Abstract
The host immune response to pathogens is a complex biological process. The majority of in vivo studies classically employed to characterize host-pathogen interactions take advantage of intraperitoneal injections of select bacteria or pathogen associated molecular patterns (PAMPs) in mice. While these techniques have yielded tremendous data associated with infectious disease pathobiology, intraperitoneal injection models are not always appropriate for host-pathogen interaction studies in the lung. Utilizing an acute lung inflammation model in mice, it is possible to conduct a high resolution analysis of the host innate immune response utilizing lipopolysaccharide (LPS). Here, we describe the methods to administer LPS using nonsurgical oropharyngeal intratracheal administration, monitor clinical parameters associated with disease pathogenesis, and utilize bronchoalveolar lavage fluid to evaluate the host immune response. The techniques that are described are widely applicable for studying the host innate immune response to a diverse range of PAMPs and pathogens. Likewise, with minor modifications, these techniques can also be applied in studies evaluating allergic airway inflammation and in pharmacological applications.
Keywords: Infection, Issue 86, LPS, Lipopolysaccharide, mouse, pneumonia, gram negative bacteria, inflammation, acute lung inflammation, innate immunity, host pathogen interaction, lung, respiratory disease
Introduction
Pulmonary infections associated with pathogenic bacteria species are a common cause of global morbidity and mortality. Determining the mechanisms that drive the host immune response to these pathogens will promote the development of novel prevention strategies and therapeutic agents that will attenuate the impact of these infections. The overall goal of the protocol described here is to provide the user with a flexible method to evaluate the host innate immune response to pathogen infection using a pathogen associated molecular pattern (PAMP) as a surrogate for live bacteria. The majority of previous studies evaluating the host innate immune response to bacteria have focused on peritoneal models due to the relative ease of execution. While these models are highly useful and have resulted in significant advances in the field of host-pathogen interactions and systemic inflammation, the data generated from these models are not always appropriate for studies involving the respiratory system. Here, a pulmonary model of acute lung inflammation is proposed as a practical and clinically relevant expansion of the classical intraperitoneal (i.p.) injection models. The proposed technique allows for the local assessment of the innate immune response in an organ specific model system.
The methods described here are designed to provide a simple and robust technique to allow users to evaluate the host immune response to LPS, which is a common PAMP. The methods are based on intratracheal (i.t.) instillation of LPS, which induces a robust innate immune response in the lungs of mice and mimics many of the pathophysiological features observed in human patients suffering from respiratory infections and acute lung injury1. A primary advantage of this technique is that it allows the user to evaluate the host immune response without the confounding factors and safety concerns associated with conducting in vivo studies using live bacteria. Likewise, the oropharyngeal i.t. administration route of exposure described in this protocol has significant advantages over other commonly utilized techniques, including intranasal (i.n.) administration and surgical i.t. administration. For example, oropharyngeal i.t. administration allows relatively accurate dosaging and lung deposition compared to i.n. administration, which typically suffers from increased variability of lung deposition due to the loss of agents in the nasal cavity and sinuses2-4. The i.t. administration route circumvents these cavities and allows direct access to the trachea and airway. Likewise, the surgical i.t. approach is a significantly more morbid administration method and requires extensive training to master. The protocols described here also include a description the common techniques and surrogate markers used to evaluate inflammation progression and end with a protocol describing the proper techniques for preparing the lungs for histopathology assessments. These protocols are focused on minimizing the number of mice required for each study by maximizing the data generated from each individual animal.
The protocols described are highly flexible and can be readily modified to evaluate a diverse range PAMPs and damage associated molecular patterns (DAMPs). Furthermore, with a few additional modifications, these protocols can also be applied to studies evaluating allergic airway disease progression or host-pathogen interactions with live bacteria, viruses or fungi5-10.
Protocol
All studies were conducted under the approval of the Institutional Care and Use Committee (IACUC) for Virginia Tech and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1. Intratracheal (i.t.) Inoculation of LPS Using Oropharyngeal Administration
Ensure that each animal is uniquely identified using either an ear punch, ear tag, or other institutionally approved method.
Record the baseline body weight and body temperature for each animal.
Prepare the working stock of LPS. Each mouse will receive a 50 µl dose of 1 mg/kg LPS in 1x phosphate buffered saline (PBS). Mock treated animals will receive a 50 µl dose of 1x PBS.
- Prepare an appropriate chamber for the administration of isoflurane using the following drop method. Institutional guidelines vary regarding the use of isoflurane and other anesthetic agents and prior to initiating any studies, persons should contact their institution's Department of Animal Care/Welfare to insure the all guidelines are adequately met. If drop method isoflurane is not an option, other anesthetic agents are acceptable alternatives.
- In an appropriate safety cabinet, apply approximately 3 ml of isoflurane to a folded absorbent paper towel and place in the bottom of a 500 ml beaker.
- Place a small piece of aluminum foil on top of the paper towel and cover the beaker with a transparent lid.
Prepare the tools and reagents that will be utilized during the i.t. inoculation. Position the rubber band that will secure the animals head on the intubation stand. This procedure will require a pair of straight forceps, a pair of angled or curved forceps, and a p200 pipette with tips.
Load 50 µl of LPS into the pipette tip and place in a readily accessible location beside the intubation stand.
Anesthetize the mouse by placing the animal into the isoflurane chamber and covering the chamber with the transparent lid. Observe the animal's breathing patterns, which will be rapid and shallow when first placed in the chamber. The animal will be sufficiently anesthetized when breathing rates approach 1 breath/2 sec, which is usually reached within 30 sec of placing in the chamber. The mouse should be anesthetized as stated in the approved animal protocol using drop method isoflurane.
Remove the anesthetized mouse from the chamber and suspend the animal on the intubation stand by its front incisors. Insure that the animal is securely restrained and the mouth and tongue are accessible.
Gently secure the tongue with the straight forceps. Grasp the tongue with the angled forceps and gently pull it out of the mouth until slight resistance is felt. This action is sufficient to block the epiglottis and gain access to the trachea.
While continuing to hold the tongue in the extended position, administer the 50 µl dose of LPS into the back of the throat and immediately cover the animal's nostrils with a gloved finger. The LPS will be visible in the back of the throat until the mouse inhales.
Continue to cover the nostrils and hold the tongue extended for 5-10 additional breaths.
Remove the mouse from the intubation stand and place the animal back into a new cage until it recovers from the anesthesia.
Monitor surrogate markers associated with disease progression. Body weight and body temperature should be monitored every 4-8 hr for the duration of the study. Likewise, evaluate behavioral characteristics and clinical symptoms associated with increased morbidity.
To evaluate disease progression, harvest mice at specific time-points following the LPS exposure. Typical time-points for harvest following LPS exposure include 0, 6, 12, 18, 24, and 48 hr. To evaluate recovery and survival, evaluate the mice for 7 days post-inoculation.
2. Serum and Bronchoalveolar Lavage Fluid (BALF) Collection
Sacrifice the mouse using an institutionally approved method.
Place the animal on its back and secure it to a necropsy board, ideally 0.5 in (1.27 cm) in height.
Wet the entire mouse with 70% ethanol.
Using a 1 ml syringe with a 27 G needle, conduct a cardiac puncture prior to making any incisions. It should be possible to withdraw 500-800 µl of whole blood from a single mouse.
Remove the needle from the syringe and transfer the whole blood to a prelabeled 1.5 ml microcentrifuge tube. For serum collection, allow the whole blood to coagulate for at least 30 min at room temperature. In addition to serum collection, this procedure can also be modified by incorporating an anti-coagulant in the tube and syringe, prior to collecting the whole blood, to study circulating immune cells.
Make a horizontal incision across the length of the peritoneal cavity and a vertical incision from the peritoneal cavity to the lower jaw of the mouse.
Using forceps, grasp both sides of the incision and gently pull the skin away from the underlying peritoneal and thoracic cavities.
Make a large incision along the length of the peritoneal cavity from the genitals to the sternum, taking care to avoid cutting the diaphragm. Gently shift the intestines in the peritoneal cavity to allow access to one of the kidneys.
Cut the renal vein leading to the kidney to serve as a drainage point for perfusion.
Nick the diaphragm using scissors, taking care to avoid the lungs and heart, to expose the left side of the heart.
Manually perfuse the heart using a 10 ml syringe with a 27 G needle and 1x PBS solution. Avoid applying excessive force during the perfusion to ensure that the saline is not forced into the lungs. The remaining blood should drain from the portal vein incision.
Carefully cut the ribcage along the sternum using blunt/blunt scissors, taking care to avoid cutting the heart and lungs. Accidentally cutting the lungs during this procedure will significantly reduce the amount of BALF collected and will result in an inability to properly inflate the lungs with fixative.
Gently isolate the heart and lungs away from the ribcage using forceps. Carefully cut each section of the ribcage using blunt/blunt scissors as close to the spine as possible and fully remove both sections.
Separate the salivary glands using forceps or remove them using scissors, to expose the trachea.
Carefully cut the muscles that overlay the trachea using scissors. It is essential that the trachea not be cut during this process.
Using scissors, separate the collarbone overlying the trachea.
Gently grasp the thymus using forceps and lift the tissue away from the heart. Remove the thymus using scissors, while taking care to avoid cutting the heart or lungs.
Make a small horizontal incision in the trachea 1-2 rings below the larynx using angled scissors (45-90º angle, sharp/sharp). The incision should be large enough to firmly secure the cannula.
Insert the cannula so that the tapered end extends 2-3 tracheal rings below the incision and secure the cannula in place using a silk suture. Ensure that the suture passes between the trachea and the esophagus and is pulled tight on the cannula.
Fill a 1 ml syringe with 1 ml of Hanks Buffered Saline Solution (HBSS) and gently insert its luer into the cannula.
Using a slow, but constant motion, inject ~900 µl into the trachea, inflating the lungs, and immediately withdraw the HBSS. Place the recovered BALF in a 15 ml conical tube.
Repeat this lavage 2 additional times to collect approximately 3 ml of BALF for future analysis. Store the BALF on ice or at 4 ºC until ready for use.
3. Histopathology Preparation
Insert a 10 ml syringe into the lung inflation stand. Assemble the tubing to the luer, the luer to the stopcock, and the stopcock to the syringe. Ensure the stopcock is in the closed position. The 10 ml syringe will serve as a gravity flow reservoir. The reservoir should be attached to the inflation stand 4.75 in above the animal and the top of the reservoir should extend 10.5 in above the animal.
Fill the syringe with neutral buffered formalin solution. Ensure that the syringe is completely filled with the fixative.
Place an absorbent towel under the open end of the tubing and open the stopcock to allow the fixative to fill the tubing. Once the fixative begins flowing from the tubing, immediately close the stopcock and refill the syringe to the top with fixative. It is important that the syringe be completely filled to provide the appropriate amount of pressure for lung inflation.
Gently pass a second piece of suture thread under the trachea, approximately 1-2 tracheal rings below the end of the cannula.
Tie a single loose knot in the suture; however, do not pull the knot tight.
Insert the tubing from the mouse inflation stand into the cannula.
Open the stopcock and allow the lungs to fill by gravity inflation.
Once the lungs reach their maximum inflation level, pull the suture tight and tie a second knot.
Close the stopcock and remove the tubing from the cannula.
Gently remove the cannula from the trachea by grasping the suture with a pair of forceps and holding the cannula with fingers. Firmly pull the suture down towards the thoracic cavity and the cannula back towards the mouse's nose.
Gently grasp the trachea or the sutures with forceps and lift the trachea away from the neck cavity.
Sever the trachea caudal to the tied suture using blunt scissors.
Once the trachea is free from the underlying tissues, begin to pull the trachea away from the mouse. Gently lift the lungs out of the thoracic cavity.
Carefully cut the connective tissue holding the lungs in the thoracic cavity. Continue pulling the trachea away from the mouse's body and apply steady upward force while cutting the underlying connections. Take care to avoid cutting the lungs. Note that the heart should be left attached to the lungs using this method.
Once the lungs have been removed, place them in 10 ml of buffered formalin.
Remove a section of mouse tail for genotyping.
Dispose of the mouse carcass following the appropriate institutional guidelines.
4. Cytokine Evaluation
Centrifuge the whole blood, collected under step 2.5, at 12,000 x g for 5 min to isolate the serum. Transfer the serum to a prelabeled 1.5 ml microcentrifuge tube and store at -80 °C.
Evaluate the serum cytokine levels by ELISA or another similar assay. Dilute the serum 1:5 - 1:20 in an appropriate dilution buffer, depending on the assay, following the manufactures instructions. These dilutions should be empirically determined prior to running the bulk of the samples.
Due to the low volume of serum collected, reduce the sample volume loaded onto the ELISA plate by half. For example, most commercial ELISAs utilize 100 μl volumes of standards and samples. To conserve specimens, load 50 μl of standards and diluted serum per well.
Centrifuge the BALF in a refrigerated tabletop centrifuge at 200 x g for 5 min. Transfer the cell free supernatant into two prelabeled 1.5 ml microcentrifuge tubes and store at -80 °C.
Evaluate BALF cytokines by ELISA or another similar assay without diluting.
Store unused serum and BALF at -80 °C.
5. Differential Staining and BAL Cellularity Evaluation
Following BALF centrifugation and complete HBSS removal, lyse the pelleted red blood cells using hypotonic saline. Resuspend the cells in 900 μl of distilled water. Immediately add 100 μl of 10x PBS.
Individually lyse each sample. If samples contain excessive amounts of red blood cells, the cells can be pelleted in the table top centrifuge at 400 x g for 5 min and the red blood cell lysis protocol can be repeated.
Determine the total BALF cellularity in the 1 ml suspension using a hemacytometer under 10X or 20 X magnification with Trypan Blue staining. Evaluate and present these data as cells/ml.
Prepare materials for the cytospin and differential staining. Label standard microscope slides using a pencil or solvent resistant pen. Secure the slides into a cytospin bracket and funnel. Secure the slide assembly in the cytospin rotor.
Cytospin 150 μl of BALF at 100 x g for 5 min. If the cell density is too great to effectively evaluate cell morphology, reduce the volume spun down onto the slides. Allow the slides to air dry overnight.
Differential stain the slides following the manufactures protocols. Allow the slides to air dry overnight. Coverslip the slides using Permount and evaluate the slides using a microscope equipped with a 20X and 40X objective.
Harvest the remaining cells for subsequent analysis, such as FACS, electron microscopy, confocal microscopy, RNA extraction for gene expression evaluation, and/or protein extraction for western blot.
6. Histopathology Evaluation
Prepare the lungs for histopathology evaluation. After 24-48 hr of formalin fixation, ventrally orient the whole inflated lungs and embedded in paraffin. Cut the resultant blocks to expose the main conducting airway.
To improve scoring accuracy, position the lungs in the same position and trim each block to yield the maximum longitudinal visualization of the intrapulmonary main axial airway. From this point, cut 5 micron serial sections and stain with hematoxylin and eosin (H&E). Additional sections can be cut and prepared for in situ hybridization using standard protocols.
Evaluate histopathology using a semi-quantitative scoring system based on the following inflammatory parameters, which are scored between 0 (absent) and 3 (severe): mononuclear and polymorphonuclear cell infiltration; airway epithelial cell hyperplasia and injury; extravasation; perivascular and peribroncheolar cuffing; and the percent of the lung involved with inflammation. Lung histopathology should be evaluated by an experienced pathologist.
Average all of the parameter scores to generate a total histopathology score or use individual scores to quantify specific aspects of disease progression. Conduct all scoring in a double blind fashion, with reviewers blinded to both genotype and treatment. This scoring system has been previously described6,7,10.
Representative Results
The cell walls of gram-negative bacteria are composed of LPS, which is highly abundant in the environment. Inhalation of LPS in sensitive human populations exacerbates airway reactivity and is capable of triggering a robust immune response11. LPS is also a common PAMP used in mouse models to elicit a robust innate immune response. In the protocol described here, the mice received an i.t. dose of LPS isolated from E. coli (serotype 0111:B4) using oropharyngeal i.t. administration. In models of LPS exposure, both body temperature and weight are typical surrogate markers of animal morbidity and disease progression. The LPS challenge will cause a significant decrease in body temperature within the first 6 hr, which will gradually increase back to baseline over the course of 24 hr (Figure 1A). However, body weight will steadily decrease over the course of 24 hr (Figure 1B), where it will peak and gradually recover over the next 48-72 hr. Thus, body temperature is a more appropriate marker to evaluate the early stages of inflammation initiation; whereas, body weight is more reliable during later stages of pathogenesis and recovery.
Airway LPS exposure results in a significant influx of leukocytes to the lungs. Within the first 6 hr, cells associated with the host innate immune response can be observed in the BALF (Figure 1C). The BALF cellularity continues to increase over the next 24-48 hr (Figure 1C), where the immune response peaks and subsequently enters a period of inflammation resolution. By 24 hr, a significant number of neutrophils are present in the lungs and can be observed in the BALF following differential staining (Figures 1D and 1E). BALF cellularity assessments provide a robust and quantifiable technique to characterize the cells associated with the host immune response in the lungs. The increase in BALF cellularity is consistent with a significant influx of neutrophils into the airways, blood vessels and in the lung parenchyma (Figures 1F and 1G). Lung histopathology can be effectively evaluated using a semi-quantitative scoring system (Figure 1F). It is possible to accurately score the histopathology at specific landmarks in the lungs of different animals when the lungs are accurately inflated to the same size with fixative and sections processed to reveal the maximum longitudinal visualization of the intrapulmonary main axial airway. The gravity based inflation protocol, described here, is designed to facilitate this scoring system. LPS induces a significant increase in perivascular, peribroncheolar and parenchymal inflammation (Figure 1G).
LPS administration induces high levels of local and systemic pro-inflammatory mediators, including several cytokines associated with the innate immune response. Local cytokine levels can be assessed in the BALF using conventional techniques, such as ELISA. Common cytokines that are up-regulated in the lungs following LPS administration include TNF-α, IL-1β, and IL-6 (Figure 2A). Systemic cytokine levels can be evaluated in the serum using the same techniques as described for the BALF assessments (Figure 2B). It is not uncommon for some mediators to be present in the local microenvironment, but absent in the serum. For example, TNF-α is routinely found at high levels in the lungs following LPS, but is not typically found systemically under the conditions described in this protocol (Figures 2A and 2B; below the level of detection).
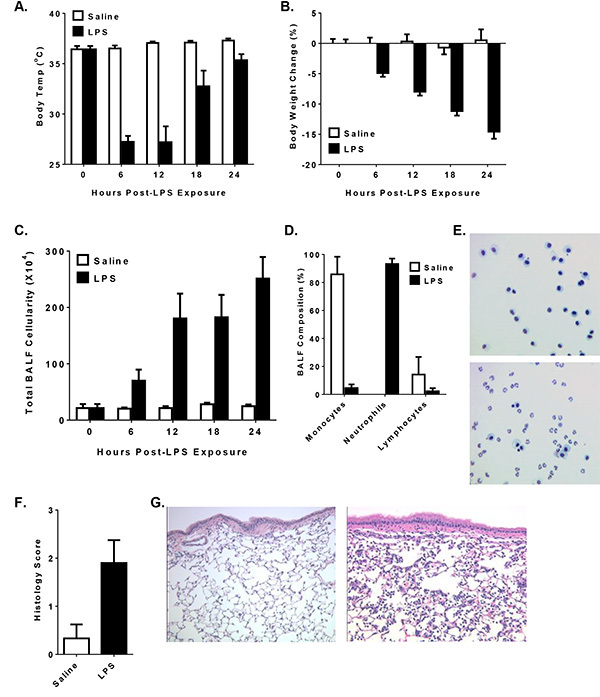 Figure 1. Oropharyngeal Intratracheal LPS Administration Increases Morbidity and Airway Inflammation in Mice. Male mice received 1 mg/kg of E. coli LPS (serotype 0111:B4) i.t. and disease pathogenesis was evaluated over the course of 24 hr. A-B) LPS induces a significant reduction in body temperature within 6 hr of administration; whereas body weight loss is more apparent at the later time points. C) LPS induces a significant increase in total BALF cellularity. D-E) Neutrophils are the dominate cell type present in the lungs 24 hr following LPS administration, as revealed by differential staining from cells isolated from the BALF. These data are typically illustrated as the percent of each cell type present in the BALF. F) Lung histopathology can be evaluated using a semi-quantitative scoring system based on two independent evaluations of the inflammation surrounding the intrapulmonary main axial airway. G) A significant amount of perivascular and peribroncheolar cuffing, mild parenchymal inflammation and some slight alveolar occlusion is typically observed 24 hr following lung LPS exposure. Click here to view larger image.
Figure 1. Oropharyngeal Intratracheal LPS Administration Increases Morbidity and Airway Inflammation in Mice. Male mice received 1 mg/kg of E. coli LPS (serotype 0111:B4) i.t. and disease pathogenesis was evaluated over the course of 24 hr. A-B) LPS induces a significant reduction in body temperature within 6 hr of administration; whereas body weight loss is more apparent at the later time points. C) LPS induces a significant increase in total BALF cellularity. D-E) Neutrophils are the dominate cell type present in the lungs 24 hr following LPS administration, as revealed by differential staining from cells isolated from the BALF. These data are typically illustrated as the percent of each cell type present in the BALF. F) Lung histopathology can be evaluated using a semi-quantitative scoring system based on two independent evaluations of the inflammation surrounding the intrapulmonary main axial airway. G) A significant amount of perivascular and peribroncheolar cuffing, mild parenchymal inflammation and some slight alveolar occlusion is typically observed 24 hr following lung LPS exposure. Click here to view larger image.
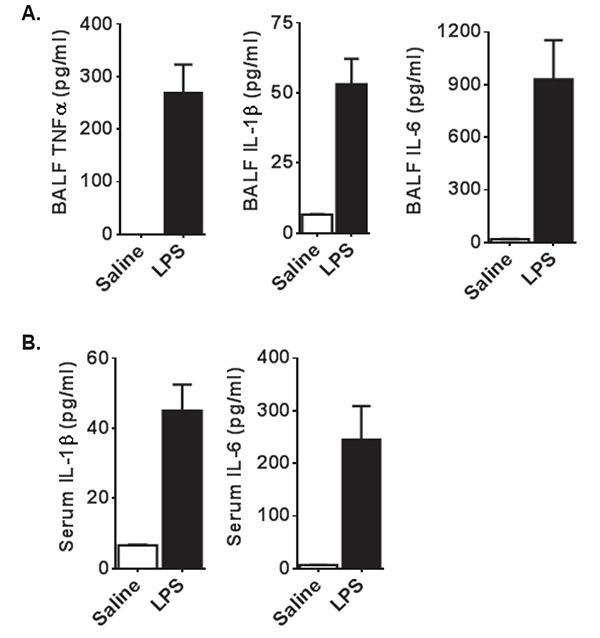 Figure 2. Airway Exposure to LPS Results in Increased Levels of Local and Systemic Cytokines. A) Following LPS exposure, local levels of a broad spectrum of pro-inflammatory cytokines can be observed in the BALF within the first 24 hr, including TNF-α, IL-1β, and IL-6. These cytokines can be evaluated using ELISA, as shown here 24 hr following LPS exposure. B) Several cytokines can also be detected systemically in the serum. However, there are some notable exceptions, including TNF-α, which are found in high levels in the BALF but not in the serum. Cytokines of interest in the serum should be evaluated empirically prior to large scale evaluation. Click here to view larger image.
Figure 2. Airway Exposure to LPS Results in Increased Levels of Local and Systemic Cytokines. A) Following LPS exposure, local levels of a broad spectrum of pro-inflammatory cytokines can be observed in the BALF within the first 24 hr, including TNF-α, IL-1β, and IL-6. These cytokines can be evaluated using ELISA, as shown here 24 hr following LPS exposure. B) Several cytokines can also be detected systemically in the serum. However, there are some notable exceptions, including TNF-α, which are found in high levels in the BALF but not in the serum. Cytokines of interest in the serum should be evaluated empirically prior to large scale evaluation. Click here to view larger image.
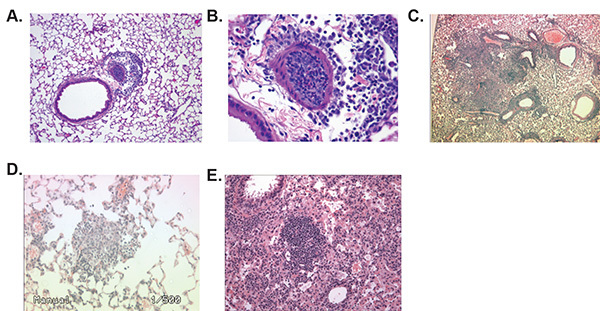 Figure 3. Lung Inflation Using Gravity Displacement Results in Reduced Histopathology Variability and Improved Visualization. The gravity displacement method of fixation described in this protocol allows for optimal histopathology evaluation compared to uninflated lungs or manual inflation. A-B) Lungs fixed by gravity inflation demonstrate uniform features allowing for accurate scoring, reduced alveolar damage, enhanced visualization, and higher resolution evaluations of inflammation. C-D) Manual inflation of the lungs typically results in nonuniform areas of inflation. C) These nonuniform areas are often partially inflated, resulting in collapsed areas that are commonly mistaken as pathological features by novice reviewers. D) Manual inflation also results in areas of the lungs that are over-inflated, which results in extensively damaged alveolar spaces. E) Uninflated lungs demonstrate collapsed alveolar spaces and areas that are difficult to resolve and evaluate without extensive training. Likewise, due to the organ being collapsed this technique does not allow visualization of the lungs as they appear in situ. Click here to view larger image.
Figure 3. Lung Inflation Using Gravity Displacement Results in Reduced Histopathology Variability and Improved Visualization. The gravity displacement method of fixation described in this protocol allows for optimal histopathology evaluation compared to uninflated lungs or manual inflation. A-B) Lungs fixed by gravity inflation demonstrate uniform features allowing for accurate scoring, reduced alveolar damage, enhanced visualization, and higher resolution evaluations of inflammation. C-D) Manual inflation of the lungs typically results in nonuniform areas of inflation. C) These nonuniform areas are often partially inflated, resulting in collapsed areas that are commonly mistaken as pathological features by novice reviewers. D) Manual inflation also results in areas of the lungs that are over-inflated, which results in extensively damaged alveolar spaces. E) Uninflated lungs demonstrate collapsed alveolar spaces and areas that are difficult to resolve and evaluate without extensive training. Likewise, due to the organ being collapsed this technique does not allow visualization of the lungs as they appear in situ. Click here to view larger image.
Discussion
The most critical steps for successfully evaluating the host immune response in mouse lungs is as follows: 1) choose the appropriate mouse strain and sex for the model being evaluated; 2) optimize PAMP delivery to the lungs; 3) correctly collect and process the BALF; and 4) properly fix and prepare of the lungs for histopathological assessments.
The choice of mouse strain is an important factor in evaluating the host immune response. C57Bl/6 mice are typically considered the optimal mouse background for studying innate immunity due to their Th1 skewing and robust response to most PAMPs. Likewise, BALB/c mice are typically used for studying allergic disease models due to their Th2 skewing. A third commonly used strain in lung models are mice on the 129SvEv background, due to their common use in generating genetically modified animals. In all cases, caution should be taken during experimental design and whenever possible, age and sex matched liter-mate control animals should be used for studies comparing genetically modified mice with wild type animals. Typically, for the LPS protocol described here, 6-12 week old sex matched C57Bl/6 mice should be used and should weigh a minimum of 20 g. It is possible that LPS exposure will result in high levels of morbidity and mortality in sensitive mouse strains or genotypes. If this occurs, it may be necessary to adjust several aspects of this protocol, including reducing the LPS dose, using larger animals, and switching the gender of the animals used in the experiment. Small scale experiments should be conducted initially to determine animal sensitivity and the conditions for each experiment should be based on the most susceptible genotype or experimental condition.
Oropharyngeal i.t. administration has been found to be more accurate than other forms of agent delivery to the lungs2. This is in large part due to the direct access to the airway and circumventing issues associated with nasal and sinus cavity deposition. However, as with all animal procedures, i.t. administration requires extensive manual dexterity and practice to achieve proficiency. Improper technique can result in inefficient lung deposition and in some cases result in animal injury. Typically, Evans Blue Dye (EBD) can be effectively utilized as either a training tool for this procedure or to troubleshoot potential issues associated with deposition4. EBD can be administered i.t. and subsequently extracted from the tissue using formamide. The quantity of EBD can be calculated using absorption levels compared against a standard curve. In our hands, typical EBD recovery ranges between 90-98%. The bulk of the unrecovered dye is expected to be associated with leakage into the esophagus. Due to its accuracy, the i.t. administration technique is ideal for delivering a diverse range of dose sensitive agents to the lungs, such as pharmaceutical agents or infectious organisms.
Analysis of the BALF and BALF cellularity can provide a significant amount of insight into the overall progression of the host immune response. Inflammatory mediators released locally in the lungs following stimulation can be effectively quantified in the BALF using common immunology techniques, such as ELISA and Western Blot. Likewise, the cellular composition of the BALF can be evaluated using either differential cell staining or flow cytometry. Developing the proper technique and manual dexterity is the most critical part of performing the bronchoalveolar lavage (BAL). One of the most common issues that occur during the BAL is failure to fully withdraw the maximum volume of fluid originally placed in the lungs. This is commonly associated with an improperly secured cannula or when needles are used in place of actual cannulas. Specialized tracheal cannulas are commercially available. It is also important that the motion and force used to insert and withdraw the saline is smooth and consistent throughout the procedure. The protocol described here has been optimized for differential staining of cells collected in the BALF. Differential staining is a modified Wright Giemsa stain and is a highly effective technique to conduct morphology based cell identification. This staining technique allows for the differentiation of neutrophils and eosinophils based on their unique granule staining properties. Monocyte derived cells are also easy to identify and are commonly observed in the BALF. These include macrophages and dendritic cells. Likewise, T-cells and B-cells are also commonly observed. However, these monocytic cells and lymphocytes are often difficult for most researchers to accurately differentiate based on morphology alone. The utilization of flow cytometry can add much higher resolution to these evaluations. However, low cell numbers recovered from control animals is often a limiting factor. Thus, if flow cytometry is to be used, the experimental design should include additional negative control animals to increase cell recovery.
Lung histopathology assessments are another critical component of this protocol and allow for the direct visualization of disease progression (Figures 3A and 3B). When the lungs are properly prepared, histopathology can be accurately evaluated, quantified, and characterized. The most critical step in preparing the lungs for histopathology is properly inflating them with fixative. Manual methods of inflation typically result in lungs that are over-inflated, under-inflated or partially-inflated, which results in suboptimal visualization and morphology assessments (Figures 3C and 3D, respectively). Likewise, lungs that are not inflated prior to fixation are very difficult to accurately evaluate and often results in highly variable histopathology scores (Figure 3E). The gravity method of inflation discussed here provides a highly reproducible method of inflation with minimal variability. Using this technique, it is possible to evaluate specific landmarks in the lungs that are consistent between experimental animals. Furthermore, gravity inflation using an inflation stand has been shown to inflate the lungs at a fixative pressure of 20 mm12. This technique allows the visualization of the lungs in their most physiologically relevant size and shape and has been shown to be highly effective for the evaluation of sensitive pathophysiological processes12. It is critical that the inflation stand be set at the proper height from the mouse to generate the proper pressure. If suboptimal inflation is observed, the height of the inflation stand should be determined empirically. The use of a commercially available small rodent lung inflation stand, which will ensure the proper height, is recommended.
This procedure has been optimized for the delivery of LPS and other PAMPs to the lungs of mice. Once these techniques are mastered, additional studies can also be initiated using modified protocols to effectively evaluate host-pathogen interactions using live pathogens. Likewise, the techniques described here are highly versatile and can be applied to any study interested in assessing the clinical and physiological relevance of an experimental or pharmaceutical agent. Because of the accuracy in the dosing, this technique is also ideal for in vivo studies that require a high level of precision in agent delivery.
This procedure has been optimized for the delivery of LPS and other PAMPs to the lungs of mice. Once these techniques are mastered, additional studies can also be initiated using modified protocols to effectively evaluate host-pathogen interactions using live pathogens. Likewise, the techniques described here are highly versatile and can be applied to any study interested in assessing the clinical and physiological relevance of an experimental or pharmaceutical agent. Because of the accuracy in the dosing, this technique is also ideal for in vivo studies that require a high level of precision in agent delivery.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
The authors thank the VA-MD Regional College of Veterinary medicine for providing core and technical support for this project. This work is supported by an NIH Career Development Award (K01DK092355).
References
- Matute-Bello G, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. respir. Cell Mol. Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger C, et al. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PloS one. 2013;8 doi: 10.1371/journal.pone.0063432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayamajhi M, et al. Nonsurgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- Revelli DA, Boylan JA, Gherardini FC. A non-invasive intratracheal inoculation method for the study of pulmonary melioidosis. Front. Cell. Infect. Microbiol. 2012;2:164. doi: 10.3389/fcimb.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, et al. Analysis of NLRP3 in the development of allergic airway disease in mice. J. Immunol. 2012;188:2884–2893. doi: 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, et al. Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PloS one. 2013. [DOI] [PMC free article] [PubMed]
- Allen IC, et al. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:1005–1017. doi: 10.1152/ajplung.00174.2006. [DOI] [PubMed] [Google Scholar]
- Kebaier C, et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA, et al. Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles. PloS one. 2013;8 doi: 10.1371/journal.pone.0062115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline JN, et al. Variable airway responsiveness to inhaled lipopolysaccharide. Am. J. Respir. Crit. Med. 1999;160:297–303. doi: 10.1164/ajrccm.160.1.9808144. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Hicks EM, Funkhouser WK, Backlund DC, Koller BH. The relationship of chronic mucin secretion to airway disease in normal and CFTR-deficient mice. Am. J. Respir. Cell Mol. Biol. 1998;19:853–866. doi: 10.1165/ajrcmb.19.6.3194. [DOI] [PubMed] [Google Scholar]


