Abstract
Erectile dysfunction (ED) is the most frequent long-term problem after radical prostatectomy. We aimed to evaluate whether the use of combination therapy with basic fibroblast growth factor (bFGF)-hydrogel on corpus cavernosum and with adipose-derived stem cells (ADSCs) and brain-derived neurotrophic factor (BDNF)-immobilized poly-lactic-co-glycolic acid (PLGA) membrane on the cavernous nerve (CN) could improve erectile function in a rat model of bilateral cavernous nerve crush injury (BCNI). Rats were randomly divided into five groups (n=15 per group): a normal group (N group), a group receiving saline application after bilateral cavernous nerve crush injury (BCNI), a group undergoing bFGF-hydrogel injection in the corpus cavernosum after BCNI (bFGF), a group receiving ADSC application covered with BDNF-membrane after BCNI (ADSC/BDNF), and a group undergoing coadministration of bFGF-hydrogel injection and BDNF-membrane with ADSCs after BDNF (bFGF+ADSC/BDNF). Four weeks postoperatively, the erectile function was assessed by detecting the ratio of intracavernous pressure (ICP) to mean arterial pressure (MAP). Smooth muscle and collagen contents were measured using Masson's trichrome staining. Neuronal nitric oxide synthase (nNOS) expression in the dorsal penile nerve was detected by immunostaining. The protein expression of the α-smooth muscle actin (α-SMA) and the cyclic guanosine monophosphate (cGMP) level of the corpus cavernosum were quantified by western blot and cGMP assay, respectively. In the bFGF+ADSC/BDNF group, the erectile function was significantly elevated compared with the BCNI and other treated groups and showed a significantly increased smooth muscle/collagen ratio, nNOS content, α-SMA expression, and cGMP level. In particular, there were no statistical differences in the ICP/MAP ratio, smooth muscle/collagen ratio, and α-SMA and cGMP levels between the bFGF+ADSC/BDNF group and normal group. Application of the BDNF-immobilized PLGA membrane with human ADSC into the CN and bFGF-incorporated hydrogel into the corpus carvernosum improved nearly normal erectile function in a rat model of postprostatectomy ED. This result suggests that a combined application of bFGF+ADSC/BDNF might be a promising treatment for postprostatectomy ED.
Introduction
Erectile dysfunction (ED) is the most frequent long-term complication after radical prostatectomy (RP), despite recent advances in anatomic knowledge and technology.1 The severity of postprostatectomy ED due to damage to the neurovascular system varies from 20% to 70% of preprocedure functioning.2 Cavernous nerve injury (CNI) is the main reason for postprostatectomy ED; therefore, researchers have focused on preventing CNI and stimulating nerve generation.3–5 As well, a rat model of cavernous nerve (CN) crush injury relating to penile erection has been developed to mimic the damage and degeneration associated with nerve-sparing RP.6,7
Stem cells may potentially be able to repair injured CNs and are considered a prospective treatment option for ED.8,9 Albersen et al. showed that injection of adipose-derived stem cells (ADSCs) improved erectile function in a rat model of bilateral cavernous nerve injury (BCNI).10 However, previous research using ADSCs or brain-derived neurotrophic factor (BDNF) for the treatment of ED have been injected on the corpus cavernosum instead of injured CNs.10,11
Despite improvements in erectile function with intracavernosal injection of stem cells, including ADSCs, this treatment has limited efficacy. Studies have suggested that growth factors could additionally increase the efficacy of stem cell transplantation and enhance stem cell proliferation.12 Neurotrophin, or BDNF, has been reported to contribute to the survival and differentiation of stem cells,13 as well as to enhance the recovery of erectile function following CNI.7,14 The increased therapeutic efficacy of coadministration of BDNF-immobilized poly-lactic-co-glycolic acid (PLGA) membrane with ADSC was also demonstrated in our previous study.15
In addition to CN damage, smooth muscle atrophy of the corpus cavernosum is considered to be one of the main causes of ED. To overcome this, we selected basic fibroblast growth factor (bFGF) as a stimulator of the recovery of venous smooth muscle. To deliver the growth factor directly into the corpus cavernosum, we used the gelatin poly(ethylene glycol)-tyramine (GPT) hydrogel in this study. Among the many currently available hydrogel systems, the GPT hydrogel has various mechanical and biological advantages, including in situ gelatinization, minimally invasive injection, and biocompatibility.16,17 In addition, the GPT hydrogel was able to maintain the sustained release of bFGF.17
In this study, we injected the bFGF-incorporated GPT hydrogel to improve smooth muscle atrophy in the corpus cavernosum and simultaneously applied a BDNF-membrane with ADSCs directly to the CNI site to ameliorate CNI through regeneration. Therefore, we aimed to evaluate whether the combined therapy of bFGF-hydrogel with ADSCs and BDNF-immobilized PLGA membrane could improve erectile function in a rat model of BCNI beyond the effects of treatment with either therapy alone.
Materials and Methods
Preparation of bFGF-incorporated GPT hydrogels and BDNF-immobilized PLGA membrane
In situ crosslinkable gelatin-GPT conjugates were synthesized as described previously.18 To prepare the bFGF-incorporated GPT hydrogels, two 3 wt% GPT solutions (1 mL), which contained horseradish peroxidase (HRP) (0.013 mg/mL) and hydrogen peroxide (0.013 wt%), respectively, were prepared, and then the bFGF (R&D Systems; 200 ng/mL) was added to the GPT solutions. Each solution was loaded into a dual syringe kit before injection into the target site.
The fabrication of the PLGA membrane containing BDNF was performed as described previously by Oh et al.19,20 BDNF was incorporated onto the surface of the PLGA membrane through heparin immobilization. The heparin-immobilized PLGA membrane was then soaked in the BDNF solution (200 ng/mL) at room temperature for 3 h, washed with phosphate-buffered saline (PBS; pH 7.4), and finally freeze-dried overnight.20
ADSC culture
FDA-approved human ADSCs (hADSCs) were provided by RNL Bio Co.19 Passage 3 hADSCs were used for this in vivo experiment. hADSCs were expanded in T-175 flasks supplemented with a nondifferentiation culture medium (Dulbecco's Modified Eagle Medium containing 10% FBS, 1% penicillin/streptomycin) before being injected into the injured site.
Animal protocol
Eight- to 10-week-old male Sprague-Dawley (SD) rats (Orient Bio Co.) were used (total number=75). All protocols were performed in accordance with the guidelines and regulations pertinent to animal experiments of the Institutional Animal Care and Use Committee of The Catholic University Medical College. Normal rats were anesthetized with a subcutaneous injection of ketamine (110 mg/kg). The urinary bladder and prostate were revealed through a lower abdominal incision. In the BCNI group, the bilateral CN below the major pelvic ganglion was identified and then compressed with a hemostat clamp for a duration of 2 min. After crushing injury of bilateral CN, bFGF-hydrogel was injected into the subcutaneous corpus cavernosum. Then, PKH-26-labeled hADSCs (cell dose: 1×106, PKH26 Red Fluorescent Cell Linker Kit; Sigma) were applied at the site of the damaged CN using a Hamilton syringe (25-gauge needle); The BDNF-membrane was then immediately applied to the site of the hADSCs. Experimental animals were randomly divided into five groups (n=15 per group): a normal (N) group, the saline application after bilateral cavernous nerve crush injury (BCNI) group, the bFGF-hydrogel injection in corpus cavernosum after BCNI (bFGF) group, the ADSC application covered with BDNF-membrane after BCNI (ADSC/BDNF) group, and the coadministration of bFGF-hydrogel injection and BDNF-membrane with ADSC after BDNF (bFGF+ADSC/BDNF) group.
Measurement of erectile function
Four weeks after the nerve crush injury, the erectile function was evaluated by measuring the change in intracavernous pressure (ICP) upon electrical stimulation of the CNs. SD rats were anesthetized using a subcutaneous injection of ketamine (110 mg/kg) and placed on a table in the supine position. The carotid artery and CN were exposed for the detection of mean arterial pressure (MAP) and ICP, respectively. PE-50 tubing was inserted into the carotid artery to measure blood pressure. At the same time, a 23-gauge butterfly needle was placed in the proximal corpus cavernosum, filled with 250 U/mL heparin solution, and then linked to a pressure transducer (model S48K; Grass Instrument Division, Astro-Med, Inc.) to measure ICP. Electrostimulation of the proximal CNI site was performed using a bipolar stainless steel electrical stimulator. Stimulus conditions were as follows: voltage, 10 V; duration, 50 s; electric current, 2.4 mA; and pulse width, 2.5 ms. The peak ICP during nerve electrostimulation was calculated by an isometric force transducer and recorded on a computer using a commercial data acquisition system (Power Lab®; AD Instruments Pty. Ltd.). ICP and MAP ratios were analyzed using Chart 5 software (AD Instruments Pty. Ltd.).
Masson's trichrome staining
After measuring MAP and ICP, the penis was removed and fixed in 4% paraformaldehyde for 24 h at 4°C. The fixed penile tissues were embedded in paraffin wax, sectioned at 4 μm thickness, and stained with Masson's trichrome staining (MTS) to evaluate muscle fibrosis. The stained sections were observed under an optical microscope (Olympus BX50). Analysis of smooth muscle and collagen was performed using Panoramic Viewer 1.14 Software (3DHISTECH Ltd.), and the cell number was counted in five fields per slide in triplicate.
Immunohistochemistry analysis
Immediately following the MAP and ICP measurements, an incision was made in the CN and penile tissue. The CN tissue was frozen with 2-methlybutane liquid nitrogen and then embedded in the OCT compound (Tissue-Tek). Serial 5-μm cross sections of NC tissues were sectioned using a cryostat (Cryostat microtomes; Leica). The frozen sections of CN and the paraffin sections of penile tissue were washed with the PBTx buffer (0.1% Triton X-100 in PBS), blocked with 1% BSA (Amresco), and then incubated in 1.5% normal goat serum (Vector Laboratories) in PBTx at 37°C for 1 h. The sections were then incubated at 4°C overnight. Frozen CN sections were immunostained with mouse neuron-specific beta-III tubulin (diluted 1: 200; Abcam) and DAPI to stain cell nuclei (Vector Labs). Paraffin sections of penile tissue were immunostained with primary antibodies against mouse neuron-specific beta-III tubulin (diluted 1: 200; Abcam) and neuronal nitric oxide synthase (nNOS) (diluted 1:100; Santa Cruz Biotechnologies). The Alexa Fluor® D488 secondary antibody was used. Digital images were acquired using an Olympus BX51 fluorescence microscope.
Western blot analysis
Harvested corpus cavernosum tissue was frozen in liquid nitrogen. After washing in PBS, the tissue was homogenized in an ice-cold lysis buffer containing 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 μm leupeptin, 20 μg/mL chymostatin and 2 mM phenylmethanesulfonyl fluoride using a homogenizer (Precellys 24; Bertin Technologies). Samples were then centrifuged at 12,000 g for 10 min at 4°C and the supernatant was collected. The protein content of samples was quantified using the Bicinchoninic Acid Protein Assay kit (Pierce). The expression of α smooth muscle actin in tissue extracts was then evaluated. Extracts (20 μg protein) were solubilized in the LDS sample buffer (Invitrogen), boiled for 10 min at 70°C, electrophoresed on NuPAGE 4–12% Bis-Tris gels (Invitrogen), and the proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% skim milk at room temperature for 1 h and then incubated with the following primary antibodies: mouse α-SMA (diluted 1:1000; Abcam) and mouse β-actin (diluted 1:2500; Santa Cruz Biotechnologies). Following incubation, membranes were washed with TBS supplemented with 0.1% Tween-20 (TBST) and then incubated with a secondary antibody conjugated to HRP for 1 h at room temperature. The ECL method (Amersham) was used to detect protein bands.
Measurement of cyclic guanosine monophosphate levels
The harvested corpus cavernosum tissue was also instantly frozen in liquid nitrogen. After PBS washing, the penile tissue was weighed and 350 μL of 0.1 M HCL with silica beads (BioSec Products, Inc.) was added for each 60 mg of sample. Samples were homogenized (Precellys 24; Bertin Technologies) and then centrifuged at 12,000 g for 10 min at 4°C, and the supernatant was collected. The cyclic guanosine monophosphate (cGMP) direct immunoassay kit (K372-100; BioVision) was used to quantify cavernous cGMP levels.
Statistical analysis
Means±SEMs were calculated for each study group, and these values were compared among groups by analysis of variance tests with the Tukey post hoc test. Two-group comparisons were performed using unpaired t-tests, and p-values less than 0.05 were considered statistically significant.
Results
Localization of fluorescently labeled ADSCs
Four weeks after the implantation of red fluorescently labeled ADSCs with BDNF-membrane, the frozen sections of CN tissue were stained with green fluorescent beta-III tubulin. In the bFGF+ADSC/BDNF group, ADSCs were identified in the CN with a red color. In particular, red fluorescent ADSCs costained with green fluorescent beta-III tubulin, resulting in a yellow color, indicating that BDNF-immobilized membrane contributed to ADSC engraftment into the damaged CN in vivo (Fig. 1).
FIG. 1.
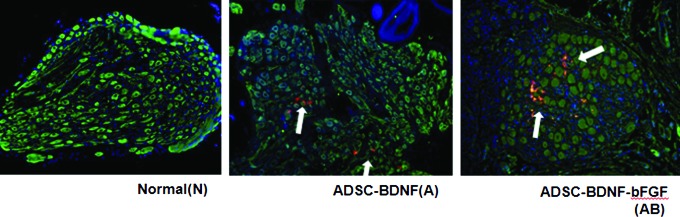
Fluorescence micrographs of the CN following bFGF+ADSC/BDNF application. Magnification is ×400. PKH26-labeled ADSCs (1×106 cells) (red) are shown around the CN. Merged ADSCs (yellow, arrow) are shown in colocalization of PKH26-labeled ADSC and CN by double immunostaining with beta-III tubulin (green). DAPI was used to stain the nuclei (blue). ADSCs, adipose-derived stem cells; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; CN, cavernous nerve. Color images available online at www.liebertpub.com/tea
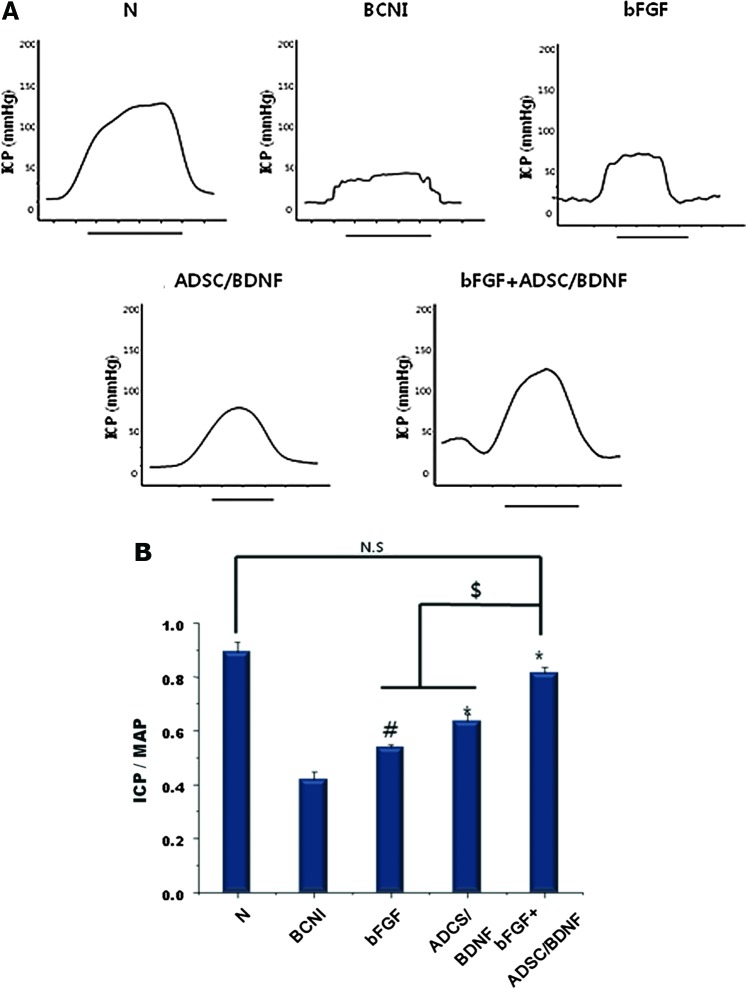
ICP/MAP ratio
To assess penile erectile function, ICP/MAP ratios in response to CN electrostimulation were measured. Graphical representations of ICP tracings for the duration of the nerve stimulation are shown in Figure 2A. No significant differences in MAP values were observed among the groups. ICP was lower in the BCNI group than the normal group, as well as lower than all the treated groups. ICP/MAP ratios in the N, BCNI, bFGF, ADSC/BDNF, and bFGF+ADSC/BDNF groups were 0.89±0.02, 0.41±0.03, 0.63±0.02, 0.53±0.04, and 0.81±0.02, respectively (Fig. 2B). The bFGF+ADSC/BDNF group showed an increase in the ICP/MAP ratio compared with the bFGF and ADSC/BDNF groups (p<0.05). In particular, there were no statistically significant differences in the ICP/MAP ratio between the bFGF, ADSC/BDNF, and normal groups.
FIG. 2.
ICP/MAP ratio change at 4 weeks following surgery. (A) Representative recordings of ICP for each group in response to CN stimulation at 10 V. The solid bars are representative ICP recordings with a stimulus interval of 50 s. (B) The ratios of ICP to MAP during electrical stimulation were calculated for each group. Each bar shows the mean ratio (±SEM) for n=15 rats per group. (#p<0.05 compared with the BCNI group; *p<0.01 compared with the BCNI group; $p<0.05; N.S., not significant). BCNI, bilateral cavernous nerve crush injury; ICP, intracavernous pressure; MAP, mean arterial pressure. Color images available online at www.liebertpub.com/tea
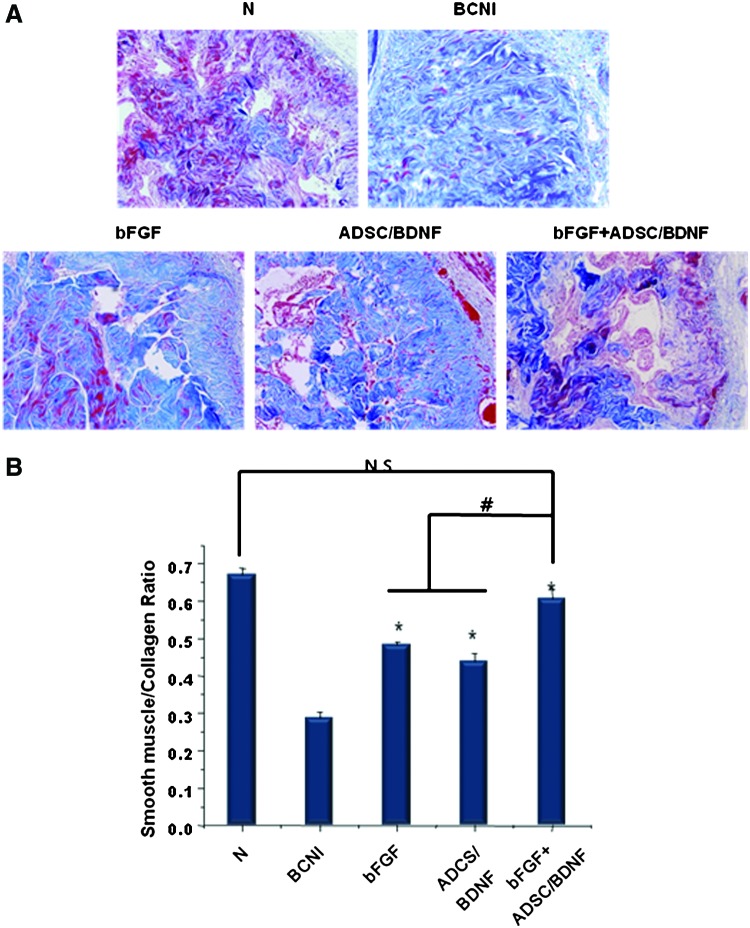
Smooth muscle/collagen ratio in the corpus cavernosum
The smooth muscle and collagen contents of the corpus cavernosum were assessed by MTS. The average smooth muscle/collagen ratios in the N, BCNI, bFGF, ADSC/BDNF, and bFGF+ADSC/BDNF groups were 35.7%, 14.4%, 28.1%, 27.7%, and 34.1%, respectively (Fig. 3B). The smooth muscle/collagen ratio was lower in the BCNI group than the normal group and all of the treated groups. In particular, the bFGF+ADSC/BDNF group had a significantly higher smooth muscle/collagen ratio than the bFGF and ADSC/BDNF groups (p<0.05). There was no significant difference in the smooth muscle/collagen ratio between the bFGF, ADSC/BDNF, and normal groups.
FIG. 3.
Histological change in smooth muscle/collagen ratios of the corpus cavernosum at 4 weeks following surgery. (A) Representative micrographs of Masson's trichrome-stained rat penile sections. Smooth muscle was stained red, and collagen fibers were stained purple-blue. Magnification is ×200. (B) Relative quantification of smooth muscle/collagen ratios of penile tissue in each group. Smooth muscle atrophy was decreased in the AB/bFGF group relative to the other treatment groups. Quantitative analysis of smooth muscle and collagen content was performed with an image analyzer. Each bar shows the mean values (±SEM) from n=15 rats per group. (*p<0.05 compared with the BCNI group; #p<0.05; N.S., not significant). Color images available online at www.liebertpub.com/tea
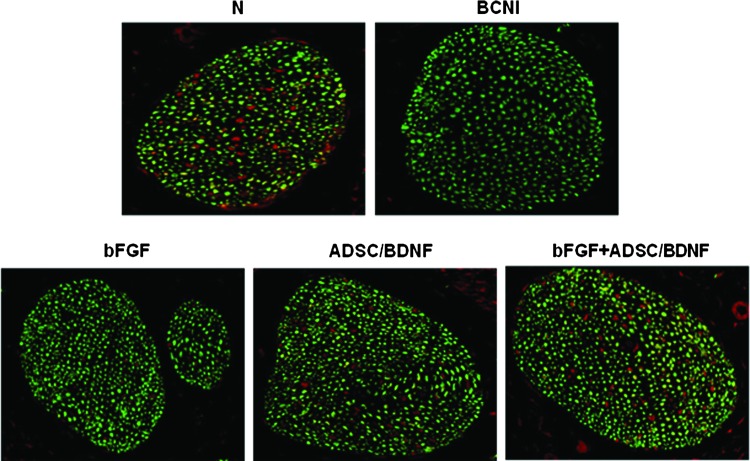
Expression of nNOS in the dorsal penile nerve
We evaluated the expression of nNOS in the dorsal penile nerve using immunohistochemical staining. Penile tissues were stained with green fluorescent beta-III tubulin and red fluorescent nNOS to identify the penile nerve fibers and their nNOS content, respectively. Figure 4 shows that the nNOS content in the BCNI group was significantly less compared with that in the normal group. bFGF+ADSC/BDNF treatment increased the number of nNOS-positive nerve fibers relative to the control group.
FIG. 4.
Double immunohistochemistry using nNOS (red) and beta-III tubulin (green) for tissue sections obtained from around the dorsal penile nerve in each group. Magnification is ×400. nNOS, neuronal nitric oxide synthase. Color images available online at www.liebertpub.com/tea
Expression of α-smooth muscle actin (α-SMA) protein in the corpus cavernosum
We investigated the protein expression of α-SMA in the corpus cavernosum by western blot analysis (Fig. 5). We used an anti-β-actin antibody as an internal control. α-SMA expression was significantly lower in the BCNI group than the normal group, suggesting that the damage to the CN induced significant apoptosis of smooth muscle cells in the corpus cavernosum. However, expression of α-SMA in all the treatment groups was significantly higher than that in the BCNI group. In particular, α-SMA expression in the bFGF-ADSC/BDNF group was significantly higher than that in the bFGF-only and ADSC/BDNF-only groups (p<0.05).
FIG. 5.
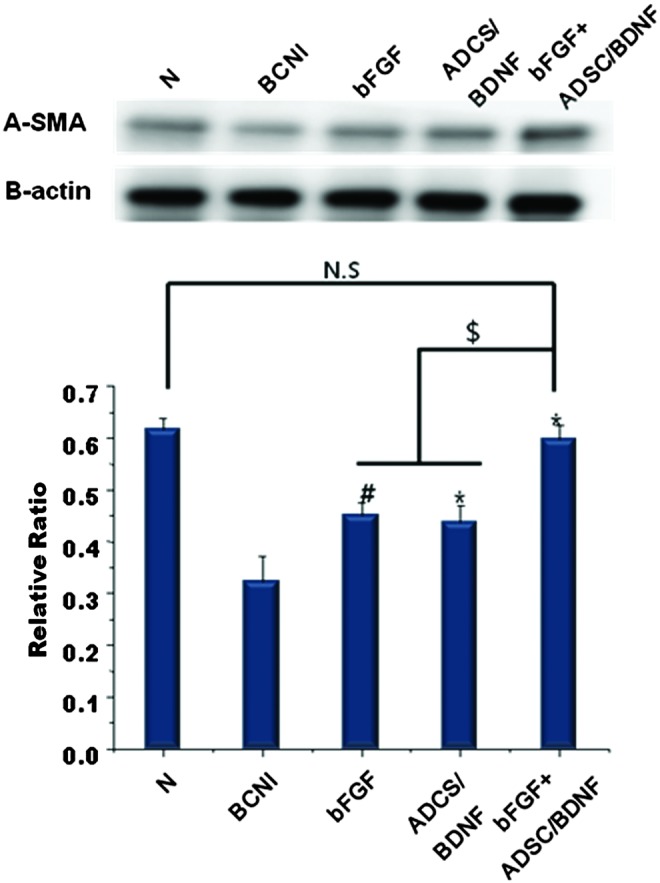
Protein expression of α-smooth muscle actin (α-SMA) in the corpus cavernosum. Expression of endogenous α-SMA protein in penile tissue was quantified by western blot. β-actin was used as an internal control to assure equal loading. The bar graph represents the relative expression of α-SMA to β-actin expression. (mean±SEM; *p<0.05 and #p<0.01 compared with the BCNI group; $p<0.01; N.S., not significant). Color images available online at www.liebertpub.com/tea
Level of cGMP in the corpus cavernosum
Corpus cavernosum concentrations of cGMP in the N, BCNI, bFGF, ADSC/BDNF, and bFGF+ADSC/BDNF groups were 40.0, 25.9, 32.5, 33.8, and 37.4 pmol/g, respectively (Fig. 6). cGMP levels increased significantly in all the treated groups compared with the BCNI group. cGMP expression in the AB/bFGF group was significantly higher than the other experimental groups and not statistically different compared with the normal group.
FIG. 6.
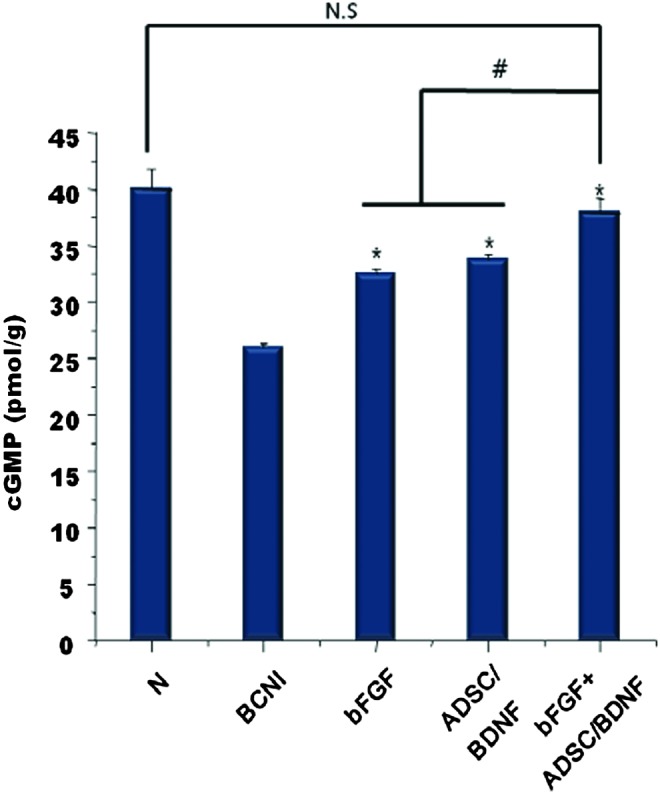
cGMP levels in the penile tissue. The cavernous cGMP level was determined by using a cGMP direct immunoassay kit (n=15 per group). The bar graph represents the mean±SEM. (*p<0.05 compared with the BCNI group; #p<0.01). cGMP, cyclic guanosine monophosphate. Color images available online at www.liebertpub.com/tea
Discussion
Even though robot-assisted laparoscopic RP and nerve-sparing techniques are performed worldwide, mechanical damage to the CN during RP is inevitable.1 For the duration of robot-assisted surgical procedures, the CN is exposed to laceration, mechanical stretching, and heat damage. Advances in anatomical knowledge and technology have decreased the rates of ED following RP; however, complete preservation of sexual potency and continence after this procedure remains a challenge. Compromised functioning of the CN after RP is the dominant cause of atrophy of the corpus cavernosum and loss of neurotransmitters and fibrosis, which together result in postprostatectomy ED.21 Neuronal damage extensively alters the microarchitecture and structure of the corpus cavernosum, significantly decreasing penile erectile function.22 However, conventional studies have only assessed direct injection of cells into the corpus cavernosum, focusing on smooth muscle atrophy in the corpus cavernosum, and have not addressed the limited efficacy thereof on CN regeneration.23 To prevent and increase the efficacy for ED treatment, we directly applied stem cells and a growth factor to injured CNs, which we deemed the ultimate cause of ED.
We used SD rats because the CN in SD rats are relatively bigger, compared with other mice, such as nude mice. While ADSCs were harvested from human adipose tissue, instead of rat adipose tissue, a previous study indicated that the hADSCs used in this study are safe in rats, and do not induce immunogenicity and tumor development.15
In the previous work, the application of ADSCs and a BDNF/PLGA membrane to the CN improved erectile function in a rat model of postprostatectomy ED.15 However, our values for ICP/MAP ratios, compared to the previous work, were quite different in the normal group (0.89 vs. 0.58), the BCNI group (0.41 vs. 0.23), and the ADSC/BDNF group (0.53 vs. 0.43). These results may be different from previous outcomes due to differences in time, places, experimenters, or other conditions. In addition, if an experimenter took a higher standard value than that in the previous experiment, the entire value of the results may be higher. Another reason for differences may result from differences in weight or other conditions of animals.
Application of ADSCs and BDNF-membranes improved the smooth muscle/collagen ratio, nNOS content, and cGMP levels. In another study, angiogenic growth factors, vascular endothelial growth factor (VEGF) and bFGF, were shown to induce endothelial proliferation and repair vascular damage.24 Furthermore, intracavernous injection of bFGF in a hypercholesterolemic rabbit model was shown to increase vasoreactivity, endothelial cell content, bFGF protein, and VEGF protein expression, despite resulting in no significant increase in corporal tissue fibrosis.25 In the present study, we demonstrated that the joint administration of ADSC/BDNF and bFGF had the greatest positive effects on erectile function in a rat model of ED.
Specifically, we measured the ICP/MAP ratio in response to electrostimulation to examine erectile function. CNI markedly decreased the ICP/MAP ratio, and significant decreases in the smooth muscle/collagen ratio and α-SMA expression were observed in the BCNI group with Masson's trichrome stain and an antibody against α-SMA, consistent with the previous findings of Iacono et al.22 It has been hypothesized that CNI in a rat model induces apoptosis of cells comprising the penile tissue, leading to subtunical smooth muscle cell damage, which in turn results in veno-occlusive dysfunction and subsequent ED.26 Not only the application of ADSC/BDNF-membrane to the CN but also the application of bFGF-hydrogel monotreatment significantly enhanced the smooth muscle/collagen ratio, compared with BCNI. However, the combination treatment with ADSC/BDNF in the CNs and bFGF in the corpus cavernosum had a synergistic effect on the smooth muscle/collagen ratio. These results indicate that nerve regeneration could be induced in human patients through periprostatic injection of ADSC/BDNF and an intracavernosal bFGF injection; these interventions may result in the prevention of corporeal smooth muscle deterioration. Our novel findings may prove helpful in enhancing early recovery from ED after prostatectomy.
In the present study, we observed that there was no statistically significant difference in the ICP/MAP ratio, smooth muscle/collagen ratio, cGMP or protein expression of α-SMA between the ADSC/BDNF and bFGF group and a normal group (p>0.05). These promising findings might be the result of the injection of the bFGF-hydrogel into the corpus cavernosum to prevent smooth muscle atrophy during nerve regeneration. Recently, several studies examined the restoration of the cavernous muscle through the injection of stem cells, phosphodiesterase 5 inhibitors, and extracorporeal shock wave therapy (ESWT). Selective inhibitors of phosphodiesterase type 5 are commonly used for ED after RP.27 Low-intensity ESWT may also be helpful for ED by improving penile hemodynamics.28 However, these interventions cannot be applied clinically immediately post-RP, although these treatments have been demonstrated to be effective in other settings.
Although we demonstrated that administration of bFGF+ADSC/BDNF improved erectile function, we cannot fully explain the mechanisms underlying the cavernous regeneration we observed with stem cell treatment. We can only speculate that hADSCs may affect cavernous recovery through a paracrine effect from cytokines and growth factors. Covering with the BDNF/PLGA membrane could promote engraftment of ADSCs into the CN. The nanopore side structure of PLGA membranes may serve as a physical barrier for prevention of connective fibrous tissue invasion and provide permeability of oxygen for tissue and nerve regeneration. The copolymerization ratio of PLGA was PLA:PGA=75:25. This molecule was dissolved within 6 months. Therefore, even though PLGA membranes dissolved at 1 month, the membrane maintained its original shape. PLGA membrane was able to maintain the sustained release of BDNF, as well as its original shape. These characteristics of PLGA membranes suggest that it is a suitable substrate for the proliferation and differentiation of ADSCs. However, further studies are required to confirm our findings. We performed our measurements at 4 weeks after stem cell implantation; it is not clear, however, how long the ADSCs and BDNF membrane will be effective at enhancing erectile function. Further long-term investigations are required to improve the understanding of this therapy.
Conclusions
Application of BDNF-immobilized PLGA membrane with hADSC into the CN and bFGF-incorporated hydrogel into the corpus cavernosum improved nearly normal erectile function in a rat model of postprostatectomy ED. The underlying mechanisms of restoration of function may involve elevation of nNOS expression in the penile nerve, enhancement of the smooth muscle/collagen ratio, eNOS phosphorylation, and increases in α-smooth muscle actin expression and cGMP levels in the corpus cavernosum. This result suggests that combined application of bFGF+ADSC/BDNF might be a promising treatment for postprostatectomy ED.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030075).
Disclosure Statement
No competing financial interests exist.
References
- 1.Salonia A., Burnett A.L., Graefen M., Hatzimouratidis K., Montorsi F., Mulhall J.P., and Stief C.Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol 62,261, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Sanda M.G., Dunn R.L., Michalski J., Sandler H.M., Northouse L., Hembroff L., Lin X., Greenfield T.K., Litwin M.S., Saigal C.S., Mahadevan A., Klein E., Kibel A., Pisters L.L., Kuban D., Kaplan I., Wood D., Ciezki J., Shah N., and Wei J.T.Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358,1250, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bella A.J., Lin G., Lin C.S., Hickling D.R., Morash C., and Lue T.F.Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med 6,347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentine H., Chen Y., Guo H., McCormick J., Wu Y., Sezen S.F., Hoke A., Burnett A.L., and Steiner J.P.Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol 51,1724, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albersen M., Fandel T.M., Zhang H., Banie L., Lin G., De Ridder D., Lin C.S., and Lue T.F.Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol 59,286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sezen S.F., Hoke A., Burnett A.L., and Snyder S.H.Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med 7,1073, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh P.S., Bochinski D.J., Lin G.T., Nunes L., Lin C.S., and Lue T.F.The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int 92,470, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bochinski D., Lin G.T., Nunes L., Carrion R., Rahman N., Lin C.S., and Lue T.F.The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int 94,904, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lin G., Banie L., Ning H., Bella A.J., Lin C.S., and Lue T.F.Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med 6,320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albersen M., Fandel T.M., Lin G., Wang G., Banie L., Lin C.S., and Lue T.F.Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7,3331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendirci M., Trost L., Bakondi B., Whitney M.J., Hellstrom W.J., and Spees J.L.Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol 184,1560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagiuchi A., Miyake H., Nomi M., Takenaka A., and Fujisawa M.Modulation of the microenvironment by growth factors regulates the in vivo growth of skeletal myoblasts. BJU Int 103,1569, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T., Ooto S., Akagi T., Amemiya K., Igarashi R., Mizushima Y., and Takahashi M.Effects of prolonged delivery of brain-derived neurotrophic factor on the fate of neural stem cells transplanted into the developing rat retina. Biochem Biophys Res Commun 309,843, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bella A.J., Lin G., Garcia M.M., Tantiwongse K., Brant W.O., Lin C.S., and Lue T.F.Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur Urol 52,574, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Piao S., Kim I.G., Lee J.Y., Hong S.H., Kim S.W., Hwang T.K., Oh S.H., Lee J.H., Ra J.C., and Lee J.Y.Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med 9,1968, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Park K.M., Lee Y., Son J.Y., Bae J.W., and Park K.D.In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjug Chem 23,2042, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Hwan J.H., Kim I.G., Piao S., Jung A.R., Lee J.Y., Park K.D., and Lee J.Y.Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials 34,6037, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Park K.M., Lee Y., Son J.Y., Oh D.H., Lee J.S., and Park K.D.In situ cross-linkable gelatin-poly (ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for tissue regenerative medicine J Mater Chem 21, 13180, 2011 [Google Scholar]
- 19.Oh S.H., Kim J.H., Kim J.M., and Lee J.H.Asymmetrically porous PLGA/Pluronic F127 membrane for effective guided bone regeneration. J Biomater Sci Polym Ed 17,1375, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Oh S.H., Kim I.G., Lee J.Y., Lee J.Y., and Lee J.H.Bioactive porous beads as an injectable urethral bulking agent: their in vitro evaluation on smooth muscle cell differentiation. Tissue Eng Part A 17,655, 2011 [DOI] [PubMed] [Google Scholar]
- 21.User H.M., Hairston J.H., Zelner D.J., McKenna K.E., and McVary K.T.Penile weight and cell subtype specific changes in a postradical prostatectomy model of erectile dysfunction. J Urol 169,1175, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Iacono F., Giannella R., Somma P., Manno G., Fusco F., and Mirone V.Histological alterations in cavernous tissue after radical prostatectomy. J Urol 173,1673, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y.C., Ning H., Shindel A.W., Fandel T.M., Lin G., Harraz A.M., Lue T.F., and Lin C.S.The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med 7,1391, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkman J.Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333,1757, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Xie D., Pippen A.M., Odronic S.I., Annex B.H., and Donatucci C.F.Intracavernosal basic fibroblast growth factor improves vasoreactivity in the hypercholesterolemic rabbit. J Sex Med 3,223, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Lue T.F.Erectile dysfunction. N Engl J Med 342,1802, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Basal S., Wambi C., Acikel C., Gupta M., and Badani K.Optimal strategy for penile rehabilitation after robot-assisted radical prostatectomy based on preoperative erectile function. BJU Int 111,658, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Goyal N.K., Garg M., and Goel A.Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 188,2018, 2012 [DOI] [PubMed] [Google Scholar]