ABSTRACT
YopM is a leucine-rich repeat (LRR)-containing effector in several Yersinia species, including Yersinia pestis and Y. pseudotuberculosis. Different Yersinia strains encode distinct YopM isoforms with variable numbers of LRRs but conserved C-terminal tails. A 15-LRR isoform in Y. pseudotuberculosis YPIII was recently shown to bind and inhibit caspase-1 via a YLTD motif in LRR 10, and attenuation of YopM− YPIII was reversed in mice lacking caspase-1, indicating that caspase-1 inhibition is a major virulence function of YopMYPIII. To determine if other YopM proteins inhibit caspase-1, we utilized Y. pseudotuberculosis strains natively expressing a 21-LRR isoform lacking the YLTD motif (YopM32777) or ectopically expressing a Y. pestis 15-LRR version with a functional (YopMKIM) or inactivated (YopMKIM D271A) YLTD motif. Results of mouse and macrophage infections with these strains showed that YopM32777, YopMKIM, and YopMKIM D271A inhibit caspase-1 activation, indicating that the YLTD motif is dispensable for this activity. Analysis of YopMKIM deletion variants revealed that LRRs 6 to 15 and the C-terminal tail are required to inhibit caspase-1 activation. YopM32777, YopMKIM, and YopMKIM deletion variants were purified, and binding partners in macrophage lysates were identified. Caspase-1 bound to YopMKIM but not YopM32777. Additionally, YopMKIM bound IQGAP1 and the use of Iqgap1−/− macrophages revealed that this scaffolding protein is important for caspase-1 activation upon infection with YopM− Y. pseudotuberculosis. Thus, while multiple YopM isoforms inhibit caspase-1 activation, their variable LRR domains bind different host proteins to perform this function and the LRRs of YopMKIM target IQGAP1, a novel regulator of caspase-1, in macrophages.
IMPORTANCE
Activation of caspase-1, mediated by macromolecular complexes termed inflammasomes, is important for innate immune defense against pathogens. Pathogens can, in turn, subvert caspase-1-dependent responses through the action of effector proteins. For example, the Yersinia effector YopM inhibits caspase-1 activation by arresting inflammasome formation. This caspase-1 inhibitory activity has been studied in a specific YopM isoform, and in this case, the protein was shown to act as a pseudosubstrate to bind and inhibit caspase-1. Different Yersinia strains encode distinct YopM isoforms, many of which lack the pseudosubstrate motif. We studied additional isoforms and found that these YopM proteins inhibit caspase-1 activation independently of a pseudosubstrate motif. We also identified IQGAP1 as a novel binding partner of the Yersinia pestis YopMKIM isoform and demonstrated that IQGAP1 is important for caspase-1 activation in macrophages infected with Yersinia. Thus, this study reveals new insights into inflammasome regulation during Yersinia infection.
INTRODUCTION
Recognition of microbial pathogens by the innate immune system is a crucial component of host defense. Pattern recognition receptors recognize conserved features of microbes, termed pathogen-associated molecular patterns (PAMPs), and mobilize host defenses against both extracellular and intracellular pathogens (1). Detection of extracellular PAMPs by Toll-like receptors (TLRs) or of cytosolic PAMPs by nucleotide-binding oligomerization domain leucine-rich repeat (LRR) receptors (NLRs) initiates cellular events important for clearance of pathogens, such as expression of proinflammatory cytokines or inflammasome formation (2, 3).
PAMPs and other danger signals associated with pathogens that access the host cytosol trigger the formation and activation of the inflammasome, a multioligomeric complex that serves as a molecular platform for the recruitment and activation of proinflammatory caspase-1 (4). Formation of the inflammasome is coordinated by protein-protein interactions between individual NLRs and in some cases an adaptor protein, adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC). Some inflammasomes (e.g., the NLRP3 inflammasome) require distinct signals for priming and activation. Induction of transcription downstream of TLR stimulation by a PAMP (signal 1) leads to the synthesis of NLRP3 and prointerleukin-1β (pro-IL-1β). The NLRP3 inflammasome is subsequently assembled and activated by a wide variety of stimuli (signal 2), such as pore-forming toxins or extracellular ATP (5, 6). In contrast, the NLRC4 inflammasome does not require a priming step and is more selective for activating stimuli such as bacterial flagellin and components of bacterial type III secretion systems (T3SS) (7, 8). While the regulation and composition of different inflammasomes are variable, they all have the ability to activate caspase-1. Caspase-1 activation by the inflammasome induces a form of cell death termed pyroptosis (9). In addition, the proinflammatory cytokines IL-1β and IL-18, synthesized as inactive precursors, rely on the activity of caspase-1 for their maturation and secretion from the host cell (4). The caspase-1-dependent processes of pyroptosis and cytokine secretion act in concert to promote innate immune responses, enabling clearance of invading pathogens. Thus, evasion of innate immune responses dependent on caspase-1 is an important strategy of pathogenic bacteria.
Pathogenic Yersinia species (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) employ a contact-dependent T3SS for the delivery of Yersinia outer protein (Yop) effectors into the cytosol of infected host cells (10). Yop effectors function to modulate key host processes to promote Yersinia pathogenesis (11). Delivery of effector Yops requires the assembly of the T3SS injectisome on the bacterial surface and insertion of the YopB/D translocon into the plasma membrane of host cells. Consequences of membrane perturbation by the T3SS translocon are assembly of inflammasomes and activation of caspase-1 during the infection of macrophages by Yersinia (12, 13). However, Yop effectors counteract the activation of inflammasomes and caspase-1. Activation of caspase-1 by the T3SS translocon is mediated primarily by the NLRP3 inflammasome and is counteracted by the function of the effector YopK, which interacts with the translocon and prevents caspase-1 activation (14). Recently, a second effector, YopM, an LRR-containing protein that is required for Yersinia virulence (15, 16), has been shown to inhibit NLRP3 inflammasome formation and activation of caspase-1 (17).
YopM consists of an N-terminal secretion signal, followed by two α-helices that fold into an LRR region, which spans most of the protein (18). The YopM protein terminates in a short, unstructured but conserved C-terminal tail of 24 amino acids. Interestingly, YopM appears to be devoid of catalytic activity, and instead, this protein acts as a scaffold to bind multiple host proteins. YopM was found to bind two host kinases, protein kinase C-related kinase 2 (PRK2) and the 90-kDa ribosomal S6 kinase (RSK1), and increase their activity toward a heterologous substrate (19, 20). RSK1 binds to the C-terminal tail of YopM, and PRK2 binds to the LRR region (21, 22). How complexes of YopM/RSK/PRK contribute to Yersinia virulence remains unknown. The LRR region and the C-terminal tail are both required for the virulence function of YopM (21, 22). Additionally, the C-terminal tail is required for the formation of high-molecular-weight complexes by YopM in macrophages (21). Each strain of Yersinia encodes one isoform of YopM, but there are a number of different isoforms found in the genus (23), and depending on the specific isoform, the LRR region contains between 13 and 21 repeats and the LRRs can be variable in sequence.
LaRock and Cookson presented evidence that YopM proteins containing 15 LRRs encoded by Y. pseudotuberculosis strain YPIII and Y. pestis strain CO92 bind directly to caspase-1 to inhibit its activity (17). These two isoforms of YopM, as exemplified by YopMYPIII, contain a consensus caspase-1 cleavage motif (Tyr-Leu-Thr-Asp or YLTD) present in the 10th LRR and appear to mimic pseudosubstrate caspase-1 inhibitors (17). YopMYPIII was shown to inhibit capsase-1 activity in vitro, to bind to the 10-kDa subunit of active caspase-1 in lysates of macrophages, and to inhibit the recruitment of caspase-1 to macromolecular complexes of NLRP3 (NLRP3 foci) in YPIII-infected macrophages (17). In contrast, a YopMYPIII variant in which the fourth position of the YLTD sequence was changed to A (D271A) was defective in all of these activities (17). LaRock and Cookson proposed that YopMYPIII uses the YLTD sequence to bind directly to caspase-1 to inhibit its activity and sequester it, resulting in the formation of arrested or “pre-NLRP3 inflammasomes” (17). Importantly, the virulence defect of a YPIII yopM mutant was reversed in mice lacking caspase-1, indicating that YopM functions to inhibit capase-1-directed immune functions in vivo (17).
The findings of LaRock and Cookson (17) provide the first molecular understanding of how a YopM protein can inhibit host innate immune responses, yet they raise a number of important questions. For example, do YopM isoforms that lack the YLTD sequence (of which there are many among different Yersinia species and strains) also inhibit the activation of caspase-1? Is the C-terminal RSK1-binding tail of YopM, which is essential for its virulence function, also required to inhibit caspase-1? Does the LRR domain of YopM target other host proteins in addition to caspase-1 to inhibit the function of the NLRP3 inflammasome?
Here, we studied two distinct YopM isoforms, YopM32777 from Y. pseudotuberculosis 32777 and YopMKIM from Y. pestis KIM. The former contains 21 LRRs and lacks the YLTD sequence, while the latter contains 15 LRRs and the YLTD sequence in LRR 10. We demonstrate that both YopM isoforms inhibit the activation of caspase-1 in mice or macrophages infected with Y. pseudotuberculosis. In addition, using deletion variants of YopMKIM, we provide evidence that both LRRs 6 to 15 and the C-terminal tail are necessary to inhibit the activation of caspase-1. Analysis of a YopMKIM D271A variant showed that the YLTD sequence is dispensable for the inhibition of caspase-1 activation in this isoform. We also identify the large scaffolding protein IQGAP1 (IQ motif-containing GTPase-activating protein 1) (24) as a novel interacting partner of the YopMKIM LRR domain and show that IQGAP1 is required for caspase-1 activation in macrophages infected with a Y. pseudotuberculosis yopM mutant. These results suggest that distinct YopM isoforms target different host proteins to inhibit caspase-1 activation and show that IQGAP1 is required for the activation of caspase-1 in macrophages infected with Yersinia.
RESULTS AND DISCUSSION
Sequence comparison of Y. pestis KIM and Y. pseudotuberculosis 32777 YopM proteins.
Two well-studied YopM isoforms, YopMKIM and YopM32777, contain 15 and 21 LRRs, respectively. Here, we have aligned the YopM32777 and YopMKIM sequences by using ClustalW (see Fig. S1 in the supplemental material). This analysis indicates that the N terminus and the C-terminal tail are conserved in YopMKIM and YopM32777 and the first two and last eight LRRs are well aligned (see Fig. S1). YopM32777 appears to have additional repeats within the region corresponding to LRRs 3 to 7 of YopMKIM. To account for these additional LRRs in YopM32777, they are numbered as subrepeats of LRRs 3, 5, and 7 of YopMKIM (see Fig. S1). The primary-structure alignment of YopMKIM and YopM32777 by this convention is shown in Fig. 1. The YLTD motif in LRR 10 of YopMKIM is absent from the YopM32777 isoform. Instead, YopM32777 has the sequence DLTD (Fig. 1; see Fig. S1).
FIG 1 .
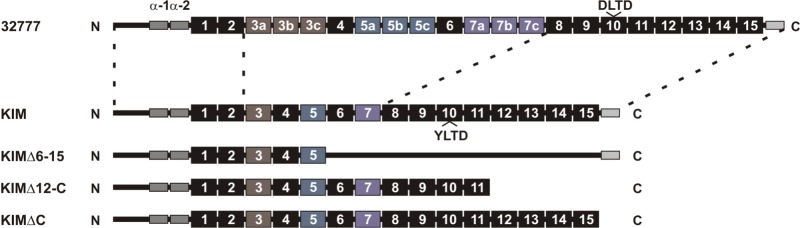
Primary-structure representation of the YopM isoforms and variants used in this study. Representations of YopM32777, YopMKIM, and three deletion variants of the KIM isoform are shown. N-terminal and C-terminal regions enclosed by dashed lines indicate the highest conservation between YopM32777 and YopMKIM, as determined by sequence alignment with ClustalW (see Fig. S1 in the supplemental material). The N-terminal gray rectangles correspond to the two α-helices, LRRs are represented by numbered boxes, and the conserved C-terminal tail is shown as a gray rectangle. Colored shading of boxes is used to indicate that additional LRRs in YopM32777 are assigned numbers as subrepeats of LRRs 3, 5, and 7 of YopMKIM. The positions of the YLTD motif in LRR 10 of YopMKIM and the corresponding DLTD motif in LRR 10 of YopM32777 are shown.
A Y. pseudotuberculosis 32777 yopM mutant is fully virulent in mice lacking caspase-1.
Y. pseudotuberculosis yopM mutants are significantly attenuated in intravenous mouse models of infection (21, 22). LaRock and Cookson showed that the virulence defect of a YPIII yopM mutant was rescued in C57BL/6 mice lacking caspase-1 (17). We used Caspase-1−/− C57BL/6 mice, which have been determined to be functionally Caspase-11−/− as well (25) (here referred to as Caspase-1/11−/− mice), to investigate if the virulence of a 32777 yopM mutant would be restored in the absence of caspase-1. Caspase-1/11−/− mice were infected with equivalent numbers of CFU of 32777 or 32777ΔyopM (Table 1), and the time to death was monitored. As expected, 32777 was virulent in Caspase-1/11−/− mice following intravenous infection (Fig. 2, solid line). We previously showed that the 32777ΔyopM mutant is attenuated in C57BL/6 mice by intravenous challenge (22), a phenotype that was confirmed in independent experiments (data not shown). A reversal of the virulence defect of the 32777ΔyopM mutant was observed in Caspase-1/11−/− mice, as all of the mice succumbed to infection (Fig. 2, dotted line). The data are consistent with those of LaRock and Cookson (17) and suggest that caspase-1-directed immune responses in vivo are responsible for the attenuation of Y. pseudotuberculosis yopM mutants. Moreover, these data point to inhibition of caspase-1 activation as a conserved function shared by distinct YopM isoforms. However, because the mice used are Caspase-1/11−/−, the possibility cannot be ruled out that caspase-11-directed responses in vivo contribute to the attenuation of Y. pseudotuberculosis yopM mutants.
TABLE 1 .
Y. pseudotuberculosis strains used in this study
| Strain | Relevant characteristic(s) | Reference |
|---|---|---|
| 32777 | Wild-type serogroup O1 strain | 41 |
| 32777ΔyopM | ΔyopM | 22 |
| 32777ΔyopK | Frame-shift mutation in yopK | This study |
| 32777ΔyopMyopB | ΔyopM, in-frame deletion of yopB (nucleotides 496–774) | This study |
| 32777ΔyopM/pMMB67EH | ΔyopM containing empty vector | 22 |
| 32777ΔyopM/pYopMKIM | ΔyopM containing pMMB67EH expressing YopMKIM under control of native promoter | 22 |
| 32777ΔyopM/pYopMKIMΔ6-15 | ΔyopM containing pMMB67EH expressing YopMKIM missing LRRs 6–15 | 22 |
| 32777ΔyopM/pYopMKIMΔ12-C | ΔyopM containing pMMB67EH expressing YopMKIM missing LRRs 12–15 and C terminus | This study |
| 32777ΔyopM/pYopMKIMΔC | ΔyopM containing pMMB67EH expressing YopMKIM missing C terminus | 22 |
| 32777ΔyopM/pYopMKIM D271A | ΔyopM containing pMMB67EH expressing YopMKIM with D271A amino acid substitution | This study |
| 32777/pGFP | Contains p67GFP3.1 | 32 |
| 32777ΔyopM/pGFP | ΔyopM containing p67GFP3.1 | This study |
FIG 2 .
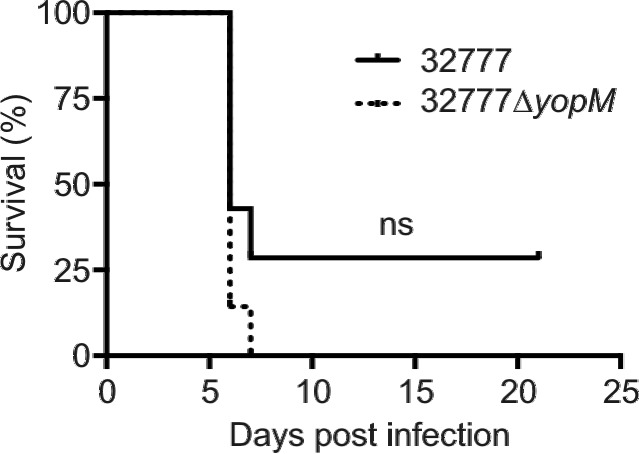
Survival assay of Caspase-1/11−/− mice infected with Y. pseudotuberculosis 32777 or 32777ΔyopM. Caspase-1/11−/− mice were infected intravenously via tail vein injection with approximately 1,500 CFU of Y. pseudotuberculosis 32777 or 32777ΔyopM, and time to death was monitored for 21 days. Results are pooled from two independent experiments with three or four mice per group (n = 7). The difference between survival curves was not significant (ns), as determined by log rank test.
YopM32777 inhibits caspase-1 activation in macrophages infected with Y. pseudotuberculosis.
To obtain more direct evidence that YopM32777 inhibits the activation of caspase-1, we infected lipopolysaccharide (LPS)-primed, bone marrow-derived macrophages (BMDMs) from C57BL/6 mice with 32777 or 32777ΔyopM and measured the activation of caspase-1. Caspase-1 activation was inhibited in C57BL/6 BMDMs infected with 32777 compared to that in 32777ΔyopM, as shown by Western blotting of macrophage lysates for cleaved (10-kDa) caspase-1 (Fig. 3A, compare lanes 1 and 5). Infection with a 32777ΔyopK mutant (Table 1) was used as a positive control for caspase-1 cleavage (Fig. 3A, lane 9). Additionally, unlike 32777, the 32777ΔyopM strain failed to inhibit IL-1β secretion (Fig. 3B) or pyroptosis (Fig. 3C), as determined by enzyme-linked immunosorbent assay (ELISA) or lactate dehydrogenase (LDH) release, respectively. Activation of caspase-1 by 32777ΔyopM required a functional T3SS, as caspase-1 was not activated when BMDMs were infected with a 32777ΔyopMyopB mutant (Table 1; see Fig. S2A in the supplemental material), as measured by Western blotting for cleaved caspase-1 (see Fig. S2B) and ELISA for secreted IL-1β (see Fig. S2C). Because caspase-11 can contribute to NLRP3-dependent activation of caspase-1 in response to cytosolic LPS (25, 26), we examined the role of caspase-11 in the response of BMDMs to infection with the 32777ΔyopM mutant. BMDMs from Caspase-1−/−, Caspase-11−/−, or Caspase-1/11−/− mice were infected and analyzed as described above. We observed similar amounts of cleaved caspase-1 in C57BL/6 and Caspase-11−/− BMDMs infected with 32777ΔyopM or 32777ΔyopK (Fig. 3A, compare lanes 5 and 8 and lanes 9 and 12). Likewise, the absence of caspase-11 did not significantly decrease the levels of secreted IL-1β in BMDMs infected with 32777ΔyopM (Fig. 3B). Interestingly, pyroptosis was partially caspase-11 dependent in BMDMs infected with the yopM mutant (Fig. 3C). As expected, IL-1β secretion (Fig. 3B) and pyroptosis (Fig. 3C) were at background levels in Caspase-1−/− and Caspase-1/11−/− BMDMs infected with 32777ΔyopM. The data show that YopM32777 inhibits caspase-1 activation, demonstrating that this activity is not restricted to the 15 LRR isoforms that contain a YLTD motif. In addition, the data show that caspase-11 is not required for the activation of caspase-1 in macrophages infected with a Y. pseudotuberculosis yopK or yopM mutant but does contribute to pyroptosis in the latter case.
FIG 3 .
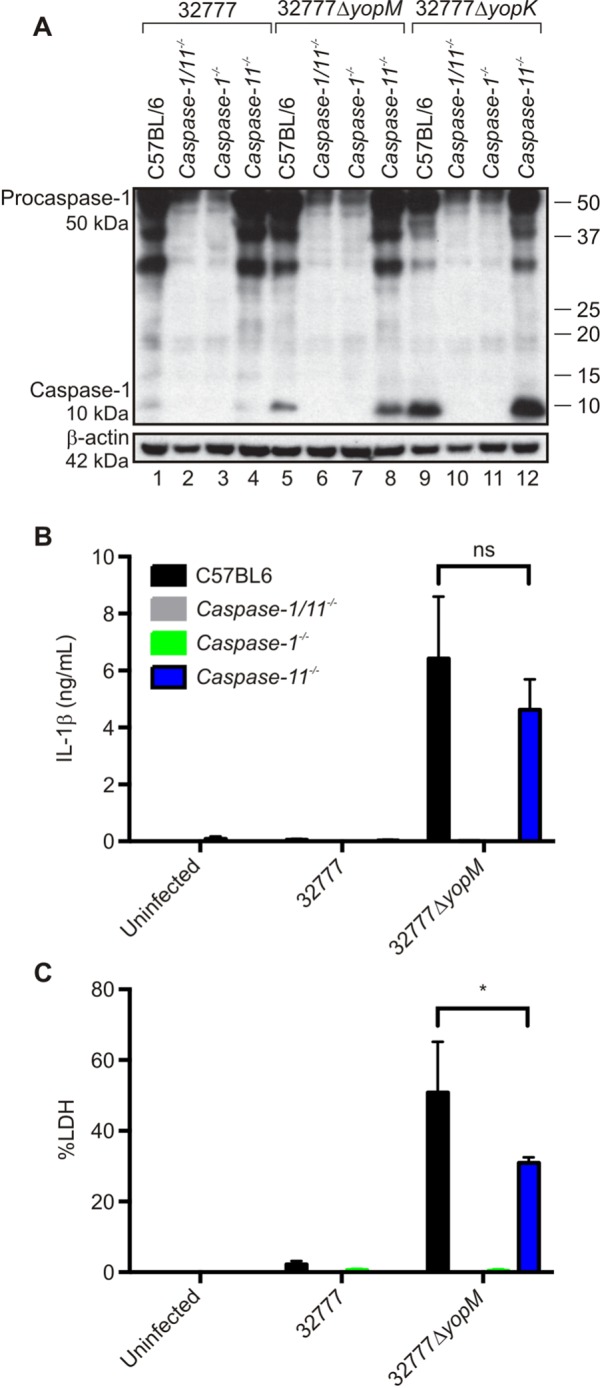
Measurement of caspase-1 activation in C57BL/6, Caspase-1−/−, Caspase-11−/−, or Caspase-1/11−/− mouse macrophages infected with Y. pseudotuberculosis. BMDMs from wild-type C57BL/6, Caspase-1−/−, Caspase-11−/−, or Caspase-1/11−/− mice were primed with 100 ng/ml LPS for 18 h and left uninfected or infected with 32777, 32777ΔyopM, or 32777ΔyopK at an MOI of 30. (A) After a 90-min infection, lysates were processed and subjected to Western blotting with an antibody against caspase-1. β-Actin was used as a loading control. Positions of 50-kDa procaspase-1, the cleaved 10-kDa caspase-1 subunit, and 42-kDa β-actin are shown on the left, and molecular size standards are shown on the right. (B) Supernatants were collected at 90 min postinfection, and secreted IL-1β was measured by ELISA. (C) Levels of cytotoxicity were measured by quantification of percent LDH release. The data in panels B and C are average values ± the standard errors of the means from three independent experiments. By two-way ANOVA comparing the C57BL/6 group to the Caspase-11−/− group infected with 32777ΔyopM, P < 0.05 (*).
Differential binding of caspase-1 by YopMKIM and YopM32777.
LaRock and Cookson showed that purified YopMYPIII bound to cleaved caspase-1 in lysates derived from BMDMs infected with the YPIII yopM mutant (17). Because YopMKIM and YopM32777 both inhibited the activation of caspase-1, we wanted to determine if both proteins bind to cleaved caspase-1. To investigate the binding of YopMKIM and YopM32777 to caspase-1, these proteins were purified as fusions to glutathione S-transferase (GST) and incubated with lysates of BMDMs treated with LPS and ATP to activate caspase-1. Western blotting was used to detect the binding of caspase-1 or RSK1 to the GST-YopM fusion proteins or GST alone as a control. As shown in Fig. 4A, GST-YopMKIM, but not GST, bound to procaspase-1 (50 kDa), as well as RSK1 (compare lanes 6 and 3). Weak binding of cleaved caspase-1 to GST-YopMKIM was also detected (Fig. 4A, lane 6). Interestingly, we did not detect binding of procaspase-1 or cleaved caspase-1 to GST-YopM32777 (Fig. 4A, lane 9). These data suggest that sequence differences account for the differential binding of YopMKIM and YopM32777 to caspase-1. We previously constructed a series of deletion variants of YopMKIM missing different numbers of LRRs or the C terminus (Fig. 1) (22). We used GST fusions of two of the YopMKIM deletion variants (Δ6-15 and Δ12-C) to delineate the regions of the protein required for binding to procaspase-1 and cleaved caspase-1. As shown in Fig. 4B, GST-YopMKIM (lane 6) and the Δ12-C variant (lane 12) bound to both procaspase-1 and cleaved caspase-1, while the Δ6-15 variant (lane 9) failed to interact with either protein. As expected, the Δ12-C variant failed to bind RSK1, while the Δ6-15 variant retained this activity (Fig. 4B, compare lanes 12 and 9). Thus, binding to both procaspase-1 and cleaved caspase-1 requires LRRs 6 to 11 of YopMKIM. Taken together, these data demonstrate differential binding of caspase-1 by YopMKIM and YopM32777 in vitro, even though both proteins inhibit the activation of caspase-1 in vivo. The use of a different isoform (YopMYPIII) or a different stimulus (infection) to activate caspase-1 in macrophages could explain why LaRock and Cookson failed to detect binding of procaspase-1 in their experiments (17).
FIG 4 .
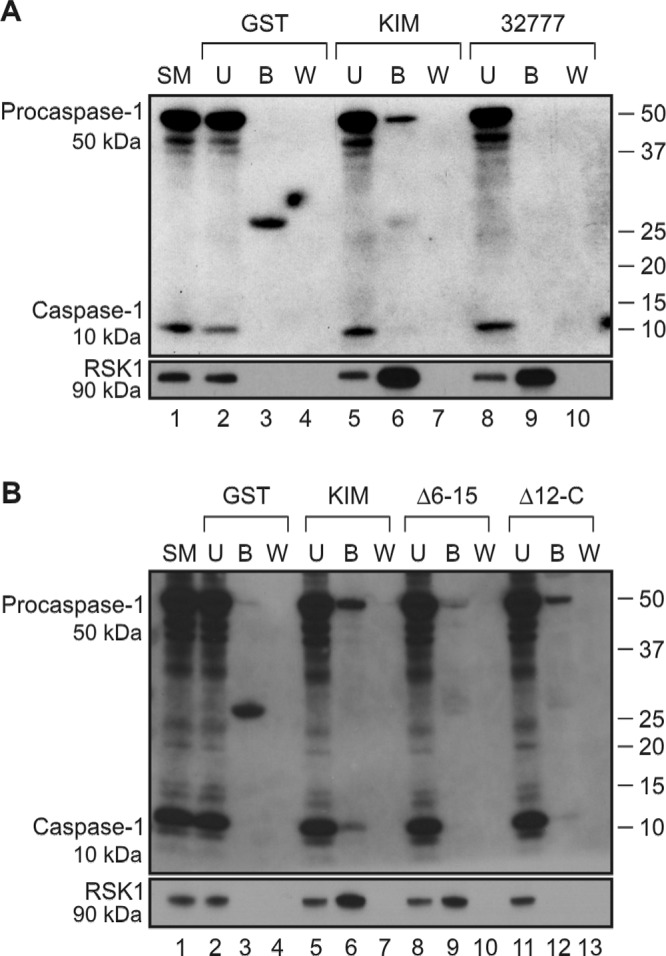
Interaction of host proteins with the GST-YopM32777, GST-YopMKIM, or GST-YopMKIM deletion variant by pulldown assay. Ten micrograms of GST, GST-YopMKIM, or the indicated GST-YopMKIM deletion variant was loaded onto GST-Bind Sepharose beads and subsequently incubated with lysates prepared from C57BL/6 BMDMs activated by treatment with LPS and ATP. Proteins bound to the beads were eluted, resolved by SDS-PAGE, and detected by Western blotting with antibodies to caspase-1 or RSK1. (A) Western blot pulldown assay of interaction of GST or the GST-YopMKIM or GST-YopM32777 fusion protein with caspase-1 or RSK1. (B) Western blot pulldown assay of interaction of GST, GST-YopMKIM or GST-YopMKIM deletion variant with caspase-1 or RSK1. SM, starting material; U, unbound; B, bound; W, wash. The positions of 50-kDa procaspase-1, the cleaved 10-kDa caspase-1 subunit, and 90-kDa RSK1 are shown on the left, and molecular size standards are shown on the right.
LRRs 6 to 15 and the C terminus of YopMKIM are necessary for inhibition of activation of caspase-1.
To investigate which domains of YopMKIM are required to inhibit the activation of caspase-1 in macrophages, the Δ6-15, Δ12-C, and ΔC deletion variants (Fig. 1) (22) and the wild-type protein were expressed in 32777ΔyopM under the control of the native YopM promoter (Table 1). A Yop secretion assay confirmed that the expression and stability of the YopMKIM deletion variants were similar to those of the wild-type protein (Fig. 5A). These strains, as well as the 32777 and 32777ΔyopM controls, were subsequently used to infect C57BL/6 BMDMs to assess whether the LRR region of the protein is sufficient to inhibit the activation of caspase-1. As expected, 32777ΔyopM was defective for inhibition of IL-1β secretion in BMDMs and this phenotype was complemented by the expression of YopMKIM (Fig. 5B). BMDMs infected with the strain expressing the Δ6-15 variant secreted an amount of IL-1β similar to that secreted by those infected with 32777ΔyopM (Fig. 5B). Interestingly, strains expressing either the Δ12-C or the ΔC variant failed to inhibit IL-1β secretion (Fig. 5B and C), suggesting that the C terminus of YopMKIM is important for inhibition of caspase-1 activation. Taken together, the data reveal that both the region comprising LRRs 6 to 15 and the C-terminal tail of YopMKIM are required for inhibition of the activation of caspase-1.
FIG 5 .
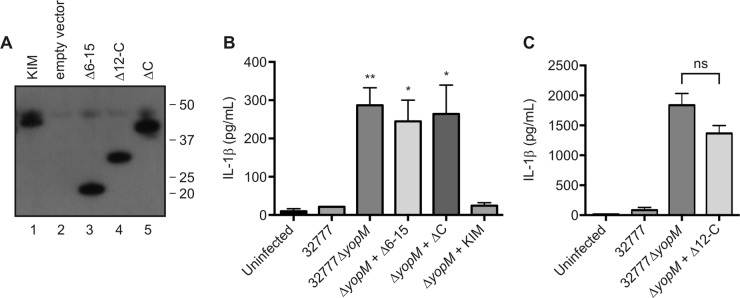
Analysis of YopMKIM deletion variant stability and inhibition of IL-1β secretion in macrophages infected with Y. pseudotuberculosis. (A) Stability of YopMKIM deletion variants as shown by a Yop secretion assay and Western blotting with a monoclonal antibody that recognizes an epitope in the N-terminal domain of the protein. The 32777ΔyopM strain encoding YopMKIM (KIM), the empty vector, or the indicated deletion variant was used for the Yop secretion assay, and samples of secreted proteins analyzed by Western blotting were normalized by the OD of the bacterial cultures. (B and C) C57BL/6 BMDMs primed with 100 ng/ml LPS for 18 h were left uninfected or infected with the indicated strains of Y. pseudotuberculosis at an MOI of 30. Supernatants were collected at 90 min postinfection and analyzed by ELISA to quantify levels of secreted IL-1β. The data are average values ± the standard errors of the means from three or four independent experiments. In panel B, a one-way ANOVA was used for comparisons with the ΔyopM + KIM group. *, P < 0.05; **, P < 0.01; ns, not significant.
A YLTD motif in YopMKIM is not required to inhibit the activation of caspase-1.
Mutation of the aspartic acid residue in the YLTD motif in YopMYPIII to an alanine (D271A) abrogated its ability to bind to and inhibit the activation of caspase-1 (17). We introduced the identical mutation into the gene encoding YopMKIM to assess if the YLTD motif is required for the Y. pestis protein to inhibit the activation of caspase-1. Figure 6A shows that the YopMKIM D271A protein is produced by 32777ΔyopM at the same levels as the wild-type protein in a Yop secretion assay (compare lanes 2 and 3). Surprisingly, when we infected C57BL/6 BMDMs with 32777ΔyopM expressing the YopMKIM D271A variant, both caspase-1 cleavage (Fig. 6B, lane 5) and IL-1β secretion (Fig. 6C) were inhibited, arguing that the YLTD motif in YopMKIM is not required to inhibit the activation of caspase-1.
FIG 6 .
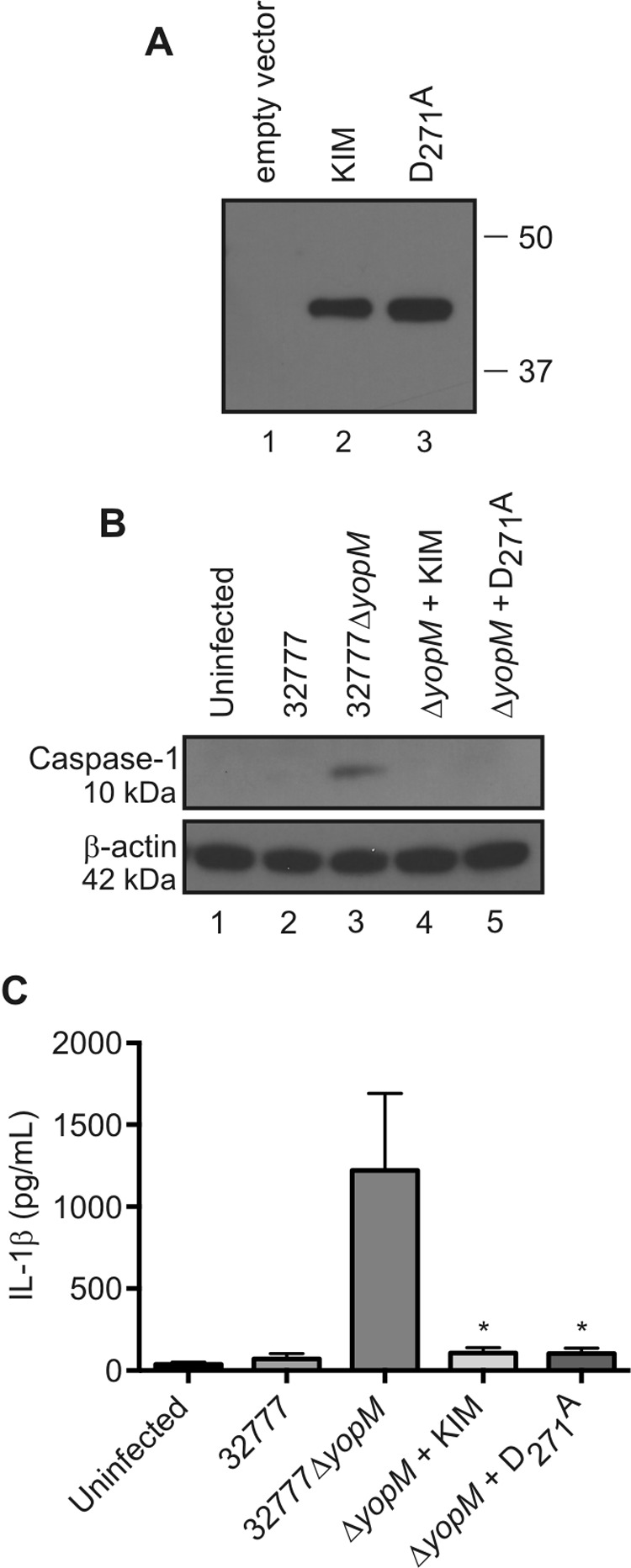
Analysis of YopMKIM D271A stability and the ability to inhibit the activation of caspase-1 in macrophages infected with Y. pseudotuberculosis. (A) Stability of the YopMKIM D271A variant as shown by a Yop secretion assay with a monoclonal antibody directed against the N terminus of YopM. 32777ΔyopM with the empty vector or expressing YopMKIM or YopMKIM D271A was used for the Yop secretion assay, and samples were normalized by the OD of the bacterial cultures. (B and C) BMDMs primed with 100 ng/ml LPS for 18 h were left uninfected or infected at an MOI of 30 with 32777, 32777ΔyopM, or 32777ΔyopM expressing YopMKIM or YopMKIM D271A. (B) At 90 min postinfection, BMDM lysates were collected and Western blotted for cleaved the 10-kDa caspase-1 subunit. β-Actin was used as a loading control (C) Supernatants were analyzed by ELISA for secreted IL-1β. The data in panel C are average values ± the standard errors of the means from three independent experiments. *, P < 0.05 (compared with the 32777ΔyopM group by one-way ANOVA).
YopMKIM interacts with IQGAP1.
To determine if novel host proteins bind to YopMKIM, pulldown assays with LPS-primed C57BL/6 BMDM lysates and GST, GST-YopMKIM, or a GST-YopMKIM Δ6-15 or Δ12-C deletion variant were carried out. Bound proteins were, eluted, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by silver staining. As shown in Fig. 7A, an ~200-kDa protein bound to GST-YopMKIM (lane 2) and the Δ12-C variant (lane 5) but not to GST (lane 1) or the Δ6-15 variant (lane 4). The ~200-kDa protein was not detected when the pulldown was done with GST-YopMKIM in lysis buffer alone (Fig. 7A, lane 3), suggesting that it was specific to the macrophage lysate. The region of the gel containing the ~200-kDa protein was excised and subjected to mass spectrometry analysis, and the corresponding protein was identified as the murine scaffolding protein IQGAP1 (24). Interestingly, IQGAP1 has previously been identified as a target of the type III effector proteins Ibe of enteropathogenic Escherichia coli (27) and SseI of Salmonella enterica serovar Typhimurium (28). In these cases, IQGAP1 was important for pedestal formation in epithelial cells by E. coli (27) or inhibition of macrophage and dendritic cell migration by S. Typhimurium (28). To verify the results obtained by mass spectrometry, samples from pulldown experiments were analyzed by Western blotting with an antibody against IQGAP1. As shown in Fig. 7B, IQGAP1 bound specifically to GST-YopMKIM (lane 5) and the Δ12-C variant (lane 11). These data indicate that YopMKIM interacts with IQGAP1 and that LRRs 6 to 11 are required for this interaction. Similar experiments performed with GST-YopM32777 showed that IQGAP1 does not bind to this isoform (data not shown). It remains to be determined whether the interaction of IQGAP1 and YopMKIM is direct or mediated by a cofactor.
FIG 7 .
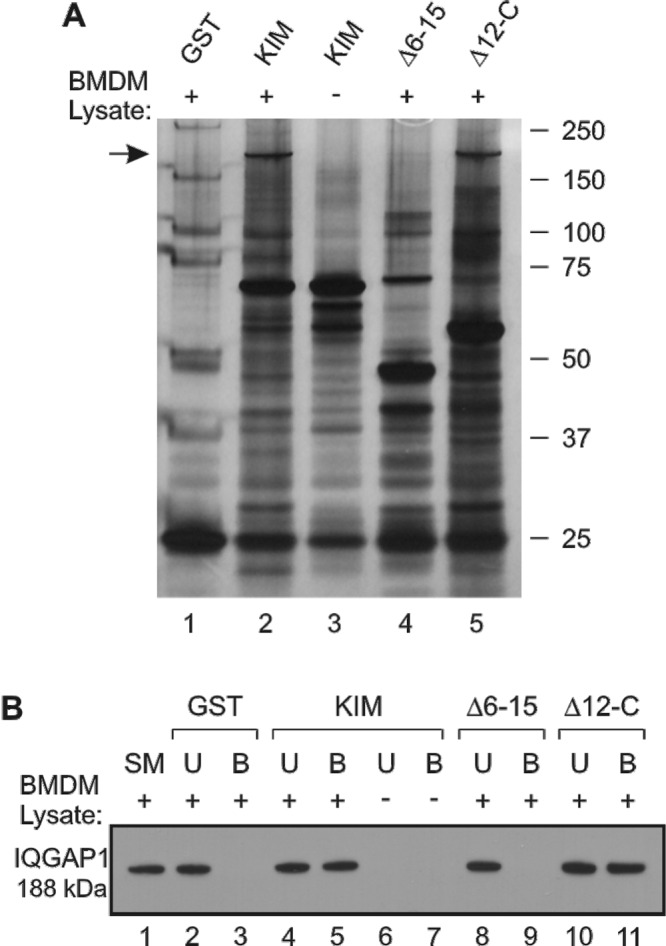
Identification of IQGAP1 as a novel binding partner of YopMKIM. Ten micrograms of GST, GST-YopMKIM, or the GST-YopMKIM deletion variant indicated was loaded onto GST-Bind Sepharose beads and subsequently incubated with lysates prepared from LPS-primed C57BL/6 BMDMs or lysis buffer alone. Unbound proteins were removed, the beads were washed, and bound proteins were eluted and resolved by SDS-PAGE. (A) Silver staining detection of eluted proteins after incubation with BMDM lysate (lanes 1, 2, 4, and 5) or lysis buffer alone (lane 3). The arrow indicates an ~200-kDa protein that was excised from the gel, fragmented by trypsin digestion, subjected to analysis by LC-MS/MS, and identified as murine IQGAP1. (B) Western blot analysis of samples (starting material [SM], unbound [U], and bound [B]) from pulldown assays with BMDM lysate (lanes 1 to 5 and 8 to 11) or lysis buffer alone (lanes 6 and 7) with an antibody against IQGAP1 (188 kDa).
IQGAP1 is important for caspase-1 activation in macrophages infected with Y. pseudotuberculosis.
The interaction between YopMKIM and the scaffolding protein IQGAP1 suggested to us that the latter protein might be important for the activation of caspase-1 in macrophages infected with Yersinia. We assessed the role of IQGAP1 in the activation of caspase-1 by infecting LPS-primed BMDMs from wild-type 129 mice or 129 Iqgap1−/− mice with Y. pseudotuberculosis and measuring caspase-1 cleavage and IL-1β secretion. There was less cleavage of caspase-1 in Iqgap1−/− BMDMs, than in BMDMs from 129 wild-type controls when they were infected with 32777ΔyopM (Fig. 8A, compare lanes 8 and 3). Additionally, there was significantly less secretion of IL-1β in Iqgap1−/− BMDMs than in the wild-type controls when they were infected with 32777ΔyopM (Fig. 8B). We noted that secretion of IL-1β by Iqgap1−/− BMDMs infected with 32777ΔyopM was not completely abolished but rather severely decreased (Fig. 8B), indicating that IQGAP1 is important but not essential for activation of caspase-1 under these conditions. Interestingly, in response to extracellular ATP, a canonical activator of the NLRP3 inflammasome, there was no noticeable difference in the amount of cleaved caspase-1 (Fig. 8A, compare lanes 5 and 10) or secreted IL-1β (Fig. 8B) between 129 and Iqgap1−/− BMDMs. Failure to secrete IL-1β was not due to a defect of YopB/D translocon insertion in Iqgap1−/− BMDMs, as there was no noticeable difference in YopM translocation between 129 and Iqgap1−/− BMDMs (Fig. 8C, compare lanes 1 and 4 and lanes 3 and 6). Additionally, there was no detectable difference between 129 and Iqgap1−/− BMDMs in the kinetics of their cell rounding response to effectors that disrupt the actin cytoskeleton (data not shown), indicating that IQGAP1 is not required for translocation. These data suggest that IQGAP1 is specifically important for activation of the NLRP3 inflammasome in macrophages during Yersinia infection but dispensable for other NLRP3-dependent stimuli that lead to caspase-1 activation. To determine if IQGAP1 is required for the formation of macromolecular complexes of NLRP3 or a later step in inflammasome assembly, we used immunofluorescence microscopy to detect NLRP3 focus formation in 129 or Iqgap1−/− BMDMs infected with Y. pseudotuberculosis. NLRP3 foci were not detected in uninfected 129 BMDMs but were detected in these cells infected with 32777 (see Fig. S3 in the supplemental material). In addition, NLRP3 foci formed in 129 and Iqgap1−/− BMDMs infected with 32777ΔyopM (see Fig. S3), suggesting that IQGAP1 functions downstream of NLRP3 activation. These results are consistent with the idea that YopM inhibits the recruitment of procaspase-1 to NLRP3 preinflammasomes (17) through targeting of IQGAP1.
FIG 8 .
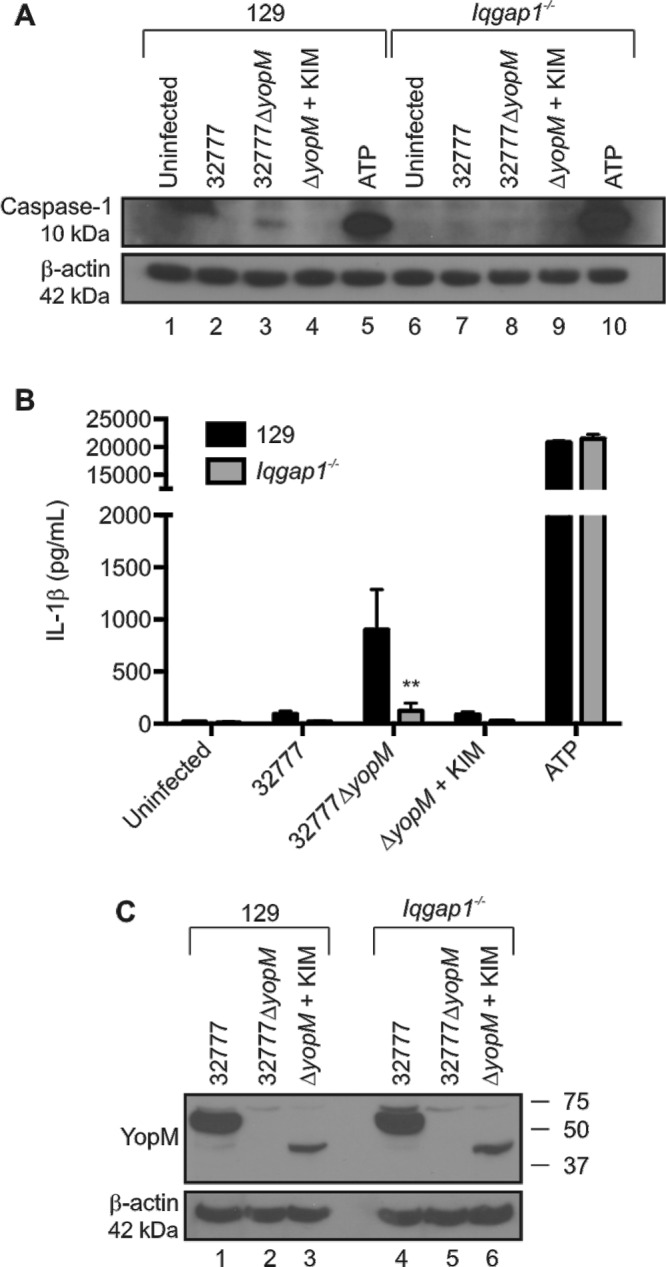
Measurement of caspase-1 activation and YopM translocation in 129 or Iqgap1−/− mouse macrophages infected with Y. pseudotuberculosis. BMDMs from 129 or Iqgap1−/− mice were primed with 100 ng/ml LPS for 18 h and left uninfected or infected for 90 min at an MOI of 30 with the indicated strains of Y. pseudotuberculosis. Alternatively, 129 or Iqgap1−/− BMDMs were treated with 100 ng/ml LPS for 4 h and then exposed to 2.5 mM ATP for 1 h to serve as a positive control for activation of caspase-1. (A) Lysates were collected and processed by Western blotting for cleaved caspase-1 or β-actin. (B) Supernatants were collected and analyzed by ELISA to quantify levels of secreted IL-1β. (C) Lysates were collected and processed by Western blotting for YopM or β-actin. The data in panel B are average values ± the standard errors of the means from three independent experiments. **, P < 0.01 (compared to 32777ΔyopM-infected 129 BMDMs by one-way ANOVA).
Summary.
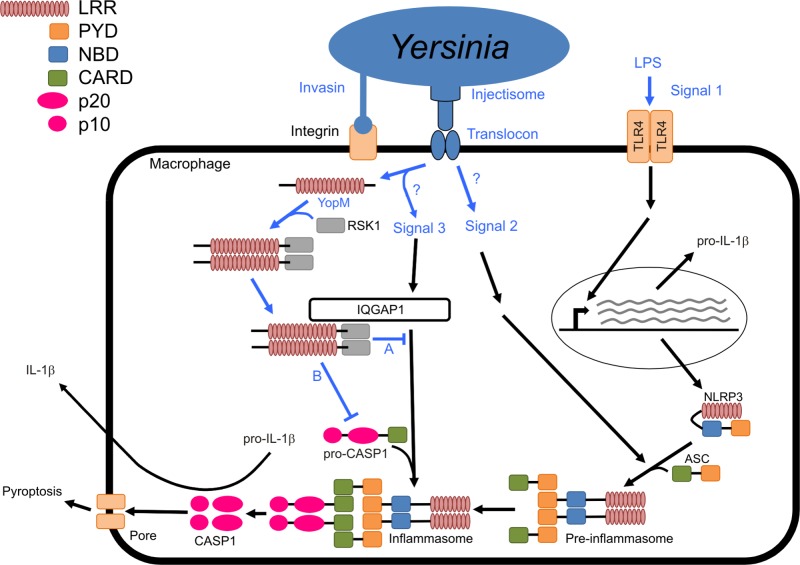
We have extended the results of LaRock and Cookson (17) by showing that (i) distinct YopM isoforms inhibit the activation of caspase-1, (ii) the LRR region and C-terminal tail are both required for this activity, and (iii) YopMKIM binds to IQGAP1, which we have identified as a novel regulator of inflammasome function in macrophages. A model of the activation of the NLRP3 inflammasome in macrophages in response to Yersinia infection and mechanisms of caspase-1 inhibition by YopM is presented in Fig. 9. We speculate that translocon insertion and effector translocation generate distinct signals that sequentially stimulate the formation of NLRP3 preinflammasomes (signal 2) and recruitment of caspase-1 to NLRP3 foci (signal 3) to complete inflammasome formation (Fig. 9). Translocon insertion could generate signal 2 by causing ion fluxes or other types of plasma membrane perturbation, while the entry of effectors, or PAMPs, into the cytosol may generate signal 3 (Fig. 9). Our data show that the C-terminal tail and LRRs 6 to 15 of YopMKIM are required to inhibit caspase-1 activation (mechanism A in Fig. 9). Preliminary results of translocation assays indicate that the Δ6-15 and Δ12-C variants exhibit reduced translocation and/or stability in infected macrophages, compared to the ΔC variant and YopMKIM (data not shown). Therefore, the Δ6-15 and Δ12-C variants may be defective for inhibition of caspase-1 activation, in part because of reduced translocation or stability. The requirement for the C terminus, which is necessary for virulence in vivo (21, 22), may reflect its role in the multimerization of YopM (Fig. 9) (21). It will be important to determine if RSK1, by promoting YopM multimerization or exhibiting kinase activity, has an important role in the inhibition of caspase-1 cleavage. We suggest that LRRs 6 to 11 in YopMKIM are essential for inhibition of caspase-1 activation because they target the protein to IQGAP1 (Fig. 9). It is unclear how IQGAP1 functions in this novel capacity to activate caspase-1, but as shown in Fig. 9, it is possible that it is important to transmit signal 3 for the recruitment of procaspase-1 to NLRP3 preinflammasomes. Using immunofluorescence microscopy we were unable to detect the localization of IQGAP1 to NLRP3 foci in macrophages infected with yopM mutant Y. pseudotuberculosis (data not shown). IQGAP1 is a ubiquitously expressed scaffolding protein that plays a role in diverse cellular processes (29, 30), and although it does not appear to localize to NLRP3 foci, it does interact with multiple host proteins, including Rho GTPases and kinases (29), that could regulate inflammasome assembly. IQGAP1 could also control caspase-1 cleavage by regulating actin organization or transcription, as these processes are known to be important for activation of the NLRP3 inflammasome. It remains to be determined if and how YopMKIM inhibits IQGAP1 function. Preliminary experiments show that steady-state levels of IQGAP1 are unaffected by the presence of YopMKIM in macrophages infected with Y. pseudotuberculosis. A final issue that remains to be addressed is the role of the YLTD motif (17), which appears to be required for YopMYPIII to inhibit procaspase-1 recruitment to NLRP3 foci, activation of caspase-1 in macrophages, and caspase-1 activity in vitro (mechanism B, Fig. 9), but is dispensable for YopM32777 and YopMKIM to inhibit the activation of caspase-1 in macrophages. YopMYPIII and YopMKIM are 99.5% identical at the amino acid level (17), making it unlikely that amino acid differences between these isoforms could explain the different results obtained. It seems more likely that in 15-LRR YopM isoforms that contain a YLTD motif, the sequence naturally plays a cryptic role in the inhibition of caspase-1, and the specific in vitro experimental conditions used by LaRock and Cookson uncovered an unusually important role for this motif. A Y. pseudotuberculosis strain expressing a 15-LRR isoform (YopMIP2666) in which repeats 10 to 11 were deleted was fully virulent in a mouse model of infection (21), arguing that the YLTD motif is dispensable for caspase-1 inhibition in vivo. A better understanding of how different YopM isoforms inhibit the activation of caspase-1 in macrophages is likely to lead to new insights into how inflammasomes are regulated and promote host protection against microbial infections.
FIG 9 .
Model of NLRP3 inflammasome activation in Yersinia-infected macrophages and mechanisms of caspase-1 inhibition by YopM. LPS activates TLR4 (signal 1), resulting in transcription and synthesis of NLRP3 and pro-IL-1β. Binding of yersiniae to macrophage β1-integrin via invasin allows injectisome activation and translocon insertion into the plasma membrane. Translocon insertion is proposed to activate signal 2, leading to activation of NLRP3 and assembly with ASC into preinflammasomes. Translocation of Yop effectors, including YopM, is proposed to activate signal 3, which is sensed by IQGAP1, resulting in the recruitment of procaspase-1 into NLRP3/ASC foci to form inflammasomes, in which active caspase-1 is generated. YopM associates with RSK1 via its C-terminal tail and multimerizes. YopMKIM binds to IQGAP1 via its LRRs and inhibits signal 3 by an unknown mechanism (A). YopMKIM’s YLTD motif may also bind directly to procaspase-1 and active caspase-1 (B) to inhibit its recruitment to inflammasomes and its activity. Abbreviations: PYD, pyrin domain; NBD, nucleotide-binding oligomerization domain; CARD, caspase activation and recruitment domain; p20, 20-kDa subunit of active caspase-1; p10, 10-kDa subunit of active caspase-1.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A description of the Y. pseudotuberculosis strains used in this study is provided in Table 1. A frame-shift mutation was introduced into yopK in 32777 as previously described (31), generating 32777ΔyopK. An in-frame deletion of yopB was constructed in the 32777ΔyopM background as previously described (31), generating 32777ΔyopMyopB. Vectors encoding YopMKIM and YopMKIM deletion variants under the control of the native YopM promoter were generated in pMMB67EH and used to transform 32777ΔyopM as previously described (22). The pYopMKIM Δ12-C vector was reconstructed in this study by the same approach and was analyzed independently of the other deletion variants, as shown in Fig. 5C. The vector pYopMKIM D271A was constructed as follows. The D271A codon change was introduced into pYopMKIM with the QuikChange site-directed mutagenesis kit (Agilent) and primers described in reference 17. The plasmids constructed in this study were verified by sequencing. 32777 and 32777ΔyopM were transformed with a plasmid expressing green fluorescent protein (GFP) by mating with E. coli s17-1λpir harboring p67GFP3.1 as previously described (32). For infection of BMDMs, Y. pseudotuberculosis strains were grown overnight in Luria broth (LB) at 28°C. The following day, cultures were diluted 1:40 in LB containing 20 mM MgCl2 and 20 mM sodium oxalate, grown at 28°C for 1 h, and then shifted to 37°C for 2 h. For analysis of secreted Yops, yersiniae were diluted and grown as described above, except at 28°C for 2 h, followed by a temperature shift to 37°C for 4 h.
Mouse infections.
Y. pseudotuberculosis cultures were grown overnight in LB at 28°C, washed twice with phosphate-buffered saline (PBS), and suspended to approximately 1.5 × 104 CFU/ml. A volume of 100 µl from this suspension was delivered by tail vein injection into Caspase-1/11−/− mice (33) (a gift from Adrianus van der Velden, Stony Brook University). Time to death was monitored for 21 days, at which point the remaining mice were euthanized. All mice were handled according to the guidelines for the humane care and use of experimental animals, and the procedures used were approved by the Stony Brook University Institutional Animal Care and Use Committee.
Bone marrow isolation and culture conditions.
Bone marrow was isolated from femur exudates of 6- to 8-week-old female C57BL/6 wild-type (Jackson Laboratories), Caspase-1−/− (34), Caspase-11−/− (35), or Caspase-1/11−/− (36) mice or 129 wild-type or Iqgap1−/− (37) mice as previously described (38). At 18 h prior to infection, BMDMs were seeded into tissue culture plates in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone), 15% L-cell-conditioned medium, 1 mM sodium pyruvate, 2 mM glutamate, and 100 ng/ml E. coli LPS (Sigma). BMDMs were used to seed six-well plates at a density of 0.8 × 106 cells/well for all of the experiments except those in Fig. 3, where 48-well plates were seeded at 2.0 × 105 cells/well.
Macrophage infection or stimulation conditions.
LPS-primed BMDMs were left uninfected or infected with Y. pseudotuberculosis grown under the conditions described above at a multiplicity of infection (MOI) of 30. Tissue culture plates were centrifuged for 5 min at 95 × g to facilitate the contact of yersiniae with BMDMs. Plates containing BMDMs were subsequently incubated at 37°C with 5% CO2. At 90 min postinfection, cell supernatants were collected and analyzed for secreted IL-1β and LDH release while cell lysates were harvested for Western blotting of host proteins. As a positive control for activation of caspase-1, BMDMs were primed with 100 ng/ml E. coli LPS for 4 h and subsequently given 2.5 mM adenosine triphosphate (ATP) for 1 h.
Western blotting of macrophage cell lysates.
BMDMs were washed twice with cold Hanks balanced salt solution to remove residual phenol red and lysed in lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl [pH 8.0], protease inhibitor cocktail [Complete, Mini, EDTA-free; Roche]). Proteins were resolved by SDS-PAGE with NuPAGE Novex 4 to 12% Bis-Tris gels (Invitrogen) unless indicated otherwise, transferred onto a polyvinylidene difluoride (PVDF) membrane, and probed with rabbit polyclonal anti-caspase-1 (Santa Cruz Biotechnologies), rabbit polyclonal anti-RSK1 (Abcam), and mouse monoclonal anti-IQGAP1 (BD Biosciences) antibodies. Horseradish peroxidase (HRP)-conjugated anti-rabbit (Cell Signaling) and anti-mouse (Jackson ImmunoResearch) antibodies were used as secondary reagents. To control for loading, Western blots were stripped and reprobed with anti-β-actin antibody conjugated to HRP (Sigma-Aldrich). Signals in Western blot assays were detected with Amersham ECL Prime Western blotting detection reagent (GE Healthcare).
ELISA.
Levels of secreted IL-1β in supernatants collected from BMDMs were analyzed with a commercially available ELISA kit (R&D Biosystems) according to the manufacturer’s instructions.
Cytotoxicity assay.
The LDH released into supernatants of BMDMs was quantified with the LDH Cytotoxicity Assay kit (Clontech) according to the manufacturer’s instructions.
Analysis of Yop secretion.
Following growth of Yersinia cultures as described above, bacteria were removed by centrifugation and trichloroacetic acid (10%, wt/vol) was added to the supernatants to precipitate and collect secreted Yops as previously described (22). Samples were subsequently processed for Western blotting with mouse monoclonal antibodies (6-1CE or 2A3.3A8.1A2) that recognize epitopes in the N-terminal region of YopM (a gift from Susan Straley, University of Kentucky), mouse monoclonal anti-YopB antibodies (76.15, 190.11, or 328.1 [unpublished data]), or mouse monoclonal anti-YopE antibodies (149.27 or 202.19 [unpublished data]).
Purification of GST-YopM proteins.
E. coli strains harboring pGEX-2T, pGEX-2T YopMKIM, pGEX-2T YopM32777, pDEST15 YopMKIMΔ6-15, or pDEST15 YopMKIMΔ12-C (22) were grown in LB at 37°C to an optical density (OD) at 600 nm of 0.3 to 0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG) was then added to a final concentration of 0.1 mM, and the cultures were grown for an additional 4 h. Bacteria were collected by centrifugation and lysed with Lysonase and Bugbuster reagents (Novagen) according to the manufacturer’s instructions. Supernatants containing bacterial proteins were added to GST-Bind Resin (Novagen) and incubated for 30 min at 4°C on a rotating shaker. Samples were washed, and bound GST and GST-fused proteins were eluted according to the manufacturer’s instructions. Purified proteins were dialyzed against PBS and used for GST pulldown assays or stored at −80°C.
GST pulldown assay.
Ten micrograms of purified GST or GST-fused protein was added to 20 µl of GST-Bind Resin (Novagen) and incubated at 4°C for 30 min. Beads were then washed three times with 1× GST Bind/Wash Buffer (Novagen). Uninfected, LPS-primed or LPS- and ATP-treated BMDMs were incubated in lysis buffer as described above, and the resulting lysates were added to the beads and incubated for 2 h at 4°C on a rotating shaker. Resin-containing bound proteins were washed four times in lysis buffer and boiled in sample buffer, and the eluted proteins were separated by SDS-PAGE. Proteins were transferred to PVDF membranes, and Western blotting was performed as described above.
Silver staining and mass spectrometry.
Samples from GST pulldown assays were resolved by SDS-PAGE. Gels were subsequently stained with the SilverQuest Stain kit (Invitrogen) according to the manufacturer’s instructions. Specific bands were excised from the gel, fragmented by trypsin digestion, and subjected to analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Yop translocation assays.
Yop translocation assays were performed as previously described (39). Briefly, infected BMDMs were incubated in lysis buffer as described above and the soluble fraction of the lysate was collected and analyzed for the presence of YopM by Western blotting as described above.
Fluorescence microscopy.
BMDMs were seeded onto glass coverslips pretreated with ethanol and acetone and sterilized to remove contaminants. BMDMs were left uninfected or infected as described above, except that the tissue culture medium was supplemented with 0.5 mM IPTG to induce GFP expression. BMDMs were washed twice with PBS, fixed with 2.5% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with bovine serum albumin as previously described (40). BMDMs were incubated with a primary rabbit anti-NLRP3 polyclonal antibody (Santa Cruz Biotechnologies). Binding of the primary antibody was visualized by the addition of Alexa Fluor 594-conjugated anti-rabbit antibody (Invitrogen), and DNA was stained by the addition of 4,6′-diamidino-2-phenylindole (DAPI) dilactate. Coverslips were mounted onto glass slides with ProLong Gold antifade reagent (Invitrogen) and imaged with an Axiovert S100 (Zeiss) with a 32× objective. Images were taken with a SPOT camera (Diagnostic Instruments) and processed with Adobe Photoshop CS 5.1.
Statistical analysis.
Experimental data analyzed for significance with GraphPad Prism 6.0 were from at least three independent experiments. Probability (P) values for IL-1β and LDH experiments were calculated by one-way analysis of variance (ANOVA) or grouped two-way ANOVA with Turkey’s multiple-comparison posttest. P values from mouse survival experiments were calculated by log rank test. P values of <0.05 were considered significant.
SUPPLEMENTAL MATERIAL
Sequence alignment of YopM proteins. The amino acid sequences of YopMKIM (NP_857953.1) and YopM32777 (YP_068456.1) were aligned with ClustalW (v 1.4). Identical and similar residues are indicated by asterisks and periods, respectively, and gaps are indicated by minus signs. α-Helix 1 (a-1), a-2, and the C-terminal tail are in bold. Each LRR is identified by a number or a number and a letter and is underlined. Additional LRRs in YopM32777 are numbered as subrepeats of LRRs 3, 5, and 7 in YopMKIM for simplicity, but this is not meant to imply sequence similarity among subrepeats. The YLTD motif in LRR 10 of YopMKIM is in red. Download
Validation of 32777ΔyopMyopB and requirement of YopB for activation of caspase-1 by 32777ΔyopM. (A) Validation of 32777ΔyopMyopB as shown by a Yop secretion assay and Western blotting with a monoclonal antibody that recognizes an epitope in the N-terminal domain of YopM, with a cocktail of anti-YopB monoclonal antibodies, or with an anti-YopE monoclonal antibody. Samples were normalized by the OD of the bacterial cultures. (B and C) BMDMs primed for 18 h with 100 ng/ml LPS were left uninfected or infected at a MOI of 30 with 32777, 32777ΔyopM, or 32777ΔyopMyopB. (B) BMDM lysates were collected and Western blotted for the cleaved 10-kDa caspase-1 subunit. β-Actin was used as a loading control. (C) Secreted IL-1β in supernatants from Yersinia-infected BMDMs as measured by ELISA. The data in panel C are average values ± the standard errors of the means of two independent experiments. Download
Visualization of NLRP3 focus formation in 129 or Iqgap1−/− mouse macrophages infected with Yersinia. BMDMs from 129 or Iqgap1−/− mice were primed with 100 ng/ml LPS and infected with GFP-expressing Y. pseudotuberculosis strains at an MOI of 30 for 90 min. Following infection, BMDMs were stained with a polyclonal antibody to NLRP3 and DAPI to label DNA and visualized by fluorescence microscopy. Panels: A, uninfected 129 BMDMs; B, 129 BMDMs infected with 32777; C, 129 BMDMs infected with 32777ΔyopM; D, Iqgap1−/− BMDMs infected with 32777ΔyopM. NLRP3, red; Yersinia, green; nuclei, blue. Download
ACKNOWLEDGMENTS
We thank Joe McPhee, Susan Straley, Jason Tam, Adrianus van der Velden, Veena Sangkhae, and Ian Hitchcock for providing reagents; Galina Romanov for preparation of macrophage cultures and strain constructions; and Patricio Mena for help with mouse infections.
This research was supported by awards from the NIH to J.B.B. (R01AI099222 and U54AI057158-Lipkin) or I.E.B. (R01AI103062 and R21AI109267), Research Scholar Grant RSG-09-033-01-CSM from the American Cancer Society to V.A.S., and shared equipment grant S10RR025072 to T.K.
Footnotes
Citation Chung LK, Philip NH, Schmidt VA, Koller A, Strowig T, Flavell RA, Brodsky IE, Bliska JB. 2014. IQGAP1 is important for activation of caspase-1 in macrophages and is targeted by Yersinia pestis type III effector YopM. mBio 5(4):e01402-14. doi:10.1128/mBio.01402-14.
REFERENCES
- 1. Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. 10.1038/nature06246 [DOI] [PubMed] [Google Scholar]
- 2. Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- 4. Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 5. Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- 6. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183:787–791. 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao EA, Warren SE. 2010. Innate immune detection of bacterial virulence factors via the NLRC4 inflammasome. J. Clin. Immunol. 30:502–506. 10.1007/s10875-010-9386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cunha LD, Zamboni DS. 2013. Subversion of inflammasome activation and pyroptosis by pathogenic bacteria. Front. Cell. Infect. Microbiol. 3:76. 10.3389/fcimb.2013.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7:99–109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornelis GR. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811–825. 10.1038/nrmicro1526 [DOI] [PubMed] [Google Scholar]
- 11. Viboud GI, Bliska JB. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69–89. 10.1146/annurev.micro.59.030804.121320 [DOI] [PubMed] [Google Scholar]
- 12. Shin H, Cornelis GR. 2007. Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of pro-inflammatory IL-1beta. Cell. Microbiol. 9:2893–2902. 10.1111/j.1462-5822.2007.01004.x [DOI] [PubMed] [Google Scholar]
- 13. Bergsbaken T, Cookson BT. 2007. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 3(11):e161. 10.1371/journal.ppat.0030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7:376–387. 10.1016/j.chom.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nemeth J, Straley SC. 1997. Effect of Yersinia pestis YopM on experimental plague. Infect. Immun. 65:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leung KY, Reisner BS, Straley SC. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaRock CN, Cookson BT. 2012. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12:799–805. 10.1016/j.chom.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evdokimov AG, Anderson DE, Routzahn KM, Waugh DS. 2001. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312:807–821. 10.1006/jmbi.2001.4973 [DOI] [PubMed] [Google Scholar]
- 19. McDonald C, Vacratsis PO, Bliska JB, Dixon JE. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278:18514–18523. 10.1074/jbc.M301226200 [DOI] [PubMed] [Google Scholar]
- 20. Hentschke M, Berneking L, Belmar Campos C, Buck F, Ruckdeschel K, Aepfelbacher M. 2010. Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5(10):e13165. 10.1371/journal.pone.0013165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCoy MW, Marré ML, Lesser CF, Mecsas J. 2010. The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infect. Immun. 78:2584–2598. 10.1128/IAI.00141-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McPhee JB, Mena P, Bliska JB. 2010. Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect. Immun. 78:3529–3539. 10.1128/IAI.00269-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boland A, Havaux S, Cornelis GR. 1998. Heterogeneity of the Yersinia YopM protein. Microb. Pathog. 25:343–348. 10.1006/mpat.1998.0247 [DOI] [PubMed] [Google Scholar]
- 24. Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. 1994. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J. Biol. Chem. 269:20517–20521 [PubMed] [Google Scholar]
- 25. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 26. Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619. 10.1016/j.cell.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buss C, Müller D, Rüter C, Heusipp G, Schmidt MA. 2009. Identification and characterization of Ibe, a novel type III effector protein of A/E pathogens targeting human IQGAP1. Cell. Microbiol. 11:661–677. 10.1111/j.1462-5822.2009.01284.x [DOI] [PubMed] [Google Scholar]
- 28. McLaughlin LM, Govoni GR, Gerke C, Gopinath S, Peng K, Laidlaw G, Chien YH, Jeong HW, Li Z, Brown MD, Sacks DB, Monack D. 2009. The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration. PLoS Pathog. 5(11):e1000671. 10.1371/journal.ppat.1000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim H, White CD, Sacks DB. 2011. IQGAP1 in microbial pathogenesis: targeting the actin cytoskeleton. FEBS Lett. 585:723–729. 10.1016/j.febslet.2011.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White CD, Erdemir HH, Sacks DB. 2012. IQGAP1 and its binding proteins control diverse biological functions. Cell. Signal. 24:826–834. 10.1016/j.cellsig.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer LE, Hobbie S, Galán JE, Bliska JB. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953–965. 10.1046/j.1365-2958.1998.00740.x [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Murtha J, Roberts MA, Siegel RM, Bliska JB. 2008. Type III secretion decreases bacterial and host survival following phagocytosis of Yersinia pseudotuberculosis by macrophages. Infect. Immun. 76:4299–4310. 10.1128/IAI.00183-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardell S, Wei F-Y, Wong W, Kamen R, Seshadri T. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401–411. 10.1016/0092-8674(95)90490-5 [DOI] [PubMed] [Google Scholar]
- 34. Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. 2013. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl. Acad. Sci. U. S. A. 110:1851–1856. 10.1073/pnas.1211521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501–509. 10.1016/S0092-8674(00)80943-5 [DOI] [PubMed] [Google Scholar]
- 36. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000–2003. 10.1126/science.7535475 [DOI] [PubMed] [Google Scholar]
- 37. Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. 2000. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol. Cell. Biol. 20:697–701. 10.1128/MCB.20.2.697-701.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Celada A, Gray PW, Rinderknecht E, Schreiber RD. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55–74. 10.1084/jem.160.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ryndak MB, Chung H, London E, Bliska JB. 2005. Role of predicted transmembrane domains for type III translocation, pore formation, and signaling by the Yersinia pseudotuberculosis YopB protein. Infect. Immun. 73:2433–2443. 10.1128/IAI.73.4.2433-2443.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pujol C, Bliska JB. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892–5899. 10.1128/IAI.71.10.5892-5899.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simonet M, Falkow S. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of YopM proteins. The amino acid sequences of YopMKIM (NP_857953.1) and YopM32777 (YP_068456.1) were aligned with ClustalW (v 1.4). Identical and similar residues are indicated by asterisks and periods, respectively, and gaps are indicated by minus signs. α-Helix 1 (a-1), a-2, and the C-terminal tail are in bold. Each LRR is identified by a number or a number and a letter and is underlined. Additional LRRs in YopM32777 are numbered as subrepeats of LRRs 3, 5, and 7 in YopMKIM for simplicity, but this is not meant to imply sequence similarity among subrepeats. The YLTD motif in LRR 10 of YopMKIM is in red. Download
Validation of 32777ΔyopMyopB and requirement of YopB for activation of caspase-1 by 32777ΔyopM. (A) Validation of 32777ΔyopMyopB as shown by a Yop secretion assay and Western blotting with a monoclonal antibody that recognizes an epitope in the N-terminal domain of YopM, with a cocktail of anti-YopB monoclonal antibodies, or with an anti-YopE monoclonal antibody. Samples were normalized by the OD of the bacterial cultures. (B and C) BMDMs primed for 18 h with 100 ng/ml LPS were left uninfected or infected at a MOI of 30 with 32777, 32777ΔyopM, or 32777ΔyopMyopB. (B) BMDM lysates were collected and Western blotted for the cleaved 10-kDa caspase-1 subunit. β-Actin was used as a loading control. (C) Secreted IL-1β in supernatants from Yersinia-infected BMDMs as measured by ELISA. The data in panel C are average values ± the standard errors of the means of two independent experiments. Download
Visualization of NLRP3 focus formation in 129 or Iqgap1−/− mouse macrophages infected with Yersinia. BMDMs from 129 or Iqgap1−/− mice were primed with 100 ng/ml LPS and infected with GFP-expressing Y. pseudotuberculosis strains at an MOI of 30 for 90 min. Following infection, BMDMs were stained with a polyclonal antibody to NLRP3 and DAPI to label DNA and visualized by fluorescence microscopy. Panels: A, uninfected 129 BMDMs; B, 129 BMDMs infected with 32777; C, 129 BMDMs infected with 32777ΔyopM; D, Iqgap1−/− BMDMs infected with 32777ΔyopM. NLRP3, red; Yersinia, green; nuclei, blue. Download