Abstract
The immune system devotes substantial resources to the life-long control of persistent pathogens, which were hypothesized to play an important role in immune aging. Specifically, the presence of latent herpesviruses has been correlated with immune exhaustion and shorter lifespan in octogenarian humans. But neither the causality nor the mechanistic link(s) were established, and the relative roles of persistent antigenic stimulation and of virus-independent homeostatic disturbances in T-cell aging remain unresolved. We longitudinally analyzed expansion, contraction and long-term maintenance of CD8+ T-cells responding to localized infection with a latent virus, HSV-1. Young mice exhibited the expected expansion and contraction of HSV-1-specific cells and the stable maintenance of memory T-cells into advanced adulthood. However, upon entry into senescence, many (>40%) animals exhibited an accumulation in Ag-specific cells (memory inflation) which in some animals was comparable to that observed in acute infection. Inflation occurred to the same extent in control mice and mice continuously treated with the anti-HSV drug famciclovir, which inhibits viral replication and was able to reduce expression of the gB protein; this was also seen in mice infected with an acute virus. The inflating cells largely maintained Ag-specific function, and exhibited typical central memory phenotype, with no signs of Ag-specific activation. They exhibited increased expression of CD122 and CD127, akin to the antigen-independent T-cell clonal expansions (TCE) found in old specific pathogen free (SPF) laboratory mice. This collectively suggests that in this model the inflating cells may be selected for high responsiveness to environmental cytokines largely in an antigen-independent manner.
INTRODUCTION
Aging of the immune system is marked by a complex set of changes that are collectively termed immune senescence (1-3). Senescence of T-cells is associated with the involution of the thymus, which drastically curtails production of naïve T-cells and reduces the renewal of the naïve T-cell compartment. Lifelong encounters with pathogens further deplete the naïve compartment, and precipitate the conversion of many naïve T-cells into memory T-cells. Therefore, there is a dramatic increase in representation of memory cells, with a concomitant decrease in representation of circulating naïve T-cells. This shift is further potentially compounded by homeostatic mechanisms. Low rate of homeostatic cycling of naïve cells ensures their long-term survival as well as the maintenance of T-cell repertoire diversity (4, 5). However, in more extreme depletion, this cycling is known to become more pronounced [homeostatic proliferative expansion (HPE)]. This mechanism is likely to maintain repertoire diversity for a while, however, HPE is known to eventually result in phenotypic and functional conversion of naïve cells into memory-phenotype cells (rev. in (5-8). Data from humans (9), and non-human primates (10) are consistent with the idea that this may further pronounce the naïve-to-memory shift with age.
While the above forces appear to be the main ones influencing T-cell aging at the population level in specific pathogen-free (SPF) laboratory mice, T-cell aging in humans is believed to be critically affected by ubiquitous persistent infections, including those by viruses from the Herpesvirus family. Cytomegalovirus (CMV) seropositivity in humans has been correlated to an age-related increase in the fraction of memory T-cells specific for CMV (rev. in (11)), a response that is unusual in its strength, breadth and complexity (12, 13). Amongst the octo- and nonagenarians, CMV seropositivity has further been correlated to shorter lifespan (14). This provided basis for suggesting a link between immune aging and persistent infections (11). However, direct causality is difficult to establish due to ethical constraints associated with human studies, and because it is difficult to demonstrate subclinical reactivation of infectious CMV in blood of asymptomatic subjects (15, 16) and mice (17-19). Therefore, mechanistic details of the interaction between a persistent virus and T-cell memory homeostasis over the lifespan of a mammal remain largely unexplored.
We were spurred by these findings to investigate the role of life-long viral infection in the age-related memory dysregulation in a defined and experimentally versatile murine model. Our findings show that despite viral persistence, many CD8+ memory T-cells specific for the virus expand in an old organism independently from viral replication, strongly suggesting that the primary and decisive role in these age-related disturbances belongs to homeostatic dysregulation.
MATERIALS AND METHODS
Mice
Female C57BL/6-NCr (B6) mice were purchased from the National Cancer Institute colony (Frederick, MD), and used young (8-12 weeks), or at adult (4-12 month -mo) or old (>20 mo) age. All animals were housed under the SPF conditions, and experiments conducted under IACUC approval and in accordance with the applicable federal, state and local regulations. Animals were inspected at necropsy and those with signs of possible tumors and gross abnormalities excluded from the study.
Viruses and viral infections
HSV-1 strain 17 obtained from Dr. D.J. McGeoch (University of Glasgow, Scotland, UK), cloned as a syn+ variant and titered on Vero cells in our laboratory, was used in all experiments. Localized (intracorneal, i.c.) infections with 106 PFU HSV-1 per mouse were performed as described (20). WNV strains NY99-crow, 31A and 385-99 were used and all virus strains yielded similar results (21). WNV strains NY99 and 385-99 were kind gifts of Drs. W. Ian Lipkin (Columbia University, New York, NY) and Robert Tesh (University of Texas Medical Branch, Galveston, TX), respectively; strain 31A was provided by the USDA reagent program (Ames, IA). Mice were immunized with WNV s.c. as described (21).
Determination of titers of replicating and reactivated virus
The amount of replicating virus in the trigeminal ganglia (TG) of acutely (day 3-5 p.i.) infected mice was determined by plaque assay of TG homogenates as previously described (20). To determine the presence of latent virus in the TG of latently infected mice (>30 days p.i.), the TG were subjected to a standard reactivation assay and the titer of reactivated virus was determined by a plaque assay or quantitative real-time PCR. Briefly, both TGs/mouse were isolated, cut into 4-6 pieces and cultured on top of Vero cell monolayer in media containing 2% FBS for 5 days in order to induce viral reactivation. At the end of the culture, the TG and the Vero cells were homogenized in the original culture media using sterile glass homogenizer, and the viral titer of the homogenate was determined by a plaque assay. The limit of detection was 10 PFU. Alternatively, the TG from latently infected or control naïve mice or from explant TG cultures were snap frozen in liquid nitrogen. Total DNA and RNA were isolated using Qiagen AllPrep DNA/RNA/Protein mini kit and were diluted to 10ng/μl. RNA was treated with DNase I (Fermentas) prior to reverse transcription. RNA was reverse transcribed using oligo (dT) primers. The 25μl real-time PCR reaction contained 8.5μl DNA or cDNA, 12.5μl TaqMan Universal PCR Master Mix (Applied Biosystems), glycoprotein B-specific primers (Invitrogen), and probe (Applied Biosystems). HSV-1 DNA was isolated from a virus stock of known titer and used to generate the standard curve. All samples were analyzed in triplicate. The PCR reaction was carried out using MyIQ PCR cycler (Bio-Rad). The PCR conditions, primer and probe sequences were as described (J Med Virol. 2005 Jul;76(3):350-5).
Famciclovir treatment
Where indicated, Famciclovir (Famvir, Novartis) was administered to mice in their drinking water at a concentration of 2mg/ml. The Famvir water was changed twice a week.
Reagents, antibodies and flow cytofluorometric (FCM) analysis
The gB-8p peptide (SSIEFARL) was purchased from SynPep Corporation (Dublin, CA), and the gB-8p :Kb tetramer was obtained from the NIH Tetramer Core Facility (Emory University, Atlanta, GA). Monoclonal antibodies anti-CD8α (clone 53-6.7), anti-CD27 (clone LG3a10), anti-IFNγ (all from BD Pharmingen, San Diego, CA); anti-CD127 (clone A7R34), anti-CD62L (clone MEL-14), anti-CD44 (Pgp-1, Ly-24), anti-CD122 (clone 5H4) (all from EBioscience, San Diego, CA); and CD43 (clone 1B11; Biolegend, San Diego, CA) were purchased from commercial sources.
FCM analysis with or without intracellular staining to detect IFNγ was performed as previously described (20, 21); in the latter case, stimulation was performed with 10-6 M gB-8p peptide for 6 h in the presence of 1.5 μg/ml brefeldin A. FCM data was acquired on FACSCalibur instrument using CellQuest 3.3 software or on the FACS LSRII instrument using the Diva software (Becton Dickinson, Mountain View, CA), and analysis performed using FlowJo software (Tree Star). At least 104 cells were analyzed per sample, with dead cells excluded by selective gating based on orthogonal and side light scatter characteristics.
CTL cultures and 51Cr-release assay
On day 8 p.i. splenocytes from infected mice were co-cultured with irradiated, gB-8p-coated syngeneic splenocytes and peptide-specific CTL activity was determined in a standard 51Cr-release assay 5 days later as described previously (22).
CDR3 length analysis (spectratyping)
Spleens of mice selected for CDR3 length analysis were enriched for CD8+ T-cells (>80% CD8+ and <2% CD4+) by depletion of CD4+ and B220+ cells by immunomagnetic sorting (MACS, Miltenyi Biotech). CDR3 length analysis was performed as described (23) on Vβ8 and 10 families, which in B6 mice account for >80% of the response to gB-8p (24).
RESULTS
Maintenance of stable anti-HSV memory becomes disrupted with age
We used ocular HSV-1 infection of B6 mice as a model to study the maintenance of memory CD8+ T-cells specific for a latent persistent virus. Ocular infection with HSV-1 results in establishment of lifelong latent infection in the trigeminal ganglia (TG) and brain. Virus-specific CD8+ T-cells are critical in limiting the viral replication during initial infection (20, 25). After termination of viral replication by day 10 post-infection (p.i.), antiviral CD8+ T-cells persist at the site of latency and they were implicated to actively survey latently infected neurons, controlling the extent of viral reactivation (26). The key feature of the anti-HSV-1 CD8+ T-cell response in B6 mice is an unusual degree of immunodominance, whereby >95% of the entire CD8 response (27-29) is directed against the glycoprotein B (gB) octapeptide 498-505, SSIEFARL, (gB-8p in the text), bound to H-2Kb (30-32) and that response is critical for antiviral protection (20, 25, 29, 33). To determine whether lifelong infection with a latent virus is accompanied by stable maintenance of systemic anti-HSV CD8+ T-cell memory, we have analyzed the frequency of gB-8p-specific CD8+ T-cells at various times post infection (p.i.) in a longitudinal study. A cohort of young mice was infected by corneal scarification, and the frequency of HSV-specific memory CD8+ T-cells was periodically assessed by FCM in circulating blood lymphocytes using the gB-8p:Kb tetramer. The infection resulted in expansion of gB-8p-specific CD8+ T-cells, which reached the peak on days 7-8 p.i. and then contracted to its memory set point by 4 months p.i. (m.p.i., Figure 1A). Thereafter, the frequency of HSV-specific CD8+ T-cells remained stable up to 7-12 m.p.i. (Figure 1A and not shown). The average frequency at set point in this experiment was 1.6% ± 0.4% of all CD8+ T-cells, ranging between 1-2.1%.
Figure 1. CD8+ T-cell memory is stable in adult but dysregulated in old animals following ocular HSV-1 infection.
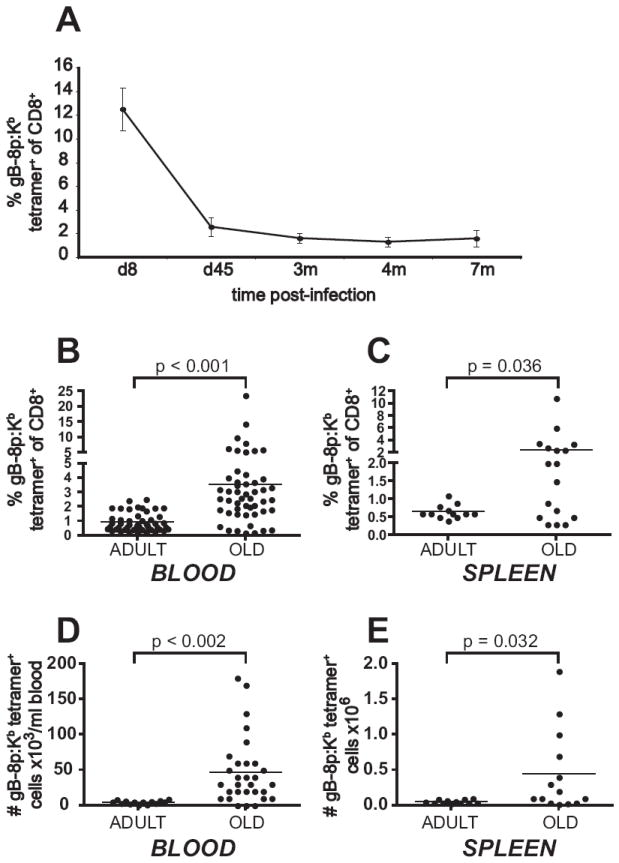
A. Stable maintenance of gB-8p specific memory CD8+ T-cells in adult animals. A cohort of young B6 mice (n = 9) was infected with 106 PFU HSV-1 (i.c. infection). Blood samples were taken at day 8, and at 1.5, 3, and 4 months p.i. and stained with anti-CD8+ and gB-8p:Kb tetramer. The values show the average percent of tetramer+ cells within CD8+ T-cells ± SEM. The data is representative of three independent experiments. B-E. Memory inflation occurs with advanced age. B. A cohort of young B6 mice (n=50) was infected as in A and followed longitudinally. Blood samples were taken from adult (4 m.p.i.) and old mice (18-24 m.p.i.), and stained as in A. The values show the percent of tetramer+ cells within CD8+ T-cells (each individual animal is represented by a dot, the dash reflects the average). The increase in the average percent of tetramer+ CD8+ T-cells in the old was significant (p<0.0001, paired Student’s t-test). C. Mice were infected as in A and select animals were sacrificed as adults (n = 12) or old (n = 17) and the frequency of gB-specific memory CD8+ T-cells was determined in their spleens as above. The increase in memory cell frequency was significant in the old (p=0.036, Student’s t-test) D and E. Absolute numbers of tetramer+ CD8+ T-cells in blood (D) and spleens (E) of mice from part C and D, respectively. Numbers were obtained from absolute counts and percentages of tetramer+ CD8+ T-cells.
To explore memory maintenance at the later time points and into senescence, we followed several mouse cohorts from the time of infection early in life (2-3 mo) until they reached old age (>18 m. p.i., corresponding to >20 months of age). We found that compared to the memory set point observed in adulthood (0.9% ± 0.6%, n =50), there was a significant increase in the frequency of memory CD8+ T-cells in the old animals (3.6% ± 4.0%, n =50, Fig. 1B). In some old mice the frequency of memory CD8+ T-cells increased to the same level or even above the extent of expansion at the peak of the primary response. Moreover, while not all of the mice exhibited dramatic increases in frequency of memory CD8+ T-cells, an increase was seen in most animals, and it was significant at a cohort level (Fig.1B, p-value < 0.001, paired Student’s t-test). Similar increase of memory cell frequencies was observed in spleens, where the average frequency of memory CD8+ T-cells increased from 0.7% (± 0.2%, n = 12) in adults to 2.2% (± 2.8%, n = 14) in the old (Fig. 1C, p-value 0.036).
Most importantly, the memory CD8+ T-cells increased in absolute number in blood (from 4.9 × 103/ml ± 2.5 × 103 in adults to 4.7 × 104/ml ± 46.8 × 104 in the old, reaching up to 1.8 × 105 cells/ml in some mice, Fig. 1D), the spleens (from 6 × 104 ± 2 ×104 in adults to 4.5 × 105 ± 5.8 × 10 5 in the old, reaching up to 1.9 × 105 cells/spleen, Fig. 1E), and the lymph nodes of individual old mice from the above study (not shown). Again, while not all animals showed pronounced absolute accumulation, more than half exhibited an absolute increase over the levels seen in adult animals.
The above results show that the CD8+ memory to localized HSV-1 infection is maintained at a stable, low frequency throughout adulthood in individual mice, but exhibits variable and in many mice significant increase from the set point level in old age, implying a loss of control in memory T-cell maintenance. This age-related memory accumulation was observed in 42% of analyzed old mice (n=50), as measured by the fraction of animals whose memory cell frequency was more than two standard deviations above the memory set point.
Memory CD8+ T-cells specific for a persisting pathogen retain functionality but show pronounced age-related variability
The uneven memory maintenance seen in the above experiments could be due to homeostatic dysregulation, to repeated viral activation, or both. We set out to distinguish between these two possibilities. In humans, many cells responding to persisting pathogens were described to have dysregulated or diminished functional capabilities (34-36), postulated to be due to exhaustion by repeated Ag stimulation. We therefore asked whether, in addition to loss of stringent control of memory CD8+ T-cell numbers, aged HSV-specific T-cells may exhibit signs of functional exhaustion. We initially compared the frequency of tetramer+ cells to frequency of cells capable of IFNγ production after short re-stimulation with the gB-8p peptide. In both adult and old mice, the frequencies of tetramer+ and IFNγ-producing cells were comparable, as shown by an excellent correlation of tetramer and IFNγ staining for each individual mouse (Figure 2A, R2 = 0.982). Thus, whereas the old mice exhibited variability in frequency of virus-specific CD8+ T-cells by tetramer staining (Fig. 1), IFNγ secretion closely mirrored this variability and the two traits correlated to each other in individual animals (Figure 2A). Therefore, cells undergoing memory inflation uniformly secreted IFNγ.
Figure 2. Functional responses of HSV-specific memory CD8+ T-cells in adult and old mice.
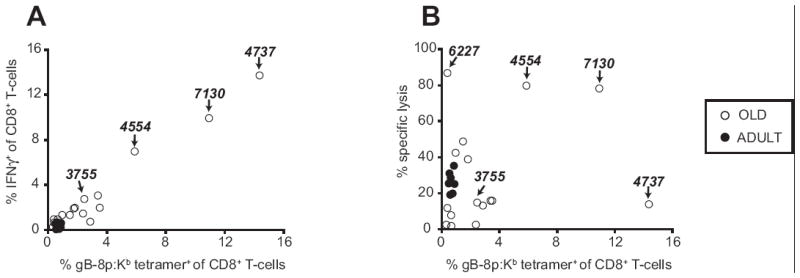
A. Most gB-8p-specific memory CD8+ T-cells produce IFNγ. Splenocytes from latently infected adult (n=7, 10 m.p.i., 12 mo) and old (n=16, 18-24 m.p.i., 20-26 mo) mice were stimulated for 6 hours with 10-6 M gB-8p peptide in presence of brefeldin A, and then stained using gB-8p:Kb tetramer, CD8 (surface) and/or intracellular IFNγ. The X values show the percent of tetramer+ cells, and the Y values shows the percent of IFNγ+ cells within total CD8+ T-cell pool of individual mice. The values for IFNγ show the net IFNγ (background IFNγ staining from unstimulated wells was subtracted; background was < 0.2%). Excellent correlation of the tetramer and IFNγ staining was observed (R2 = 0.982). B. Cytotoxic activity of memory CD8+ T-cells from mice at various times post HSV-1 infection. Splenocytes harvested from mice in A were cultured for 5 days in presence of gB-8p:Kb – pulsed stimulators. On day 5 of culture the cytotoxic function of HSV-specific CTLs was tested in a standard 51Cr release assay. The CTLs (effectors) were mixed with gB-8p:Kb – pulsed EL-4 cells that were intracellularly labeled with 51Cr (targets). Background lysis values obtained with peptide-negative targets (<8%) were subtracted. The X axis values show the percent of tetramer+ cells at day 0 of culture, and the Y axis values show the percent of specific lysis at effector-to- target ratio of 80:1. The numbers on the graph indicate the IDs of specific mice as discussed in the results.
We also examined the lytic capacity of these cells after 5-day in-vitro restimulation. Again, tightly clustered lytic activity of adult animals was replaced by high variability in the CTL responses of old animals (Figure 2B), with some exhibiting strong and others poor CTL reactivity. Correlation between lytic activity and the frequency of memory cells at the start of the 5-day culture was less tight in old animals, and several different phenotypes were observed (Fig. 2A and B): low accumulation and brisk CTL responses (mouse #6227); low inflation, but poor CTL responsiveness; some or massive accumulation, with good IFNγ response, but poor CTL responses (mouse #3755, and, in particular, 4737), and finally, accumulation with good CTL activity (mouse # 4554 and 7130). These data suggest that CTL activity correlates poorly to memory accumulation during lifelong HSV-1 infection, and fail to provide decisive evidence for antigen-driven exhaustion in this model.
Surface phenotype of CD8+ T-cells undergoing late memory inflation suggests no recent or repeated activation by antigen
The above data are somewhat reminiscent of the phenomenon originally described by Reddehase’s (37, 38) and subsequently by Klenerman’s groups (39, 40) (christened by the latter group “memory inflation”) in mice infected with the murine cytomegalovirus (mCMV). However, these groups described a much earlier onset of memory inflation (3-4 months p.i.), whereas our results suggested that the loss of numerical control of the memory CD8+ T-cell pool occurs only with advanced age during lifelong HSV-1 infection. In a separate study (A. Lang et al, in preparation), we have shown that this is not a consequence of fundamental differences between HSV-1 and mCMV, but rather a likely difference in the latent viral loads, which can be largely equalized if HSV-1 is administered systemically. Our present study, however, sought to understand the interplay of aging and a latent, lifelong infection in the ocular model, which faithfully mimics the fundamental features of natural human infection. To that effect, we proceeded to test whether the accumulating gB-specific CD8+ T-cell population bore signs of recent or prolonged contact with antigen.
Multicolor FCM analysis was performed to assess the expression of acute and chronic activation markers. We found no evidence of Ag-specific acute activation as judged by the lack of expression of CD25 and CD69 (not shown). Moreover, the markers which are downregulated with repeated or prolonged activation (CD62L, CD127 and CD27) were all expressed highly on gB-8:Kb tetramer+ cells (Figure 3). In addition, these cells expressed high levels of CD44 and Ly-6C, and intermediate levels of CD43, exhibiting the CD25-CD69-CD44hiCD62LhiCD27hiCD127hiLy-6ChiCD43int phenotype, typical of central (resting) memory cells (Fig. 3 and not shown). These results provided further evidence against the possibility that memory accumulation in this model may be driven by persistent antigenic stimulation.
Figure 3. Expanded gB-8p-specific memory cells in old HSV-1 infected mice uniformly exhibit central memory phenotype.
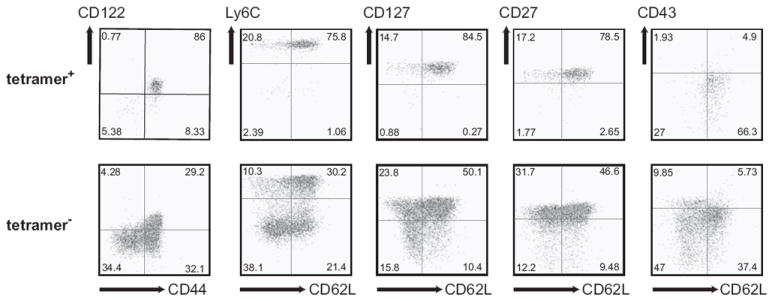
Splenocytes (shown) or lymphocytes isolated from blood (not shown) from old HSV-1 infected mice with age-related expansions of gB-8p-specific memory CD8+ T-cells were stained with CD8, tetramer and a panel of surface markers (CD44, CD62L, Ly6C, CD127, CD122, CD43, CD27). The staining of tetramer+ (top panel) and tetramer- (bottom panel) CD8+ T-cells from a representative mouse is shown. The same phenotype was observed in CD8+ T-cells isolated from blood (not shown).
Antiviral treatment has no influence upon late-age memory inflation
All the above evidence does not formally exclude the possibility that periodic viral reactivation may have stimulated expansion and increased functional responses of memory cells in some mice. To further test the role of latent virus reactivation in the above age-related memory dysregulation, we took advantage of a soluble, oral anti-HSV drug famciclovir (Famvir®), an oral form of acyclovir, which has been shown to inhibit viral DNA polymerase and thereby abrogate viral replication ((41, 42) and references therein). A question critical for the interpretation of our data was whether Famvir was able to restrict the extent of expression of viral late genes, including gB, during reactivation from latency. Viral genes can be expressed even in the absence of DNA synthesis (43, 44), and since Famvir interferes with the replication cycle at the DNA synthesis level, we expected that low level of gB expression will be possible even during Famvir treatment. With that in mind, we did not expect to completely block gB expression, but rather to prevent full viral reactivation and the associated viral spread with increased gene, and the resulting gB antigen, expression. Consistent with these expectations, we were able to show that Famvir, when administered prior to ocular infection, was able to abrogate acute viral replication (Fig. 4A), prevent establishment of latency in TG (Fig. 4B), and abrogate the antiviral memory CD8+ T cell generation (Fig. 4C).
Figure 4. A-C. Famvir blocks viral replication and decreases the extent of gB transcription.
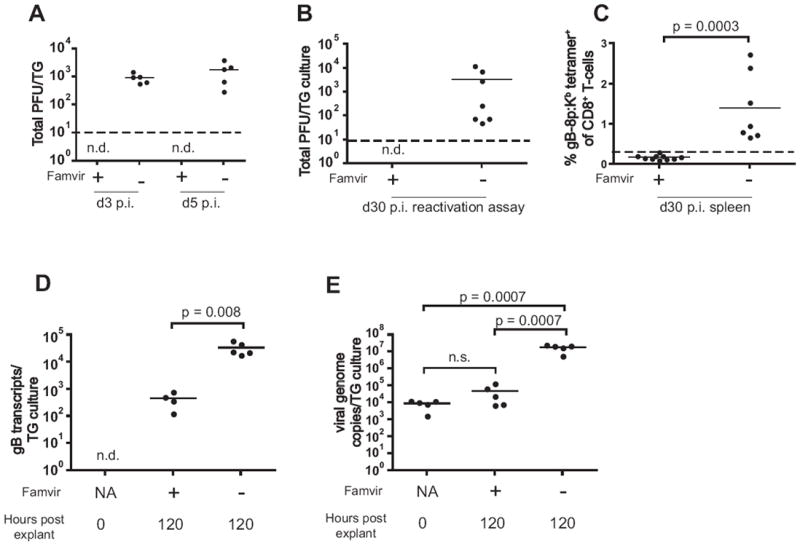
Famvir treatment during acute infection abrogates viral replication and lowers the latent viral load to undetectable level. Young mice were infected i.c. with HSV-1 as described in Materials and Methods. Some mice (n=5) received Famvir continuously in the drinking water starting on day (-7). A. On days 3 and 5 p.i. TGs of Famvir-treated (n=5) and untreated (n=5) mice were isolated and tested for presence of infectious virus by plaque assay. Dashed line represents the level of reliable detection. B. On day 30 p.i. the TGs from untreated (n=7) or Famvir-treated (n=10) mice were isolated and subjected to a reactivation assay as described in Materials and Methods. The total titer of the virus reactivated from TG culture from each mouse is shown. No virus was detected in the cultures from Famvir-treated mice (n.d., not detected). Dashed line represents the level of reliable detection. C. Splenocytes from mice in B were stained with anti-CD8 and gB-8p:Kb tetramer. Dashed line represents levels found in control, uninfected animals. D-E. Famvir decreases the extent of gB transcription and prevents viral DNA synthesis during reactivation from latency. TGs were isolated from latently infected (4 m.p.i.) mice and subjected to standard TG explant reactivation assay in vitro. Quantitative real-time PCR was performed to detect the level of gB transcription (D) and viral DNA load (E). RNA and DNA were isolated from TGs ex-vivo (n = 5), or after 5 day TG explant culture in control media (n= 5) or media containing 1 mM Famvir (n = 5), as described in Materials and Methods. Mice whose TGs were cultured in presence of Famvir were given Famvir in their drinking water for 7 days prior to organ harvest to allow for drug’s immediate access to TGs upon initiation of explant culture. The data represents the total number of gB transcripts (D) or viral genome copies (E) per individual mouse TG. The data is representative of 2 independent experiments.
It was also possible that the memory inflation observed in old mice was caused by decreased functional responses of CD8 T cells present in the TG, permitting increased frequency and magnitude of viral reactivation from latency, even though data from Fig. 2 suggested that functional CD8 T-cell characteristics did not correlate to memory accumulation. If so, one would expect that old ganglia may show increased gB transcription, and that Famvir should be able to decrease transcription levels. To that effect, we analyzed gB transcription in latently infected TGs during standard ex-vivo reactivation assay. TGs isolated from latently infected mice were cultured for 5 days in media containing 1mM Famvir or in control media, and gB transcription was determined at the end of culture by quantitative real-time PCR. We found that Famvir significantly decreased gB transcription (Fig. 4D), and prevented viral DNA synthesis, as expected (Fig. 4E). We conclude that Famvir treatment indeed leads to prevention of viral DNA synthesis, reduction of viral spread and diminished gB transcription.
Having demonstrated the efficacy of Famvir in mice, we initiated longitudinal experiments where Famvir was administered continuously in drinking water starting on day 14 post ocular infection. In the ocular infection model, productive viral replication ends between days 8-10 p.i., and from then on the virus remains latent in the TG and brain, from where it can periodically reactivate. Therefore, initiation of Famvir treatment on day 14 p.i. allows for establishment of latency (not shown), but should prevent viral reactivation. Figures 5A and B show that Famvir treatment did not prevent the age-related memory accumulation despite being able to block DNA replication and to reduce viral spread and gB transcription (Fig. 4). Importantly, this was true both at the level of relative representation (Fig. 5A) and the absolute numbers (Fig. 5B). These results further argued against the possibility that viral reactivation and restimulation of the virus-specific CD8+ T-cells cause memory accumulation in this model. Finally, if viral factors played a role in memory accumulation, one would expect that animals with pronounced CD8 T-cell memory accumulation may have larger viral loads in the ganglia. Figure 5C shows that this is not the case, as we found no difference in latent viral loads between animals with large accumulation of gB-8p-specific memory CD8+ T-cells and those without memory inflation.
Figure 5. Effect of viral reactivation on development of age-related memory CD8+ T-cell expansions.
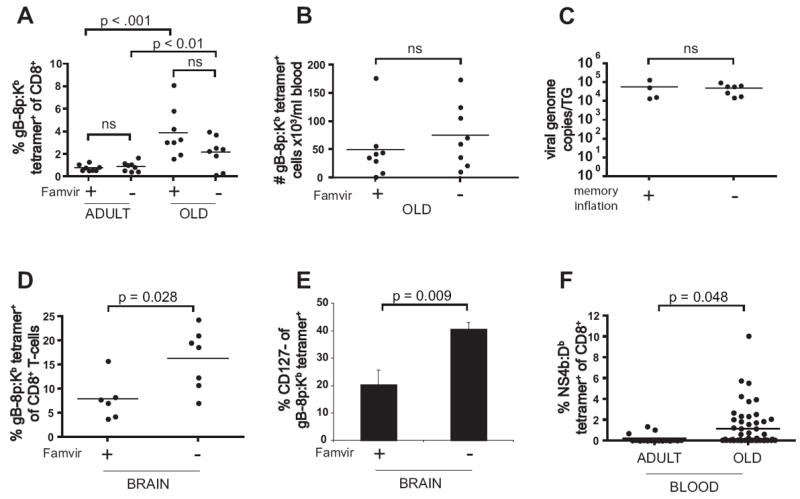
A-C. Continuous Famvir treatment of latently infected mice does not prevent the development of age-related memory CD8+ T-cell expansions. Young mice (n=16) were infected i.c. with HSV-1 and their gB-8p-specific CD8+ T-cell responses were followed longitudinally. Half of the cohort was continuously treated with Famvir starting on day 14 p.i. The same mice were screened for frequency of their HSV-specific memory CD8+ T-cells as adults and old. Age-related memory inflation was observed both in control and Famvir-treated group, and there were no significant differences in the frequency (A) or absolute numbers (B) of gB-8p-specific memory CD8+ T-cells in the old mice from either group. Mice undergoing memory inflation had the same latent viral load as the memory inflation-free mice, as determined by real-time PCR at the time of sacrifice at 20 m.p.i. (C). The data is representative of two independent experiments. D-E. Continuous Famvir treatment of latently infected mice affects the size and phenotype of memory CD8+ T-cell response in brain. Mice were infected with HSV-1 as adults and continuously treated with Famvir from day 14 p.i. as in A. At 22 m.p.i. the mice were sacrificed, and lymphocytes isolated from their brains were stained with anti-CD8 and tetramer (D) and CD127 (E). F. Age-associated CD8+ memory inflation in old mice infected with acute virus. Young mice (2-4 mo) were infected with WNV. Peripheral blood lymphocytes were stained with anti-CD8 and NS4b:Db tetramer in adult (n = 17) or in old mice (18-22 m.p.i., n = 50).
Several published studies (25, 26, 45) and our data (not shown) demonstrated ongoing presence of tetramer+ cells exhibiting activated phenotype in the latently infected TG throughout the lifelong infection. Little is known about the exchange of memory cells between the latently infected ganglia and uninfected lymphoid and non-lymphoid organs, and our data does not exclude the possibility that the increase in frequency and numbers of gB-8p-specific memory CD8+ cells present in the blood and spleen of old mice was a result of influx of recently stimulated cells from the infected TG. However, we believe that this explanation is unlikely for several reasons. First, we detected an increase in tetramer+ memory CD8+ T-cells exclusively in old mice, and if the TG and the brain were an ongoing supplier of recently activated cells, one would expect to observe memory inflation earlier in life. Second, we found that Famvir treatment during latency resulted in decreased frequency and numbers of gB-8p-specific CD8+ T cells at the site of latency (brain, Fig. 5D and not shown), and smaller percentage of the memory cells present in brain had activated CD127- phenotype (Fig. 5E). Despite that, Famvir treatment did not prevent memory inflation in peripheral lymphoid compartments (blood, spleen), and there was no correlation between the number of memory cells in the brain and in blood/spleen of old mice (not shown). Third, we and others have found in transfer experiments that at least 90 days are needed for CD62L to reappear on the surface of repeatedly stimulated CD8 T-cells ((46, 47) and A.L. et al., in preparation). If the accumulated T-cells were supplied from the activated CD8 T-cell pool in TG and brain, a much larger fraction of the systemic pool would at least have to be CD62Llo, which we did not find in our experiments. Finally, we found that age-related memory CD8+ T-cell inflation is not restricted to infection with persistent virus, as we observed a similar phenomenon in old mice which were infected in youth with the West Nile Virus (WNV) (Fig. 5E). WNV is an acute virus which is completely cleared from the organism within 10 days p.i. (21, 48). We found that old WNV-infected mice had a significant increase in percentage of memory CD8+ T-cells specific for the dominant NS4b epitope (21) from set point observed in adults (Fig. 5E). Similarly to old HSV-1 infected mice, a large proportion (32%) of the analyzed old WNV-infected mice (n=50) had expanded NS4b-specific memory CD8+ T-cell populations, as defined by a frequency at least two standard deviations above the memory set point observed in adult infected mice. In addition, the expanded WNV-specific memory CD8+ T-cell populations had a uniform central-memory phenotype (not shown), similar to that found in memory cell expansions in old HSV-infected mice. In conclusion, the sum of our data is most consistent with the explanation that old age-associated accumulation of memory CD8+ T cells can occur independently of antigenic stimulation.
HSV-specific memory CD8 T-cells undergoing age-related accumulation resemble spontaneously arising T-cell clonal expansions (TCE)
Another well-described age-related phenomenon bears resemblance to the above situation. Mice older than 18 months were described to develop spontaneous age-related T-cell clonal expansions (TCE) in variable percentages (usually 40-50%) (23, 49), and the same phenomenology has been previously described in humans (50, 51). These CD8+ expansions are monoclonal, can usurp up to 80% of the total CD8+ T-cell repertoire (rev in. (52)) and exhibit a uniform central memory phenotype, being CD8+CD25-CD69-CD44hiCD62LhiCD27hiCD127hiLy-6ChiCD43intCD122hi (53). Their onset can be accelerated and the incidence elevated by lymphopenia (54), and they express higher levels of CD127 and CD122 compared to their normal central memory counterparts that are not monoclonal nor expanded (53). Given those characteristics and the fact that they do not seem to be selected by antigen (they appear to stochastically express different TCRVαβ protein combinations and were shown to exhibit no particular reactivity pattern), we proposed that these cells are selected by the ability to preferentially respond to homeostatic cytokines and have provisionally classified them as antigen-independent (AI)-TCE (53, 54) and Messaoudi, I, et al, in preparation). We note that the antigen-independent designation refers to their expansion and maintenance (i.e. their becoming TCE), and not necessarily that these cells were never in contact with antigen.
We have already noted marked similarities between the AI-TCE and the expanded memory cells described in this study in the time of onset (both diagnosable after 18 mo of age) and cell surface phenotype. To test whether the expanded memory cells seen in aging mice after life-long ocular HSV-1 infection share other cardinal features of TCE, we first performed TCR Vβ repertoire and CDR3 length analysis on several animals exhibiting pronounced memory inflation, taking advantage of the fact that the anti-gB-8:Kb CD8+ T-cells preferentially utilize TCRVβ8 and 10 elements (24). Upon TCRVβ staining, we saw the largely expected frequencies of TCR Vβ8 and 10, suggesting that both families are being used in the response, with neither fully dominating, which would be expected if the response was clonal (not shown). Figure 5A shows the results of CDR3 length analysis. Here too we failed to see the pronounced TCE-like clonality amongst the cells undergoing MI, although several of them exhibited signs of restricted, even oligoclonal, CDR3 profiles (Fig. 6A). Therefore, it appeared that the expanded memory cells, while clearly antigen-selected (tetramer+), were not monoclonal. We next examined in more detail the levels of surface expression of cytokine receptors CD122 (IL15Rβ) and CD127 (IL7Rα), which were previously shown to be elevated on AI-TCE as compared to non-TCE cells in the same mice (53). Remarkably, the expanded gB-specific cells exhibited increased cytokine receptor expression as compared to other central memory (CD44+ CD62L+) CD8+ T-cells in the same mice (Figure 6B). Specifically, nearly all of them expressed CD122 and CD127, and they had an increased per cell expression of CD127, expressing 25% more of it than other central memory cells (Figure 6B). All of the above results suggest that memory cells undergoing late-age memory inflation are selected for expansion and survival in an antigen-independent manner, perhaps based upon their ability to utilize cytokine resources present in the old environment, an issue further discussed below.
Figure 6. Clonal composition and expression of IL7Rα and IL15Rβ by expanded gB-8p-specific memory CD8+ T-cells in old mice.
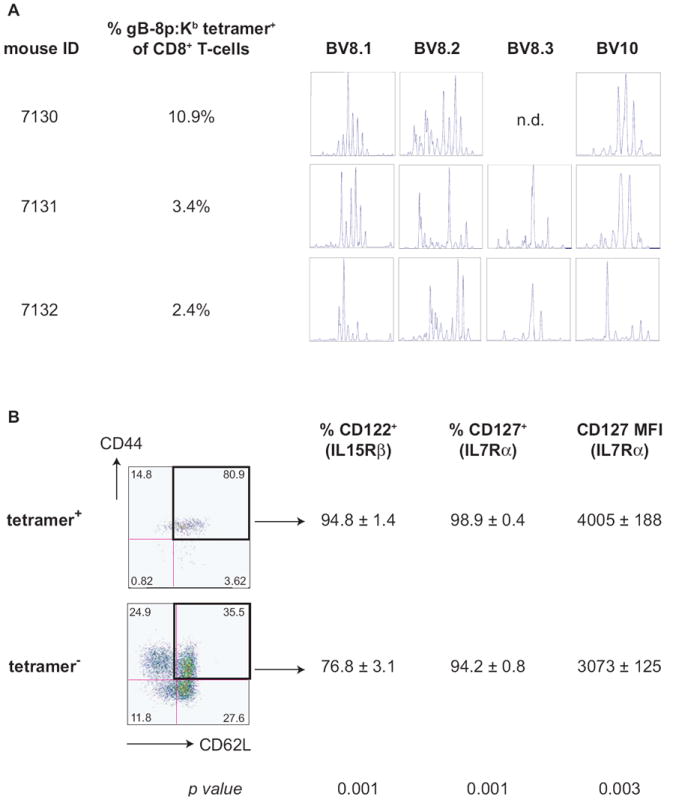
A. CDR3 length analysis of the BV segments involved in gB-8p-specific CD8+ T cell response in mice undergoing late-age memory inflation. CD8+ T-cells from mice exhibiting old-age memory inflation were purified using magnetic bead separation, the RNA was extracted and the diversity of CDR3 lengths of BV8 (8.1, 8.2 and 8.3) and BV10 segments was determined as described in Materials and Methods. CDR3 profiles from three representative mice are shown. B. Elevated expression of IL7Rα (CD127) and IL15Rβ (CD122) on expanded gB-8p-specific memory cells in old mice. Splenocytes from old latently infected mice (n=6) undergoing old-age memory inflation were stained with CD8, CD44, CD62L, tetramer, CD127 and CD122. The percentage of CD122+ and CD127+ cells as well as the mean fluorescent intensity (MFI) of CD127 was compared between central memory phenotype (CD44+ CD62L+) tetramer+ (top panels) or tetramer- (bottom panel) CD8+ T-cells. The values represent the average ± SD (first two rows) or the p-value (obtained from paired Student’s t-test comparing the values from the tetramer+ and tetramer- fraction of cells of individual mice), and the data is representative of two independent experiments.
DISCUSSION
Latent viruses – initiators or drivers of age-related memory dysregulation?
Accumulating evidence has linked persistent (viral) infections and T-cell senescence (rev. in (11). Here, we used the term “persistent” to indicate that the virus remains associated with the host, without any reference to the status of viral replication. Defined as such, persistent viruses can be either chronic (continuously replicating; e.g. HIV, HCV) or latent (HSV, VZV, CMV). Given that chronic viruses often produce disease even in immunocompetent hosts, and that they are not present in the majority of aging individuals, they are considered not to be associated with normal aging.
Latent viruses, on the other hand, coexist with immunocompetent hosts for life, and are therefore considered as potential partners of the aging process. HSV-1 is a persistent, latent virus, that initially replicates at the site of epithelial/mucosal entry, enters the sensory nerves, and travels to the nearest sensory ganglia and the CNS, where it is ultimately contained by the CD8+ T-cells (20, 55) and references therein). Once controlled, the virus establishes latency for the life of the organism, remaining in the ganglia, and, possibly, the brain (56, 57). In mice, HSV never shows clinical signs of spontaneous reactivation (ref. (58, 59), and this lack of clinical reactivation may be due to CD8+ T-cells, which were proposed to control the virus in the ganglia prior to manifest clinical reactivation (26, 45, 57).
So is this virus contributing to CD8 T-cell aging and how? We show that following localized (ocular) infection in youth, HSV-1 memory is maintained at stable, low levels throughout the adulthood in mice. However, old age leads to improper memory maintenance, marked by an accumulation of memory cells specific for the original immunodominant peptide antigen, gB-8p. One possibility is that memory accumulation arises due to a loss of antiviral CD8+ T-cell function (primary or due to exhaustion by stimulation), which results in viral reactivation, feeding into a positive feedback loop, whereby the virus continuously stimulates the immune system and contributes to the accumulation of memory T-cells. The CD8+ T-cells found at the sites of latency (trigeminal ganglia, brain) bear the effector-memory phenotype (20, 26, 57), A.L. & J.N-Z., Fig. 5E and unpublished observations), whereas the HSV-specific CD8+ T-cells which accumulate with aging exhibit uniform central memory phenotype. This suggests that the exchange of cells between the latently infected organs and the systemic virus-specific CD8+ T-cell pool, is probably numerically small, although this issue remains to be directly addressed experimentally. Neither the levels of latent virus nor the inhibition of viral replication by antiviral drugs, with the consequent reduction (but no abrogation) in viral Ag levels, had any impact upon the extent of the late-life memory cell accumulation. Moreover, the accumulating cells were highly positive for the receptors for common γ chain cytokines IL-7 and IL-2/15. In addition, memory inflation was also observed in old mice infected with acute virus (WNV, Fig. 5F). Finally, even an acute virus (WNV) was able to produce similar memory cell accumulation (Fig. 6). The sum of our results, therefore, argues against the Ag-driven accumulation in this model of localized viral infection, which is similar to the localized HSV infection in humans.
Homeostatic disturbances are critically involved in T-cell memory dysregulation
It is pertinent to compare and contrast the above results with other cases of dysregulation of the CD8+ memory T-cell compartment. Systemic infection with mCMV was shown to induce rapid (detectable in 2-4 m.p.i.) and vigorous memory inflation, (38, 39). Such cells bear all the characteristics of repeated antigenic stimulation and the phenotype of effector memory T-cells (CD62Llo, CD27int/lo), and can accumulate to impressive levels, up to 30-40% of the total CD8 compartment. Accumulating cells in our current study, in a model that closely mimics natural human infection with HSV-1, share very few common characteristics with these mCMV-specific cells, differing in phenotype (central vs. effector memory), time of onset (>18 mo, and 12-16 m.p.i., vs. 2-4 m.p.i.) and the extent of accumulation (2.4-23% vs. 20-30% of CD8+ T-cells).
This prompted us to compare and contrast the properties of expanded memory cells from this study to another dysregulated memory T-cell type, the spontaneously arising TCE. Spontaneously arising TCE are clonal in nature (2, 49-51), they exhibit the central memory phenotype (54, 60), can make up to 80% of the total CD8+ T-cell pool, their onset can be accelerated and incidence increased by lymphopenia and/or increased turnover(54) and they express elevated levels of the receptors for key cytokines that regulate survival, maintenance and homeostatic proliferation of T-cells, IL-7 and IL-15 (53). The sum of characteristics of HSV-specific cells undergoing late-onset memory inflation in this study is reminiscent of the spontaneously arising TCE. Two features differed between these cells and TCE: most gB-specific expanded cell populations did not appear to be fully clonal in nature (although evidence for oligoclonality was present), and their upper level of expansion documented so far (23% of total blood CD8+ T-cells) fell short of the very large spontaneous TCE (which can make up 80% or more of CD8+ T-cells). All other characteristics were either highly similar or super imposable between the two.
Based upon the above discussion, we propose the following scenario for the generation of expanded T-cell populations in old age. Memory T-cell pool will be formed by antigenic contact, generating larger or smaller Ag-specific subsets. From these subsets, the aging microenvironment will select cells based on their ability to survive and accumulate in response to homeostasis-regulating cytokines. Thus, antigenic stimulation plays a role only inasmuch as it contributes to the generation of the total memory T-cell population. Because this process is not antigen-driven, the selected cells will carry random repertoire of T-cell receptors. The most successful selectees will accumulate to large numbers, and will become large TCE. This is consistent with the results of Callahan et al. (49), as well as our own data (23), where no enrichment for particular T-cell specificity or TCRVβ segment could be linked to the “spontaneously” arising TCE. Consistent with that, memory CD8 T-cell pools specific for acute pathogens such as Sendai virus (61) and WNV (Figure 5) can provide the substrate for AI-TCE. Accordingly, we believe that the gB-8p:Kb-specific CD8 T-cells that accumulate with aging have been selected stochastically from the available pool of memory cells. We would predict that in a model where memory inflation is even more pronounced (such as is the case with systemic mCMV infection (38, 39)), the dysregulation of memory homeostasis in old age would include an even higher frequency of mCMV-specific T-cell expansions. The mouse model of aging and infection with HSV, mCMV and other persistent pathogens should be highly conducive to conclusively test this hypothesis.
Acknowledgments
The authors would like to thank the NIH Tetramer Production Facility (Atlanta, GA) for outstanding reagent preparation, Ms. M. Fischer and Mr. T. Totonchy for excellent technical assistance, and the members of the Nikolich laboratory for assistance and stimulating discussion.
Supported by the USPHS Awards AG20719 (to J. N-Z.), NEI T32EY07123 (A.L), T32 AI007472 (J.B & I.M.) and RR0163 (to the ONPRC) from the National Institute on Aging, National Institute of Allergy and Infectious Diseases and the National Institute for Research Resources, NIH.
ABBREVIATIONS
- AI-TCE
Antigen-independent T-cell clonal expansions
- AR-TCE
Ag-responding TCE
- ATX
adult thymectomy
- gB-8p
immunodominant H-2-Kb-binding HSV-1 epitope from the glycoprotein B (gB498-505, SSIEFARL)
- FCM
flow cytofluorometry
- KO
knockout, carrying targeted disruption of indicated molecules
- rVV-gB-8p
recombinant vaccinia virus carrying HSV-1 gB-8p epitope
- TCE
T-cell clonal expansions
- TCR
T-cell receptor
Footnotes
The authors report no financial conflict of interest nor commercial affiliations.
References
- 1.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 2.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 3.Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev. 2005;205:5–6. doi: 10.1111/j.0105-2896.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Regulation of naive and memory T-cell homeostasis. Microbes Infect. 2002;4:51–56. doi: 10.1016/s1286-4579(01)01509-x. [DOI] [PubMed] [Google Scholar]
- 5.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 6.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murali-Krishna K, Ahmed R. Naive T cells masquerading as memory cells. Journal of Immunology. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 9.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 10.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 14.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 15.Mocarski ES, C CT. Cytomegaloviruses and their replication. In: Knipe DM, Howley PM, editors. Fields - Virology. Lippincot Williams & Wilkins; Philadelphia: 2001. pp. 2629–2674. [Google Scholar]
- 16.Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. 2002;5:403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- 17.Pollock JL, Virgin HWt. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddehase MJ, Podlech J, Grzimek NK. Mouse models of cytomegalovirus latency: overview. J Clin Virol. 2002;25(Suppl 2):S23–36. doi: 10.1016/s1386-6532(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 19.Reddehase MJ. The immunogenicity of human and murine cytomegaloviruses. Curr Opin Immunol. 2000;12:390–396. doi: 10.1016/s0952-7915(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 20.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 21.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 22.Messaoudi I, LeMaoult J, Metzner BM, Miley MJ, Fremont DH, Nikolich-Zugich J. Functional evidence that conserved TCR CDR alpha 3 loop docking governs the cross-recognition of closely related peptide:class I complexes. Journal of Immunology. 2001;167:836–843. doi: 10.4049/jimmunol.167.2.836. [DOI] [PubMed] [Google Scholar]
- 23.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. Journal of Immunology. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 24.Cose SC, Kelly JM, Carbone FR. Characterization of a diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. Journal of Immunology. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 26.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. Immunity. 2003;18:593–608. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyall R, Messaoudi I, Janetzki S, Nikolic-Zugic J. MHC polymorphism can enrich the cell repertoire of the species by shifts in intrathymic selection. 2000;164:1695–1698. doi: 10.4049/jimmunol.164.4.1695. [DOI] [PubMed] [Google Scholar]
- 29.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T-cell avidity and diversity in immune defense. Science. 2002;298:1797–1801. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 30.Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasilakos JP, Michael JG. Herpes simplex virus class I-restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+ T cells. Journal of Immunology. 1993;150:2346–2355. [PubMed] [Google Scholar]
- 32.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H- 2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 33.Blaney JE, Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu T-M, Kawaoka Y, Tevethia SS. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang Q, Wagner WM, Wikby A, Remarque E, Pawelec G. Compromised interferon gamma (IFN-gamma) production in the elderly to both acute and latent viral antigen stimulation: contribution to the immune risk phenotype? Eur Cytokine Netw. 2002;13:392–394. [PubMed] [Google Scholar]
- 35.Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 36.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term Cytomegalovirus infection leads to significant changes in the composition of the CD8 T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005 doi: 10.1128/JVI.79.6.3675-3683.2005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol. 2000;74:7496–7507. doi: 10.1128/jvi.74.16.7496-7507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 40.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 41.Field HJ, Tewari D, Sutton D, Thackray AM. Comparison of efficacies of famciclovir and valaciclovir against herpes simplex virus type 1 in a murine immunosuppression model. 1995;39:1114–1119. doi: 10.1128/aac.39.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thackray AM, Field HJ. Further evidence from a murine infection model that famciclovir interferes with the establishment of HSV-1 latent infections. 2000;45:825–833. doi: 10.1093/jac/45.6.825. [DOI] [PubMed] [Google Scholar]
- 43.Pesola JM, Zhu J, Knipe DM, Coen DM. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J Virol. 2005;79:14516–14525. doi: 10.1128/JVI.79.23.14516-14525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawtell NM. Quantitative Analysis of Herpes Simplex Virus Reactivation In Vivo Demonstrates that Reactivation in the Nervous System Is Not Inhibited at Early Times Postinoculation. J Virol. 2003;77:4127–4138. doi: 10.1128/JVI.77.7.4127-4138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 47.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, LeFrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 48.Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. Journal of Immunology. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 50.Hingorani R, Choi I-H, Akolka P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. Journal of Immunology. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 51.Posnett DN, Sinha S, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. Journal of Experimental Medicine. 1994;179:609–617. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 53.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 54.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 55.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36:119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 56.Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunological Research. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Khanna KM, Chen X-P, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. Journal of Experimental Medicine. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thackray AM, Field HJ. Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. 1996;173:291–299. doi: 10.1093/infdis/173.2.291. [DOI] [PubMed] [Google Scholar]
- 59.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological Stress Compromises CD8+ T Cell Control of Latent Herpes Simplex Virus Type 1 Infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ku CC, Kappler J, Marrack P. The Growth of Very Large CD8+ T cell Clones in Older Mice is Controlled by Cytokines. Journal of Immunology. 2001;166:2186–2193. doi: 10.4049/jimmunol.166.4.2186. [DOI] [PubMed] [Google Scholar]
- 61.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]


