistic effects of matrix stiffness and VEGF rapidly drove MSCs to transdifferentiate into cells that expressed several mature endothelial markers after only 24 h. Furthermore, our results suggest that the combined use of mechanical and biochemical stimuli enhance the specificity of vascular differentiation. MSCs seeded on 2 kPa+VEGF showed minimal expression of the early stage smooth muscle marker SMA, while showing significant amounts of the functional endothelial marker eNOS. In contrast, MSCs seeded on rigid substrates with or without VEGF showed a significant amount of SMA expression with low eNOS and vWF expression. Taken together, our results have shown that mechanical and biochemical microenvironmental factors act together to guide MSCs to regenerate healthy vascular tissue through transdifferentiation and paracrine signaling mechanisms. The present study also suggested that MSC transdifferentiation into ECs and MSC paracrine signaling to promote activities of ECs might be inherently linked; both MSC transdifferentiation and paracrine signaling are enhanced by the synergistic effects of soft matrix elasticity and VEGF factor.
When utilizing MSCs for vascular regeneration it is critical to direct cell differentiation to a healthy, specific endothelial phenotype. We found that MSCs on soft matrices with VEGF expressed no SMA protein, while expressing several mature endothelial markers. Interestingly, the sole use of VEGF led MSCs to express a significant amount of SMA protein and increase proliferation while upregulating an early endothelial marker. The presence of both endothelial and smooth muscle markers is a sign of a dysfunctional, proliferative cellular phenotype that can be found in diseased vascular tissues such as neointimal hyperplasia.27,28 Our results are consistent with previous findings in vivo and in vitro, showing proliferating SMA+ cells were present at the sites of neointimal hyperplasia where a significant amount of exogenous VEGF was found.31 Additionally, Park et al. found that MSCs seeded with TGF-β for 24 h on polyacrylamide gels with an elasticity of 1 kPa showed significantly less SMA and calponin-1 than those seeded on stiff (>15 kPa) substrates.32 Therefore, it is the combination of mechanical and chemical factors that drive MSC transdifferentiation to healthier endothelial phenotypes with increased phenotypic-specific function.
Though a number of studies have explored the individual effects of vascular mechanical and biochemical factors on MSC differentiation, few have attempted to simultaneously modulate both stimuli in a 3D matrix as occurs in vivo. To that end, the present study uses a 3D matrix to model the stiffness of the in vivo vascular intima and VEGF to model the biochemical environment. The sole use of biochemical factors such as VEGF requires at least a 7-day culture time to induce the endothelial differentiation of MSCs.9 Additionally, the independent use of biomechanical factors such as soft matrices may expedite the differentiation process, inducing MSCs to display early to intermediate endothelial markers in 1- to 3-day culture times, as shown by our previous study and others.8,18 Results from this study demonstrate that the combination of appropriate biomechanical (elasticity of ∼2 kPa) and biochemical (a VEGF concentration of 10 ng/mL) stimuli lead to rapid endothelial differentiation of MSCs and more mature, specific endothelial phenotype, compared with the independent use of these stimuli. MSC differentiation toward endothelial lineage in vivo is regulated by a combination of biochemical and biomechanical factors. We have shown that simultaneous regulation of biochemical and biomechanical factors in vitro is an effective way to quickly perpetuate the differentiation of MSCs into cells that express both mature and functional endothelial markers (Flt, vWF, PECAM, and eNOS) of vascular ECs. The experimental setup here utilized the PS and VEGF conditions to ensure the methods and results of this research are consistent with and comparable to many previous studies using growth factors on rigid two-dimensional (2D) substrates. The conclusion of this work clearly demonstrates that MSCs on soft matrices with VEGF show more mature EC markers than MSC on soft matrices without VEGF. Further, MSCs on soft matrices demonstrated a more rapid, specific commitment to endothelial lineage than cells on 2D substrates with or without VEGF. Therefore, the responses of stem cells to chemical growth factors drastically vary with their physical environment. Experiments examining the impact of chemical growth factors on stem cell behavior in standard 2D rigid environment might not accurately predict the behavior of stem cells in either the in vivo microenvironment or a 3D biomimetic cell scaffold.
Recent studies have highlighted a major biological function of MSCs is their capability to secrete a large number of paracrine signaling factors, which in turn affect neighboring cells. The signaling factors include inflammatory factors and angiogenic factors that promote surrounding ECs to proliferate, migrate, and organize into capillaries.19,22,33 The injection of MSCs into an ischemic myocardium was shown to improve myocardial function by increasing vascularity6,29 Furthermore, the sole use of signaling factors released from MSC can improve vascularization.34,35 Recent studies suggest that microenvironments around MSCs can influence their signal secretory functions. For example, Seib et al. demonstrated that decreasing substrate elasticity reduced MSC secretion of IL8, an inflammatory chemical.19 Currently, it is largely unknown how chemical and mechanical factors in the cellular microenvironment influence MSC paracrine signaling. The present study showed that conditioned media from MSCs seeded on soft matrices with VEGF resulted in increased EC migration and formation of capillary-like structures. It seems this combination of chemical and mechanical stimuli increased MSC capabilities of secreting angiogenic factors. Our results also suggest that IGF might partially contribute to the enhanced paracrine signaling from MSCs. Interestingly, Linke et al. and Sang et al. have also linked the IGF gene to both germ cell and cardiac stem cell migration.36,37
Conclusion
This study has demonstrated that both mechanical and biochemical factors are critical to direct MSC differentiation into healthy endothelial phenotypes. Mechanical and biochemical factors also act synergistically to enhance MSC secretion of paracrine signals, improving EC angiogenesis capabilities. This research highlights the importance of precisely controlling both mechanical and chemical factors when designing cell therapies or tissue engineered constructs.
Acknowledgments
We would like to acknowledge Dr. Stephanie Bryant's lab for providing PEGDM 3000 material and Dr. Evgenia Gerasimovskaya's lab for providing endothelial cells and protocol for migration and angiogenesis assays. The research is partly funded by NIH (K25-HL097246 and R01 HL119371 to W.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Soliman E.Z., Sorlie P.D., Sotoodehnia N., Turan T.N., Virani S.S., Wong N.D., Woo D., and Turner M.B.Heart Disease and Stroke Statistics—2012 Update A Report From the American Heart Association. Circulation 125,e2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang N.F., and Li S.Mesenchymal stem cells for vascular regeneration. Regen Med 3,877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lith R., and Ameer G.A.Biohybrid strategies for vascular grafts. In: Pallua N., and Suschek C.V., eds. Tissue Engineering. Heidelberg: Springer Berlin, 2011, pp. 279–316 [Google Scholar]
- 4.Park J., Huang N., Kurpinski K., Patel S., Hsu S., and Li S.Mechanobiology of mesenchymal stem cells and their use in cardiovascular repair. Front Biosci 12,5098, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Riha G.M., Lin P.H., Lumsden A.B., Yao Q., and Chen C.Review: application of stem cells for vascular tissue engineering. Tissue Eng 11,1535, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Silva G.V., Litovsky S., Assad J.A.R., Sousa A.L.S., Martin B.J., Vela D., Coulter S.C., Lin J., Ober J., Vaughn W.K., Branco R.V.C., Oliveira E.M., He R., Geng Y.-J., Willerson J.T., and Perin E.C.Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111,150, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Caplan A.I., and Dennis J.E.Mesenchymal stem cells as trophic mediators. J Cell Biochem 98,1076, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Zhang G., Drinnan C.T., Geuss L.R., and Suggs L.J.Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater 6,3395, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Oswald J., Boxberger S., Jorgensen B., Feldmann S., Ehninger G., Bornhauser M., and Werner C.Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22,377, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hocking A.M., and Gibran N.S.Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 316,2213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashi C.K., Zhu Y., Yang G.-Y., Young W.L., Hsiao B.S., Wang K., Chu B., and Li S.Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A 104,11915, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Shea C.A., Hynes S.O., Shaw G., Coen B.A., Hynes A.C., McMahon J., Murphy M., Barry F., and O'Brien T.Bolus delivery of mesenchymal stem cells to injured vasculature in the rabbit carotid artery produces a dysfunctional endothelium. Tissue Eng Part A 16,1657, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Xu Q.Stem cells and transplant arteriosclerosis. Circ Res 102,1011, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Sata M., Saiura A., Kunisato A., Tojo A., Okada S., Tokuhisa T., Hirai H., Makuuchi M., Hirata Y., and Nagai R.Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8,403, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Engler A., Sen S., Sweeney H., and Discher D.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Peloquin J., Huynh J., Williams R.M., and Reinhart-King C.A.Indentation measurements of the subendothelial matrix in bovine carotid arteries. J Biomech 44,815, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Liliensiek S.J., Nealey P., and Murphy C.J.Characterization of endothelial basement membrane nanotopography in Rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A 15,9,2643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingate K., Bonani W., Tan Y., Bryant S.J., and Tan W.Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelial or smooth muscle cell markers. Acta Biomater 8,1440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seib F.P., Prewitz M., Werner C., and Bornhäuser M.Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs). Biochem Biophys Res Commun 389,663, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Cheng A.S., and Yau T.M.Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg 20,94, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Bajpai V.K., and Andreadis S.T.Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev 18,405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirotsou M., Jayawardena T.M., Schmeckpeper J., Gnecchi M., and Dzau V.J.Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50,280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong M.L., and Medrano J.F.Real-time PCR for mRNA quantitation. Biotechniques 39,75, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Jiang Y., Hao H., Gupta K., Xu J., Chu L., McFalls E., Zweier J., Verfaillie C., and Bache R.J.Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol 293,H1760, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Luo Z., Wang G., Wang W., Xiao Q., and Xu Q.Signalling pathways that regulate endothelial differentiation from stem cells. Front Biosci 16,472, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Park C., Lugus J.J., and Choi K.Stepwise commitment from embryonic stem to hematopoietic and endothelial cells. Curr Top Dev Biol 66,1, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Yeager M.E., Frid M.G., and Stenmark K.R.Progenitor cells in pulmonary vascular remodeling. Pulm Circ 1,3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K., Sata M., Natori T., Kim-Kaneyama J.-R., Nose K., Shibanuma M., Hirata Y., and Nagai R.Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. FASEB J, 22,428, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., Mu H., Noiseux N., Zhang L., Pratt R.E., Ingwall J.S., and Dzau V.J.Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11,367, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Fleissner F., and Thum T.The IGF-1 receptor as a therapeutic target to improve endothelial progenitor cell function. Mol Med 14,235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhardwaj S., Roy H., Heikura T., and Ylä-Herttuala S.VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest 35,669, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Park J.S., Chu J.S., Tsou A.D., Diop R., Tang Z., Wang A., and Li S.The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 32,3921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potente M., Gerhardt H., and Carmeliet P.Basic and therapeutic aspects of angiogenesis. Cell 146,873, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Angoulvant D., Ivanes F., Ferrera R., Matthews P.G., Nataf S., and Ovize M.Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant 30,95, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Herrmann J.L., Wang Y., Abarbanell A.M., Weil B.R., Tan J., and Meldrum D.R.Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 33,24, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Linke A., Müller P., Nurzynska D., Casarsa C., Torella D., Nascimbene A., Castaldo C., Cascapera S., Böhm M., Quaini F., Urbanek K., Leri A., Hintze T.H., Kajstura J., and Anversa P.Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 102,8966, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sang X., Curran M.S., and Wood A.W.Paracrine insulin-like growth factor signaling influences primordial germ cell migration: in vivo evidence from the zebrafish model. Endocrinology 149,5035, 2008 [DOI] [PubMed] [Google Scholar]