Abstract
No metazoan cell survives on its own, absent the signals and support of its milieu. For multicellular life with specialized tissues to persist, organization is everything and so defining the association of position with cell state is critical to understanding how tissues function, maintain and repair. This review focuses specifically on place for progenitor and stem cells with a special emphasis on hematopoietic cells where free movement is often considered a defining trait and where concepts of regulatory interrelationships have been shown with some precision. It reviews classical and emerging concepts of the niche, particularly considering how niche functions may participate in neoplastic disease.
Introduction
Position in the society of life is an anthropologic concept that may be rightly if loosely applied to the interactive communities of cells that comprise our bodies. There are hierarchies of function, of differentiation, of responsiveness and production of signals and of participation in disease. While the molecular processes defining cell states are defined with increasing and quantifiable precision by genome-wide inventories of chromatin structure and gene expression, the characterization of cell interactions remains largely qualitative. Yet, the principles of how cells engage to create and maintain tissue are increasingly evident mainly through genetic models where select subpopulations of cells are modified or eliminated. The majority of these examples concern adult tissues and assess how tissue homeostasis and repair are conducted. Therefore, they largely reveal the governance of stem and progenitor cells. This review discusses the changing landscape of stem and progenitor regulation including how their position and the interactions that influence them may participate in the evolution of cancer.
Historic background
Radiation biology was of particular concern following the advent of nuclear weapons in World War II as protecting populations from radiation exposure was a paramount public health goal. Combined efforts by physicists and biologists included the first experimental definition of a stem cell in the classic and ingenious experiments of Till, a biophysicist, and McCulloch, a physician and cell biologist (Becker et al., 1963; Till and Mc, 1961). They defined the power of a single cell to regenerate a tissue destroyed by radiation. Places like the University of Manchester and the affiliated Holt Radium Institute assembled hematology researchers including T. Michael Dexter who developed stromal co-cultures as a means of maintaining hematopoietic stem cells in vitro and demonstrated the dependence of hematopoietic stem cells on support from populations of non-hematopoietic cells in the bone marrow (Dexter et al., 1977); Brian Lord who championed the concept of an architectural organization to the bone marrow demonstrating regionalization of stem and progenitor cells in vivo (Lord et al., 1975); and Raymond Schofield who formally proposed the stem cell niche articulating the functional attributes of a specialized microenvironment on stem cell function in vivo (Schofield, 1978). Together, they provided the intellectual underpinnings for much of what has subsequently developed in niche biology.
Schofield laid out a theory that included more than the postulate that stem cells were located in physical sites where they were uniquely regulated (stem cells were not autonomous, as conventional wisdom suggested), but that the niche had additional functions including the ability to impose the stem cell state on more differentiated cells (Figure 1). “The stem cell daughter is a CFU-S [colony forming unit – in spleen]. However, if it can find and occupy a niche it will itself become a stem cell” (Schofield, 1978). He thereby proposed that the niche can effectively drive cell state. He also noted that “a fixed [in place] haematopoietic stem cell may be not only the means by which its immortality is achieved but also the means by which the number of mutational errors is minimized” (Schofield, 1978). A cell in its niche has self-renewal capacity, but he hypothesized that there are features of the niche that prevent the natural consequence of self-renewal, namely accumulation of genetic damage, from occurring. The niche therefore could limit genetically altered stem cells from corrupting normal hematopoiesis. The niche concept was just that, however, as Schofield carefully noted that “no direct evidence for this actually exists” (Schofield, 1978).
Figure 1. Elements of a stem cell niche as originally proposed by Raymond Schofield.

Image of Schofield provided by his colleague Brian Lord. Note the background drawing of the blind men and the elephant parable: an appropriate cautionary reminder of the need for integration of partial information for full understanding of niche biology.
Ecologic niche
Schofield “invoked the postulate of an environment…to explain the unlimited proliferation and failure to mature of …stem cells” (Schofield, 1978) with clear reference to environmental constructs used in organismal biology. The ecological concept of a niche to which he referred had features that were articulated at the time by his contemporary, PJ Darlington as a place of “extended competition in action” (Darlington, 1972). He viewed the niche as different than “pre-existing pigeonholes with boundaries” but rather as a setting where “pressures and processes” influenced the relative abundance of subsets of occupants (Darlington, 1972). While much of the definition of niche biology within complex organisms has focused on defining the components of a pigeonhole, a more dynamic view of the niche is now emerging and may speak to Schofield’s conception of a place where mutations are minimized. These features again refer to ecologic concepts of niches and niche functions.
For example, the ecologic niche may be viewed as a basis for determining the diversity of inhabitants within a given setting. That is, under a particular set of conditions within a given environment of specified nutrient availability and temperature, the range of species occupying it will be based on their relative competitive advantage. With stable environmental conditions, sub-species with particular traits will predominate and limit sub-species diversity by virtue of competitive exclusion (Levine and HilleRisLambers, 2009) (Figure 2). That concept explains how an equilibrium that emerges in a particular niche will be one of limited diversity where sub-optimal occupants are progressively lost from the occupant pool. If however, the environment shifts, leading to an increase in niche ‘breadth,’ the ability for previously disadvantaged sub-species to thrive increases and with it sub-species diversity. The presence of a variegated niche may then lend itself to the ‘coexistence’ of previously excluded sub-populations and the increase in diversity may permit different competitive relationships between populations to emerge. Niche breadth is a driver of diversity.
Figure 2. Niche effects on inhabitant diversity from ecologic models.
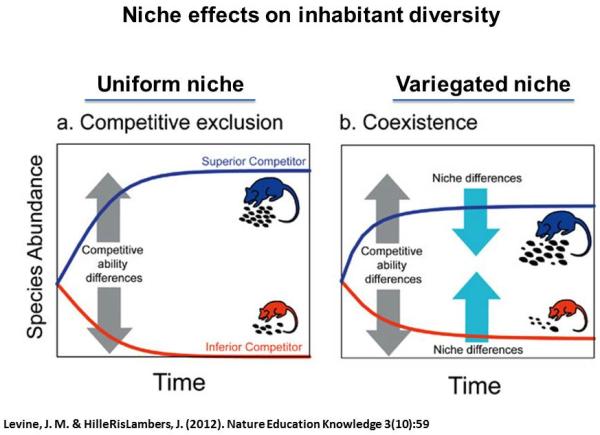
Settings of stable environmental conditions enable dominance of a competitively advantaged population to the progressive exclusion of less fit populations (left). However, in the context of increased variability of niche conditions, the number of subpopulations supported in a given microenvironment increases resulting in a greater diversity of inhabitants (right).
Ecologic concepts for tissue biology
Applying these concepts to cell populations within the context of a tissue may be a useful means of considering the emergence of dysplastic and neoplastic cells within previously healthy tissue. If niche breadth in cell biology determines the diversity of clonal sup-populations of cells within tissue, it would follow that the persistence of the altered cells that constitute dysplastic or neoplastic tissues would be favored if there was a cooperating change in the niche. Is there evidence for cooperativity between the niche and the parenchymal cells they support in fostering abnormal tissue?
Testing this issue requires experiments where alterations in niche cells can be well controlled. The modification of specific subsets of cells constituting niche components has been limited, largely because defining those cell populations with specificity is still very restricted. This limitation is changing accompanied by intriguing results about the role of microenvironmental ‘support’ cells. In a model where it is not clear if stem/progenitor cells are involved, TGF-beta II receptor deletion in stromal cells expressing the putative fibroblast specific promoter (FSP1) results in invasive squamous cell cancer of the stomach and intraepithelial neoplasia in the prostate (Bhowmick et al., 2004). Other studies showed that inducing overexpression of FGF10 in prostate mesenchymal cells results in prostate adenocarcinoma and overexpression of a chromatin remodeling protein encoding gene (Hmga2) in prostate stromal cells and altered Wnt signaling and neoplasia in prostate epithelial cells (Memarzadeh et al., 2007; Zong et al., 2012). The genetic alterations are accomplished by viral transduction in the latter experiments so the cell type altered may not be selective, but the expression data does indicate that the transgene is not in the epithelial cells themselves. Whether the epithelial cells have genetic alterations is not defined.
Environments enabling neoplasia
In hematopoiesis, genetic mutations in the marrow microenvironment results in myeloproliferative neoplasia in the mouse. Specifically retinoic acid receptor-gamma (RARg) deficient animals develop myeloproliferation, but this does not occur if the RARg−/− hematopoietic cells are transplanted into a wild-type bone marrow. Rather, genetically wild-type hematopoietic cells transplanted into a RARg−/− host results in myelproliferation (Walkley et al., 2007a). The myeloproliferation is not clearly associated with any genetic alteration in the hematopoietic cells. However, in the context of retinoblastoma (Rb) deletions, myeloproliferation only develops in hematopoietic cells if complemented by Rb deletion in the microenvironment (Walkley et al., 2007b). Neither cell type alone is sufficient to induce the phenotype. It has also been shown that the neoplastic phenotype can be markedly affected by the genetics of the microenvironment. Acute myeloid leukemia induced by transduction of the oncogenic human fusion of MLL-AF9 into human CD34+ HSPC has very different manifestations dependent on the immunocompromised mouse strain into which the cells are transplanted (Wei et al., 2008). Thus, the microenvironmental context can enable and can modify hematopoietic neoplasia.
Focusing on more specific subsets of cells, clearer evidence of microenvironmental changes participating in the development of neoplasia has been generated. Deletion of a number of microRNA processing enzymes or the ribosomal protein associated with human disease, Shwachman Bodian Diamond Syndrome (SBDS), in early but not mature osteolineage mesenchymal cells, results in disordered hematopoiesis (Raaijmakers et al., 2010). In that model, there is the rare outgrowth of cells that have acquired new, multiple genetic lesions and have transformed into lethal acute myeloid leukemia. Of the three leukemias that could be studied in detail, it is notable that two of them have a shared region of chromosomal copy number change suggesting a non-random outgrowth of cells. Further, bone marrow cells derived from animals with the altered microenvironment have evidence of altered Akt phosphorylation. Additional studies modifying β-catenin in mouse osteoblasts have recently shown the emergence of acute myeloid leukemias with common chromosomal alterations and increased Notch signaling. Corresponding molecular alterations were seen in osteoblasts in ~38% of human AML patients (Kode et al., 2014). Furthermore, in some patients who have undergone allogeneic transplantation for AML, they have relapsed with leukemia of donor cell origin strongly suggesting that they have a leukemia-fostering microenvironment (Wiseman, 2011). These data indicate that perturbations of cells in the environment can alter the signals provided to the parenchymal cells they support and can enable - if not induce - the expansion of an altered sub-population of cells that can eventually become dominant and deadly. Much like in ecology, a shift in the conditions of the niche can permit subspecies to thrive, in this case, leukemic cells.
While examples indicate that altered mesenchymal cells can result in a neoplastic phenotype in the parenchymal cells they support, the relevant studies do not define if this is due to a competitive selection process or simply altered growth support by cells in the microenvironment. However, other models indicate that the interface between a niche and its occupants can select for specific characteristics. This is the case in at least one classic example in hematopoiesis, the W/Wv mouse. In that animal, a spontaneously occurring mutant tyrosine kinase receptor gene, c-kit, impairs the hematopoietic cells and specifically renders them incapable of successfully competing against cells of wild-type c-kit (Bernstein and Russell, 1959). Wild type stem cells readily replace the W/Wv cells and provide durable hematopoiesis. Therefore competitive disadvantage has been demonstrated. Pharmacologic manipulation of pathways implicated in niche occupancy has also resulted in alteration of competitive relationships among stem and progenitor cells. The use of non-steroidal anti-inflammatory drug (NSAID) eicosanoid inhibitors alters the expression of chemokine receptors such as CXCR4, a known mediator of HSC-niche interactions. HSC in which the eicosanoid pathway is inhibited by specific NSAIDS are disadvantaged when competing with cells not exposed to that drug (Hoggatt et al., 2013). Therefore, select perturbations can sufficiently affect stem cell-niche interactions to change the competitive dynamics of cell populations within a tissue.
Niche variability
The likelihood that niche cells acquire genotypic changes that introduce variability into the niche and affect the conditions for parenchymal cells is in part dependent on the dynamics of niche cell populations. For example, if niche cells are long-lived and only replace themselves via mature cell division, the potential for an acquired change in a niche cell to combine with a complementary change in a parenchymal cell to result in neoplasia would be low. A niche cell might be mutated, but it would not be expected to create a major ‘field’ unless it was fully transformed. However, if niche cells were more dynamically turning over and depend on a self-renewing pool of stem cells for replenishment, mutations could accumulate in a pool of niche cells creating a field defect in the niche. This abnormal field may change the parameters of the niche that foster or select against parenchymal cell occupants. By forming an abnormal niche ‘field’ rather than single modified niche cells, the potential for complementary abnormalities enabling abnormal parenchymal cells to establish themselves hypothetically increases (Figure 3). The combination of stem cells contributing to cells on either side of the niche-stem cell interface would provide a context in which genetic alterations in each stem cell population could accumulate and, if both sides turnover with some rapidity, a pairing of complementary phenotypes would be more likely (Figure 4).
Figure 3. How population dynamics of stromal support populations may influence the relative likelihood of contributing to a neoplastic outgrowth.
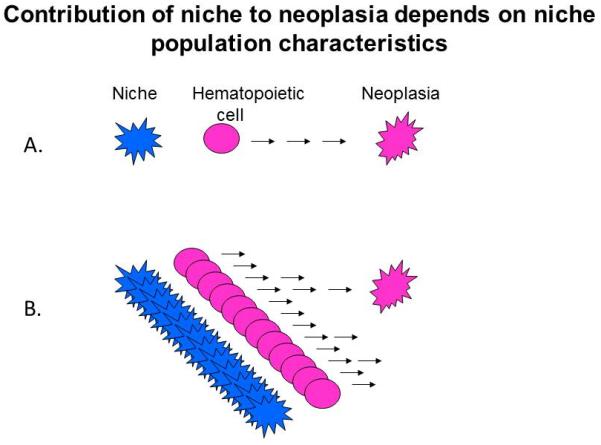
Abnormal hematopoietic subpopulations that depend upon stromal support will persist provided there is a ‘match’ between the supportive context the stroma provides and the needs of the mutant hematopoietic stem/progenitor cells. The likelihood such cooperating pairing will occur is low if the stromal cells rarely turnover or are replenished by mature cell division (left). In such a setting, the abnormal hematopoietic cell may be lost by competitive exclusion. However, if stromal cells are dynamic with frequent cell replenishment occurring by production of mature stromal cells from stem cells, then a cooperating alteration in a stromal ‘field’ supporting abnormal hematopoietic populations would be more likely to occur and the abnormal clone to persist (right).
Figure 4. Dynamics of genetic changes in the stem cell niche.
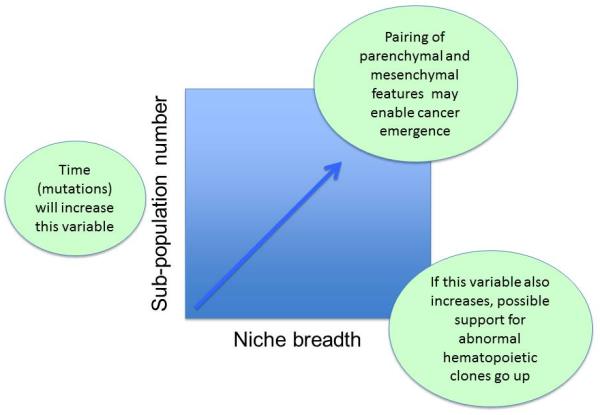
Diversity of subpopulations introduced by accumulating genetic changes may increase over time in both the support and supported cells leading to an increased likelihood of cooperation between cell types to enable neoplasia.
Mesenchymal cell dynamics in the bone marrow
Defining the cell dynamics of niche cells depends on well-defined niche cell identities and having genetic tools available to accomplish pulse-chase experiments. This has been done in osteolineage cells in the bone marrow and shown that the turnover kinetics of these cells is strikingly high (measured in weeks to months) and the cells are replenished by a stem/progenitor population (Park et al., 2012). Of note, primitive, self-renewing mesenchymal cells with ‘stem cell’ characteristics in that study can be serially transplanted and have the capacity to both migrate locally and translocate via the blood. Population dynamics and function would therefore suggest that, if mutations arise in the population that affects hematopoietic cell support, they can result in a field-like alteration of the bone marrow. Whether this would be sufficient to accomplish a so called “field cancerization” effect of a stem/progenitor niche is not known, but the characteristics of the cells are commensurate with what would be required (Slaughter et al., 1953).
Descendent cells altering the niche
Co-existence of genetically modified niche and parenchymal cells may occur in another setting where the genetic events may not be independent. Daughter cells descending from stem cells have been shown to become niche cells in a number of tissues. In those cases, genetically aberrant stem cells may create offspring that affect the fitness constraints for their parents. For example, in the small intestine the Paneth cell is a Lgr5+ stem cell descendent and plays a critical niche role in regulating Lgr5+ intestinal stem cells (Sato et al., 2011). This cell type has been shown to alter stem cell growth as discussed in greater detail below. In the hair follicle, K6+ inner bulge stem cells enter into cell cycle, differentiate and rather than contributing to the generation of hair, revert back to the bulge where they contribute to the stem cell niche (Hsu et al., 2011). They do not revert to a stem cell state, but can regulate the proliferation of stem cell neighbors. With descendant cells playing central roles in the niche, alterations in the stem cell have the potential to result in alterations in the niche.
In hematopoiesis, three different types of mature hematopoietic cells have been implicated in modifying the local stem cell environment. Macrophages alter stem cell localization and regulatory T cells provide immune sanctuary (Chow et al., 2011; Fujisaki et al., 2011; Winkler et al., 2010). In addition, recent work indicates that megakaryocytes provide multiple signals that affect mesenchymal components of the stem cell niche and the cell cycle status of stem cells, inducing quiescence (Heazlewood et al., 2013; Olson et al., 2013)(Paul Frenette personal communication). In this context, the megakaryocyte provides feedback whereby adequate numbers of those more mature cells keeps the less mature stem cell from excessive activation. It is possible therefore that an abnormal stem cell could generate offspring incapable of constraining self-renewing stem cell proliferation: a set-up for oncogenesis. This model remains hypothetical at this time, but the prominence of abnormal megakaryocytes in malignant human syndromes such as myelodysplasia assures that it will be tested in short order.
Bidirectional communication within the niche in disease
The dynamic nature of the cells in the niche may include other mechanisms by which an abnormal population of niche residents may shape the environment to their favor. This has been documented in one study where a leukemia cell line influenced the interaction of normal hematopoietic stem/progenitor cells with the bone marrow microenvironment by changing their mobilization capability (Colmone et al., 2008). More definitively, genetic changes introduced into hematopoietic cells induce secondary changes in microenvironmental cells that foster support of the abnormal hematopoietic populations (Schepers et al., 2013). Using a model of BCR/ABL induced myeloproliferative neoplasia (MPN), it was demonstrated that this primary modification of hematopoietic cells is accompanied by a decrease in osteolineage mesenchymal cell expression of molecules that support normal hematopoiesis (such as CXCL12 and kit ligand). The result is a marked disadvantage to normal hematopoietic stem and progenitor cells while providing a fully supportive environment for leukemic cells. The competitive balance was therefore shifted toward malignant cells, not simply due to intrinsic characteristics of the BCR/ABL transformed cells. Rather, the BCR/ABL cells effectively induce a re-modeling of the niche to their advantage accompanied by a secondary compromise of their normal cell competitors: ruthless neighbors.
This two-way conversation may not be restricted to mesenchymal cells as neural populations participating in the niche are also affected in settings of tissue dysfunction. Emerging data indicates that the neural crest derived nonmyelinating Schwann cells associate with the sympathetic nerve cells in the bone marrow become abnormal in the context of a myeloproliferative neoplasia associated with the JAK2-V617F mutation. Those neoplastic hematopoietic cells impair activity of the sympathetic nervous system. Intervening with β3 agonists to overcome the neural deficit partially reverses the hematopoietic phenotype ( S. Méndez-Ferrer, personal communication). The abnormal occupant of the niche can thereby engage in a functional symbiosis with its corresponding niche cells.
The implication of this model is that interventions to arrest neoplasia need not be restricted to the putative neoplastic cell itself. Rather, if neoplasia is fostered by a coordinated corruption of both niche and niche occupant, attacking the niche can theoretically provide benefit. The concept of cancer as a disease of tissue and not just a particular cell type has long been argued and the models above provide support for that perspective. They do not negate the critical importance of cell autonomous drivers of transformation, but they do suggest that cooperativity may be of sufficient impact in some settings to explore those, at least for some cancers like leukemia. Evidence for intervening to specifically alter the niche has been provided by recent data showing that inactivation of the parathyroid hormone receptor in osteolineage mesenchymal cells in the bone marrow, genetically or with drugs, results in a reduction of leukemia stem-like cells in vivo (Krause et al., 2013). Therefore, the ongoing and highly plastic relationship between niche cell and occupant may ultimately unveil new biologically driven approaches to some cancers.
Niche as a driver of cell state
The conversation between niche and stem cell is clearly two-way and it is not just the niche cell that can be molded by its occupant. The niche can be dominant and impose stem cell features on occupying cells as envisioned by Schofield (Schofield, 1978). This ability of the niche to impose a stem cell state first gained experimental support in Drosophila (Brawley and Matunis, 2004). Two studies demonstrated that the germ cell niche can revert maturing cells to germ cell like features. In males, an empty testis niche occupied by prospermatogonia results in the reversion of those cells to a germ cell stem cell state (Brawley and Matunis, 2004). Ovarioles similarly are shown to have replacement of lost germ cells by stem cell descendants who are capable of re-acquiring stem cell features and competing with other stem cells for niche occupancy (Nystul and Spradling, 2007). In animals with long intervals to sexual maturity like most mammals, it was thought that such a model would be disadvantageous and may be selected against since enabling cells to re-acquire self-renewal would be a set-up for cancer. The ‘transient amplifying’ pool of progenitors was considered a means of enabling cell expansion while reducing the durability of any acquired mutation. If the cells could become self-renewing by occupying a vacant niche, they would no longer be transient and the risk of the multiple genetic alterations associated with cancer would be increased. Recent data argues against the prediction that microenvironments in mammals are not capable of reverting maturing cells to a stem cell state.
Using elegant lineage tracing methods in the mouse, induction of stemness has been demonstrated in several experimental systems. Short-lived multipotent epithelial cells in the small intestinal crypt express Dll1 and that promoter was used to label and track the fate of the progenitor cells (van Es et al., 2012). Under conditions of epithelial injury, the cells provide long-term multilineage reconstitution in vivo, consistent with microenvironment-induced reversion to a stem cell state. In the skin, it has been shown that a vacant hair follicle stem cell niche created by laser ablation can be effectively repopulated with a non-stem cell population that takes on stem cell functions (Rompolas et al., 2013). The function of stem cells is highly regionalized with cells positioned at the upper regions of the bulge being quiescent, lower bulge cells proliferating and yielding outer root sheath cells and those stem cells below it, in the hair germ, generating differentiating populations. These position/function correlations resemble what has been documented in the intestine where rapidly cycling and quiescent stem cells reside in distinct locations at the base of the intestinal crypt (Li and Clevers, 2010). In the skin, specific locations could be vacated using laser ablation. Specifically, bulge cell loss results in the translocalization of other epidermal cells, cells that bear lineage marks of epithelial cells from the interfollicular region or other non-bulge sites (Rompolas et al., 2013). Yet, those cells can generate and regenerate hair and re-organized the position/function relationships of the intact follicle. The niche seemed to be able to drive cell fate converting one population to another simply by occupancy. Such plasticity of cell fate and reversion to a tissue specific multipotential cell state has been experimentally achieved with genetic manipulation in the past (Nutt et al., 1999). But, the ability of such reprogramming to occur based on signals of a niche environment belies a new level of plasticity.
It is apparent that the dedifferentiation effect of some locations may be more generalizable. By depleting stem cells in the lung, Rajagopal and colleagues showed that loss of basal cells, a multipotent stem cell of airway epithelia, results in the lineage reversion of mature secretory cells (Clara cells) into functional stem cells that can then go on to repopulate both Clara and ciliated cells (Tata et al., 2013). Studying the stomach, Clevers et al found similarly that mature cells, in this case fully differentiated chief cells that retained expression of stem cell associated genes including troy, can adopt a stem cell-like function (Stange et al., 2013). The latter model is more consistent with a ‘facultative’ adoption of stem cell features by mature cells, rather than a dedifferentiation process (Yanger and Stanger, 2011). This model would argue for a reserve population of cells with stem cell capacity essentially at the ‘ready’ in times of need. These examples of plasticity of cell fate with re-acquisition or resumption of stem cell functions respectively, argue for context providing marked shifts in cell state. What molecular mechanisms modulate these changes is not entirely clear, though in the case of the stomach, Wnt signals, perhaps from mesenchymal cells found in pits near the troy+ chief cells, may provide essential cues. In the case of the lung, a very interesting dependence on other cell types is observed.
Reversion to a stem cell-like state in the lung is prevented by the presence of neighboring stem cells. Ex vivo, even a single basal cell was sufficient to restrain the secretory Clara cells from undergoing a state conversion to a stem cell (Tata et al., 2013). These data would therefore argue that vacancy in a niche could enable dedifferentiation, but within rather severely imposed constraints imposed by other stem cells. If a stem cell can confine the ability of a differentiated cell to revert to ‘stemness’, then occupants of niches may not be merely filling an otherwise vacant, stem cell-enabling space. Rather, they may ‘feed-forward’ inhibitory signals on their progeny restricting the number that can re-acquire stem cell features. The ability of the niche to induce the stem cell state is influenced by existing stem cells.
Tissue logic in the niche
If the stem cell state can be imposed by the niche, and yet the niche can be shaped by the stem cell, what are the boundaries that keep niche and stem cell numbers constant? Directing organizers must exist. Exploring this in adult tissues is complicated, but the hematopoietic system does offer some insight, particularly from the higher order architecture that is now being defined in the bone marrow, where cells have freedom of movement not seen in most organs. Quiescent hematopoietic stem cells have been defined to be in close proximity to arterioles with Nestin-hi mesenchymal cells that are abundant in the endosteal region (Kunisaki et al., 2013). Such a physical association is highly non-random in contrast to proximity to sinusoidal vessels that have other mesenchymal cells in association with them (Ding et al., 2012; Kunisaki et al., 2013). Hematopoietic stem cells that are transplanted also appear to favor periarteriolar sites near the endosteum (Spencer et al. in press) and a number of studies have indicated the preferential localization of HSC near the endosteal region of trabecular bone in both mouse and human (Ellis et al., 2011; Guezguez et al., 2013; Nombela-Arrieta et al., 2013). The nestin+ cells have been reported to modify stem cell function (Mendez-Ferrer et al., 2010). Ablation of those niche cells reduces HSC number. High resolution imaging of transplanted subsets of cells indicates that stem cells and progenitors cells locate distinctly in the bone marrow; position was cell state dependent (Lo Celso et al., 2009). This organizing of cells, pairing cell states with particular locations defined by macroanatomic structures like arterioles or trabecular endosteum, implies an architectural or tissue level of control. That is, that niche organization reflects the same influences that shape the morphogenesis of a tissue or the vasculature that sustains it. These include biomechanical forces that have now been experimentally demonstrated to influence the differentiation and proliferative features of skeletal muscle, hematopoietic and multiple other stem cell types (Gilbert et al., 2012; Lutolf et al., 2009; Shin et al., 2013).
Niche as interlocutor of tissue and organismal state
The homeostatic and reparative functions of stem cells require that they be regulated in a manner fitting the physiologic context. As such, what regulates them must have the ability to gain inputs reflective of the tissue and organismal state and make those coherent to the stem cell in terms of proliferative, differentiation and survival signals. The niche then can be viewed as an integrator and translator of information from the tissue home of the stem cell and from more distant sites (Figure 5). Combined, these functions make it unlikely that any single cell type comprises the niche for complex, rapidly turning over tissues like blood, skin, intestine and airway. Within the bone marrow, the need to incorporate tissue and organismal input for blood cell production makes it unsurprising that the niche includes cells of the circulatory, nervous and immune systems.
Figure 5. Niche as an interlocutor of organismal needs.
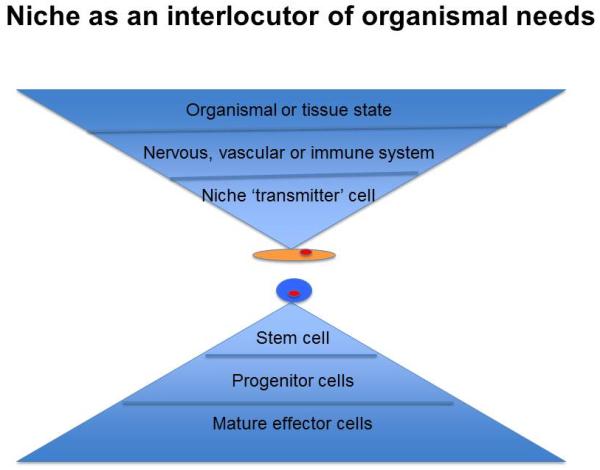
How the niche must incorporate signals indicating the state of the tissue and organism to properly regulate cell production under homeostatic and stress conditions.
Experimental evidence has been generated in support of a role for each of these systems. For the circulatory system, endothelial cells expressing Cre recombinase under the control of the Tie2 promoter was used to conditionally delete kit ligand or CXCL12 and resulted in a decrease in HSC in the bone marrow indicating the necessity of this cell type for the persistence of hematopoietic stem cells (Ding et al.) (Ding and Morrison, 2013; Ding et al., 2012; Greenbaum et al., 2013). Further, VEGFR2 expressing sinusoidal endothelial cells have been shown to be necessary for hematopoietic stem and progenitor cell engraftment post irradiation (Hooper et al., 2009). Protection of bone marrow vascular endothelium improves survival and regeneration of hematopoiesis after radiation (Doan et al., 2013), while addition of endothelial progenitors to a stem cell graft enhanced engraftment efficiency (Salter et al., 2009). These studies demonstrate the participation of endothelium in regulating stem and progenitor cells, though how they may alter that regulation to reflect tissue or organismal state has not been experimentally examined.
Nervous system cells are more clearly integrators of information from a distance. Evidence for nervous system contribution to HSC regulation comes from several experimental contexts. First, sympathetic neurons affect stem cell localization mediated by β3 adrenergic receptor activation (Katayama et al., 2006). The presence of altered sympathetic neurons in the marrow in models of diabetes is associated with altered ability of HSPC to be mobilized by G-CSF and cytotoxic chemotherapy related sympathetic neuron injury adversely affected hematopoietic recovery (Ferraro et al., 2011; Lucas et al., 2013). Second, glial cells that serve as non-myelinating Schwann cells activate TGFβ and thereby regulate HSC quiescence as evident from adrenergic neuron disruption in marrow resulting in loss of HSC (Yamazaki et al., 2011).
Evidence for immune system participating in stem cell regulation includes T lymphocytes contributing to HSC engraftment (Adams et al., 2003) and T cell depletion associating with engraftment failure clinically (Ash et al., 1991). With engraftment, regulatory T cells provide an immune sanctuary in the bone marrow for HSC (Fujisaki et al., 2011). These three systems are in a sense professional integrators of information at an organismal level and input from them can provide a means by which the HSC then ‘reads-out’ on a single cell level the needs of the organism.
Evidence for the HSC reflecting organismal state is seen in several contexts. First, the β3-adrenergic cells regulation of stem cell localization has been linked to central nervous circadian rhythms (Mendez-Ferrer et al., 2009). Hematopoietic stem cells migrate into and out of the bone marrow throughout adulthood with varying frequency on a daily basis mediated by sympathetic nervous system cells and the efficiency of HSC transplants is affected by circadian cycle (Mendez-Ferrer et al., 2009) (Scheiermann et al. 2012). This connection of neural input to hematopoietic activity is not just seen in mammals as Drosophila have been shown to alter hematopoietic progenitor differentiation in response to GABA released upon olfactory nerve stimulation by food odors (Shim et al., 2013).
As an example of acute responses to a tissue or organismal need, HSC proliferate in response to immune triggers like interferon alpha (Essers et al., 2009). In a model of systemic infection with Mycobacterium, HSC change proliferative activity in response to interferon gamma (Baldridge et al., 2010) and with Pseudomonas sepsis, they decrease myeloid differentiation capacity in a TLR4 dependent manner (Rodriguez et al., 2009). Other systemic inputs also influence stem and progenitor pools. Drosophila modulates hematopoietic progenitor differentiation directly in response to amino acid levels, increasing the differentiation and thereby the loss of progenitors under conditions of amino acid deficiency altering mTor activity (Shim et al., 2012). With intestinal stem cells (ISC), it has been elegantly demonstrated that nutritional state affects stem cells not directly, but through the niche (Yilmaz et al., 2012). Paneth cells are key participants in ISC regulation (Sato et al., 2011). Calorie restriction decreased mTorc1 signaling resulting in increased levels of the ectoenzyme, bone stromal antigen-1, that produces ADP-ribose (Yilmaz et al., 2012). ADP-ribose then acted in a paracrine manner to augment ISC self-renewal. In this way, the niche translates organismal nutritional status to activity of the stem cell.
Niche subtypes regulate specific subsets of stem and progenitor cells
It is also likely that the information needed for the regulated differentiation of stem and progenitor cells is governed at multiple levels. It is the progenitor or transient amplifying pool that may be regarded as the most nimble cell population in terms of cell production in response to changing needs of a rapidly turning over tissue. That population is highly replicative and highly responsive to cytokines and progenitors have been shown to feed back to stem cells. For example, it has been shown that differentiating hematopoietic cells in Drosophila negatively regulate extracellular adenosine levels by expression of an adenosine deaminase (Mondal et al., 2011). Since adenosine is a proliferative signal to more immature cells, the maturing progenitor cells effectively restrict further generation of themselves. In mammals, some K6+ descendants of hair stem cells become negative regulators of stem cell function by expressing BMP6 and FGF18 (Hsu et al., 2011) thereby also restricting the activity of stem cell production.
There is also evidence that progenitor differentiation choice is regulated by stem cells. For example, in Drosophila, mid-gut intestinal stem cells differentially express vesicular Delta to affect the relative generation of enterocytes and enteroendocrine cells (Ohlstein and Spradling, 2007).
Progenitor regulation is then reasonable to consider as also responsive to cells in place, to a localized niche. This has now been demonstrated in several settings including mammalian hematopoiesis. In mouse bone marrow, there are a number of perivascular mesenchymal cells that have been defined by markers such as Nestin, LeptinR, PRX1 and CXCL12 (Ding et al., 2012; Greenbaum et al., 2013; Mendez-Ferrer et al., 2010; Sugiyama et al., 2006). None of these markers are entirely specific and studies defining the role of cells defined by them all have limitations, but it does not appear that the populations are entirely overlapping (Kunisaki et al., 2013). Heterogeneity in identity extends beyond that of the markers defining them as the cells have different functions. For example, the Nestin-GFPhigh cells appear to be those residing in close proximity to and regulating quiescent HSC (Kunisaki et al., 2013). LepR+ cells express high levels of kit ligand that has been shown to be critical for maintaining the number of HSC. Nestin-Cre cells do not express kit ligand at the levels reported for LepR+ cells and deleting kit ligand in Nestin-Cre cells had little impact on HSC number (Ding et al., 2012). Other cells that express markers indicating osteolineage specification, appear to be more relevant for regulating hematopoietic progenitors rather than stem cells. Indeed, in animals where CXCL12 is deleted in cells expressing Cre recombinase under control of a collagen 1.1 2.3kb promoter (active in osteoblastic cells), HSC function is not different in SDF1−/− compared with heterozygote CXCL12-/+ animals (Ding and Morrison, 2013). Rather, lymphoid cells are the most affected.
Specific regulatory microenvironments then appear to exist for progenitor as well as stem cells. As the distinct populations of cells within the marrow microenvironment are better defined it is likely that distinct roles will become evident. For example, conducting cell depletion experiments using conditional expression of the diphtheria toxin receptor restricted to particular populations, the loss of osteocalcin expressing cells altered the number and function of a very specific, lineage restricted cell of T lymphoid precursors (D Scadden, unpublished data). Combined with the SDF1 deletion in osteoblastic cells suggests that there may be a fine grained distinction between mesenchymal cells in terms of the hematopoietic cells they support. Some more immature osteolineage cells affecting lymphoid progenitors broadly and more mature osteolineage cells affecting specific subsets of lymphoid progenitors. The specific pairing of mesenchymal and hematopoietic cells is suggested by these findings and raises the potential for gaining a directory of regulatory cell types in niches and their regulated partners. Should this be borne out, it offers the potential of being able to tune the production of particular hematopoietic cells by targeting their niche companion. Accomplishing this will require an intensive assessment of just how diverse populations of mesenchymal and endothelial cells are. Heterogeneity among these cell types clearly exists. But disambiguating the collection into a hierarchical ordering in terms of whom they influence, is an essential next step in the field both for the purposes of engineering particular outcomes and in terms of exploring the role of particular interactions in the development of disease.
Concluding remarks
The term stroma is no longer a suitable means of simply lumping all the subpopulations of mesenchymal cells regulating hematopoiesis or other stem and progenitor populations, but the constituent list of what we now call stroma remains very incomplete. At present, select promoters are used to either delete selected genes or selected cell populations, but more unbiased approaches are needed. In the skin, efforts to address this by segregating fibroblasts based on their position relative to epidermis has been informative (FM Watt personal communication). That is, deep dermal fibroblasts had the capacity to support hair follicle formation while superficial fibroblasts did not. Therefore, it may be reasonable to start by looking at cells based on proximity to the cells of interest. Evaluating this in the hematopoietic system by examining osteolineage cells at endosteal surface that reside in close proximity to the HSPC localizing there after transplant has been performed. Using a single cell isolation and RNA sequencing approach it is clear that proximate mesenchymal cells bear a distinct molecular expression signature compared with cells at a distance (D Scadden, unpublished data). This has led to identification of cell surface molecules that then can be used to either isolate such cells or evaluate them in situ by immunohistochemistry. It can also identify cell surface or secreted molecules that may play a regulatory role as has been recently validated.
While the proximity principal may help identify those cells most likely to have a regulatory effect, it excludes cells that may act at a distance and requires some degree of prior bias to label the cells. It is reasonable therefore to begin to take truly unbiased approaches by efforts such as single cell RNAseq on nonhematopoietic cells broadly to begin to catalogue the full complexity of the cells comprising the stem and progenitor cell microenvironment. While collecting specimens seems more like field biology, it is likely to be a fruitful endeavor in tissue biology so that experiments can be designed to test the lineage relationships among the cells of stroma and to then systematically evaluate their functional consequence. By having better classification schemas for cell sub-populations and matching their effects on hematopoietic cells, a systems biology of hematopoietic tissue will become a possibility. It is the systems approach that will ultimately permit understanding the circuitry of stem and progenitor cell responses. With such information in hand, it may be possible to provide more targeted interventions to achieve better outcomes in regeneration or neoplasia.
Acknowledgements
The contributions of Elizabeth Scadden, Jay Rajagopal, Rushdia Yusuf, Vionnie Yu, Borja Saez and Jonathon Hoggatt are gratefully acknowledged for their thoughtful input, editing and help with figures. Members of the Scadden lab have been invaluable in shaping this work and being the best partners in discovery a lab chief could ever imagine. There is much outstanding work in the field that is not included in this piece and I ask the forbearance of my colleagues for omissions of their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GB, Chabner KT, Foxall RB, Weibrecht KW, Rodrigues NP, Dombkowski D, Fallon R, Poznansky MC, Scadden DT. Heterologous cells cooperate to augment stem cell migration, homing, and engraftment. Blood. 2003;101:45–51. doi: 10.1182/blood-2002-02-0486. [DOI] [PubMed] [Google Scholar]
- Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC, Henslee PJ, Kolb HJ, Lowenberg B, Masaoka T, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7:443–452. [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Bernstein SE, Russell ES. Implantation of normal bloodforming tissue in genetically anemic mice, without x-irradiation of host. Proc Soc Exp Biol Med. 1959;101:769–773. doi: 10.3181/00379727-101-25089. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Darlington PJ., Jr Competition, competitive repulsion, and coexistence. Proc Natl Acad Sci U S A. 1972;69:3151–3155. doi: 10.1073/pnas.69.11.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter TM, Allen TD, Lajha LG. Conditions controlling the proliferation of hemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan PL, Russell JL, Himburg HA, Helms K, Harris JR, Lucas J, Holshausen KC, Meadows SK, Daher P, Jeffords LB, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31:327–337. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–1524. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Corbel S, Doyonnas R, Havenstrite K, Magnusson KE, Blau HM. A single cell bioengineering approach to elucidate mechanisms of adult stem cell self-renewal. Integr Biol (Camb) 2012;4:360–367. doi: 10.1039/c2ib00148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A, Bhatia M. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell stem cell. 2013;13:175–189. doi: 10.1016/j.stem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, Nilsson SK. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 2013;11:782–792. doi: 10.1016/j.scr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM, Hu P, Poteat BA, Stilger KN, Ferraro F, et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell stem cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014 doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, Lezeau S, Attar E, Wu JY, Lin HY, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nature medicine. 2013;19:1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nature medicine. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, Witte ON. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell stem cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M, Horwitz EM. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell stem cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA, et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064–4076. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, Russell L, Chen B, Chao NJ, Chute JP. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, Wagers AJ, Hsiao EC, Passegue E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell stem cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Mondal BC, Liu T, Young GC, Wijewarnasuriya DP, Banerjee U. Olfactory control of blood progenitor maintenance. Cell. 2013;155:1141–1153. doi: 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, Leon C, Gachet C, Dingal PC, Ivanovska IL, et al. Contractile Forces Sustain and Polarize Hematopoiesis from Stem and Progenitor Cells. Cell stem cell. 2013 doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, et al. Differentiated troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007a;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007b;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, Zheng Y, Cancelas JA, Gu Y, Jansen M, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Wiseman DH. Donor cell leukemia: a review. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:771–789. doi: 10.1016/j.bbmt.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yanger K, Stanger BZ. Facultative stem cells in liver and pancreas: fact and fancy. Dev Dyn. 2011;240:521–529. doi: 10.1002/dvdy.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI, Chada KK, Witte ON. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E3395–3404. doi: 10.1073/pnas.1217982109. [DOI] [PMC free article] [PubMed] [Google Scholar]


