Abstract
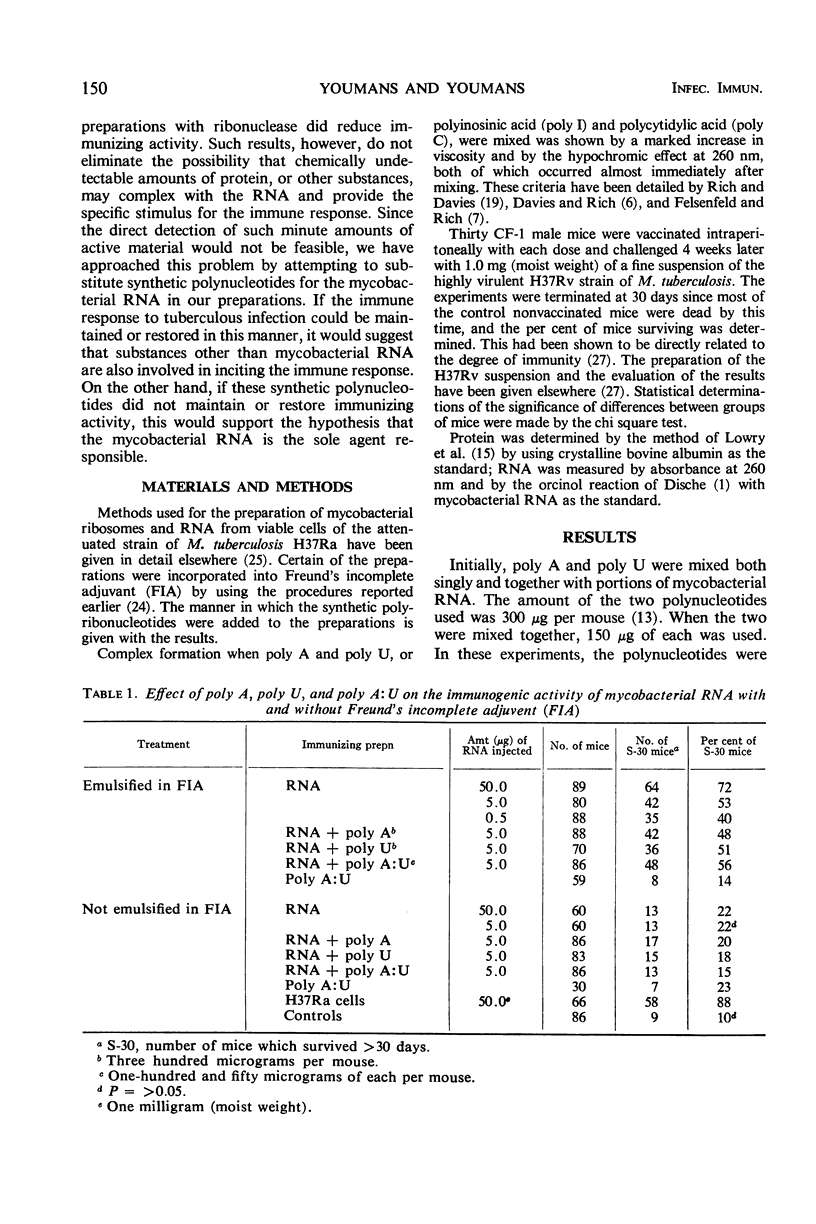
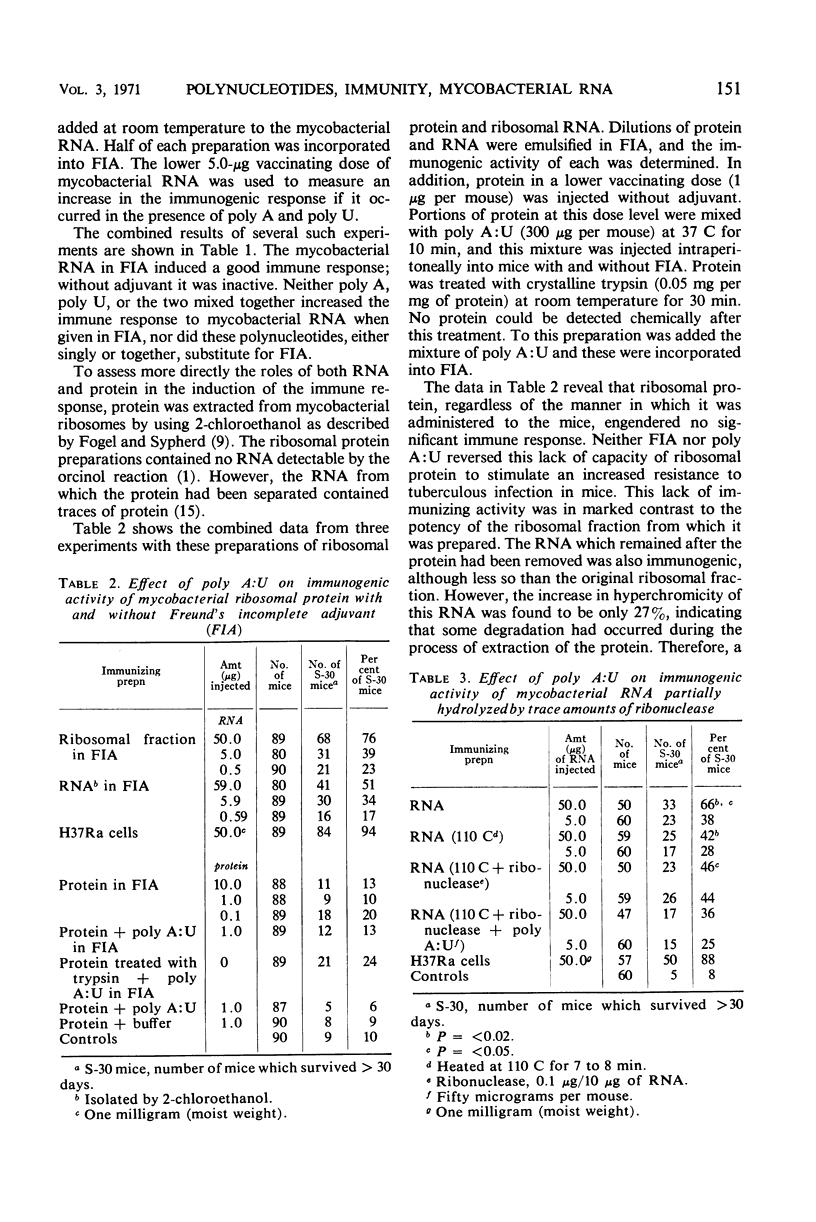
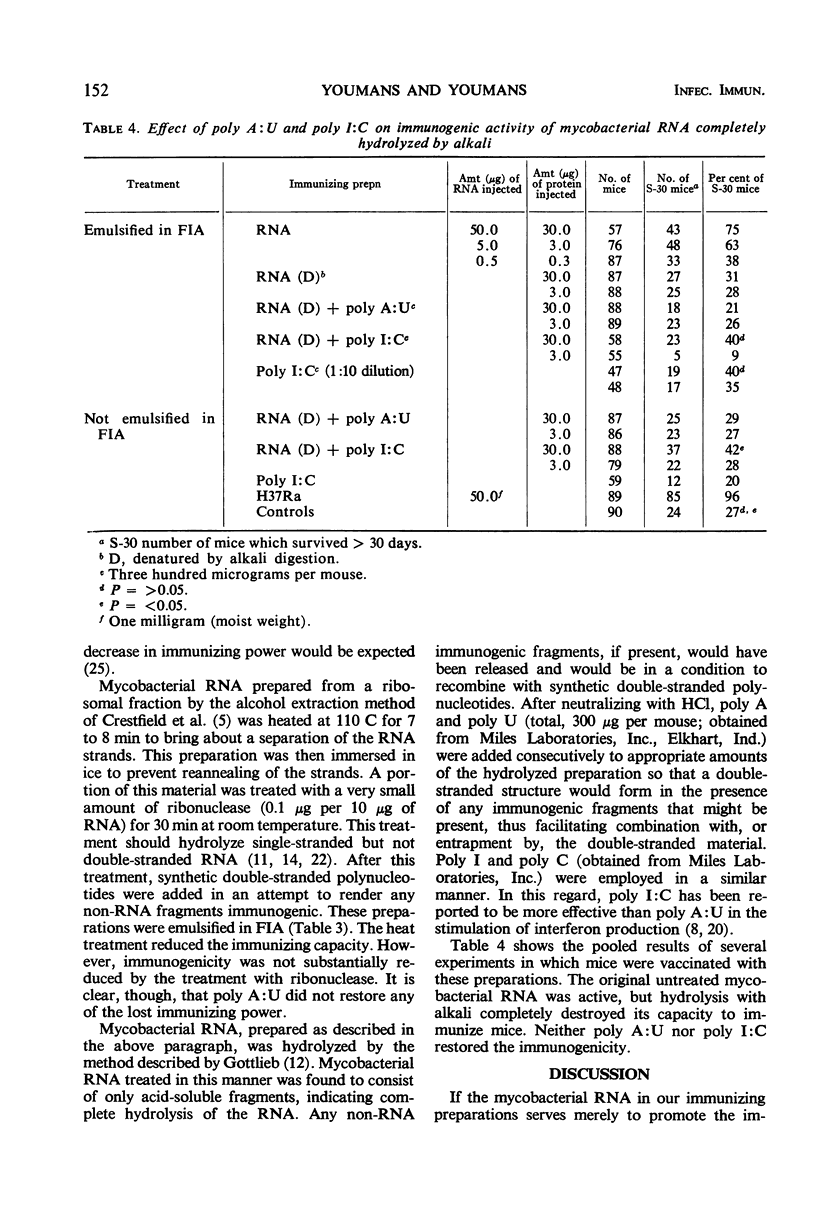
The synthetic polyribonucleotides adenylic acid (poly A), uridylic acid (poly U), cytidylic acid (poly C), and inosinic acid (poly I), whether single- or double-stranded (poly A:U, poly I:C), cannot replace mycobacterial ribonucleic acid (RNA) in the production of a high immune response in CF-1 mice against tuberculous disease. These conclusions are based on the results of several types of experiments. (i) Poly A and poly U, used either singly or in combination, did not increase the immunogenicity of mycobacterial RNA preparations whether emulsified in Freund's incomplete adjuvant (FIA) or not emulsified. (ii) Mycobacterial ribosomal protein, extracted with 2-chloroethanol, was not immunogenic; the addition of poly A:U to the protein did not produce an immune response and FIA did not affect these results. (iii) The RNA left after the protein was extracted was partially immunogenic when emulsified in FIA even though it was partially degraded. (iv) Mycobacterial RNA prepared with ethyl alcohol and partially degraded with ribonuclease had a significantly lower immunogenic activity, and the original higher immune response was not restored by the addition of poly A:U. (v) Mycobacterial RNA totally degraded by weak alkali was not immunogenic, the original immunogenic activity was not restored by the addition of poly A:U or poly I:C, and FIA again did not influence the results. These findings suggest that (i) protein, polypeptides, or other antigenic fragments, if present, are not the specific immunogens; and (ii) mycobacterial RNA is responsible for the high immunogenic activity of mycobacterial ribosomal and RNA preparations. In addition, since the double-stranded forms of these synthetic polynucleotides markedly potentiate the formation of circulating antibodies, these results also reemphasize the lack of correlation between conventional antibody formation and immunity against tuberculosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., RHODES J. M. IMMUNOGENICITY OF ANTIGEN-CONTAINING RIBONUCLEIC ACID PREPARATIONS FROM MACROPHAGES. Nature. 1965 Jan 30;205:470–474. doi: 10.1038/205470a0. [DOI] [PubMed] [Google Scholar]
- Braun W., Nakano M. Antibody formation: stimulation by polyadenylic and polycytidylic acids. Science. 1967 Aug 18;157(3790):819–821. doi: 10.1126/science.157.3790.819. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., SMITH K. C., ALLEN F. W. The preparation and characterization of ribonucleic acids from yeast. J Biol Chem. 1955 Sep;216(1):185–193. [PubMed] [Google Scholar]
- FELSENFELD G., RICH A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim Biophys Acta. 1957 Dec;26(3):457–468. doi: 10.1016/0006-3002(57)90091-4. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Extraction and isolation of individual ribosomal proteins from Escherichia coli. J Bacteriol. 1968 Aug;96(2):358–364. doi: 10.1128/jb.96.2.358-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. P., Stavitsky A. B., Solomon J. M. Induction in vitro of antibodies to phage T2: antigens in the RNA extract employed. Science. 1965 Sep 3;149(3688):1106–1107. doi: 10.1126/science.149.3688.1106. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A. A. Macrophage ribonucleoprotein: nature of the antigenic fragment. Science. 1969 Aug 8;165(3893):592–594. doi: 10.1126/science.165.3893.592. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. VI. ENHANCEMENT OF ANTIBODY FORMATION BY NUCLEIC ACIDS. J Immunol. 1965 Mar;94:416–422. [PubMed] [Google Scholar]
- Pinchuck P., Fishman M., Adler F. L., Maurer P. H. Antibody formation: initiation in "nonresponder" mice by macrophage synthetic polypeptide RNA. Science. 1968 Apr 12;160(3824):194–195. doi: 10.1126/science.160.3824.194. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Shiu G., Neubauer R. H., Baron S., Huebner R. J. Virus-induced sarcoma of mice: inhibition by a synthetic polyribonucleotide complex. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1046–1051. doi: 10.1073/pnas.62.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner W., Chan S. P., Chirigos M. A. Stimulation of humoral and cellular antibody formation in mice by poly Ir:Cr. Proc Soc Exp Biol Med. 1970 Jan;133(1):334–338. doi: 10.3181/00379727-133-34469. [DOI] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation and effect of different adjuvants on the immunogenic activity of mycobacterial ribosomal fraction. J Bacteriol. 1967 Oct;94(4):836–843. doi: 10.1128/jb.94.4.836-843.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]


