Abstract
Evaluating the effects of presession drug administration on intertemporal choice in nonhumans is a useful approach for identifying compounds that promote impulsive behavior in clinical populations, such as those prescribed the dopamine agonist pramipexole (PPX). Based on the results of previous studies, it is unclear whether PPX increases rats’ impulsive choice or attenuates aspects of stimulus control. The present study was designed to experimentally isolate behavioral processes fundamental to intertemporal choice and challenge them pharmacologically with PPX administration. In Experiment 1, the hypothesis that PPX increases impulsive choice as a result of enhanced sensitivity to reinforcer delays was tested and disconfirmed. That is, acute PPX diminished delay sensitivity in a manner consistent with disruption of stimulus control whereas repeated PPX had no effect on delay sensitivity. Experiments 2 and 3 elaborated upon this finding by examining the effects of repeated PPX on rats’ discrimination of response–reinforcer contingencies and reinforcer amounts, respectively. Accuracy of both discriminations was reduced by PPX. Collectively these results provide no support for past studies that have suggested PPX increases impulsive choice. Instead, PPX impairs stimulus control over choice behavior. The behavioral approach adopted herein could be profitably integrated with genetic and other biobehavioral models to advance our understanding of impulsive behavior associated with drug administration.
Keywords: impulsive choice, pramipexole, stimulus control, lever press, rat
An intertemporal choice typically requires that an organism choose between a small magnitude reinforcer available immediately (smaller–sooner reinforcer, or SSR) and a larger magnitude reinforcer available after a delay (larger–later reinforcer, or LLR). Exaggerated preference for SSRs is correlated with substance abuse (for a review, see Mackillop, Amlung, Few, Ray, Sweet, & Munafó, 2011) and probability of relapse to drug taking in humans (Stanger, Ryan, Fu, Landes, Jones, Bicket, & Alan, 2011; Washio, Higgins, Heil, McKerchar, Badger, Skelly, & Dantona, 2011; Yoon, Higgins, Heil, Sugarbaker, Thomas, & Badger, 2007) and, in nonhumans, is often predictive of acquisition, escalation, and relapse of drug self-administration (for a review, see Stein & Madden, in press). By comparison, the extant literature regarding the effects of presession drug administration on SSR preference is somewhat less consistent, potentially due in part to a number of variations in intertemporal choice procedures (for reviews, see Mitchell & de Wit, 2010; Stein & Madden, in press). While presession drug administration has most often been employed to investigate behavioral effects of illicit substances, it may also assist in clarifying the role of clinically prescribed compounds associated with increased levels of impulsive behavior.
Pramipexole (PPX) is a dopamine (DA) agonist medication with partial affinity for D2/D3 receptor subtypes, which densely populate the mesolimbic, or “reward,” pathway (Beaulieu & Gainetdinov, 2011). Clinical populations, such as those diagnosed with Parkinson’s disease, restless legs syndrome, fibromyalgia, or treatment-resistant depression, that are prescribed PPX and other selective DA agonists report development of impulsive behaviors such as pathological gambling (e.g., Dodd, Klos, Bower, Geda, Josephs, & Ahlskog, 2005; Driver-Dunckley, Samanta, & Stacy, 2003), hypersexuality (e.g., Giladi, Weitzman, Schreiber, Shabtai, & Peretz, 2007; Klos, Bower, Josephs, Matsumoto, & Ahlskog, 2005), and compulsive eating (e.g., Hassan, Bower, Kumar, Matsumoto, Fealey, Josephs, & Ahlskog, 2011; Khan & Rana, 2010) or shopping (Cornelius, Tippmann-Peikert, Slocumb, Frerichs, & Silber, 2010); onset and offset of these impulsive behaviors appears to coincide with initiation and termination of the drug regimen (Ávila, Cardona, Martín-Baranera, Bello, & Sastre, 2011; Mamikonyan, Siderowf, Duda, Potenza, Horn, Stern, & Weintraub, 2008). Point prevalence estimates for these impulsive behaviors in Parkinson’s disease range from 7.1% (Voon et al., 2011) to 13.6% (Weintraub et al., 2010); in a majority of these clinical reports, PPX is the prescribed DA agonist (e.g., Perez-Lloret, Bondon-Guitton, Rascol, Montastruc, & French Association of Regional Pharmacovigilance Centers, 2010; Szarfman, Doraiswamy, Tonning, & Levine, 2006).
Informed by these clinical findings, researchers have begun to examine the effects of acute PPX in the context of nonhuman intertemporal choice. Madden, Johnson, Brewer, Pinkston, and Fowler (2010, Experiment 1), for example, administered PPX (0.1, 0.18, & 0.3 mg/kg) prior to sessions in which rats chose repeatedly between SSRs and LLRs. In their “self-control” baseline, the delay to the LLR was adjusted until SSR choice was infrequent (≤ 20%). Here, PPX significantly and dose-dependently increased the frequency of SSR choices. In the “impulsive” baseline (delay adjusted until ≥ 80% SSR choice), PPX did not affect choice, suggesting that the drug did not disrupt choice nonspecifically (e.g., attenuate stimulus control) in the “self-control” baseline. However, using a different procedure in which delays to the LLR increased within session and across trial blocks (Evenden & Ryan, 1996), Madden et al. (Experiment 2) and Koffarnus, Newman, Grundt, Rice, and Woods (2011) reported that PPX did not increase impulsive choice; instead, preference shifted toward indifference at higher doses, an effect suggestive of an attenuation of stimulus control over choice behavior.
In sum, the effects of acute PPX on nonhuman intertemporal choice appear to depend upon the type of procedure used. Conceptually, this disparity is counterintuitive because, although intertemporal choice procedures may differ structurally, they are assumed to recruit similar behavioral processes. Given this assumption, one approach to reconciling these disparate findings is to investigate experimentally those processes likely to be fundamental to the drug effect and to challenge them pharmacologically with PPX. In doing so, a common behavioral mechanism could be identified that is generally interpretable across procedures. Because intertemporal choice is a complex discriminated operant, however, the present study focused on providing only an initial survey of behavioral processes that, if negatively affected by PPX, could have contributed to previous findings of PPX-induced impulsive choice, as well as disruption of stimulus control.
Experiment 1 used a concurrent-chains preparation to measure preference (i.e., initial-link response allocation) for differently delayed but equally sized food reinforcers (see Pitts & Febbo, 2004; Ta, Pitts, Hughes, McLean, & Grace, 2008). Across a range of terminal-link delays to reinforcement, response allocation was fitted by the generalized matching equation (Baum, 1974) to provide an index of delay sensitivity. Sensitivity estimates from saline sessions were then compared to those from drug sessions to describe the manner in which PPX affected delay sensitivity. Within this framework, if PPX enhances sensitivity to differences in delay to reinforcement (i.e., increases preference for relative immediacy), then such an effect would manifest as increased preference for the SSR, as in Madden et al.’s (2010) Experiment 1. Alternatively, if PPX diminishes delay sensitivity, then preference would shift toward the LLR (assuming sensitivity to differences in reinforcer amount remains intact). If, however, PPX diminishes sensitivity to differences in reinforcer delay and amount then choice would shift toward indifference (as was observed at higher doses in Madden et al., Experiment 2; and Koffarnus et al., 2011). To explore these possibilities, Experiment 1 quantified the effects of acute and repeated (i.e., once daily) PPX on sensitivity to reinforcer delay in the concurrent-chains preparation. Experiments 2 and 3 employed different procedures to evaluate the effects of PPX on response–reinforcer contingency discrimination (Davison & Jenkins, 1985) and discrimination of differences in reinforcer amount, respectively.
Repeated dosing was examined because clinical populations administer PPX at least once daily to achieve therapeutic effects (Antonini & Calandrella, 2011) and because repeated dosing has not yet been explored in nonhuman PPX studies of intertemporal choice. In addition, acute PPX administration significantly reduces activity in presynaptic DA neurons in the ventral tegmental area, whereas repeated PPX administration restores such activity to near-baseline levels and increases postsynaptic activity at projections in the prefrontal cortex (Chernoloz, El Mansari, & Blier, 2009, 2012; Maj, Rogóz, Margas, Kata, & Dziedzicka-Wasylewska, 2000). Such effects may influence the presence or absence of any nonspecific drug effects, such as locomotor slowing (Chang, Breier, Yang, & Swerdlow, 2011; Riddle, Rokosik, & Napier, 2012) or attenuation of stimulus control. To assess whether tolerance had developed during extended exposure to the drug, we also compared behavioral measures from the first four and the last four sessions of the repeated PPX assessment
Experiment 1
Methods
Subjects
Twelve experimentally naïve male Wistar rats served as subjects. Rats arrived in the colony weighing approximately 325–350 grams (~ 9 weeks) and were housed individually in polycarbonate cages in a room maintained on a 12/12-programmed light/dark cycle. With the exception of experimental sessions, which were conducted 7 days per week, water was continuously available. At least 2 hr after each session, supplementary chow was provided in order to maintain weights of 375 grams.
All rats having completed the acute assessment served as subjects in the repeated assessment (n = 11, see below). With the exception of the drug administration regimen, all environmental conditions—experimental and extraexperimental—were identical across assessments. Animal use was in accordance with the Institutional Animal Care and Use Committee (IACUC) of the University of Kansas.
Apparatus
Experimental sessions were conducted in six identical operant conditioning chambers (24.1 cm × 30.5 cm × 21.0 cm; Med Associates Inc., St. Albans, VT). The intelligence panel of each chamber featured two low-profile retractable side levers (ENV-112CM, Med Associates Inc.) spaced horizontally 11 cm apart. A 28-volt DC cue light was located 6 cm above each lever. Positioned 1 cm above the floor and centered between the side levers was a pellet receptacle into which nutritional grain-based rodent pellets could be delivered (45 mg; Bio-Serv, Frenchtown, NJ). A speaker generated white noise to mask extraneous sound and a fan ventilated the sound-attenuating cubicle in which each chamber was located. Experimental sessions were executed by a PC running MED-PC® IV software in an adjacent room.
Behavioral procedure
Lever pressing was initially trained using an autoshaping procedure. Once reliable responding had been established, a concurrent-chain procedure was introduced for 40-trial sessions. Each trial began with both levers inserted into the chamber and the stimulus light above each lever lit. During the initial link, a dependent concurrent VI 30-s VI 30-s schedule was in effect with the distributions programmed according to the Fleshler and Hoffman (1962) method. Each reinforcer was randomly assigned to either the left or right lever with two restrictions: (1) The same lever could not produce terminal-link access on more than three consecutive trials and (2) left and right levers were selected an equal number of times per session (20 each). A 3-s changeover delay (COD) prevented responses emitted during the COD from producing terminal-link access.
When a lever press granted terminal-link access, the levers were retracted, the stimulus light above the unselected lever was extinguished, and a delay to reinforcement was initiated. The duration of the terminal-link delay depended upon the lever selected and the experimental condition (see Table 1). After the terminal-link delay, the light above the selected lever was extinguished and two food pellets were delivered to the receptacle regardless of which alternative produced terminal-link access. A postreinforcer blackout was arranged so that initial-link levers were inserted every 100 s (or multiples thereof, if the time of reinforcer delivery occurred after a 100-s interval). Sessions ended once all 40 reinforcers had been earned or 2 hr had elapsed, whichever came first.
Table 1.
Sequence of delay conditions and sessions to stability for individual rats in the acute and repeated PPX assessments of Experiment 1
| Rat | Delay (s) (Left/Right) | Acute
|
Repeated
|
||
|---|---|---|---|---|---|
| Condition Order | Sessions to Stability | Condition Order | Sessions to Stability | ||
| G1 | 7.5/7.5 | 1 | 23 | 3 | 25 |
| 12/3 | 2 | 24 | 2 | 23 | |
| 3/12 | 3 | 30 | 1 | - | |
| G2 | 7.5/7.5 | 1 | 22 | 3 | 56 |
| 12/3 | 2 | 23 | 2 | 22 | |
| 3/12 | 3 | 30 | 1 | - | |
| G3 | 7.5/7.5 | 1 | 22 | - | - |
| 12/3 | 2 | 20 | 2 | 37 | |
| 3/12 | 3 | 26a | 1 | - | |
| G4 | 7.5/7.5 | 1 | 20 | 3 | 39 |
| 12/3 | 2 | 20 | 2 | 34 | |
| 3/12 | 3 | 40 | 1 | - | |
| R1B1 | 7.5/7.5 | 1 | 22 | 3 | 23 |
| 12/3 | 2 | 24 | 2 | 27 | |
| 3/12 | 3 | 44 | 1 | - | |
| R1B2 | 7.5/7.5 | 1 | 20 | 3 | 33 |
| 12/3 | 2 | 22 | 2 | 28 | |
| 3/12 | 3 | 34 | 1 | - | |
| R1B3 | 7.5/7.5 | 1 | 23 | 3 | 30 |
| 12/3 | 3 | 47 | 1 | - | |
| 3/12 | 2 | 23 | 2 | 27 | |
| P2 | 7.5/7.5 | 1 | 20 | 3 | - |
| 12/3 | 3 | 45 | 1 | - | |
| 3/12 | 2 | 21 | 2 | 26 | |
| B1R1 | 7.5/7.5 | 1 | 20 | 3 | 23 |
| 12/3 | 3 | 47 | 1 | - | |
| 3/12 | 2 | 20 | 2 | 25 | |
| B1R2 | 7.5/7.5 | 1 | 20 | 3 | 33 |
| 12/3 | 3 | 42 | 1 | - | |
| 3/12 | 2 | 24 | 2 | 27 | |
| B1R3 | 7.5/7.5 | 1 | 23 | 3 | 23 |
| 12/3 | 3 | 46 | 1 | - | |
| 3/12 | 2 | 22 | 2 | 28 | |
Occurred following remediation of left-lever bias (see text for details).
Response allocation was investigated in three conditions in which the terminal-link delays were manipulated. In the first condition, both terminal-link delays were 7.5 s. In subsequent conditions, left/right terminal-link delays were 12 s/3 s and 3 s/12 s (order counterbalanced across subjects; see Table 1).
Baseline (no-injection) sessions continued in each condition for at least 20 sessions and until (1) the mean initial-link response proportion (left/total) from the last three sessions deviated by ≤ .05 from the mean of the previous three sessions and (2) no monotonic trend was visually apparent over the last six sessions. After response allocation met these stability criteria, the acute dosing assessment began.
Drug procedure
PPX hydrochloride (N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride) was synthesized and provided by Drs. Shaomeng Wang and Jianyong Chen (University of Michigan, Ann Arbor, MI). PPX was dissolved in physiological saline (0.9% NaCl) and was administered subcutaneously (s.c.) at a volume of 1.0 ml/kg. Ten minutes prior to every fifth session, saline or PPX (0.03, 0.1, 0.18, & 0.3 mg/kg) was administered in a descending dose order beginning with saline. The sequence of doses was assessed twice in each delay condition.
Four days following completion of the acute dosing assessment, the repeated dosing assessment began without changing the delay condition. Subjects experienced repeated dosing with either saline or PPX (0.18 mg/kg) for at least 14 consecutive sessions in an order counterbalanced across subjects. Dosing procedures (e.g., s.c. 10 min prior to session) were identical to those used in the acute assessment. Four-day washout periods separated saline and PPX regimens. For the remainder of the repeated assessment, the order of delay conditions was reverse that of the acute assessment. Except for the first delay condition, stability was assessed according to the criteria from the acute assessment.
Data analysis
The logarithm of the response allocation ratio for each dose was plotted as a function of the logarithm of the terminal-link delay ratio in each experimental condition. Log response allocation ratios were calculated separately using the means of the two dosing series from the acute assessment and the respective means of the first and last four sessions of the dosing regimen from the repeated assessment. Linear regressions were performed using the generalized matching equation (Baum, 1974):
| (1) |
Sensitivity to relative reinforcer delay (ad) and bias (log b) estimates were analyzed using one-way repeated-measures analysis of variance (ANOVA) with Dose (saline, 0.03, 0.1, 0.18, & 0.3 mg/kg) as the within-subject factor (IBM SPSS Statistics 20). Because we did not hypothesize a priori that PPX would affect bias in a systematic manner, we used the absolute value of log b in this and all subsequent statistical analyses involving bias. Repeated saline and PPX estimates were compared using paired-samples t tests.
While acute PPX increases response latencies in both humans (Hamidovic, Kang, & de Wit, 2008) and nonhumans (Johnson, Madden, Brewer, Pinkston, & Fowler, 2011; Koffarnus et al., 2011; Madden et al., 2010), repeated administration may normalize these sedative effects (Riddle et al., 2012). We therefore examined the drug effect on mean left- and right-lever latencies in both assessments using three-way repeated-measures ANOVA (Delay Condition, Dose, Lever). A left- or right-lever latency was defined as the time from the onset of a trial (insertion of both levers) to the first response on a lever (i.e., only one latency, left or right, per trial). In 5 out of 330 cases (2%; 4 saline, 1 0.03 mg/kg) in the acute assessment, 2 rats (G1 & G2) never pressed the right lever first at the onset of a trial and, as a result, did not produce right-lever latencies. Missing data were imputed using the between-subjects mean for that condition, and this did not alter the outcome of the analysis compared to listwise deletion (i.e., removing a subject with missing data from the analysis; Allison, 2001). Right-lever latencies were also absent in 5 out of 132 cases (3.7%; 4 saline, 1 0.18 mg/kg) for 4 rats (G1, G2, R1B1, & R1B2) in the repeated assessment and were treated similarly.
In addition, we explored if PPX-induced increases in SSR choice could have been due to perseverative or rate-dependent effects. Reports of D2/D3 DA agonist-induced response perseveration are not uncommon (Boulougouris, Castañé, & Robbins, 2009; Haluk & Floresco, 2009); therefore, mean bout length (i.e., number of responses preceding a changeover event) was evaluated using two-way repeated-measures ANOVA (Delay Condition, Dose). Dopamine agonists (e.g., amphetamine) have also been shown to produce rate-dependent effects on responding (e.g., by simultaneously decreasing high nondrug rates and increasing low nondrug rates below and above control levels, respectively; Dews, 1958; Lucki & DeLong, 1983). Local response rates from the acute assessment (expressed as a percentage of saline rates) were therefore examined for rate dependency using two-way repeated-measures ANOVA (Terminal-link Delay, Dose). Local response rates for the common 0.18 mg/kg dose were also compared between the acute and repeated assessments. We hypothesized that relative local response rates would be affected by terminal-link delays (i.e., higher local rates on the lever with the shorter delay to reinforcement). If PPX decreases high rates and increases low rates, then rate-dependent changes in choice may explain previous findings of PPX-induced changes in SSR preference.
In the acute assessment, 1 rat (P1) fell ill, was euthanized, and was excluded from all analyses. A 2nd rat (G3) developed an extreme right-lever bias in nondrug sessions in the final delay condition (3/12). This bias toward the lever on which was programmed the longer delay was remediated in 10 sessions in which reinforcers were only available by pressing the left lever. Subsequently, stability was reestablished for this rat (see Table 1). As a result of these remedial procedures, G3 did not begin the final delay condition of the repeated assessment (7.5/7.5). Also in the repeated assessment, 1 rat (P2) fell ill before completing the final delay condition and was euthanized. The incomplete data collected with the latter 2 rats were sufficient to confidently assess delay sensitivity, but not bias. However, the volume of missing data for these 2 rats prevented their inclusion in other statistical analyses comparing acute and repeated assessments (e.g., latency).
Pairwise comparisons were evaluated using Bonferroni-corrected alphas. Effects sizes for repeated-measures ANOVA were calculated as generalized eta squared (see Bakeman, 2005); Cohen’s d was used for paired-samples t tests. For cases where data violated assumptions of normality, Greenhouse-Geisser adjusted degrees of freedom were used to estimate criterion for significance. All effects and interactions were significant at the p < .05 level.
Results
Rats required a mean of 27.55 (SD = 10.16) and 29.45 (SD = 4.96) sessions to achieve stability during the acute and repeated PPX assessments, respectively (Table 1). For rats completing both assessments, significantly more sessions were required to achieve stability in the repeated assessment, .
Delay sensitivity and bias
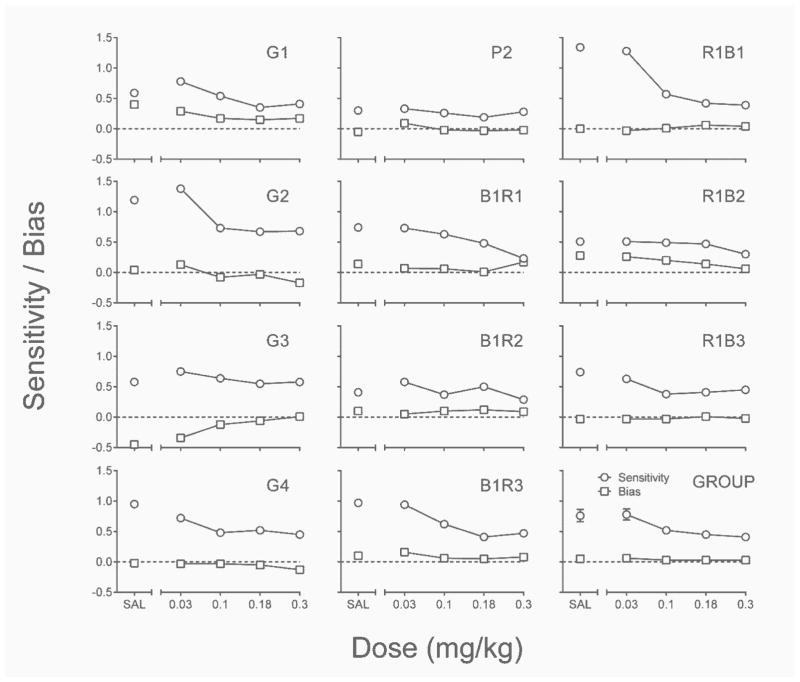
Figure 1 depicts dose-effect curves for delay sensitivity and bias estimates from the best-fitting linear regressions to individual subject and group data from the acute PPX assessment. Increases in PPX dose above 0.03 mg/kg tended to decrease sensitivity to relative reinforcer delay below saline levels, significant main effect: ; with rats G3, P2, B1R2, and R1B2 being exceptions to this rule. Pairwise comparisons revealed that the only acute PPX dose at which delay sensitivity was significantly lower than saline was 0.3 mg/kg (p = .02). Delay sensitivity in saline sessions tended to be higher among rats that showed the most systematic dose effect.
Fig. 1.
Individual-subject and group delay sensitivity and bias estimates from the best-fitting linear regressions of response allocation from the acute PPX assessment of Experiment 1. SAL = saline. Error bars represent ±SEM.
With the exception of 3 rats (G1, G3, & R1B2), there was no clear indication of lever bias in saline sessions in the acute PPX assessment. For these 3 rats, acute PPX appeared to attenuate these biases. This observation was not supported statistically, however, as absolute bias was unaffected by acute PPX at the group level (p > .12).
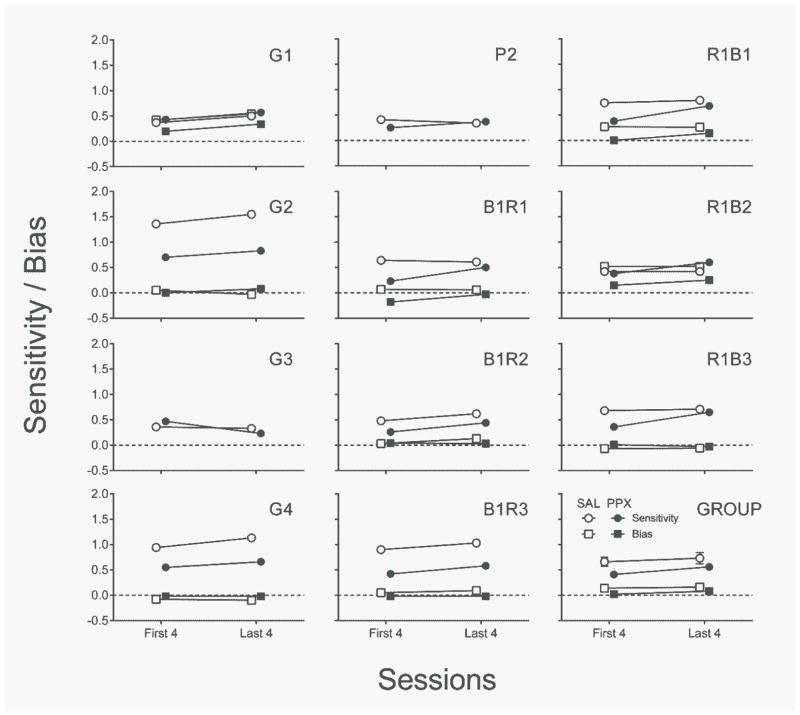
Figure 2 shows delay-sensitivity and bias estimates for individual rats (and the group) in the first and last four sessions of the repeated PPX assessment. In the first four sessions, PPX tended to decrease sensitivity to delay, t(10) = 3.65, p < .01, d = 1.10; rats G1, G3, P2, and R1B2 being exceptions to this rule. In the final four PPX sessions, however, sensitivity had largely returned to levels observed over the last four saline sessions (p = .06); rats G2, G4, and B1R3 being exceptions. The latter increase in delay sensitivity with repeated PPX dosing, t(10) = 3.44, p < .01, d = 1.04, is suggestive of drug tolerance.
Fig. 2.
Individual-subject and group delay sensitivity and bias estimates from the best-fitting linear regressions of response allocation from the first four and last four sessions of repeated saline (open symbols) and PPX (closed symbols) assessments of Experiment 1. SAL = saline. PPX = pramipexole (0.18 mg/kg). Error bars represent ±SEM.
Bias was present for 3 rats (G1, R1B1, & R1B2) in the repeated saline assessment. Repeated PPX reduced the severity of these biases, and this was true statistically of the difference between absolute levels of bias over the last four sessions of the saline and PPX regimens, t(8) = 2.97, p < .02, d = .99. Absolute bias was not significantly affected by repeated dosing (first vs. last four PPX, p = .34).
Response latencies
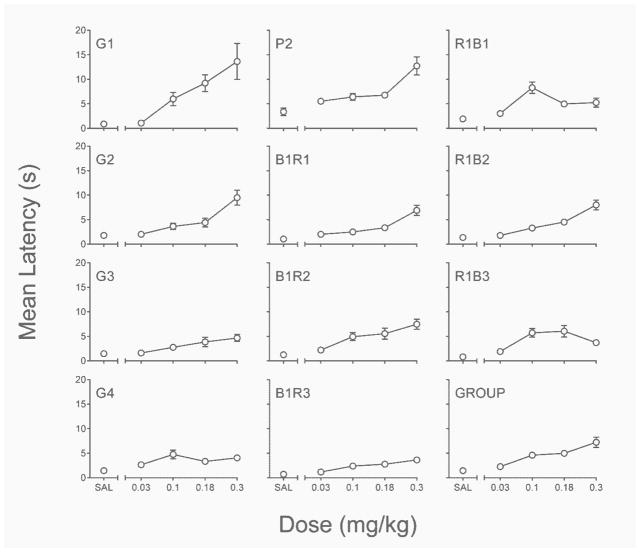
Figure 3 depicts the effects of acute PPX on rats’ mean latencies to emit a lever press in the initial link of the concurrent-chains schedule. Data are collapsed across terminal-link delay conditions and levers (left vs. right) as neither of these within-subjects factors was significantly related to latency measures (all main effect and interaction p’s > .18).
Fig. 3.
Individual-subject and group mean latency from the acute PPX assessment of Experiment 1. SAL = saline. Error bars represent ±SEM.
As in previous reports, acute PPX significantly increased rats’ latencies, main effect of dose, . For a majority of the rats there was a positive monotonic relation between acute PPX dose and response latencies; G4, R1B1, and R1B3 being exceptions. Pairwise comparisons conducted at the group level confirmed the general finding that latencies at all PPX doses were significantly elevated above saline latencies (all p’s ≤ .01).
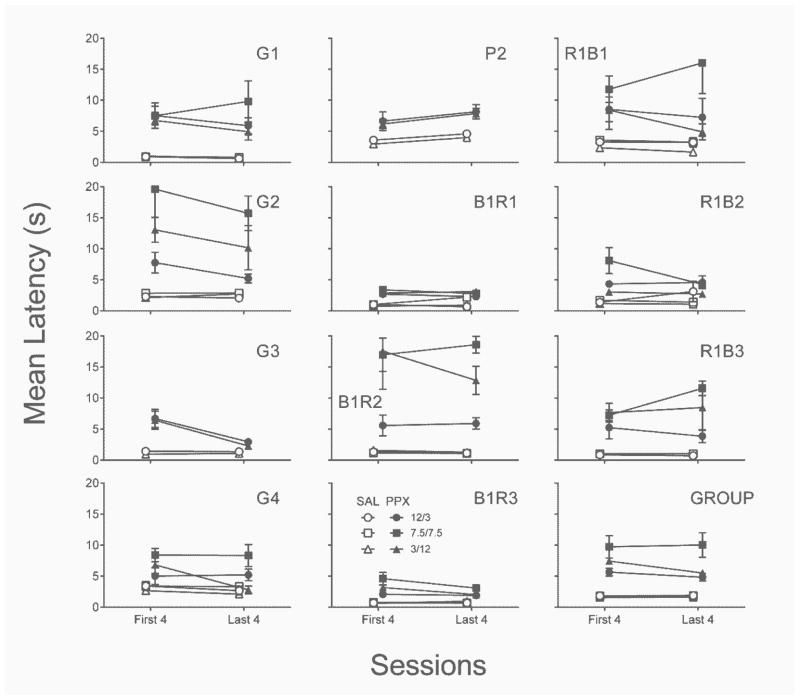
Response latencies were also generally increased by repeated PPX (Figure 4). Due to the presence of a significant Dose x Delay Condition interaction, , latencies are collapsed only across the within-subject factor of Lever. Across conditions and rats, there was little evidence for consistent PPX sensitivity or tolerance developing from the first four until the last four sessions of the repeated assessment. Compared to the acute assessment, rats were slower to respond in this assessment, and this was due in part to the elevated latencies in the final delay condition (7.5/7.5), significant Assessment x Delay Condition interaction, .
Fig. 4.
Individual-subject and group mean latency from the first and last four sessions of each delay condition of the repeated saline (open symbols) and PPX (closed symbols) assessments of Experiment 1. SAL = saline. PPX = pramipexole (0.18 mg/kg). Error bars represent ±SEM.
Response perseveration
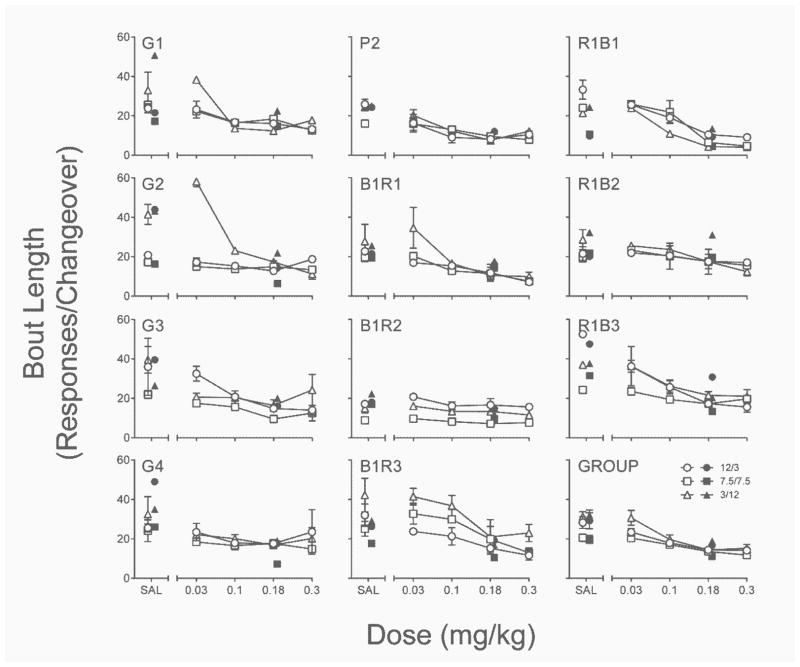
Figure 5 depicts the effects of acute and repeated PPX on response perseveration. Although the Dose x Delay Condition interaction in the acute PPX assessment was significant, , this was largely due to the behavior of 3 rats (G1, G2, & B1R1) that, in the 3/12 condition at the 0.03 mg/kg dose, tended to emit more responses on a lever before a changeover event. At the group level, however, increasing the acute PPX dose tended to decrease bout length. Post-hoc comparisons were therefore not conducted on the simple main effect of dose because in only 14 out of 132 cases (10.6%, almost exclusively at the 0.03 mg/kg dose) did acute PPX increase bout length above saline levels (i.e., induce response perseveration).
Fig. 5.
Individual-subject and group mean bout length (responses per changeover) from each delay condition in the acute (open symbols) and repeated (closed symbols) PPX assessments of Experiment 1. SAL = saline. Error bars represent ±SEM.
Figure 5 also shows that, relative to repeated saline, repeated PPX significantly reduced bout length, main effect of dose, . Although for some rats the decrease in bout length was less clear (G1, B1R2, & R1B2), the effect for most rats was consistent with the group finding and did not differ statistically from the level of decreases observed in the acute PPX assessment (p = .47). Bout length was also generally more robust against this suppressive effect of repeated PPX in the final delay condition (7.5/7.5) compared to other delay conditions, significant Dose x Delay Condition interaction, . Most importantly for the hypothesis that PPX increases the likelihood of response perseveration, in neither assessment did PPX significantly increase bout length.
Rate dependency
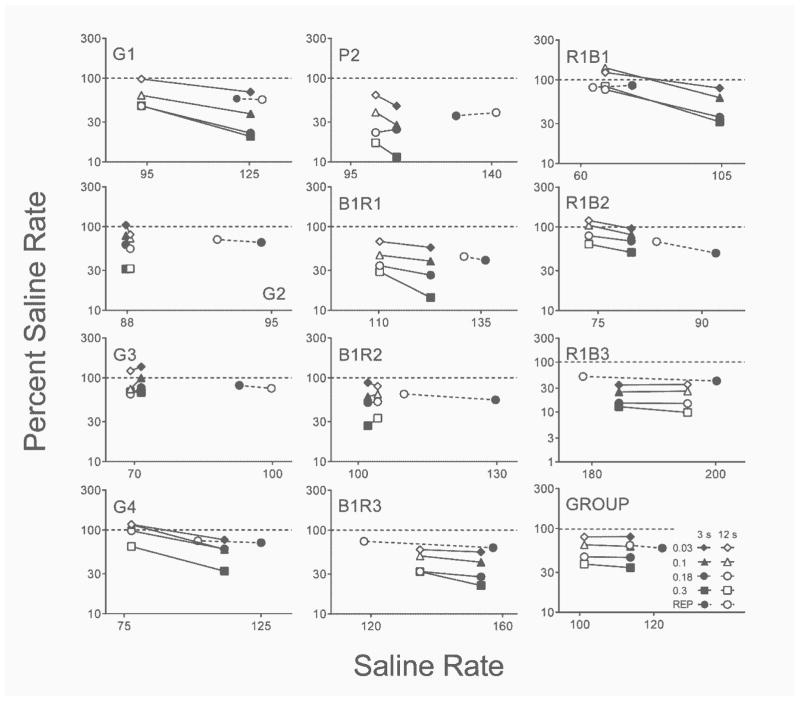
Figure 6 shows individual-subject and group rate-dependency plots from the acute and repeated PPX assessments. Because local initial-link response rates for the 3-s and 12-s terminal-link delays did not differ significantly between the 3/12 and 12/3 delay conditions (i.e., no main effect or interaction involving Lever; all p’s ≥ .06) data were collapsed across lever assignments. Mean response rates are expressed as a percentage of the corresponding saline response rate (responses per min) and plotted as a function of saline response rate for acute and repeated PPX assessments.
Fig. 6.
Individual-subject and group mean local response rate (responses per minute; expressed as percent saline rate) in initial links corresponding to 3 s (closed symbols) and 12 s (open symbols) terminal-link delays as a function of local response rate in saline sessions in the acute (solid lines) and repeated (dashed lines) PPX assessments in Experiment 1. The horizontal dashed lines indicate no change from saline performance. Log–log axes are scaled differently to optimize presentation for each subject. REP = repeated PPX assessment.
In saline sessions in the acute PPX assessment, local initial-link response rates were higher for the 3-s terminal-link delay than for the 12-s delay for 8 out of 11 rats, a difference that was significant at the group level, t(10) = 2.50, p = .03, d = .76. The primary effect of acute PPX was to decrease local response rates relative to saline rates (i.e., data points below the dashed line; ). This was true also for all rats in the repeated PPX assessment. These decreases in the percent saline rate tended to be more pronounced in the initial link that had previously maintained higher local response rates (i.e., negative slope). Of 44 pairs of rates (four per rat) in the acute assessment, only seven (15.91%) showed a rate-dependent effect consistent with previous effects of dopamine agonists (i.e., above-baseline increases in lower rates and below-baseline decreases in higher rates); there were no cases of similar rate-dependent effects of PPX in the repeated assessment.
Discussion
The primary finding of Experiment 1 was that sensitivity to differences in reinforcer delay in a concurrent-chains arrangement decreased as acute PPX doses increased. Recall that Madden et al. (2010, Exp. 1) observed PPX-induced increases in SSR choice, whereas Madden et al. (2010, Exp. 2) and Koffarnus et al. (2011) reported that indifference emerged at higher PPX doses, suggestive of nonspecific attenuation of stimulus control. Our primary finding of diminished sensitivity to delay following acute PPX doses is incompatible with the findings of Madden et al.’s Experiment 1 in which acute PPX dose-dependently increased SSR choice. That is, diminished sensitivity to relative reinforcer delay (present experiment) should either shift preference in an intertemporal choice context toward more LLR choices (if choice remains sensitive to relative reinforcer amounts) or toward indifference (if amount sensitivity is similarly disrupted). The primary finding of the present experiment is consistent with the findings of Madden et al.’s Experiment 2 and Koffarnus et al., wherein PPX shifted preference toward indifference in a manner resembling an attenuation of stimulus control over choice behavior.
Repeated PPX (0.18 mg/kg) did not significantly affect sensitivity to relative reinforcer delay. While the drug did not significantly affect absolute levels of bias in the acute assessment (i.e., relative to saline), repeated PPX did attenuate biases observed under repeated saline conditions. As in previous reports, acute PPX increased response latencies, and this effect extended to the repeated assessment. Alternative explanations for the effects of PPX in previous intertemporal choice studies, namely response perseveration and rate-dependent increases in selection of the SSR, were not supported. Finally, tolerance to the effects of PPX was evident for delay sensitivity, but not for absolute bias or response latencies when comparing the first and last four days of repeated PPX administration.
Although concurrent-chains preparations might be viewed as simpler than intertemporal choice tasks because they equalize one dimension (e.g., reinforcer amount) while varying another (e.g., reinforcer delay), the performances they generate are still complex in that they require the organism to discriminate aspects of the choice situation that are more ambiguous (i.e., fewer discriminative stimuli) than in an intertemporal choice. For instance, contingencies relating responses and the reinforcers they produce must be discriminated by the organism (Davison & Jenkins, 1985); something that can be difficult when both responses produce the same reinforcer in the same location. If PPX impairs the discrimination of response–reinforcer contingencies, then responding may become undifferentiated, which in the context of concurrent-chain schedules and intertemporal choice situations would manifest as indifference.
Delaying reinforcement may also negatively affect discrimination of response–reinforcer contingencies. However, because only one alternative in most intertemporal choice studies is delayed (i.e., the LLR alternative), it is possible that PPX more severely impairs discrimination of the LLR response–reinforcer contingency. If so, then the LLR contingency would less effectively control choice relative to the SSR contingency, an outcome that should shift preference more toward the SSR. If PPX disrupts an organism’s ability to discriminate response–reinforcer contingencies, then this hypothesis could explain the pattern of results in Madden et al.’s (2010) Experiment 1. Specifically, in their self-control baseline, rats were predominantly choosing the LLR and PPX increased preference for the SSR. If PPX disrupted response–reinforcer contingency discriminations in this condition in which these discriminations were difficult to make because of the delay, then this would shift choice toward the SSR alternative. However, in Madden et al.’s impulsive baseline, where choice was predominantly for the SSR and, presumably, contingency discriminations were easily made, PPX had no effect on choice. If response–reinforcer contingency discriminations were easily made in the impulsive baseline (because there was no delay between response and reinforcer), then the absence of a PPX effect may have more to do with response–reinforcer temporal contiguity than to the absence of indirect effects of PPX (the interpretation forwarded by Madden et al.).
This hypothesis could also account for the shift toward indifference reported by Koffarnus et al. (2011): At longer delays to the LLR, differential impairment of the discrimination of response–reinforcer contingencies would shift preference away from LLR choices. This hypothesis does not, however, provide a coherent account of the results of Madden et al.’s (2010) Experiment 2. In that experiment, rats preferred SSRs at the longest delay to the LLR (30 s) under saline conditions. The differential-impairment hypothesis described above predicts that following PPX administration, as delays to the LLR increase, discriminating the LLR response–reinforcer contingency would become increasingly difficult. If PPX disrupts this contingency discrimination, then we would expect PPX to increase choice of the SSR as delays to the LLR increase in duration. This was not observed. Instead, choice shifted toward indifference regardless of the delay (including 0-s delays), a pattern of results consistent with undifferentiated impairment in discrimination of all response–reinforcer contingencies.
These different outcomes provided the rationale for Experiment 2: to examine the effects of PPX on discrimination of response–reinforcer contingencies. A spatial matching to sample (SpMTS) procedure was used because we were interested in signal detection (Jones & Davison, 1998). That is, we were interested in the effects of PPX on the effectiveness of rats’ own behavior (left or right lever press) to serve as a sample stimulus that occasioned comparison responding on a spatially-related operandum (left or right nose poke). Repeated PPX dosing was arranged so as to reduce the interference of nonspecific drug effects (e.g., locomotor slowing).
Experiment 2
Methods
Subjects
Twelve experimentally naïve male Wistar rats served as subjects and were treated identically to subjects serving in Experiment 1. Animal use was in accordance with the research protocol approved by the IACUC at Utah State University.
Apparatus
Sessions were conducted in standard operant conditioning chambers housed within sound-attenuating cubicles (Med Associates Inc., St. Albans, VT). The front wall of each chamber was identical to that described in Experiment 1. On the opposite wall were two nose-poke operanda (left and right sides). A central food receptacle was located between the nose pokes and was serviced by an additional 45-mg pellet dispenser (Coulbourn Instruments, Whitehall, PA). Chambers were equipped with a white noise speaker and ventilation fan. All experimental events were coordinated and recorded via a PC.
Behavioral procedure
As in Experiment 1, lever pressing was initially trained using an autoshaping procedure. Once reliable responding had been established, experimental sessions began. Sessions consisted of 40 trials. For the first part of each trial (sample period), one of the levers was selected randomly without replacement and inserted into the chamber accompanied by illumination of its stimulus light (20 trials per lever). A single response on this lever retracted the lever, extinguished its stimulus light and resulted in the delivery of one food pellet to the front receptacle. If a sample response did not occur within 15 s of lever insertion, the trial was terminated and counted as an omission.
Immediately following reinforcer deliveries to the front receptacle, discrimination of the response that produced reinforcement was assessed (comparison period). First, the stimulus lights located within the rear nose pokes were illuminated. Next, a conditional discrimination was required such that the rat had to make a single nose poke to the nose poke operandum spatially associated with the sample response (e.g., if the pellet was earned by pressing the left lever, choose the left nose poke). Failure to emit a comparison response within 15 s of illumination of the nose poke lights resulted in trial termination and the trial being counted as an omission.
Correct nose-poke responses extinguished all stimuli and resulted in the delivery of one food pellet to the rear receptacle. Incorrect responses produced the same series of events with a 0.5-s blackout taking the place of pellet delivery. Following each trial, a 30-s ITI occurred during which all stimuli were in the “off” state.
For the first 20 sessions of the experiment, a correction procedure was implemented. During this period, trials in which samples were not identified correctly were repeated indefinitely until the correct discrimination was made. Sessions ended once 40 correct discriminations were made or two hr had elapsed, whichever came first. The correction procedure was then removed for 10 sessions and for the remainder of the experiment in which the effects of repeated PPX were assessed.
Drug procedure
PPX (0.18 mg/kg) or saline vehicle was administered subcutaneously 10 min prior to every session for 14 consecutive sessions. After a 6-day no-injection washout period, a second repeated dosing regimen was initiated with the compound not administered in the first regimen (order counterbalanced across subjects).
Data analysis
Accuracy of rats’ discriminations of sample responses was calculated as log d using the signal-detection model forwarded by McCarthy, Davison, and Jenkins (1982):
| (2) |
in which RLeftLeft is the number of left nose pokes following a reinforcer from the left lever (correct discrimination) and RLeftRight is the number of right nose pokes having just obtained a reinforcer from the left lever (incorrect discrimination); the same nomenclature apply to reinforcers earned from the right lever. Log d was calculated for the first sessions of the saline and PPX administration, as well as over the course of the last four sessions of these regimens. Perfect discrimination between the contingencies (i.e., the correct nose poke was always chosen) was indicated by ceiling log d values of 1.91 (one session) and 2.51 (four sessions); chance responding always resulted in a log d of 0. A correction to Equation 2 suggested by Brown and White (2005) in which 0.25 is added to each response count was adopted. Compared to a percent correct measure, log d expresses accuracy proportionately and is, therefore, less likely to be influenced by the total number of trials considered in the calculation. Bias (i.e., favoring a particular comparison response over another due to nonexperimenter programmed variables) was calculated as log b:
| (3) |
Repeated PPX increased response latencies in Experiment 1 and we hypothesized that this effect, if manifested in the SpMTS task as prolonged retention intervals, could be a mechanism by which discrimination accuracy is reduced (for a review, see White, 2013). We therefore assessed rats’ latencies to emit comparison responses (correct nose poke only), as well as latencies to engage in the sample response (lever press) and the frequency of omissions (response latencies > 15 s) using paired-samples t tests. The analysis of latencies was restricted to those preceding correct comparison responses because half of the rats failed to produce incorrect response latencies during repeated saline dosing. Finally, to quantify the potential relation between comparison response latencies and discrimination accuracy (calculated as percent correct), we examined correlations between these variables in the first session and last four sessions of repeated PPX administration relative to saline performance.
Where data failed to satisfy assumptions of normality (i.e., significantly nonnormal according to D’Agostino-Pearson omnibus normality test; GraphPad Prism 5.00 for Windows, GraphPad Software, La Jolla, California, USA, www.graphpad.com), related-samples Wilcoxon signed-rank tests (nonparametric equivalent of paired-samples t test) were used to test for PPX-induced differences in the accuracy with which rats discriminated response–reinforcer contingencies (log d) and bias (log b). To evaluate the acute PPX effect, we examined performances in the first repeated session in which PPX was administered. Where appropriate, data from the first four repeated PPX sessions were compared to data from the last four sessions to evaluate tolerance as in Experiment 1. For nonparametric tests, effect sizes were calculated according to the method described by Field (2009).
Results
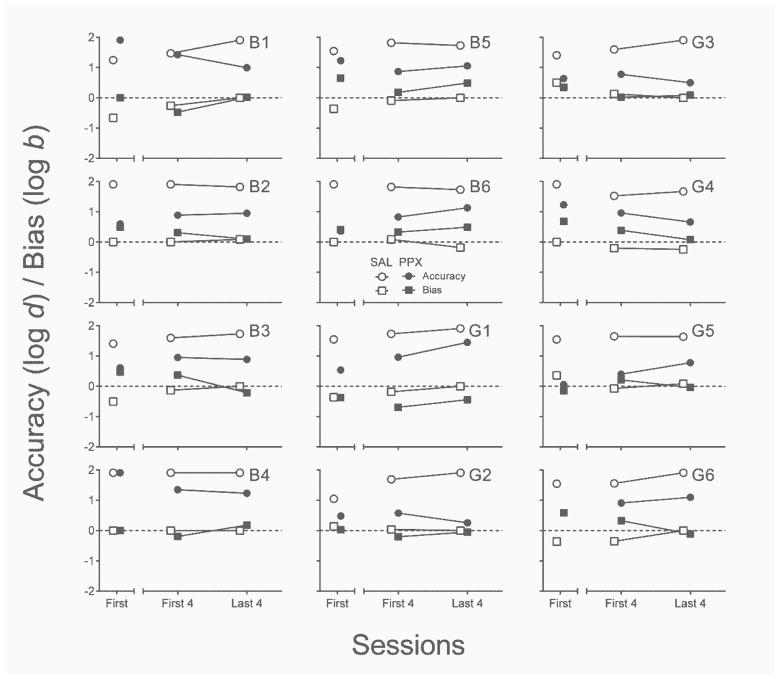
Accuracy and bias
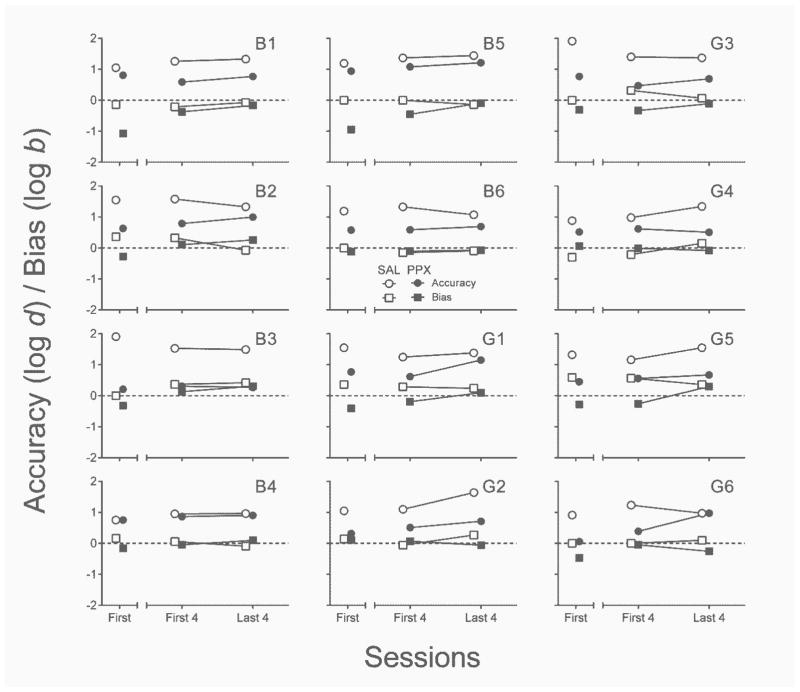
Because maximum log d and log b values differ depending on the number of sessions analyzed, Figure 7 displays accuracy and bias measures in two ways. The left segment of each x-axis shows data collected in the first saline session and the first PPX session. The right segment of each x-axis illustrates mean per-session values from the first four and last four sessions of repeated saline and PPX. Nearly all rats discriminated response–reinforcer contingencies with perfect accuracy in the first saline session, as well as over the first and last four saline sessions. With the exceptions of B1 and B4, the first administration of PPX reduced the accuracy of response–reinforcer contingency discrimination relative to the first saline administration, z = −2.67, p < .01, ES = .55. All rats discriminated response–reinforcer contingencies less well in the last four sessions of repeated PPX relative to the last four sessions of saline, z = −3.06, p < .01, ES = .62. There was, however, no across-subject consistent effect of PPX on accuracy from the first four to the last four sessions of the repeated assessment (p = .70).
Fig. 7.
Individual-subject accuracy (log d) and bias (log b) estimates from the first session (left segment of x-axis) and first and last four sessions (right segment of x-axis) of the repeated saline (open symbols) and PPX (closed symbols) assessments in Experiment 2. SAL = saline. PPX = pramipexole (0.18 mg/kg).
In the first saline session, bias (log b) was near zero for 5 rats (B2, B4, B6, G2, & G4) and unsystematic across the other 7 (left nose poke bias: G3 & G5; right nose poke bias: B1, B3, B5, G1, & G6) across rats. There was a tendency for biases to resolve over the course of repeated saline administration. The effect of first-session PPX on bias relative to first-session saline was similarly unsystematic. Over the course of the repeated PPX regimen, absolute levels of bias observed in the first four sessions were reduced in most cases, but exacerbated in others (B5 & B6); at the group level, the difference was not significant (p = .37). Likewise, absolute bias observed over the last four PPX sessions was not significantly different from absolute bias in either the first session (p = .46) or last four sessions (p = .58) of repeated saline administration.
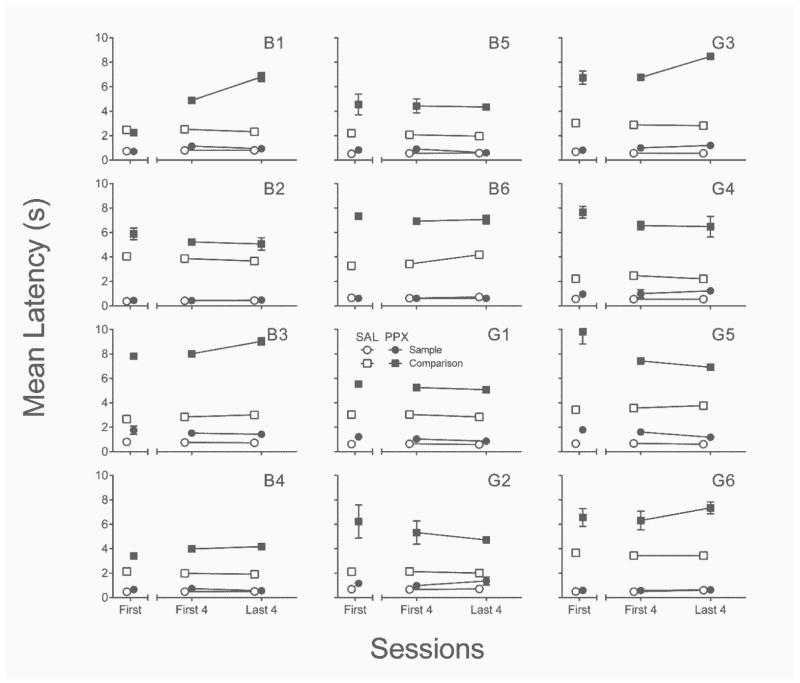
Response latencies
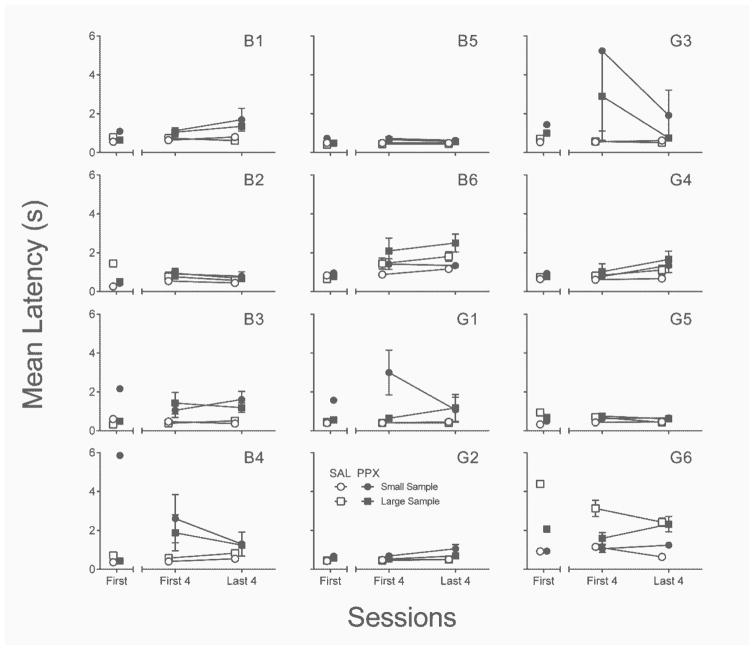
Figure 8 depicts the effects of PPX on mean latencies to emit sample (lever) and correct comparison (nose poke) responses. Neither latency differed significantly between left or right levers, regardless of saline or PPX administration; latencies are therefore collapsed across levers. First-session PPX increased nominally sample–response latencies in about half of the rats, t(11) = 3.23, p < .01, d = .93. The same was true of sample–response latencies when compared between the last four sessions of repeated saline and PPX, t(11) = 3.39, p < .01, d = .98. The difference in sample–response latencies between the first four and last four sessions of repeated PPX was not significantly different (p = .55).
Fig. 8.
Individual-subject mean sample and comparison latencies from the first session (left segment of x-axis) and first and last four sessions (right segment of x-axis) of the repeated saline (open symbols) and PPX (closed symbols) assessments in Experiment 2. SAL = saline. PPX = pramipexole (0.18 mg/kg). Error bars represent ±SEM.
Figure 8 also shows mean correct-comparison response latencies. With the exception of rat B1, these latencies were increased above saline levels by first-session PPX, t(11) = 6.02, p < .001, d = 1.74. A more consistent effect was observed in the last four sessions of repeated PPX, t(11) = 8.31, p < .001, d = 2.40. There was no consistent across-subjects effect of repeated dosing (first four vs. last four sessions) on comparison latencies (p = .17). PPX also increased the mean number of omissions (i.e., latencies ≥ 15 s) from zero in saline sessions to 7.00 (SD = 8.18) in the first session, t(11) = 3.04, p = .01, d = .88, and 7.42 (SD = 8.73) over the last four sessions of repeated dosing, t(11) = 2.94, p = .01, d = .85 (data not shown).
Accuracy-Latency Correlations
Figure 9 depicts correlations between changes in accuracy (calculated as percent correct) as a function of changes in correct comparison latency in the first session and last four sessions of repeated PPX administration. When analyzed in terms of absolute change from baseline (i.e., saline), neither change in latencies nor change in accuracy differed significantly between left or right levers and so the data were collapsed across levers.
Fig. 9.
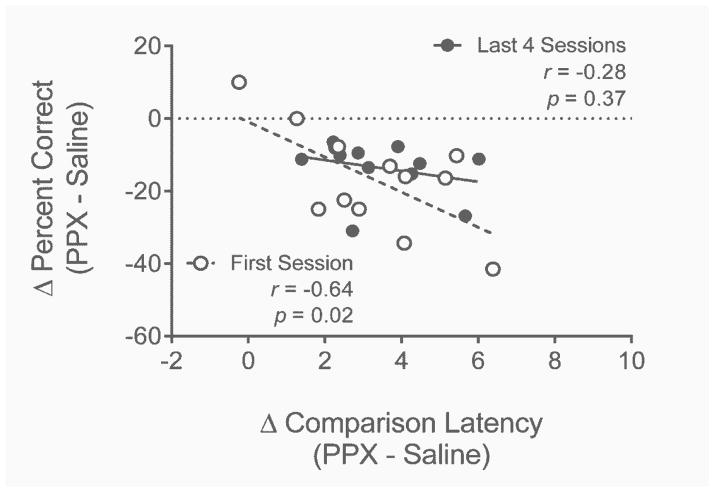
Correlations between changes in percent correct and changes in comparison latencies in the first session (open symbols) and last four sessions (closed symbols) of PPX administration in Experiment 2. The dashed horizontal line indicates no change from saline performance. Statistical outcomes are inset.
Relative to the first saline session, the first session of PPX administration increased correct comparison response latencies by an average of 3.29 s (SD = 1.89) and decreased percent correct discriminations by an average of 16.84% (SD = 14.22). A significant negative correlation between these change measures was observed (i.e., longer latencies were correlated with greater decrements in accuracy, r = −0.64, p = .02).
Repeated PPX (last four sessions) increased correct comparison latencies by an average of 3.44 s (SD = 1.44) and decreased percent correct discriminations by an average of 13.62% (SD = 7.60) relative to the last four sessions of repeated saline administration. Neither measure differed significantly between the first session and the last four sessions of repeated dosing (both p’s ≥ .28). There was no significant correlation between latency and accuracy over the last four sessions, r = −0.28, p = .37.
Discussion
Experiment 2 explored the possibility that PPX disrupts rats’ discrimination of response–reinforcer contingencies. Rats were trained to spatially relate samples (left or right levers) to comparisons (left or right nose poke operanda). Accuracy of rats’ discrimination of the response–reinforcer contingencies was generally high in the first saline session and perfect for half of the subjects over the last four repeated saline sessions. PPX (0.18 mg/kg) administration reduced the accuracy of the discrimination in the first session and increased latencies to emit sample and comparison responses. Each of these effects was sustained throughout the repeated PPX regimen. Unlike in Experiment 1, there was no statistical evidence that tolerance had developed over the course of repeated PPX administration for any measure.
In addition to a potential direct effect of PPX on discrimination accuracy, we also hypothesized that a prolonged comparison latency resulting from the drug’s documented sedative properties could decrease accuracy by effectively acting as a delay between the sample stimulus and production of the comparison response. If this indirect drug effect were responsible for the results of Experiment 2, then one might predict a negative correlation between increases in comparison latencies and decreases in accuracy during the PPX regimen. This hypothesis was supported in our analysis of data from the first PPX session, our proxy for acute administration in the repeated regimen. While this finding is intriguing, it should be interpreted cautiously as it does not definitively indicate a causal role for increased latencies in the reduction of accuracy. A plausible alternative explanation, for example, is that PPX directly impaired accuracy, which resulted in a decrease in reinforcement rate, which in turn led to an increase in comparison latencies.
Over the last four sessions of repeated PPX administration, increases in comparison latencies were not significantly related to decreases in discrimination accuracy. That is, regardless of any between-subjects differences in the potential sedative effect of repeated PPX, rats discriminated response–reinforcer contingencies with a similar degree of accuracy, a correlation supportive of a direct negative effect of the drug.
The findings of Experiment 2 suggest that PPX-induced changes in intertemporal choice (Koffarnus et al., 2011; Madden et al., 2010) may have been affected by disruptions in response–reinforcer contingency discrimination. Because PPX was administered acutely in these previous studies, it is possible that the significant negative correlation identified in the first PPX session of the present experiment could have influenced previous outcomes, regardless of the direction of causation between latencies (i.e., delay) and discrimination accuracy. However, as evidenced by the lack of a correlation between these behavioral measures following repeated PPX, we cannot rule out the involvement of a direct effect of PPX on impairment of contingency discrimination in previous studies. A more compelling case for a direct effect could be made in a preparation unaffected by locomotor slowing (i.e., no change in comparison latencies), which we unsuccessfully attempted to achieve in Experiment 2 through repeated PPX administration. Such an experiment would also be capable of evaluating the alternative explanation discussed above that increases in comparison latencies are the consequence, rather than the cause, of decreases in accuracy.
An additional explanation for the findings of previous intertemporal choice experiments is that PPX impaired rats’ discrimination of different reinforcer amounts. For instance, in Koffarnus et al. (2011) and in Madden et al.’s (2010) Experiment 2, high PPX doses (0.1–0.32 mg/kg) increased SSR choice in the initial trial block in which rats chose between small and large reinforcer amounts, both available immediately. Because nondrug choice favored almost exclusively the LLR in both studies, the direction of the PPX-induced shift in preference for this alternative suggests that discrimination of reinforcer amounts may have been compromised. Furthermore, given the results of Experiment 1 of the present study (i.e., decreased delay sensitivity following PPX), if amount discrimination were intact in previous studies, then intertemporal choice would have favored the LLR (i.e., rats would have preferred more to less food). That PPX has never been shown to increase LLR choice strongly suggests that reinforcer amounts are discriminated poorly following PPX administration.
Impaired amount discrimination may also interact with and enable the disruption in response–reinforcer contingency discrimination observed in Experiment 2 of the present study. More specifically, if discrimination of response–reinforcer contingencies is impaired, then differences in reinforcer amount (e.g., one vs. three pellets in an intertemporal choice task) may serve a discriminative function that aids in contingency discrimination. However, if amount discrimination is also impaired by PPX, then there are no additional discriminative stimuli available to guide subsequent choice behavior.
To investigate the hypothesis that PPX impairs rats’ discrimination of different reinforcer amounts, Experiment 3 used a symbolic MTS (SyMTS) procedure similar to the SpMTS employed in Experiment 2 (McCarthy & Davison, 1986). The experimental question could not be evaluated using the concurrent-chains procedure of Experiment 1 because impaired amount discrimination is predicted to decrease the slope of the matching function in a manner formally identical to an attenuation of stimulus control, an outcome that would fail to dissociate the two accounts. As discussed below, the SyMTS procedure does not require rats to discriminate the source of reinforcement—only the reinforcer amount obtained—and is therefore less confounded by impairments of other relevant discriminations. In our experiment, rats received response-independently either small or large food amounts (one or three food pellets), which served as the sample stimulus. Following consumption, rats selected a left or right lever to report which sample was provided. The resulting measure of accuracy provided an individualized baseline performance against which the effects of repeated PPX were then compared.
Experiment 3
Methods
Subjects and apparatus
The subjects and apparatus were those used in Experiment 2. A head entry detector (ENV-254-CB, Med Associates Inc., St. Albans, VT) was installed in the front pellet receptacle between Experiments 2 and 3 to precisely coordinate the onset of comparison stimuli. Animal use was in accordance with the IACUC of Utah State University.
Behavioral procedure
Experimental sessions consisted of 40 trials. For the first part of each trial, one of two food reinforcer amounts (one or three pellets) was selected randomly without replacement to be delivered response-independently into the front receptacle (20 trials per reinforcer amount; sample period). The time from a rat’s initial entry into the food receptacle until it had exited the receptacle served as a measure of consumption duration. Once the rat refrained from reentering the receptacle for 0.2 s, the SyMTS task was initiated to assess discrimination of the one- and three-pellet reinforcer amounts (comparison period). First, the left and right levers were inserted and their associated stimulus lights were illuminated. Next, a conditional discrimination was required such that the rat had to press the lever symbolically associated with the sample reinforcer amount (e.g., if three pellets were delivered, choose left lever); symbolic relations were counterbalanced across rats. Correct responses extinguished all stimuli and resulted in the delivery of one food pellet to the front receptacle. Incorrect responses produced the same series of events with a 0.5-s blackout taking the place of pellet delivery. Following each trial, a 30-s ITI occurred during which all stimuli were in the “off” state. Failure to emit a comparison response within 15 s of lever activation resulted in trial termination and the trial being counted as an omission.
For the first 10 sessions of the experiment, a correction procedure was implemented. During this period, trials in which samples were not identified correctly were repeated indefinitely until the correct discrimination was made. Sessions ended once 40 correct discriminations were made or 2 hr had elapsed. The correction procedure was then removed for 10 sessions, after which the repeated PPX assessment began regardless of baseline accuracy.
Drug procedure and data analysis
With the exception of the order of saline and PPX regimens (opposite those experienced in Experiment 2), drug procedures and analytical techniques were identical to those used in Experiment 2. Log d and log b calculations were modified from Equations 2 and 3 to yield amount-specific formulations:
| (4) |
| (5) |
In Equations 4 and 5, RSmallSmall and RLargeLarge correspond to trial counts for correct discriminations of small and large samples, respectively; RSmallLarge and RLargeSmall are trials on which subjects reported incorrectly small and large samples.
Three temporal measures were of interest in Experiment 3: the latency to enter the pellet receptacle to consume the sample, the duration of sample consumption (i.e., head entry duration), and the latency to emit a correct comparison response (i.e., left or right lever press). Based on the results of previous experiments, we hypothesized that repeated PPX would increase these temporal measures. As in Experiment 2, we examined correlations between changes in comparison latencies and discrimination accuracy (calculated as percent correct).
Tolerance was assessed by comparing behavioral measures from the first four sessions to those from the last four sessions of repeated PPX. Nonparametric statistical comparisons (log d and absolute log b) used Wilcoxon signed-ranks tests with an alpha level of .05. Temporal measures met assumptions of normality and were analyzed using paired-samples t tests.
Results
Accuracy and bias
As in Experiment 2, Figure 10 depicts per-session log d and log b measures to facilitate comparison with first-session accuracy and bias. With one exception (B4), first-session PPX administration reduced the accuracy with which rats discriminated differences in amount relative to first-session saline performance, z = −2.93, p < .01, ES = .49. Repeated PPX administration (last four sessions) also reduced accuracy relative to repeated saline for most rats, z = −2.63, p < .01, ES = .54; B4 and G6 being the exceptions. In half of the subjects (B1, B2, G1, G2, G3, & G6), repeated PPX (last four sessions) attenuated decrements observed in the first four sessions of the regimen, while 5 rats (B3, B4, B5, B6, & G5) showed no effect and 1 rat (G4) was slightly less accurate at the end of the regimen. Overall, there was insufficient evidence for the development of tolerance in this measure (p = .13).
Fig. 10.
Individual-subject accuracy (log d) and bias (log b) estimates from the first session (left segment of x-axis) and first and last four sessions (right segment of x-axis) of the repeated saline (open symbols) and PPX (closed symbols) assessments in Experiment 3. SAL = saline. PPX = pramipexole (0.18 mg/kg).
During the first saline session, 5 rats (B2, B4, G1, G2, & G5) were biased towards reporting that the sample amount was small (i.e., positive log b); the remaining rats showed the opposite or no bias. By the last four saline sessions, the grouped (nonabsolute) bias was significantly greater than zero, z = −2.24, p = .03, d = .46. For all but 2 rats (G2 & G4), the first PPX administration shifted bias in favor of reporting that the sample was large, although absolute levels of bias did not differ significantly between saline and drug sessions (i.e., rats were no more biased in PPX than in saline sessions; p = .12). Repeated PPX administration did not affect absolute bias systematically when compared to the last four sessions of saline, (p = .52). Relative to the first four sessions of PPX administration, repeated PPX also did not significantly affect absolute bias (i.e., no tolerance, p = .85).
Response latencies
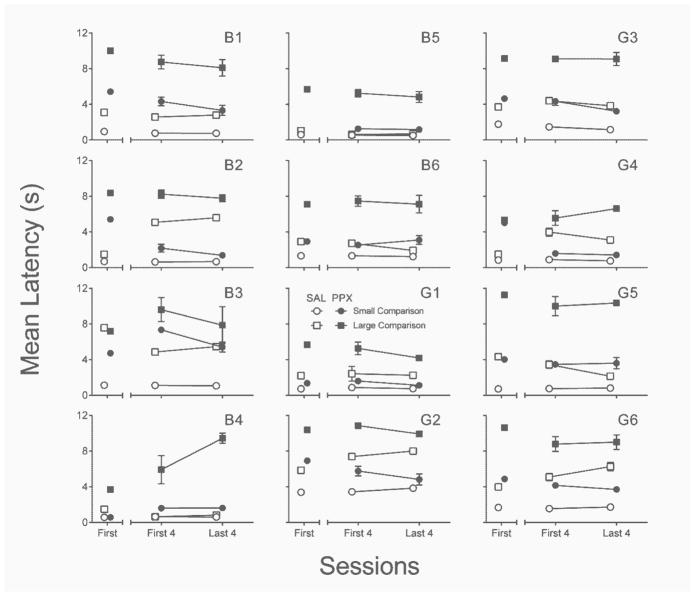
Figure 11 depicts the effects of PPX on mean head-entry latencies to enter the food receptacle to consume the sample pellets. With four exceptions (B2, B6, G5, & G6), rats did not respond to small and large trials differentially in saline sessions, and this was consistent at the group level (all p’s ≥ .13). Neither small- nor large-sample latencies were significantly affected by the first PPX administration relative to the first saline administration (p = .06 and .25, respectively), although at the individual-subject level both B3 and B4 responded more slowly for small than for large samples. Repeated PPX administration (last four sessions), however, significantly increased head entry latencies on both small-, t(11) = 5.61, p < .001, d = 1.62, and large-, t(11) = 4.36, p = .001, d = 1.26, sample trials. Relative to the first four PPX sessions, repeated PPX had no systematic effect on small- or large-sample latencies (both p’s ≥ .29).
Fig. 11.
Individual-subject mean sample latencies from small- and large-sample trials in the first session (left segment of x-axis) and first and last four sessions (right segment of x-axis) of the repeated saline (open symbols) and PPX (closed symbols) assessments in Experiment 3. SAL = saline. PPX = pramipexole (0.18 mg/kg). Error bars represent ±SEM.
Consumption durations (i.e., head-entry durations; data not shown) were significantly increased by the first PPX administration, small: t(11) = 8.02, p < .001, d = 2.31; large: t(11) = 3.26, p < .01, d = .94, as well as by repeated PPX, small: t(11) = 7.32, p < .001, d = 2.11; large: t(11) = 4.61, p < .001, d = 1.33. Small-sample, t(11) = 5.49, p < .001, d = 1.59, but not large-sample consumption durations (p = .31) were significantly lower following repeated PPX administration.
As shown in Figure 12, with few exceptions, latencies to emit a correct comparison response were of shorter duration for small-sample trials compared to large-sample trials. First-session PPX administration elevated both comparison latencies, small: t(11) = 4.45, p = .001, d = 1.28; large: t(11) = 7.44, p < .001, d = 2.15, as did repeated PPX (relative to repeated saline), small: t(11) = 4.96, p < .001, d = 1.43; large: t(11) = 6.40, p < .001, d = 1.85. The statistical analysis suggested that small-sample comparison latencies declined from the first four to the last four sessions, t(11) = 2.65, p < .03, d = .76, but this difference was often not observed at the level of individual rats. No such decrease was observed in large-sample comparison latencies (p = .91). The frequency of omissions per session also increased above saline levels with first, t(11) = 2.34, p < .04, d = .68, and repeated PPX administration, t(11) = 2.81, p < .02, d = .81, with no indication of tolerance (p = .42; data not shown).
Fig. 12.
Individual-subject mean comparison latencies from small- and large-sample trials in the first session (left segment of x-axis) and first and last four sessions (right segment of x-axis) of the repeated saline (open symbols) and PPX (closed symbols) assessments in Experiment 3. SAL = saline. PPX = pramipexole (0.18 mg/kg). Error bars represent ±SEM.
Accuracy-latency correlations
Figure 13 depicts correlations between changes in accuracy (calculated as percent correct and shown separately for small- and large-sample trials) as a function of changes in correct comparison latency following the first session and last four sessions of PPX administration. Unlike in Experiment 2, absolute change in accuracy differed significantly by sample-trial type (small vs. large) following the first PPX administration, t(11) = 3.45, p < .01, d = 1.00, but not over the course of the last four PPX sessions (p = .43). Absolute change in small- and large-sample correct comparison latencies also differed significantly at each time point, first: t(11) = 2.98, p = .01, d = .86; last four: t(11) = 3.76, p < .01, d = 1.09. For these reasons, accuracy-latency correlations were evaluated separately for each sample-trial type.
Fig. 13.
Correlations between changes in percent correct and changes in comparison latencies for small- and large-sample trials in the first session (open symbols) and last four sessions (closed symbols) of PPX administration in Experiment 3. The dashed horizontal lines indicate no change from saline performance. Statistical outcomes are inset.
In the first PPX session (left panel), correct comparison latencies on small-sample trials increased by an average of 2.31 s (SD = 1.80) and accuracy on these trials decreased by an average of 32.90% (SD = 19.25). A significant negative correlation between these change measures was observed (i.e., longer latencies were correlated with greater decrements in accuracy, r = −0.68, p = .01). On large-sample trials, latencies were increased by an average of 4.24 s (SD = 1.97) and accuracy decreased by an average of 6.57% (SD = 11.56). For these trials, the correlation between the change measures did not achieve conventional levels of significance, r = .49, p = .10.
With repeated PPX administration (right panel), correct comparison latencies on small-sample trials were increased above repeated saline levels by an average of 1.67 s (SD = 1.17) and accuracy was decreased by 11.26% (SD = 8.82). Repeated PPX change measures were significantly less extreme when compared to first-session PPX change measures (both p’s ≤ .03), and this difference was manifest in the nonsignificant negative correlation on small-sample trials. On large-sample trials, repeated PPX increased correct comparison latencies by an average of 4.28 s (SD = 2.32) and decreased accuracy by 7.76% (SD = 12.27). Unlike small-sample trials, neither of these change measures differed significantly from those calculated using first-session data (both p’s ≥ .93). The correlation between accuracy and latency changes was also nonsignificant for large-sample trials following repeated PPX administration.
Discussion
Experiment 3 was conducted to assess the effects of PPX administration on rats’ discrimination of small and large reinforcer amounts. Both the first session and last four sessions of PPX administration reduced rats’ accurate discrimination of the reinforcer amounts (log d). Nondrug bias (log b) in favor of reporting a small sample was reversed in the first PPX session, although absolute levels of bias remained unaffected by PPX. Temporal characteristics of responding such as latencies to enter the food receptacle, durations of sample consumption, and latencies to correctly report the sample (i.e., emit a comparison response) were generally elevated by initial and repeated PPX administration. Of these measures, only two (small-sample consumption duration and small-sample correct comparison latency) decreased over the course of repeated PPX dosing (i.e., showed evidence for tolerance).
As in Experiment 2, we hypothesized that PPX-induced increases in latencies to emit a comparison response could negatively affect discrimination accuracy. This was true of first-session small-sample trials, which may reflect an effect of delay on discrimination accuracy, or it may be the result of lower reinforcement rates produced by poor accuracy. The nonsignificant correlation between changes in latencies and accuracy on large-sample trials does not support the latter interpretation. In fact, the trend in these trials was for rats to show greater decrements in accuracy at shorter comparison latencies (i.e. positive relation).
One plausible explanation for these differences across sample-trial types is that rather than the relative reinforcer amounts serving as the sample stimuli, rats responded under the stimulus control of relative reinforcer consumption durations. That is, under saline conditions, small samples accompanied relatively shorter consumption durations than large samples. When PPX increased consumption durations for both samples, the time spent eating a small sample more closely approximated the time spent eating large samples during saline sessions. As such, as more time was spent eating the small sample, the more likely was the rat to report that it was a large sample (hence the negative correlation in the left panel of Figure 13). Consistent with this account, in the last four sessions of repeated PPX, consumption duration and correct comparison latencies were significantly reduced, relative to first-session levels, in small- but not large-sample trials following repeated PPX administration. When consumption durations returned to levels approximating those in saline sessions this may have improved accuracy through restoration of the discriminative stimuli that previously controlled SyMTS performance.
Regardless of the mechanism involved, the finding that PPX reduced discrimination of different reinforcer amounts—or different reinforcer consumption durations—in the present experiment supports the hypothesis that previous intertemporal choice studies may have been affected by poor amount discriminations. Compared to the magnitude of the disruptions in choice observed by Madden et al. (2010) and Koffarnus et al. (2011)—that is, disruptions sufficient to shift choice toward indifference—the disruption of amount discrimination in Experiment 3 was modest. Thus, impaired amount discrimination alone cannot likely account for the disruptions in choice evident in those studies.
General Discussion
Across three experiments, putative behavioral mechanisms underlying the effects of acute and repeated PPX on intertemporal choice were investigated in an effort to provide an explanation for the divergent findings of Madden et al. (2010) and Koffarnus et al. (2011). Those findings are as follows:
Madden et al. (2010, Exp. 1) reported that PPX (0.1–0.3 mg/kg) increased SS choice in a baseline condition of nondrug LLR preference (“self-control” baseline), but not in a control condition of nondrug SSR preference (“impulsive” baseline), suggesting that the drug did not affect choice nonspecifically (i.e., attenuate stimulus control);
Koffarnus et al. (2011) and Madden et al. (2010, Exp. 2) reported that PPX (0.1, 0.18, 0.3, & 0.32 mg/kg) increased preference for the SSR primarily when the larger reinforcer was not delayed (0-s trial block of the increasing-delay procedure) and generally shifted preference toward indifference (50% choice) in subsequent trial blocks, two findings suggesting that PPX attenuated aspects of stimulus control over choice behavior.
In the present study, behavioral processes relevant to intertemporal choice were experimentally isolated to quantify the effect of PPX on each process independently. To the extent that the effects observed across these experiments generalize to the more complex procedural arrangements characterizing intertemporal choice studies, the present research may help to explain why PPX produces the divergent behavioral outcomes summarized above.
We hypothesized that Finding 1 could have been the product of PPX-enhanced sensitivity to LLR delays. That is, increased delay sensitivity would manifest as increased preference for the SSR. This account was not supported by the results of Experiment 1. Instead, sensitivity to relative reinforcer delay was diminished by acute PPX, whereas repeated PPX did not affect sensitivity to delay. This finding suggests PPX attenuates stimulus control over choice behavior, an account consistent with Finding 2.
Experiments 2 and 3 of the present study investigated aspects of stimulus control that may have been disrupted by PPX. Experiment 2 revealed that PPX—both initially and following repeated dosing—decreased the accuracy of rats’ discrimination of a spatial response–reinforcer contingency. If rats are unable to discriminate the source of reinforcers (left or right lever) then this would be expected to shift intertemporal choice toward indifference (Finding 2). Likewise, Experiment 3 revealed that PPX modestly disrupted discrimination of reinforcer amounts. If reinforcer amounts are discriminated imperfectly, then intertemporal choice should be governed increasingly by differences in reinforcer delay (i.e., shifting preference toward the SSR, Finding 1). However, this requires that delay discrimination remains intact, an outcome not supported by the results of our Experiment 1. Given the profile of outcomes of the present experiments, it would appear that the mechanism of PPX’s effects on intertemporal choice is a disruption in stimulus control; that is, control by reinforcer delays (Experiment 1), response–reinforcer contingencies (Experiment 2), and reinforcer amount (Experiment 3).
Although our findings implicate PPX in the attenuation of aspects of stimulus control over choice behavior, it is possible that the drug may have also affected reinforcer efficacy. Diminishing reinforcer efficacy could explain the increased response latencies observed in our experiments and the often reported sedative effects of the drug (e.g., Hamidovic et al., 2008; Johnson et al., 2011; Koffarnus et al., 2011; Madden et al., 2010). In rats, D3-preferring dopamine agonists like PPX (e.g., 7-OH-DPAT) decrease consumption of freely available food unless longer durations of deprivation are arranged (e.g., McQuade, Benoit, Woods, & Seeley, 2003). To our knowledge, there are no examinations of PPX’s effects on reinforcer efficacy but data from Experiment 3 suggest decreased reinforcer efficacy was not a factor in our study. Here, food reinforcers were provided response-independently as samples, and in our proxy for examining acute drug effects (i.e., first-session PPX), the drug did not significantly increase head-entry latencies (see Figure 11). Further, while repeated PPX did significantly increase these latencies, the average increase for both sample-trial types was 0.50 s (SD = 0.36). That rats would continue to respond quickly relative to saline latencies suggests that the negative effects of acute or repeated PPX on reinforcer efficacy are minimal. This interpretation, however, does not exclude the possibility that previous intertemporal choice studies or the other experiments in the present study were not influenced by PPX-induced changes in reinforcer efficacy.
Across three experiments, the effects of PPX administration on choice appeared to be mediated by impairing stimulus control. Confidence in this account should be tempered by five limitations of the present line of research. First, the procedures used were designed to isolate single behavioral processes; as such, the results of these experiments may not reveal the interactions between these processes that contributed to the findings of previous intertemporal choice studies. Second, the concurrent-chains procedure used in Experiment 1 was designed to isolate the effects of relative reinforcer delays on response allocation, but as demonstrated in Experiment 2 was likely also influenced by negative effects of PPX on discrimination of response–reinforcer contingencies. As such, the procedure may not have provided a valid index of the drug effect on delay sensitivity independent of other behavioral perturbations. Use of a SyMTS procedure with delays as sample stimuli or a temporal bisection task (Church & Deluty, 1977) could have addressed this procedural shortcoming and resulted in an unadulterated measure of delay discrimination. Third, rats were significantly biased in favor of reporting a small sample under repeated saline conditions in Experiment 3. This may have been the result of providing reinforcement of an identical magnitude for correct reinforcer-amount discriminations. Whether this bias interacted with the drug effect to reduce accuracy is unknown, but remains a possibility that could have been avoided through the use of reinforcement differing qualitatively from the sample stimuli. Fourth, the repeated PPX dose of 0.18 mg/kg was chosen for examination because this dose produced behavioral effects in previous studies and with fewer omissions than the highest PPX dose (0.3 mg/kg). Investigation of repeated PPX is important for its resemblance to the regimens of clinical patients and should be parametrically examined across a wider dose range to accurately describe its effects at both low and high doses. Finally, Experiments 2 and 3 were conducted using the same subjects, a decision which may have reduced baseline accuracy in the amount discrimination task that was completed after the contingency discrimination task. Within-subject manipulations allow researchers to reduce the number of subjects used, but may also compromise behavioral performances if dependent measures are prone to interference from historical variables.
In sum, the present research was conducted to identify candidate behavioral mechanisms underlying the effects of PPX in previous nonhuman intertemporal choice studies. In light of these efforts, however, the determinants of clinically aberrant impulsive behaviors associated with PPX and other DA medications in human populations remain elusive. Recent neurobiological evidence suggests that dysregulation of corticostriatal dopaminergic circuitry may place certain individuals prescribed DA agonists at risk for development of impulsive behavior (Ray et al., 2012). On a more molecular level, these functional irregularities may be the product of polymorphisms of genes responsible for regulating D2 and D3 receptor subtypes (Lee, Lee, Park, Lim, Kim, Kim, & Jeon, 2009; but, see Vallelunga, Flaibani, Formento-Dojot, Facchini, & Antonini, 2012). In these instances, nonhuman approaches not unlike the one presented herein may be uniquely positioned to illuminate the complex interactions between biology, pharmacology, and behavior. Future research may expand upon our efforts through pretreatments with selective antagonists or through the use of DA receptor knockout models to further elucidate the biobehavioral bases of impulsivity associated with drug administration.
Acknowledgments
The first author thanks dissertation committee members Timothy A. Shahan, Amy L. Odum, Andrew L. Samaha, and Timothy A. Gilbertson. This research was supported by a grant from the National Institute on Drug Abuse (1R01DA029605) awarded to the last author.
Footnotes
Portions of these data were presented at the 2010 meeting of the Association for Behavior Analysis International in San Antonio, TX, the 2011 Behavioral Economics Conference in Chicago, IL, and the 2012 meeting of the Society for the Quantitative Analysis of Behavior in Seattle, WA. This report is based on the first author’s dissertation in partial fulfillment of a Ph.D. in Psychology at Utah State University.
References
- Allison PD. Missing data. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Antonini A, Calandrella D. Once-daily pramipexole for the treatment of early and advanced idiopathic Parkinson’s disease: Implications for patients. Neuropsychiatric Disease and Treatment. 2011;7:297–302. doi: 10.2147/NDT.S10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila A, Cardona X, Martín-Baranera M, Bello J, Sastre F. Impulsive and compulsive behaviors in Parkinson’s disease: A one-year follow-up study. Journal of the Neurological Sciences. 2011;310:197–201. doi: 10.1016/j.jns.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behavioral Research Methods. 2005;37:379–384. doi: 10.3758/BF03192707. [DOI] [PubMed] [Google Scholar]
- Baum WM. On two types of deviation from the matching law: Bias and undermatching. Journal of the Experimental Analysis of Behavior. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J, Gainetdinov R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castañé A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: Investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Brown GS, White KG. The optimal correction for estimating extreme discriminability. Behavior Research Methods. 2005;37:436–449. doi: 10.3758/BF03192712. [DOI] [PubMed] [Google Scholar]
- Chang WL, Breier MR, Yang A, Swerdlow NR. Disparate effects of pramipexole on locomotor activity and sensorimotor gating in Sprague-Dawley rats. Pharmacology Biochemistry and Behavior. 2011;99:634–638. doi: 10.1016/j.pbb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34:651–661. doi: 10.1038/npp.2008.114. [DOI] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Long-term administration of the dopamine D3/2 receptor agonist pramipexole increases dopamine and serotonin neurotransmission in the male rat forebrain. Journal of Psychiatry and Neuroscience. 2012;37:113–121. doi: 10.1503/jpn.110038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:216–228. doi: 10.1037/0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: A case-control study. Sleep. 2010;33:81–87. [PMC free article] [PubMed] [Google Scholar]
- Davison M, Jenkins PE. Stimulus discriminability, contingency discriminability, and schedule performance. Learning & Behavior. 1985;13:77–84. doi: 10.3758/BF03213368. [DOI] [Google Scholar]
- Dews PB. Analysis of effects of psychopharmacological agents in behavioral terms. Federation Proceedings. 1958;17:1024–1030. [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Archives of Neurology. 2005;62:1–5. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61:422–423. doi: 10.1212/01.WNL.0000076478.45005.EC. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. London: Sage Publications Ltd; 2009. [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Weitzman N, Schreiber S, Shabtai H, Peretz C. New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson’s disease: The role of dopamine agonist treatment and age at motor symptoms onset. Journal of Psychopharmacology. 2007;21:501–506. doi: 10.1177/0269881106073109. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of Clinical Psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Hassan A, Bower JH, Kumar N, Matsumoto JY, Fealey RD, Josephs KA, Ahlskog JE. Dopamine agonist-triggered pathological behaviors: Surveillance in the PD clinic reveals high frequencies. Parkinsonism and Related Disorders. 2011;17:260–264. doi: 10.1016/j.parkreldis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Johnson PS, Madden GJ, Brewer AT, Pinkston JW, Fowler SC. Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology (Berl) 2011;213:11–18. doi: 10.1007/s00213-010-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BM, Davison M. Reporting contingencies of reinforcement in concurrent schedules. Journal of the Experimental Analysis of Behavior. 1998;69:161–183. doi: 10.1901/jeab.1998.69-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Rana AQ. Dopamine agonist induced compulsive eating behavior in a Parkinson’s disease patient. Pharmacology World and Science. 2010;32:114–116. doi: 10.1007/s11096-009-9358-0. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism and Related Disorders. 2005;11:381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behavioural Pharmacology. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, Jeon BS. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Movement Disorders. 2009;24:1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- Lucki I, DeLong RE. Control rate of response or reinforcement and amphetamine’s effect on behavior. Journal of the Experimental Analysis of Behavior. 1983;40:123–132. doi: 10.1901/jeab.1983.40-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafó MR. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS, Brewer AT, Pinkston JW, Fowler SC. Effects of pramipexole on impulsive choice in male Wistar rats. Experimental and Clinical Psychopharmacology. 2010;18:267–276. doi: 10.1037/a0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Rogóz Z, Margas W, Kata M, Dziedzicka-Wasylewska M. The effect of repeated treatment with pramipexole on the central dopamine D3 system. Journal of Neural Transmission. 2000;107:1369–1379. doi: 10.1007/s007020070022. [DOI] [PubMed] [Google Scholar]
- Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, Weintraub D. Long-term follow-up of impulse control disorders in Parkinson’s disease. Movement Disorders. 2008;23:75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D, Davison M. Delayed reinforcement and delayed choice in symbolic matching to sample: Effects on stimulus discriminability. Journal of the Experimental Analysis of Behavior. 1986;46:293–303. doi: 10.1901/jeab.1986.46-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D, Davison M, Jenkins PE. Stimulus discriminability in free-operant and discrete-trial detection procedures. Journal of the Experimental Analysis of Behavior. 1982;37:199–215. doi: 10.1901/jeab.1982.37-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JM, Benoit SC, Woods SC, Seeley RJ. 7-OH-DPAT selectively reduces intake of both chow and high fat diets in different food intake regimens. Pharmacology Biochemistry and Behavior. 2003;76:517–523. doi: 10.1016/j.pbb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Mitchell S, de Wit H. Drug effects on delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. pp. 213–241. [DOI] [Google Scholar]
- Perez-Lloret S, Bondon-Guitton E, Rascol O, Montastruc JL French Association of Regional Pharmacovigilance Centers. Adverse drug reactions to dopamine agonists: a comparative study in the French Pharmacovigilance Database. Movement Disorders. 2010;25:1876–1880. doi: 10.1002/mds.23204. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Febbo SM. Quantitative analyses of methamphetamine’s effects on self-control choices: Implications for elucidating behavioral mechanisms of drug action. Behavioural Processes. 2004;66:213–233. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ray N, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, Strafella AP. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiology of Disease. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle JL, Rokosik SL, Napier TC. Pramipexole- and methamphetamine-induced reward-mediated behavior in a rodent model of Parkinson’s disease and controls. Behavioral Brain Research. 2012;233:15–23. doi: 10.1016/j.bbr.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Alan J. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2011;20:205–212. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Madden GJ. Delay discounting and drug abuse: Empirical, conceptual, and methodological considerations. In: Mackillop J, de Wit H, editors. The Wiley-Blackwell handbook of addiction psychopharmacology. Oxford, UK: Wiley-Blackwell; (in press) [Google Scholar]
- Szarfman A, Doraiswamy PM, Tonning JM, Levine JG. Association between pathologic gambling and parkinsonian therapy as detected in the Food and Drug Administration Adverse Event database. Archives of Neurology. 2006;63:299–300. doi: 10.1001/archneur.63.2.299-b. [DOI] [PubMed] [Google Scholar]
- Ta WM, Pitts RC, Hughes CE, McLean AP, Grace RC. Rapid acquisition of preference in concurrent chains: Effects of d-amphetamine on sensitivity to reinforcement delay. Journal of the Experimental Analysis of Behavior. 2008;89:71–91. doi: 10.1901/jeab.2008.89-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallelunga A, Flaibani R, Formento-Dojot P, Facchini S, Antonini A. Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism and Related Disorders. 2012;18:397–399. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Stacy M. Impulse control disorders in Parkinson disease: A multicenter case-control study. Annals of Neurology. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- Washio Y, Higgins ST, Heil SH, McKerchar TL, Badger GJ, Skelly JM, Dantona RL. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Experimental and Clinical Psychopharmacology. 2011;19:243–248. doi: 10.1037/a0023617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Lang AE. Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Archives of Neurology. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- White KG. Remembering and forgetting. In: Madden GJ, Dube WV, Hackenberg T, Hanley GP, Lattal KA, editors. APA handbooks in psychology: APA handbook of behavior analysis, Vol. 1: Methods and principles. Washington, DC: American Psychological Association; 2013. pp. 411–437. [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15:176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]