Abstract
Background
Approximately 15% of women have caesarean sections (CS) and while the rate varies, the number is increasing in many countries. This is of concern because higher CS rates do not confer additional health gain but may adversely affect maternal health and have implications for future pregnancies. Active management of labour has been proposed as a means of reducing CS rates. This refers to a package of care including strict diagnosis of labour, routine amniotomy, oxytocin for slow progress and one to one support in labour.
Objectives
To determine whether active management of labour reduces CS rates in low-risk women and improves satisfaction.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (February 2008), MEDLINE (1966 to December 2007), EMBASE (1980 to 2007), MIDIRS (1985 to 2007) and CINAHL (1982 to 2007).
Selection criteria
Randomised controlled trials comparing low-risk women receiving a predefined package of care (active management) with women receiving routine (variable) care. Trials where slow progress had been diagnosed before entry into the trial were excluded.
Data collection and analysis
At least two review authors extracted data. We assessed included studies for risk of bias.
Main results
We included seven trials, with a total of 5390 women. The quality of studies was mixed. The CS rate was slightly lower in the active management group compared to the group that received routine care, but this difference did not reach statistical significance (RR 0.88, 95% CI 0.77 to 1.01). However, in one study there was a large number of post-randomisation exclusions. On excluding this study, CS rates in the active management group were statistically significantly lower than in the routine care group (RR 0.77 95% CI 0.63 to 0.94). More women in the active management group had labours lasting less than twelve hours, but there was wide variation in length of labour within and between trials. There were no differences between groups in use of analgesia, rates of assisted vaginal deliveries or maternal or neonatal complications. Only one trial examined maternal satisfaction; the majority of women (over 75%) in both groups were very satisfied with care.
Authors’ conclusions
Active management is associated with small reductions in the CS rate, but it is highly prescriptive and interventional. It is possible that some components of the active management package are more effective than others. Further work is required to determine the acceptability of active management to women in labour.
Medical Subject Headings (MeSH): *Labor, Obstetric; Cesarean Section [trends; *utilization]; Labor, Induced [*methods]; Oxytocics; Oxytocin; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
The global caesarean section (CS) rate is estimated as 15%, with CS rates over 20% in many developed countries, Latin America and the Caribbean (Betran 2007). CS rates in many countries continue to increase. In the USA, the CS rate is estimated to have increased by 3% from 2005 to 2006 reaching a record high of 31.1% (NVSR 2007). The rate in England increased from 21.5% in 2000 (Thomas 2001) to 23.5% in 2006 (NHS 2007). In Brazil, about one in two births are by CS (CS rate 51%) (Najmi 2000) and across Latin American countries overall CS rates are 33% (Villar 2007). While the optimum rate of CS remains controver-sial, countries with increasing CS rates have focused on strategies to reduce its use due to concern that higher CS rates do not confer additional health gain, but may increase maternal risks, have negative implications for future fertility and subsequent pregnancies, and have resource implications for health services (Betran 2007; NICE 2004; Thomas 2001; Villar 2007; Walker 2002).
In England and Wales, 20% of CSs were carried out for poor progress in labour or ‘failure to progress’ (Thomas 2001). In 1970, the use of a labour ward protocol for low-risk women called ‘active management of labour’ was described with the aim of reducing the length of labour and the numbers of women who have CS (O’Driscoll 1970).
Active management of labour as originally described by O’Driscoll (O’Driscoll 1970; O’Driscoll 1973) includes:
one-to-one support in labour (continual presence of a nurse during labour);
routine amniotomy (artificial rupture of the amniotic membranes);
the use of the intravenous drug oxytocin;
strict criteria for the diagnosis of labour;
strict monitoring of progress in labour (e.g. by plotting on a partogram);
strict criteria for identifying slow progress and fetal compromise;
peer review of assisted deliveries.
Active management is a complex package of interventions. In the original description of active management the aim was to ensure that women experienced a shorter labour. This included strict criteria for the diagnosis of labour based on the woman’s assessment and confirmed by a senior member of staff using objective evidence (i.e. ‘show’, spontaneous rupture of membranes, complete effacement of the cervix). Once diagnosed, both amniotomy and oxytocin were used to improve or augment the progress of labour by increasing the intensity and frequency of uterine contractions. During the first stage of labour acceptable rate of progress was set at 1 cm cervical dilatation per hour and during the second stage progress was measured in terms of descent and subsequent rotation of the head. A maximum of 10 hours was allowed for the first stage of labour and two hours for the second stage. Observational studies by the initiators of active management showed lower CS rates, less prolonged labour, better neonatal outcomes and maternal satisfaction (O’Driscoll 1984). However, subsequent observational studies did not have similar results (Peaceman 1993; Thornton 1994) and, hence, it has remained an area of controversy. The disadvantage of active management is related to the need for more interventions during labour, possibly with invasive monitoring resulting in a more medicalised birth process in which women have less control and less satisfaction.
Recent meta-analyses have included randomised controlled trials evaluating the effectiveness of single interventions (oxytocin administration only or amniotomy only) on reducing the CS rate. Neither of these single interventions was effective for reducing CS rates (Bidgood 1984; Bugg 2008; Fraser 2003). The use of partogram alone is currently being considered in a separate review (Lavender 2005).
A systematic review of randomised controlled trials that evaluated the effectiveness of one-to-one support in labour (without other interventions) demonstrated its effect in reducing CS rates (Hodnett 2003). Therefore, it is possible that the effectiveness of the active management of labour package could be due to this component alone. Alternatively, it is possible that the effect on CS rates from a policy of active management occurs as a result of the combination of interventions rather than because of individual interventions.
OBJECTIVES
To determine whether a predefined package of interventions during childbirth such as ‘active management of labour’ can reduce the CS rate in low-risk women and improve women’s satisfaction.
METHODS
Criteria for considering studies for this review
Types of studies
All randomised controlled trials, published or unpublished, including cluster-randomised controlled trials. Quasi-randomised trials are not included.
Types of participants
Healthy pregnant women with an uncomplicated singleton pregnancy in spontaneous labour at term. Studies where women had been diagnosed with delay in labour at randomisation are not included as these studies are considered in a related review (Wei 2008).
Types of interventions
Any intervention in which a predefined interventionist package of care during childbirth has been compared to non-interventionist care during childbirth. To be included in the review the predefined package of childbirth care had to be clearly described and had to include some (more than two) or all of the key elements described traditionally as active management of labour: routine amniotomy and early augmentation with oxytocin; strict criteria for the diagnosis of labour; abnormal progress in labour and fetal compromise; continual presence of a midwife/nurse during labour; peer review of assisted deliveries and progress of labour plotted using a graph (O’Driscoll 1970). The comparative care also needed to be clearly described for a study to be included.
Types of outcome measures
Primary outcomes
CS
Maternal satisfaction (measured quantitatively using validated questionnaires)
Secondary outcomes
Length of labour
Incidence of prolonged labour
Analgesia
Neonatal outcomes of Apgar scores, umbilical cord pH, neurological morbidity, admission to special care baby units
Subgroups
Primiparous women versus multiparous women
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Coordinator (February 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Coordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Coordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched MEDLINE (1966 to December 2007), EMBASE (1980 to 2007), MIDIRS (1985 to 2007) and CINAHL (1982 to 2007) using the search strategy detailed in Appendix 1 We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Assessing eligibility for inclusion
We obtained the full text of all potentially relevant studies and two review authors independently applied the inclusion criteria. We have given reasons for excluding any trial in the table of exclusions. We did not assess trials blindly, as we were aware of the author’s name, institution and the source of publication. We resolved any disagreement regarding eligibility by discussion between three review authors (H Brown, S Paranjothy and T Dowswell) until consensus was reached.
Data extraction and management
We designed a form to extract data. At least two review authors (H Brown(HB), S Paranjothy(SP), T Dowswell (TD)) extracted the data from each included study using the agreed form. We resolved discrepancies through discussion, and when required a third person was consulted. One of the review authors (TD) entered data into Review Manager software (RevMan 2008) and a second author (SP) then checked for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved disagreement by discussion or by involving a third person.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the methods as:
adequate (e.g. random number table; computer random number generator);
inadequate (odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and have assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We assessed methods as:
adequate (e.g. central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We have described for each included study the methods used (if any) to blind study participants and personnel from knowledge of which intervention a participant received. We have noted where blinding was not possible or was not used (and this is likely to be the case in interventions where different styles of care are compared).
We assessed the methods as:
adequate, inadequate or unclear for women taking part in studies;
adequate, inadequate or unclear for clinical staff;
adequate inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts and protocol deviations)
We have described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers (compared with the total randomised participants), reasons for attrition/exclusion (where reported), and any re-inclusions in analyses which we have undertaken.
We have assessed the methods as:
adequate (e.g. where there was no missing data or low levels of missing data, and where reasons for missing data were balanced across groups);
inadequate (e.g. where there were high levels of missing data or where attrition was not balanced across groups);
unclear (e.g. where there was insufficient reporting of attrition or exclusions to permit a judgement to be made).
(For outcomes measured in labour we would expect levels of missing data to be less than 10% for a study to be judged adequate.)
(5) Selective reporting bias
We have described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could not be used; where the study failed to include results of a key outcome that would have been expected to have been reported or where only statistically significant results are included);
unclear.
(6) Other sources of bias
We have described for each included study any important concerns we had about other possible sources of bias. For example, where there was a potential source of bias related to the specific study design, where the experimental protocol changed part-way through, where there was extreme baseline imbalance, or where we had serious concerns that the study data were duplicated from another study.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We have made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above we assessed the likely magnitude and direction of bias and whether we considered it was likely to impact on findings.
We have set out assessments of bias for included studies in the risk of bias tables. Where studies were excluded on grounds of serious risk of bias/poor study quality, we have indicated this in the table of excluded studies.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed-effect meta-analyses for combining data in the absence of heterogeneity. For outcomes where there was considerable heterogeneity (e.g. outcomes relating to length of labour) we used random-effects meta-analyses.
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We have reported where there was evidence of non-normally distributed data.
Unit of analysis issues
Cluster-randomised trials
We did not identify any cluster-randomised trials on this topic.
Dealing with missing data
For included studies we have noted levels of attrition.
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, using the I2 statistic. Where high levels of heterogeneity between the trials was identified, we explored this using random-effects meta-analyses.
Assessment of reporting biases
Where we suspected reporting bias or where missing data were thought to introduce serious bias this has been reported.
Subgroup analysis and investigation of heterogeneity
We had intended to conduct sub-group analysis for parity (nulliparous versus multiparous women) for the main outcome measure - rate of caesarean sections. However, this was not possible as all of the included studies recruited only nulliparous women.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The search strategy identified 18 studies for possible inclusion in the review.
Included studies
We included seven studies comparing groups of women receiving a package of care for the active management of labour. These studies were carried out in hospital settings in several countries, three in the USA (Frigoletto 1995; Lopez-Zeno 1992; Rogers 1997) and one each in New Zealand (Sadler 2000), Europe (Cammu 1996), Thailand (Somprasit 2005), and Nigeria (Tabowei 2003).
Although we intended to include studies recruiting both nulliparous and multiparous women, all the included studies stated nulliparity as an inclusion criteria.
The included studies all had a pre-defined package of care during labour and childbirth (intervention arm) which was compared to less interventionist or routine care. The intervention packages included all or several of the elements associated with active management. All of the packages included routine amniotomy soon after labour diagnosis (usually within one hour) irrespective of any delay being diagnosed, strict monitoring of progress and augmentation with oxytocin if progress was deemed slow or delayed (more than 1 cm cervical dilatation in the first stage of labour or delayed descent in the second). Oxytocin was typically administered at high doses in the intervention groups (e.g. 6 mU/min increasing by 6 mU every 15 minutes to a maximum of 36 or 40 mU per minute unless uterine hyperstimulation or fetal distress were identified). Most of the packages included frequent vaginal examinations to identify slow progress (see Table 1). Special antenatal childbirth education was a feature of the active management package in two trials (Frigoletto 1995; Tabowei 2003). One-to-one care was part of active care where resources permitted (Cammu 1996; Frigoletto 1995; Lopez-Zeno 1992; Sadler 2000; Tabowei 2003), otherwise one-to-two care was offered (Rogers 1997; Somprasit 2005).
Table 1. Characteristics of Active Management Protocols and Routine Care.
| Study | Routine Amniotomy-time from admission | Criteria for diagnosing delay-cm-per-hour cervical dilatation | IV-oxytocin-dosage | One-to-one care |
|---|---|---|---|---|
| Cammu 1996 | AM-routine within 1 hr of admission RC-only for arrest or at 2nd stage |
AM-hrly vaginal exam. Delay= < 1cm/hr RC-Delay= < 0.5 cm/hr up to 4cm, then <1cm/hr |
AM-2mU/min, 4mU at 20min, 6mU 40 min up to 30mU at 120min RC-as above |
One to one care in both groups |
| Frigoletto 1995 | AM-routine within 1hr of admission RC-variable |
AM-Delay=< 1cm/hr RC-variable |
AM-4mU/min increasing by 4mU every 15 min to max 40mU/min RC-1-2mU/min increasing every 15 min by 1-2mU/min |
AM-one to one care in separate unit RC-one to two care |
| Lopez-Zeno 1992 | AM-routine within one hour of admission RC-variable |
AM-hourly vaginal examination for 3 hrs. Delay = < 1cm/hr RC-at discretion of clinician, generally 1cm/hr |
AM-6mU/min increasing 6mU every 15min to max of 36mU/min RC-1mU/min increasing 1 or 2mU/min every 15min |
Not clear |
| Rogers 1997 | AM-routine within 2hrs admission RC-not clear |
AM-Delay= < 1cm/hr RC-Delay= <1.25cm/hr |
AM-6mU/min increasing 6mU every 15min to max of 36mU/min RC-1mU/min increasing by 1mU/min every 30-40min |
One to two care in both groups |
| Sadler 2000 | AM-amniotomy offered on diagnosis of labour RC-variable |
AM - 2hrly vaginal exam. Delay= < 1cm/hr cervical dilatation RC-variable |
AM-6mU/min increasing by 6mU per min up to max of 36mU/min RC-1mU/min doubling every 10 min to 8mU/min then increase of 2mU/min to 40mU/min |
One to one care in both groups |
| Somprasit 2005 | AM - routine within 1hr admission RC-variable/not clear |
AM-Delay= <1cm/hr RC-variable/not clear |
AM-6mU/min increasing by 2mU/min every 30 min to max of 40mU/min RC-variable/not clear |
Both groups -aimed for 1:1 but actually 1:2 |
| Tabowei 2003 | AM - routinely at labour diagnosis RC-when indicated/variable |
AM - Delay <1cm/hr | AM-6mU/min increasing by 6mU per min up to max of 36mU/min RC-2mU/min increasing 2mU every 30min to 36mU |
AM - one to one in separate ward RC-not clear |
AM: active management group
cm: centimetre
hr: hour
hrly: hourly
IV: intravenous
max: maximum
min: minutes
mU: milli-unit
RC: routine care group
The comparison groups had more variable care. Routine care depended on the care setting, local labour ward management protocols, and the variable practice of clinicians. Routine amniotomy was generally not performed unless delay was identified. The criteria for identifying delay was less stringent and, with the exception of the study by Cammu 1996, when delay was identified, oxytocin was administered at lower doses than in the actively managed groups (e.g. 1 mU per minute increasing every 30 minutes unless contraindicated).
When interpreting the results of this review it is important to bear in mind the considerable variability in care offered to women in both the intervention and comparison groups across the studies. Table 1 shows the variation in management protocols for women in the two study groups in the different studies. Table 2 shows the rate of interventions in women in the active management groups compared to those receiving routine care in the included studies. Similar facilities were available to both intervention and comparison group members in terms of access to pain relief and electronic fetal monitoring. The availability of epidural analgesia and more sophisticated monitoring devices depended on the resources available in the study setting rather than on study group allocation.
Table 2. Active Management and Routine Care - Interventions in labour.
| Study | Amniotomy-performed n (%) | Oxytocin-augmentation n (%) |
|---|---|---|
| Cammu 1996 | AM-86 (91%) RC-56 (57%)* *In first stage (not clear for later in labour) |
AM-80 (53%) RC-41 (27%) |
| Frigoletto 1995 | Not clear AM-61% RC - 51% |
Not clear AM-70% RC-56% |
| Lopez-Zeno 1992 | Not clear | AM-250 (71.2%) RC-234 (66.1%) |
| Rogers 1997 | Not clear - spontaneous rupture of membranes: AM-28 (14%) RC-41 (20%) |
AM-112 (56%) RC-105 (51%) |
| Sadler 2000 | AM - 231 (72%) RC-209 (63%) |
AM-168 (53%) RC -129 (39%) |
| Somprasit 2005 | Not clear | AM - 178 (55.6%) RC-305 (47.7%) |
| Tabowei 2003 | AM - 171 (77%) RC-114 (50%) |
AM - 130 (59%) RC-89 (39%) |
AM: active management group
RC: routine care group
See the ‘Characteristics of included studies’ tables for more information on the packages of care offered to study participants.
All of the studies included caesarean section (CS) rates and duration of labour as pre-specified study outcomes. Other outcomes included assisted delivery rates, uptake of interventions, and adverse maternal and fetal events. Only one study (Sadler 2000) included maternal satisfaction with care in labour as a study outcome.
Excluded studies
Ten studies identified by the search strategy were excluded. The reasons for exclusions are set out in the Excluded studies table. Some studies were excluded because they did not focus on the review intervention (package of care) or participants (low-risk women before the identification of delay). Several exclusions were made for the following reasons; papers were reviews rather than reports of clinical trials; results were not reported by randomised group allocation; or the study methods used did not result in random group allocation (e.g. alternate allocation).
One study is awaiting assessment of eligibility (Abo 2001). We were concerned that this study duplicated data from an earlier study by a different research team included in this review (Cammu 1996); this is being investigated.
Risk of bias in included studies
Overall, the quality of included studies was variable.
Allocation
Studies where group allocation was not random were excluded. The random sequence generation was mainly adequate in the included studies (e.g. computer-generated random numbers) but in two studies the method was not clear (Cammu 1996; Lopez-Zeno 1992). Allocation concealment was judged to be adequate in all of the studies.
Blinding
In studies comparing different styles of care it is not feasible to blind women, clinicians or those assessing outcomes to group allocation. In some studies group allocation was indicated by stickers on notes. We judged that blinding was not feasible, and thus inadequate, for all included studies. It is important to recognise that the lack of blinding is likely to introduce bias, and this must be kept in mind when interpreting the results of the review.
Incomplete outcome data
The levels of loss to follow up and exclusions were low in all but two of the studies (Frigoletto 1995; Sadler 2000).
The study by Frigoletto 1995 randomised women in the third trimester. Between randomisation and the onset of labour more than a third of the original sample became ineligible. The reasons for post randomisation exclusions were justified by the study authors, and appeared balanced between intervention and comparison groups. However, the exclusion of women who became ineligible after randomisation is a serious design flaw, and this level of exclusion is likely to introduce bias. For this study, it was only possible to include post-randomisation exclusions in an intention-to-treat analysis for the main review outcome - CS see Data and analyses comparison 1.1 CS rates- all women).
In the New Zealand study described by Sadler 2000 there was a large number of exclusions between recruitment to the study and onset of labour with 46% of those recruited in the third trimester no longer eligible by the time they went into labour. Those excluded before labour were reported as being different in a number of respects from those remaining eligible (e.g. eligible women that remained in the study tended to be younger and less likely to be of European origin). In this study however, the exclusions were done before randomisation which took place at the onset of labour and data were available for most of those women randomised. Therefore there is low risk of bias from this study for outcomes measured in labour. However, results from this study included data from maternal questionnaires collected at 6 weeks post-partum and for outcomes measured at this time (including maternal satisfaction and breast feeding) there was considerable attrition. Overall response rates were 72% (28% attrition). Further, there were differences in the response rates for the active management (76% responded) and the control group (68% responded). The high level of attrition overall, and the different response rates in the two arms of the trial, are likely to introduce bias for outcomes measured in the postnatal period.
In the remaining five studies randomisation took place at the onset of labour and attrition rates were relatively low.
Selective reporting
All of the studies reported findings for the main outcomes and included statistically non-significant results. Several studies included results on neonatal and maternal outcomes which had not been pre-specified and these studies may have been under-powered to detect differences between groups for these additional outcomes.
Other potential sources of bias
There was little information in some studies about the numbers of eligible women who were approached but, for whatever reasons, had not been recruited to the trial.
In some studies the recruitment period was long; more than three years in the studies by Rogers 1997 and Sadler 2000. In such cases the changing social and medical context could affect the treatment effect and the incidence of outcomes. The difference between the groups could also be affected by other aspects of care. For example, the changing clinical context led to a change in study protocol part-way through one study (Frigoletto 1995).
Details of group allocation methods, attrition etc in the individual included studies are set out in the ‘Characteristics of included studies’ risk of bias tables.
Effects of interventions
Active management versus routine care (seven studies including 5390 women)
Primary outcomes
Caesarean section
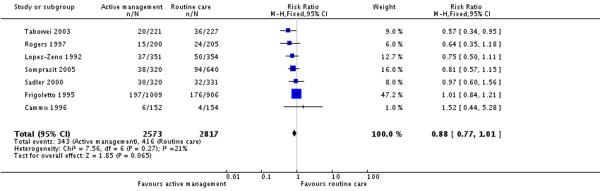
All seven included studies (5390 participants) reported on CS as an outcome. Women randomised to active management in labour group were less likely to have CS, but this did not reach statistical significance (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.77 to 1.01). The overall (unweighted) CS rate in the routine care group was 14.8%.
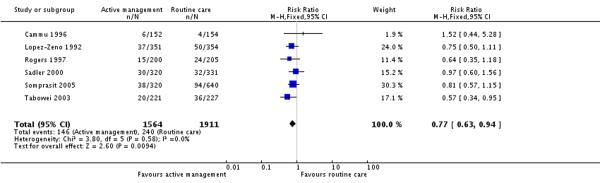
One large study randomised women at 30 weeks, and approximately a third of the women were excluded (in both intervention and control groups) before the onset of labour (Frigoletto 1995). Due to the high number of post randomisation exclusions, analysis was repeated excluding the Frigoletto 1995 study to examine the effect of this study (Analysis 1.2). In this analysis of six studies (3475 participants), there was a reduction in the CS rate for women in the actively managed group compared with the routine care group and this result was statistically significant (RR 0.77, 95% CI 0.63 to 0.94). The overall (unweighted) CS rate in the routine care group was 12.3%.
Maternal satisfaction
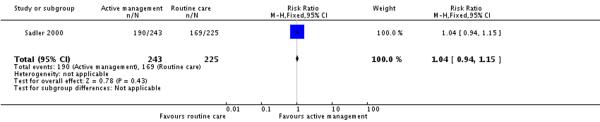
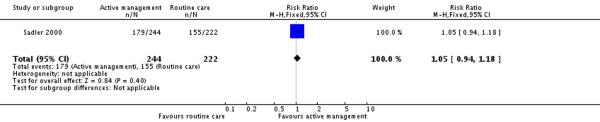
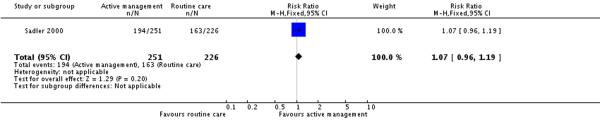
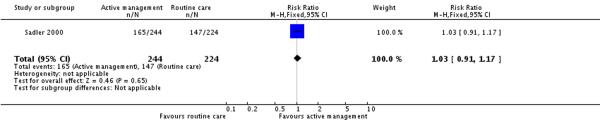
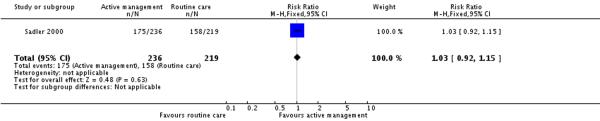
Only one of the included studies provided information on maternal satisfaction with care in labour (Sadler 2000) and as we have noted earlier, these results need to be interpreted with caution as there were high levels of attrition for this outcome, and differences in response rates for women in the intervention and control groups. In this study (477 participants at postnatal follow up), the majority of women had positive views about their care. A high proportion of women in the routine care group (75%) reported that they were very satisfied with their care overall. Women in both the active management group and those in the routine care group had reported similar levels of satisfaction (RR 1.04, 95% CI 0.94 to 1.15)(Analysis 2.1). Similarly, high levels of satisfaction were reported for other measures of satisfaction, and none of the differences between groups reached statistical significance. Most women (at least two thirds) in both groups reported that they would choose the same style of care again, had a strong sense of achievement, had adequate pain relief and thought that the staff caring for them explained what was happening.
Secondary outcomes
Duration of labour
Women in the active management (intervention) group tended to have shorter labours than those receiving routine care. However, there was considerable variation reported in the duration of labour both within and between studies. In view of the heterogeneity between trials, we used random-effects meta-analyses for all of these outcomes. Further, in three studies it was not clear whether women who had CS were excluded from analyses relating to length of labour (Cammu 1996; Lopez-Zeno 1992; Tabowei 2003). Thus, the results relating to the length of labour should be interpreted with caution.
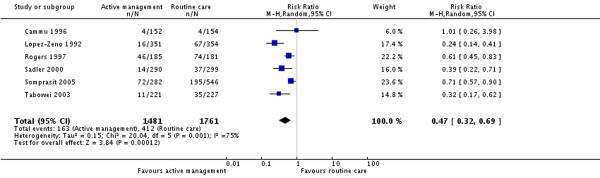
Six studies (3242 participants) reported on prolonged labour (labour lasting more than 12 hours) as an outcome. There was a lower proportion of women with prolonged labour in the actively managed group compared to the routine care group (Analysis 3.1) (RR 0.47, 95% CI 0.32 to 0.69). The overall rate of prolonged labour in the routine care group (unweighted) was 23.4%.
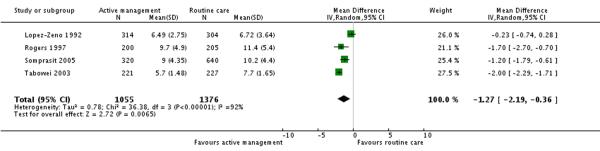
Four studies (2431 participants) reported length of labour as an outcome. Length of labour from admission to delivery was statistically significantly shorter in the active management group compared to the routine care group (mean difference (MD) −1.27 hours, 95% CI −2.19 to −0.36) (Analysis 3.2).
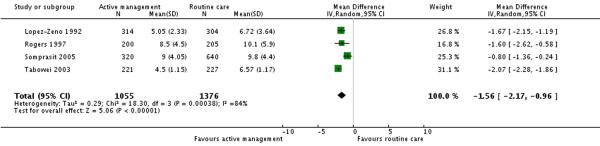
Four studies (2431 participants) reported duration of first stage of labour as an outcome. This was shorter in the active management group compared to the routine care group (MD −1.56 hours 95% CI −2.17 to −0.96)(Analysis 3.3).
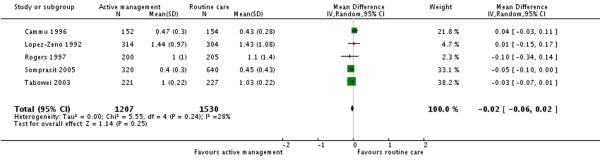
Five studies (2737 participants) reported on the duration of second stage of labour. There was no significant difference in this outcome between the two groups (MD −0.02 hours 95% CI −0.06 to 0.02) (Analysis 3.4).
Analgesia
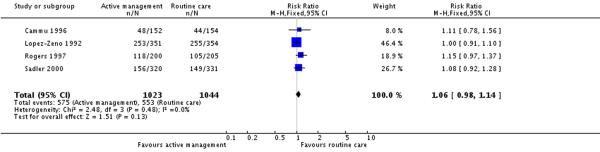
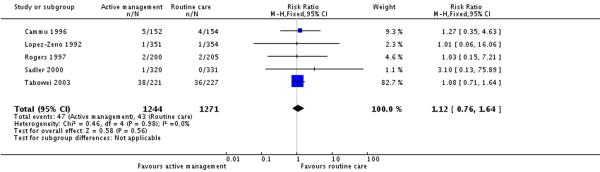
Four studies (2067 participants) reported on use of epidural analgesia. There were no statistically significant differences in the proportion of women receiving epidural analgesia between the intervention and control groups (RR 1.06, 95% CI 0.98 to 1.14) (Analysis 4.1).
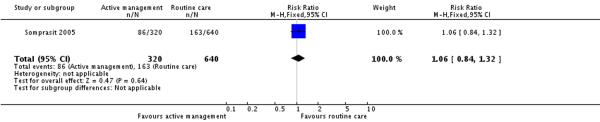
In one study (Somprasit 2005)(where epidural analgesia was not available) information was provided about other analgesia. In this study (960 participants) the proportions of women receiving meperidine (pethidine) were similar in the actively and routinely managed groups (RR 1.06, 95% CI 0.84 to1.32) (Analysis 5.1). In this study 25.4% of women in the routine care group received meperidine.
Assisted vaginal deliveries
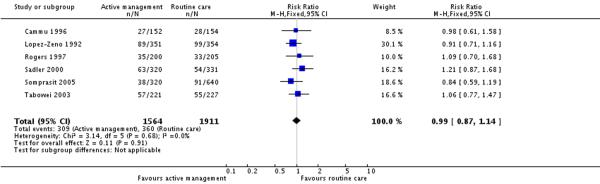
Six studies (3475 participants) reported on this outcome.There was no statistically significant difference in the rate of assisted vaginal deliveries between the active management and routine care groups (RR 0.99, 95% CI 0.87 to 1.14). The overall rate of assisted vaginal deliveries in the routine care group (unweighted) was 18.8% (Analysis 6.1).
Neonatal outcomes
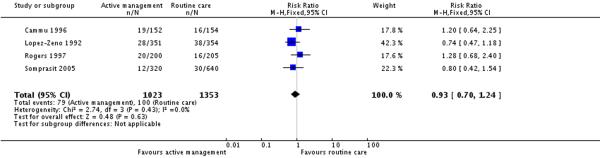
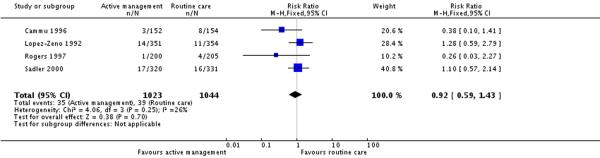
Six studies reported on neonatal outcomes such as low Apgar score at five minutes, meconium staining and admission to special care baby units. There were no statistically significant differences between groups for any of these outcomes (Analysis 7.1; Analysis 7.2; Analysis 7.3).
Maternal complications
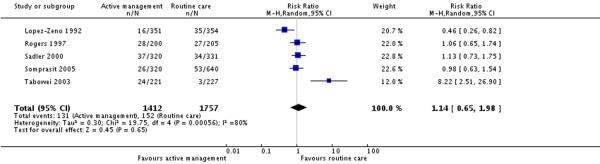
Five studies (3169 participants) reported on maternal infection rates.There was no statistically significant difference between groups in terms of rates of maternal infection (RR 1.14, 95% CI 0.65 to 1.98)(Analysis 8.1). The overall maternal infection rate in the routine care group (unweighted) was 8.6%.
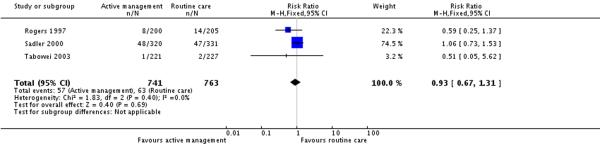
Three studies (1504 participants) reported on postpartum haemorrhage (blood loss greater than 500 ml) rates. There was no statistically significant difference between groups in terms of rates of postpartum haemorrhage (RR 0.93, 95% CI 0.67 to 1.31)(Analysis 8.2). The overall postpartum haemorrhage rate in the routine care group (unweighted) was 8.2%.
Breastfeeding
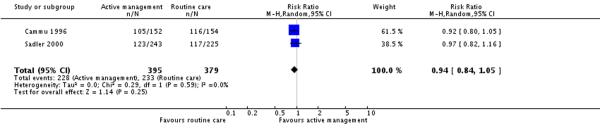
Two studies (774 participants) reported on this outcome. In the Sadler 2000 study this outcome was measured at 6 weeks postpartum, in the Cammu 1996 study it was not clear when this outcome was measured. There was no difference in breastfeeding rates between women in the actively managed versus routine care groups (RR 0.94, 95% CI 0.84 to 1.05)(Analysis 9.1). The overall breastfeeding rate in the routine care group (unweighted) was 48%.
DISCUSSION
Overall, in these studies active management was associated with a small reduction in CS rates and a shorter length of labour (<12 hours) but had little impact on most other outcomes. Compared to routine care, active management of labour did not have an effect in either direction on morbidity for mothers or babies.There was no difference in maternal satisfaction between the two groups but only one trial evaluated this outcome (Sadler 2000). This trial was set in Australia, and it is unclear if these findings are generalisable to other settings. Furthermore there was high loss to follow up rate in this study which could introduce bias into the results.
Interpretation of the results from included studies is not simple; first, there was variation between studies in what was included within an active management package; second, there was considerable variation in the care received by women in the comparison groups within and between studies; and third, for some outcomes e.g. duration of labour, there was considerable heterogeneity in study findings.
The primary outcome in this review was delivery by CS. The study by Frigoletto 1995 had a serious design flaw that resulted in a large number of women who were already randomised to receive either active management or routine care becoming ineligible for the study at a later date. Although data were provided such that the analysis for CS could be carried out for all randomised women, such a large rate of exclusions meant that a large number of women in the active management group would not have received the intervention, which therefore introduces a bias towards the null in the results. Consequently, we repeated the analysis excluding this study to investigate its effect on the results. Having excluded this study, the CS rate in the active management group was statistically significantly lower compared to the usual care group (RR 0.77 95% CI 0.63 to 0.94). In this analysis, the overall CS rate in the control group was 12% which is the same rate as that recorded for women in England and Wales who had a CS after going into labour (Paranjothy 2005).
Active management is associated with modest reductions in the CS rate. With interventions to combat rising CS rates being highlighted as a priority in many countries, even modest reductions may have a public health impact. The recent NICE guidance on intrapartum care does not recommend the routine use of active management of labour for practice within the UK context (NICE 2007). However, their analysis included only four (Frigoletto 1995; Rogers 1997; Sadler 2000; Tabowei 2003) of the seven trials that we included. Furthermore the inclusion of the Frigoletto 1995 trial, may not be appropriate as the high rate of post randomisation exclusions within the trial introduces bias into the results towards the null, as we have demonstrated in the resuts of this review. Some individual components of active management are effective in reducing the CS rate, such as continuous support for women in labour (RR 0.91 95%CI 0.83-0.99) (Hodnett 2003). Our review aimed to investigate the effect on CS rates from a policy of active management and our findings suggest that active management is associated with modest reductions in the CS rate (RR 0.77 95%CI 0.63 to 0.94). However, further research is required to investigate women’s satsfaction with the use of active management of labour.
AUTHORS’ CONCLUSIONS
Implications for practice
The evidence base so far suggests that the use of a policy of a package of interventions (active management of labour) is likely to result in modest reductions in the CS rate. The benefits of small reductions in the CS rate should be balanced against the risk of increasing interventions in the management of low-risk pregnancies and further evaluation would be needed to investigate this. Further work is also required to determine the acceptability of, and women’s satisfaction with, such policies.
Implications for research
While studies collected information on adverse events most did not have the power to detect differences between groups. There is little information on maternal satisfaction in different settings and future studies should evaluate this.
PLAIN LANGUAGE SUMMARY.
A package of care to actively manage labour in women who are at low risk of complications to reduce caesarean section rates
Many countries have an increasing rate of caesarean section. Higher rates do not always give additional health gains, they can increase maternal risks and affect subsequent pregnancies. Active management of labour has been proposed to reduce the number of caesarean births. Active management includes routine amniotomy (artificial rupture of the membranes), strict rules for diagnosing slow progress, use of the intravenous drug oxytocin to increase contractions of the uterus and one-to-one care. The disadvantages of active management are that it can possibly lead to more invasive monitoring, more interventions and a more medicalised birth in which women have less control and less satisfaction. The review included seven trials involving 5390 women. These studies show that women who received active management were slightly less likely to have a caesarean section and were more likely to have shorter labours (less than 12 hours). There was no difference in the number of assisted deliveries, nor was there any difference in complications for mothers or their babies when comparing women in the active management group with those receiving routine care.
ACKNOWLEDGEMENTS
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
National Institute for Health Research (NIHR), UK.
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT. | |
| Participants | Study in hospital serving urban middle-class women. Participants: 306 women. Inclusion criteria: nulliparous women in spontaneous labour at or over 37 weeks’ gestation, singleton fetus, cephalic presentation, normal cardiotocogram and clear amniotic fluid, maternal height >150 cm, attended at least 1 antenatal appointment |
|
| Interventions | Active management group: amniotomy within 1 hr of admission; hourly vaginal assessments of progress; oxytocin for delayed progress (< 1 cm cervical dilatation per hour) - incremental doses up to 30 mU per min after 120 mins unless contra-indicated Comparison group: selective management, amniotomy only after arrest of dilatation, oxytocin if 2 hrs behind schedule (at same dose as intervention group) One-to-one care and electronic fetal monitoring in both groups |
|
| Outcomes | Caesarean section rates, assisted vaginal delivery rates, duration of labour | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information provided. |
| Allocation concealment? | Yes | Numbered, sealed, opaque envelopes. |
| Blinding? Women |
No | Different treatment regimes compared. |
| Blinding? Clinicians |
No | Group allocation indicated on notes. |
| Blinding? Outcome assessor |
No | Group allocation indicated on notes. |
| Incomplete outcome data addressed? All outcomes |
Yes | Small number of post-randomisation exclusions (2% attrition) |
| Free of selective reporting? | Yes | Main outcomes reported. |
| Free of other bias? | Unclear | Unclear how many women were eligible. |
| Methods | RCT (stratified by site with block randomisation). | |
| Participants | Boston, USA. Participants: 1915 women. Inclusion criteria: Nulliparous, English-speaking women 18 years of age or over Exclusion criteria: women at increased risk of caesarean delivery (eg cervical incompetence, diabetes, pregnancy-induced hypertension) Women randomised at 30 weeks - only eligible for study protocol if spontaneous onset of labour, at term, vertex presentation, singleton fetus, without medical or obstetric complications |
|
| Interventions | Active management group: attended special childbirth classes; 1:1 care in separate unit, standard criteria for labour diagnosis, amniotomy within 1 hr of labour diagnosis, cervical assessments every 2 hrs, oxytocin for delayed progress 4mU per min increasing every 15 mins to a maximum dose of 40 mU unless contraindicated Usual care group: payment to attend childbirth education of choice, no protocol, amniotomy unusual Fetal monitoring and access to analgesia in both groups. |
|
| Outcomes | Ceasarean section rate, assisted vaginal delivery rates, duration of labour, complications and neonate adverse events | |
| Notes | Randomisation at 30 weeks, therefore large numbers of women became ineligible between randomisation and the onset of labour. Attrition between randomisation and labour was similar (in numbers and reasons) in both study groups | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random numbers in blocks. |
| Allocation concealment? | Yes | Randomisation by coordinating centre or sealed opaque, sequentially numbered envelopes |
| Blinding? Women |
No | Different intervention packages. |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
No | Large number of post randomisation exclusions, outcome data for exclusions were only given for CS but not for the other outcomes. (Attrition rates: less than 1% for the CS outcome, 35% attrition between randomisation and labour, this study has been excluded for these other outcomes) |
| Free of selective reporting? | Unclear | Only main outcome reported for whole randomised sample. |
| Free of other bias? | Unclear | Change in protocol during study. |
| Methods | RCT (permuted block design). | |
| Participants | Chicago hospital, USA. Participants: 705 women. Inclusion criteria: nulliparous women admitted in spontaneous labour = to or > 37 weeks’ gestation Exclusion criteria: multiple pregnancy, non-cephalic presentation, previous uterine surgery, amniotomy or oxytocin before labour diagnosis |
|
| Interventions | Active management group: amniotomy within 1 hr of labour diagnosis, vaginal examinations hourly for 3 hrs and then every 2 hrs, oxytocin augmentation for delay (cervical dilatation < 1 cm per hr) 6 mU per min increasing by 6 mU per min every 15 mins to maximum of 36 mU unless contraindicated Traditional management group: care varied at the discretion of the attending obstetrician, oxytocin augmentation when used at a lower dose than the actively managed group Electronic fetal monitoring, cord blood gas analysis at delivery and monitoring of uterine efficiency and pressure in both groups |
|
| Outcomes | Dystocia and caesarean section rates. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Permuted block design, but it was not clear whether block size was random |
| Allocation concealment? | Yes | Sequentially numbered, opaque, sealed envelopes. |
| Blinding? Women |
No | Different intervention packages. |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | Small number of post-randomisation exclusions (2% attrition) |
| Free of selective reporting? | Yes | Main outcomes reported. |
| Free of other bias? | Yes | |
| Methods | RCT. | |
| Participants | New Mexico, USA hospital serving hispanic, white and native Americans Participants: 405 women. Inclusion criteria: nulliparous women at term who had attended for antenatal care, cephalic presentation, no known medical or obsteric complications or fetal abnormality Exclusion criteria: multiple pregnancies, obstetric complications, previous uterine surgery |
|
| Interventions | Active management group: strict diagnosis of labour, amniotomy within two hrs of admission, oxytocin for delay (< 1 cm cervical dilatation in the 1st stage or < 1 cm descent in 2nd stage) 6 mU per min increased by 6 mU every 15 mins to max of 36 mU per min unless contraindicated, vaginal examinations every 2 hrs Control group: less strict labour diagnosis and delay, oxytocin at lower doses (1 mU per min increased every 30-40 mins), amniotomy at the discretion of attending clinician Continuous fetal monitoring and 1:2 care in both groups. |
|
| Outcomes | Caesarean section rate and length of labour. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated list of random numbers. |
| Allocation concealment? | Yes | Sealed, opaque envelopes. |
| Blinding? Women |
No | |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | Low numbers of post randomisation exclusions (less than 1% attrition) |
| Free of selective reporting? | Yes | |
| Free of other bias? | Unclear | 4 year recruitment period and not clear how many eligible women participated |
| Methods | RCT. | |
| Participants | Women attending large New Zealand hospital recruited in 3rd trimester Participants: 651 women. Inclusion criteria (to be eligible for protocol): nulliparous women, singleton pregnancy, cephalic presentation in sponateous labour Exclusion criteria: cardiac disease, uterine scar, contracted pelvis, fetal distress on admission |
|
| Interventions | Active management group: amniotomy offered at diagnosis of labour, oxytocin augmentation for delay (< 1 cm cervical dilatation/hr in 1st stage or absence of descent for 30 mins in 2nd stage or contractions < 1 in 5 mins) 6 mU per min increasing by 6 mU every 15 mins to max of 36 mU per min unless contraindicated Routine care group: no written guideline, care at the discretion of the attending clinician. Where given, oxytocin at lower dose (1 mU per min doubled every 10 mins to 8 mU per min then increasing by 2 mU to max of 40 mU per minute) Both groups had 1: 1 care and same antenatal care |
|
| Outcomes | Ceasarean section and operative delivery rates, prolonged labour, maternal and neonate complications and maternal satisfaction with care | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated random numbers. |
| Allocation concealment? | Yes | Opaque sealed envelopes. |
| Blinding? Women |
No | |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
No | Low levels of attrition for labour outcomes, but for postnatal outcomes (breastfeeding and maternal satisfaction) attrition rates were 28% and attrition was greater in the control group (32%) than in the intervention group (24%) |
| Free of selective reporting? | Yes | |
| Free of other bias? | Unclear | Not clear how many women were eligible for the study. Some missing data for some outcomes (eg maternal satisfaction questionnaire) |
| Methods | RCT. | |
| Participants | Teaching hospital in Thailand. Participants: 960 women. Inclusion criteria: nulliparous women in spontaneous labour with single, term fetus with no evidence of fetal distress. No known medical or surgical complications Exclusion criteria: vaginal delivery or oxytocin augmentation contraindicated, diatetes, cervical incompetence or pregnancy-induced hypertension |
|
| Interventions | Active management group: strict criteria for labour diagnosis, amniotomy within 1 hr of admission, 2 hrly vaginal examinations, oxytocin augmentation for delay (< 1 cm cervical dilatation per hr) 6 mU, increasing by 2 mU every 30 mins to max 40 mU per min) Conventional management group: variable care depending on clinician Intermittent fetal auscultation and 1:2 care both groups. Epidural not available to either group. |
|
| Outcomes | Caesarean section rate, duration of labour. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Numbered, opaque, sealed envelopes. |
| Blinding? Women |
No | |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | Some post-randomisation exclusions but analysis by intention to treat (1.5% attrition) |
| Free of selective reporting? | Yes | Reported on main outcomes. |
| Free of other bias? | Unclear | No clear description of the mangement of the comparison group |
| Methods | RCT. | |
| Participants | District hospital, Nigeria. Participants: 448 women. Inclusion criteria: nulliparous women with singleton, cephalic, term pregnancies with no contraindications for vaginal delivery or medical complications |
|
| Interventions | Active management group: antenatal classes to reduce anxiety, diagnosis of labour, 1:1 care in separate area, 2 hourly vaginal examinations, delayed progress (< 1 cm hr in 1st stage or no descent for 1 hr in 2nd stage) led to high-dose oxytocin augmentation (6 mU per min increasing by 6 mU every 15 mins to maximum of 36 mU unless contraindicated) Usual care group: amniotomy only when indicated, low-dose oxytocin for failure to progress (2 mU per min increasing by 2 mU every 30 mins to maximum of 36 mU) |
|
| Outcomes | Caesarean section rate. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated random numbers. |
| Allocation concealment? | Yes | Opaque sealed envelopes. |
| Blinding? Women |
No | Different treatment packages. |
| Blinding? Clinicians |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Unclear | Some post-randomisation exclusions (11.7% attrition). |
| Free of selective reporting? | Yes | |
| Free of other bias? | Unclear | Number of women eligible for recruitment not stated. |
cm: centimetre
hr: hour
min: minutes
mU: milli-unit
RCT: randomised controlled trials
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cohen 1987 | Study only included women already diagnosed with poor progress in labour |
| Hinshaw 1995 | The intervention was not a package of care. A single intervention was compared between groups |
| Hogston 1993 | Not RCT. |
| Jyoti 2006 | Not RCT (alternate allocation). |
| Lieberman 2000 | Subgroup analysis not according to random group allocation. |
| Pattinson 2003 | The intervention tested was not a package of care during labour. The control and intervention groups differed only in terms of the type of partogram used and frequency of associated vaginal examinations. Neither group had any other component of an active management of labour package such as continuous support during labour, higher dose of oxytocin or early amniotomy |
| Peaceman 1993a | Not RCT - review of other trials. |
| Serman 1995 | Not RCT. Group allocation by patient file number. |
| Treisser 1981 | Not RCT - study focusing on dystocia. |
| Wood 1974 | Intervention limited to the second stage of labour. |
RCT: randomised controlled trials
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | This study is currently being investigated. Methods and findings were very similar to those reported in an included study (Cammu 1996). |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
DATA AND ANALYSES
Comparison 1. Active management versus routine care: caesarean section rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section rate - all women | 7 | 5390 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 2 Caesarean section rate (Sensitivity analysis: Frigoletto (1995) study excluded) | 6 | 3475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
Comparison 2. Active management versus routine care: maternal satisfaction with care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall satisfaction with care | 1 | 468 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 2 Maternal views - would choose this style of care again | 1 | 466 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 3 Maternal views - mother feels strong sense of achievement | 1 | 477 | Risk Ratio (M-H, Fixed, 95% CI) | 1.07 [0.96, 1.19] |
| 4 Maternal views - staff always explained enough | 1 | 468 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 5 Maternal views - pain relief was adequate | 1 | 455 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
Comparison 3. Active mangement versus routine care: duration of labour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged labour (> 12 hours) | 6 | 3242 | Risk Ratio (M-H, Random, 95% CI) | 0.47 [0.32, 0.69] |
| 2 Length of labour (admission to delivery) | 4 | 2431 | Mean Difference (IV, Random, 95% CI) | −1.27 [−2.19, −0.36] |
| 3 Duration of first stage of labour (hours) | 4 | 2431 | Mean Difference (IV, Random, 95% CI) | −1.56 [−2.17, −0.96] |
| 4 Duration of second stage (hours) | 5 | 2737 | Mean Difference (IV, Random, 95% CI) | −0.02 [−0.06, 0.02] |
Comparison 4. Active management versus routine care: epidural analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women having epidural analgesia | 4 | 2067 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
Comparison 5. Active management versus routine care: use of analgesia (other than epidural).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of analgesia (other than epidural) | 1 | 960 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.84, 1.32] |
Comparison 6. Active management versus routine care: assisted vaginal deliveries.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assisted vaginal delivery rates | 6 | 3475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.87, 1.14] |
Comparison 7. Active management versus routine care: neonatal outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low APGAR score at 5 minutes | 5 | 2515 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.76, 1.64] |
| 2 Meconium staining | 4 | 2376 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.70, 1.24] |
| 3 Admission to special care (various definitions) | 4 | 2067 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.59, 1.43] |
Comparison 8. Active management versus routine care: maternal complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infection (various definitions) | 5 | 3169 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.65, 1.98] |
| 2 Postpartum haemorrhage (blood loss > 500 ml) | 3 | 1504 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.67, 1.31] |
Comparison 9. Active management versus routine care: breastfeeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breastfeeding (various measurement points) | 2 | 774 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.84, 1.05] |
Analysis 1.1. Comparison 1 Active management versus routine care: caesarean section rate, Outcome 1 Caesarean section rate - all women.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 1 Active management versus routine care: caesarean section rate
Outcome: 1 Caesarean section rate - all women
 |
Analysis 1.2. Comparison 1 Active management versus routine care: caesarean section rate, Outcome 2 Caesarean section rate (Sensitivity analysis: Frigoletto (1995) study excluded).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 1 Active management versus routine care: caesarean section rate
Outcome: 2 Caesarean section rate (Sensitivity analysis: Frigoletto (1995) study excluded)
 |
Analysis 2.1. Comparison 2 Active management versus routine care: maternal satisfaction with care, Outcome 1 Overall satisfaction with care.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 2 Active management versus routine care: maternal satisfaction with care
Outcome: 1 Overall satisfaction with care
 |
Analysis 2.2. Comparison 2 Active management versus routine care: maternal satisfaction with care, Outcome 2 Maternal views - would choose this style of care again.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 2 Active management versus routine care: maternal satisfaction with care
Outcome: 2 Maternal views - would choose this style of care again
 |
Analysis 2.3. Comparison 2 Active management versus routine care: maternal satisfaction with care, Outcome 3 Maternal views - mother feels strong sense of achievement.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 2 Active management versus routine care: maternal satisfaction with care
Outcome: 3 Maternal views - mother feels strong sense of achievement
 |
Analysis 2.4. Comparison 2 Active management versus routine care: maternal satisfaction with care, Outcome 4 Maternal views - staff always explained enough.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 2 Active management versus routine care: maternal satisfaction with care
Outcome: 4 Maternal views - staff always explained enough
 |
Analysis 2.5. Comparison 2 Active management versus routine care: maternal satisfaction with care, Outcome 5 Maternal views - pain relief was adequate.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 2 Active management versus routine care: maternal satisfaction with care
Outcome: 5 Maternal views - pain relief was adequate
 |
Analysis 3.1. Comparison 3 Active mangement versus routine care: duration of labour, Outcome 1 Prolonged labour (> 12 hours).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 3 Active mangement versus routine care: duration of labour
Outcome: 1 Prolonged labour (> 12 hours)
 |
Analysis 3.2. Comparison 3 Active mangement versus routine care: duration of labour, Outcome 2 Length of labour (admission to delivery).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 3 Active mangement versus routine care: duration of labour
Outcome: 2 Length of labour (admission to delivery)
 |
Analysis 3.3. Comparison 3 Active mangement versus routine care: duration of labour, Outcome 3 Duration of first stage of labour (hours).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 3 Active mangement versus routine care: duration of labour
Outcome: 3 Duration of first stage of labour (hours)
 |
Analysis 3.4. Comparison 3 Active mangement versus routine care: duration of labour, Outcome 4 Duration of second stage (hours).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 3 Active m angem ent versus routine care: duration of labour
Outcome: 4 Duration of second stage (hours)
 |
Analysis 4.1. Comparison 4 Active management versus routine care: epidural analgesia, Outcome 1 Number of women having epidural analgesia.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 4 Active m anagem ent versus routine care: epidural analgesia
Outcome: 1 Number of women having epidural analgesia
 |
Analysis 5.1. Comparison 5 Active management versus routine care: use of analgesia (other than epidural), Outcome 1 Use of analgesia (other than epidural).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 5 Active management versus routine care: use of analgesia (other than epidural)
Outcome: 1 Use of analgesia (other than epidural)
 |
Analysis 6.1. Comparison 6 Active management versus routine care: assisted vaginal deliveries, Outcome 1 Assisted vaginal delivery rates.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 6 Active m anagem ent versus routine care: assisted vaginal deliveries
Outcome: 1 Assisted vaginal delivery rates
 |
Analysis 7.1. Comparison 7 Active management versus routine care: neonatal outcome, Outcome 1 Low APGAR score at 5 minutes.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 7 Active m anagem ent versus routine care: neonatal outcome
Outcome: 1 Low APCAR score at 5 minutes
 |
Analysis 7.2. Comparison 7 Active management versus routine care: neonatal outcome, Outcome 2 Meconium staining.
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 7 Active management versus routine care: neonatal outcome
Outcome: 2 Meconium staining
 |
Analysis 7.3. Comparison 7 Active management versus routine care: neonatal outcome, Outcome 3 Admission to special care (various definitions).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 7 Active m anagem ent versus routine care: neonatal outcome
Outcome: 3 Admission to special care (various definitions)
 |
Analysis 8.1. Comparison 8 Active management versus routine care: maternal complications, Outcome 1 Maternal infection (various definitions).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 8 Active m anagem ent versus routine care: maternal complications
Outcome: 1 Maternal infection (various definitions)
 |
Analysis 8.2. Comparison 8 Active management versus routine care: maternal complications, Outcome 2 Postpartum haemorrhage (blood loss > 500 ml).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 8 Active management versus routine care: maternal complications
Outcome: 2 Postpartum haemorrhage (blood loss > 500 ml)
 |
Analysis 9.1. Comparison 9 Active management versus routine care: breastfeeding, Outcome 1 Breastfeeding (various measurement points).
Review: Package of care for active management in labour for reducing caesarean section rates in low-risk worn en
Comparison: 9 Active management versus routine care: breastfeeding
Outcome: 1 Breastfeeding (various measurement points)
 |
Appendix 1. Search strategy
MEDLINE
exp Labor, Obstetric/or labour OR labor OR intrapartum OR parturition
active management OR partogra* OR amniotomy OR oxytocin OR Oxytocin/OR augmentation OR support OR companion*
exp Cesarean Section/OR caesarean OR CS OR cesarean OR vaginal birth OR vaginal delivery OR assisted delivery OR length of labour OR duration of labour OR prolonged labour OR analgesia OR death OR Mortality/OR mortality OR pH OR APGAR OR satisfaction OR breastfeeding OR Breast Feeding/OR intensive care OR high care OR high dependency OR ICU OR ITU OR HDU
#1 AND #2 AND #3
WHAT’S NEW
Last assessed as up-to-date: 28 February 2008.
| Date | Event | Description |
|---|---|---|
| 12 May 2009 | Amended | Description of sequence generation assessment added to Risk of bias table for Lopez-Zeno 1992. |
HISTORY
Protocol first published: Issue 3, 2004
Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 20 December 2007 | Amended | Converted to new review format. |
Footnotes
DECLARATIONS OF INTEREST: None known.
References to studies included in this review
- Cammu 1996 {published data only} .Cammu H, Van Eeckhout E. A randomised controlled trial of early vs delayed use of amniotomy and oxytocin infusion in nulliparous labor. British Journal of Obstetrics and Gynaecology. 1996;103:313–8. doi: 10.1111/j.1471-0528.1996.tb09734.x. [DOI] [PubMed] [Google Scholar]
- Frigoletto 1995 {published data only} .Frigoletto FD, Lieberman E, Lang JM, Cohen A, Barss V, Ringer S, et al. A clinical trial of active management of labor. New England Journal of Medicine. 1995;333:745–50. doi: 10.1056/NEJM199509213331201. [DOI] [PubMed] [Google Scholar]
- Lopez-Zeno 1992 {published data only} .Lopez-Zeno JA, Peaceman AM, Adashek JA, Socol ML. A controlled trial of a program for the active management of labor. New England Journal of Medicine. 1992;326:450–4. doi: 10.1056/NEJM199202133260705. [DOI] [PubMed] [Google Scholar]; *
- Lopez-Zeno JA, Peaceman AM, Socol ML. Active management of labor (AMOL) - an evaluation of its efficacy. American Journal of Obstetrics and Gynecology. 1991;164:306. [Google Scholar]
- Rogers 1997 {published data only} .Rogers R, Gilson G, Kammerer-Doak D. Epidural analgesia and active management of labor: effects on length of labor and mode of delivery. Obstetrics & Gynecology. 1999;93(6):995–8. doi: 10.1016/s0029-7844(98)00564-x. [DOI] [PubMed] [Google Scholar]
- Rogers R, Gilson GJ, Miller AC, Izquierdo LE, Curet LB, Qualls CR. Active management of labor: does it make a difference? American Journal of Obstetrics and Gynecology. 1997;177(3):599–605. doi: 10.1016/s0002-9378(97)70152-2. [DOI] [PubMed] [Google Scholar]; *
- Rogers RG, Gardner MO, Tool KJ, Ainsley J, Gilson G. Active management of labor: a cost analysis of a randomized controlled trial. Western Journal of Medicine. 2000;172:240–3. doi: 10.1136/ewjm.172.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler 2000 {published data only} .Sadler LC, Davison T, McCowan LM. A randomised controlled trial and meta-analysis of active management of labour. BJOG: an international journal of obstetrics and gynaecology. 2000;107(7):909–15. doi: 10.1111/j.1471-0528.2000.tb11091.x. [DOI] [PubMed] [Google Scholar]; *
- Sadler LC, Davison T, McCowan LME. Maternal satisfaction with active management of labor: a randomized controlled trial. Birth. 2001;28(4):225–35. doi: 10.1046/j.1523-536x.2001.00225.x. [DOI] [PubMed] [Google Scholar]
- Somprasit 2005 {published data only} .Somprasit C, Tanprasertkul C, Kamudhamas A. Reducing cesarean delivery rates: an active management labor program in a setting with limited resources. Journal of the Medical Association of Thailand. 2005;88(1):20–5. [PubMed] [Google Scholar]
- Tabowei 2003 {published data only} .Tabowei TO, Oboro VO. Active management of labour in a district hospital setting. Journal of Obstetrics and Gynaecology. 2003;23(1):9–12. doi: 10.1080/0144361021000043137. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Cohen 1987 {published data only} .Cohen GR, O’Brien WF, Lewis L, Knuppel RA. A prospective randomized study of the aggressive management of early labor. American Journal of Obstetrics and Gynecology. 1987;157:1174–7. doi: 10.1016/s0002-9378(87)80288-0. [DOI] [PubMed] [Google Scholar]
- Hinshaw 1995 {published data only} .Hinshaw K. [accessed 2005];Oxytocin augmentation in the management of primary dysfunctional labour in nulliparous women - effect on caesarean section rate: a randomised controlled trial. The Research Findings Register. Summary number 1244. Retrieved 11 January 2005, from http://www.ReFeR.nhs.uk/ViewRecord.asp?ID=1244. [Google Scholar]
- Hinshaw K, Moustafa A, Wilson K, Boyd P, Fawzi H, Kumarendran K. Oxytocin augmentation versus conservative management for primary dysfunctional labour in nulliparous women: a randomised controlled trial. 27th British Congress of Obstetrics and Gynaecology; Dublin, Ireland. 1995; Jul 4-7, p. 207. 1995. [Google Scholar]; *
- Hogston 1993 {published data only} .Hogston P, Noble W. Active management of labour - the Portsmouth experience. Journal of Obstetrics and Gynaecology. 1993;13:340–2. [Google Scholar]
- Jyoti 2006 {published data only} .Jyoti M, Singhal P, Choudhary D, Programed labor Journal of Obstetrics and Gynaecology of India. 2006;56(1):53–5. [Google Scholar]
- Lieberman 2000 {published data only} .Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105(1 Pt 1):8–13. doi: 10.1542/peds.105.1.8. [DOI] [PubMed] [Google Scholar]
- Pattinson 2003 {published data only} .Howarth GR, Pattinson RC, Mdluli WPP, Macdonald AP, Funk M, Makin J. Aggressive or expectant management of labour; a randomised controlled trial; 20th Conference on Priorities in Perinatal Care in Southern Africa; KwaZulu-Natal, South Africa. 2001; Mar 6-9, 2001. [Google Scholar]
- Pattinson RC, Howarth GR, Mdluli W, Macdonald AP, Makin JD, Funk M. Aggressive or expectant management of labour: a randomised clinical trial. BJOG: an international journal of obstetrics and gynaecology. 2003;110(5):457–61. [PubMed] [Google Scholar]; *
- Peaceman 1993a {published data only} .Peaceman AM, Lopez-Zeno J, Minogue JP, Socol ML. Factors that influence route of delivery - active vs traditional labor management. American Journal of Obstetrics and Gynecology. 1993;169:940–4. doi: 10.1016/0002-9378(93)90031-d. [DOI] [PubMed] [Google Scholar]
- Serman 1995 {published data only} .Serman VF, Benavides AC, Sandoval ZJ, Pazols JR, Bermedo SJ, Fuenzalida FR, et al. Active management of labour in primiparas Prospective study [Manejo activo del parto en primiparas, estudio prospectivo] Revista Chilena de Obstetricia y Ginecologia. 1995;60:6–11. [PubMed] [Google Scholar]
- Treisser 1981 {published data only} .Treisser A, Breart G, Blum F, Jouhet P, Pigne A, Barrat J. Dystocia at the onset of labour. An evaluation of the different treatments available. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 1981;10:91–8. [PubMed] [Google Scholar]
- Wood 1974 {published data only} .Wood C, Ng KH, Hounslow D, Benning H. A control trial demonstrates that speeding birth favourably affects cord blood Ph. [abstract] Journal of Reproduction and Fertility. 1974;36(2):472–3. doi: 10.1530/jrf.0.0360472. [DOI] [PubMed] [Google Scholar]
- Wood C, Ng KH, Hounslow D, Benning H. Time - an important variable in normal delivery. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1973;80:295–300. doi: 10.1111/j.1471-0528.1973.tb11194.x. [DOI] [PubMed] [Google Scholar]; *
References to studies awaiting assessment
- Abo 2001 {published data only} .Abo Omar A. Active versus more selective management of labor. Journal of the Bahrain Medical Society. 2001;13(3):139–42. [Google Scholar]
Additional references
- Betran 2007 .Betran AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, Wagner M. Rates of caesarean section: analysis of global, regional and national estimates. Paediatric and Perinatal Epidemiology. 2007;21(2):98–113. doi: 10.1111/j.1365-3016.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- Bidgood 1984 .Bidgood KA, Steer PJ. A randomised control study of oxytocin augmentation of labour. 1. Obstetric outcome. British Journal of Obstetrics and Gynaecology. 1987;94(6):512–7. doi: 10.1111/j.1471-0528.1987.tb03142.x. [DOI] [PubMed] [Google Scholar]
- Bugg 2008 .Bugg GJ, Siddiqui F, Thornton JG. Oxytocin versus placebo or no treatment for slow progress in the first stage of spontaneous labour. Cochrane Database of Systematic Reviews. 2008;(2) Art. No.: CD007123. DOI: 10.1002/14651858.CD007123. [Google Scholar]
- Fraser 2003 .Fraser WD, Turcot L, Krauss I, Brisson-Carrol G. Amniotomy for shortening spontaneous labour (Cochrane Review) Cochrane Database of Systematic Reviews. 2003;(Issue 2) doi: 10.1002/14651858.CD000015. Art. No.: CD000015. DOI: 10.1002/14651858.CD000015.pub2. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008] In: The Cochrane Library. John Wiley and Sons, Ltd; Chichester, UK: 2008. [Google Scholar]
- Hodnett 2003 .Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth (Cochrane Review) Cochrane Database of Systematic Reviews. 2003;(3) doi: 10.1002/14651858.CD003766. Art. No.: CD003766. DOI: 10.1002/14651858.CD003766.pub2. [DOI] [PubMed] [Google Scholar]
- Lavender 2005 .Lavender T, O’Brien P, Hart A. Effect of partogram use on outcomes for women in spontaneous labour at term. Cochrane Database of Systematic Reviews. 2005;(3) DOI: 10. 1002/14651858 CD005461. [Google Scholar]
- Najmi 2000 .Najmi RS, Rehan N. Prevalence and determinants of caesarean section in a teaching hospital in Pakistan. Journal of Obstetrics and Gynaecology. 2000;20(5):479–83. doi: 10.1080/014436100434640. [DOI] [PubMed] [Google Scholar]
- NHS 2007 .The Information Centre [accessed 2007];National Statistics: NHS Maternity Statistics, England: 2005-06. http://www.ic.nhs.uk/webfiles/publications/maternity0506/nhsMaternityStatsEngland200506.
- NICE 2004 .National Collaborating Centre for Women’s and Children’s Health . Caesarean Section, Clinical Guidelines. Commissioned by the National Institute for Clinical Excellence. RCOG Press; London: Apr, 2004. [DOI] [PubMed] [Google Scholar]
- NICE 2007 .National Institute for Health and Clinical Excellence [accessed 2007];Intrapartum care: care of healthy women and their babies during childbirth. NICE clinical guideline 55. www.nice.org.uk.
- NVSR 2007 .Centre for Disease Control Births: preliminary data for 2006. National Vital Statistics Report, National Centre for Health Statistics. 2007 Dec;56(7):18. [Google Scholar]
- O’Driscoll 1970 .O’Driscoll K, Jackson RJ, Galagher JT. Active management of labour and cephalopelvic disproportion. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1970;77(5):385–9. doi: 10.1111/j.1471-0528.1970.tb03539.x. [DOI] [PubMed] [Google Scholar]
- O’Driscoll 1973 .O’Driscoll K, Stronge JM, Minogue M. Active management of labour. BMJ. 1973;3:135–7. doi: 10.1136/bmj.3.5872.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll 1984 .O’Driscoll K, Foley M, MacDonald D. Active management of labour as an alternative to cesarean section for dystocia. Obstetrics & Gynecology. 1984;63(4):485–90. [PubMed] [Google Scholar]
- Paranjothy 2005 .Paranjothy S, Frost C, Thomas J. How much variation in CS rates can be explained by case mix differences? BJOG: an International Journal of Obstetrics and Gynaecology. 2005;112:658–666. doi: 10.1111/j.1471-0528.2005.00501.x. [DOI] [PubMed] [Google Scholar]
- Peaceman 1993 .Peaceman AM, Lopez-Zeno JA, Minogue JP, Socol ML. Factors that influence route of delivery - active versus traditional labor management. American Journal of Obstetrics and Gynecology. 1993;169(4):940–4. doi: 10.1016/0002-9378(93)90031-d. [DOI] [PubMed] [Google Scholar]
- RevMan 2008 .The Cochrane Collaboration . Review Manager (RevMan). 5.0 for Windows. The Nordic Cochrane Centre: The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Thomas 2001 .Thomas J, Paranjothy S. The National Sentinel Ceasarean Section Audit Report. RCOG; London: 2001. [Google Scholar]
- Thornton 1994 .Thornton JG, Lilford RJ. Active management of labour: current knowledge and research issues. BMJ. 1994;309(6951):366–9. doi: 10.1136/bmj.309.6951.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar 2007 .Villar J, Carroli G, Zavaleta N, Donner A, Wojdyla D, Faundes A, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. BMJ. 2007;335(7628):1025. doi: 10.1136/bmj.39363.706956.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker 2002 .Walker R, Turnbull D, Wilkinson C. Strategies to address global caesarean section rates: a review of the evidence. Birth. 2002;29(1):28–39. doi: 10.1046/j.1523-536x.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- Wei 2008 .Wei SQ, Wo BL, Xu HR, Roy C, Turcot L, Fraser WD. Early amniotomy and early oxytocin for delay in first stage spontaneous labor compared with routine care. Cochrane Database of Systematic Reviews. 2008;(1) [Google Scholar]
- *Indicates the major publication for the study


