ABSTRACT
The new medicinal compound bedaquiline (BDQ) kills Mycobacterium tuberculosis by inhibiting F1Fo-ATP synthase. BDQ is bacteriostatic for 4 to 7 days and kills relatively slowly compared to other frontline tuberculosis (TB) drugs. Here we show that killing with BDQ can be improved significantly by inhibiting cytochrome bd oxidase, a non-proton-pumping terminal oxidase. BDQ was instantly bactericidal against a cytochrome bd oxidase null mutant of M. tuberculosis, and the rate of killing was increased by more than 50%. We propose that this exclusively bacterial enzyme should be a high-priority target for new drug discovery.
IMPORTANCE
A major drawback of current TB chemotherapy is its long duration. New drug regimens with rapid killing kinetics are desperately needed. Our study demonstrates that inhibition of a nonessential bacterial enzyme greatly improves the efficacy of the latest TB drug bedaquiline and emphasizes that screening for compounds with synergistic killing mechanisms is a promising strategy.
Observation
The rapid global increase of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) cases makes new drugs with new mechanisms of action an urgent need. Bedaquiline (BDQ) is the new silver lining on the horizon for MDR and XDR TB patients. This FDA-approved drug specifically inhibits the F1Fo-ATP synthase of mycobacteria and leads to rapid depletion of cellular ATP (1–3). However, BDQ kills relatively slowly compared to other frontline drugs, like isoniazid, and is bacteriostatic for only the first 4 to 7 days of treatment (4). This delayed onset of killing was suggested to be the result of a global remodeling in energy metabolism in response to the drug (4). Following the discovery of BDQ, the respiratory chain of M. tuberculosis was widely recognized as a new important target for drug discovery. This has already yielded a new compound called Q203, which targets the bc1 cytochrome reductase (5). However, this compound was also shown to be slow acting in the first 2 weeks of treatment (5).
Inhibition of the ATP synthase stops proton influx, thereby creating back pressure on the proton-pumping components of the electron transport chain (ETC) (4, 6). M. tuberculosis encodes two proton-pumping complexes: NADH dehydrogenase I and an aa3-type cytochrome c oxidase (7). While NADH dehydrogenase I is dispensable for growth and is not expressed in vivo (8), the aa3-type cytochrome c oxidase is an essential component of M. tuberculosis’s ETC (9). Nevertheless, it was shown recently that cytochrome bd oxidase, a high-affinity terminal oxidase, can partially compensate for the loss in activity of the bc1-aa3 complex (10). In addition, cytochrome bd oxidase was strongly induced in M. tuberculosis treated with BDQ (4). This suggests that expression of the non-proton-pumping cytochrome bd oxidase relieves the back pressure on the ETC and allows the bacterium to prolong maintenance of its membrane potential in the absence of ATP synthase activity. Therefore, we hypothesized that a strain lacking cytochrome bd oxidase should be hypersensitive to BDQ and that no delay of killing should be observed.
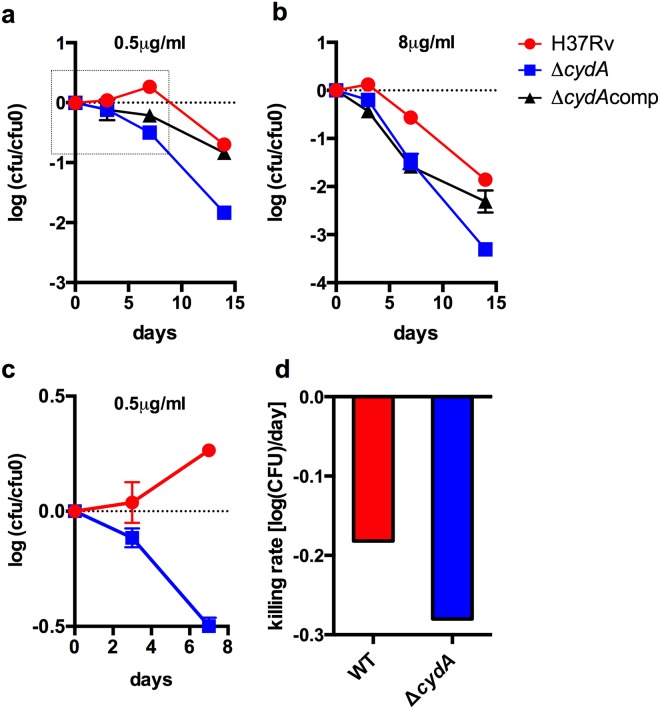
To test the hypothesis of hypersensitivity, we created a cytochrome bd oxidase knockout of M. tuberculosis H37Rv by replacing the first gene, cydA, in the cydAB operon with a hygromycin cassette by using specialized transduction. As expected, this mutant showed no growth defect under standard aerobic growth conditions (see Fig. S1 in the supplemental material). Susceptibility to different concentrations of bedaquiline (0.5 and 8 µg/ml) was tested in 5-ml cultures in ink well bottles starting at a concentration of 5 × 105 CFU. To determine initial killing kinetics, numbers of CFU were monitored over a period of 14 days. Killing by BDQ set in immediately in the mutant cultures, while for the wild type (WT), we saw the expected delay in killing of up to 1 week (Fig. 1c). In addition, a 55%-increased killing rate was detected for the mutant strain (Fig. 1d), with an up to 70-fold difference in numbers of surviving cells after 14 days of treatment with 8 µg/ml BDQ (Fig. 1b). A mutant strain harboring a plasmid (pMV361::cydA) expressing cydA from the HSP60 promoter could partially complement the phenotype (Fig. 1a and b). This partial complementation is likely due to copy number differences and the fact that respiratory enzymes, as part of the electron transport chain complex, need fine-tuned expression control to guarantee optimal electron flux.
FIG 1 .
Killing assay with 0.5 µg/ml (a) or 8 µg/ml (b) BDQ comparing the H37Rv wild type (red) with the ΔcydA mutant (blue) and the ΔcydAcomp complemented mutant (black). cfu0, CFU at time 0. (c) Closeup of the initial 7 days of exposure of the WT and ΔcydAto strain to 0.5 µg/ml BDQ (box in panel a). Cells were grown in 7H9 OADC medium to exponential phase. Then, cultures were diluted to yield a starting CFU concentration of around 106 and challenged with BDQ. Data were normalized to the starting CFU concentration for a proper comparison of killing kinetics. (d) First-order rate kinetics of kill curves at 8 µg/ml BDQ. Rates were calculated by linear regression from data points in panel b at 3, 7, and 14 days. Killing rates for the ΔcydAcomp strain at 8 µg/ml BDQ are not depicted because the kill curve was biphasic (b) and first-order rate kinetics do not apply.
Cytochrome bd oxidase as a drug target would likely be missed in drug screens because this enzyme is not essential for growth due to the presence of the aa3 cytochrome c oxidase. Our data show that inhibition of cytochrome bd oxidase in M. tuberculosis leads to hypersensitivity to BDQ and rapid killing. An essential role of CydAB in vivo was indicated recently. Mutants of M. tuberculosis with transposon insertions in cytochrome bd oxidase genes were underrepresented in mouse lungs after 48 days of infection (11). This fits with cytochrome bd oxidase’s purported role as a high-affinity terminal oxidase that is crucial for the adaptation of M. tuberculosis to hypoxia in vivo (12, 13). These results combined with the fact that cytochrome bd oxidase is found exclusively in bacteria make it a very attractive drug target.
To prevent rapid evolution of drug resistance, new compounds should be part of a completely new drug regimen. Ideally, these antibiotics have synergistic effects leading to exponential increases in killing efficacy. Our results predict that a specific inhibitor of cytochrome bd oxidase combined with BDQ (and likely also with Q203) would have such a multiplicative effect. Moreover, the phenotype reported here will allow us to elucidate the yet-unknown mechanism of killing of BDQ as well as the role of cytochrome bd oxidase in the respiratory chain of M. tuberculosis.
Bacterial strains and growth conditions.
All bacterial strains, plasmids, and primers used in this study are listed in Table S1 in the supplemental material. Mycobacterial strains were grown in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 10% (vol/vol) OADC enrichment (0.5 g oleic acid, 50 g albumin, 20 g dextrose, 0.04 g catalase, 8.5 g sodium chloride in 1 liter water), 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) tyloxapol (Sigma). Selective media contained 75 µg/ml hygromycin B and/or 20 µg/ml kanamycin. The gene cydA (Rv1623c) was deleted in M. tuberculosis H37Rv by specialized transduction as described previously (14). Mutations were confirmed by 3-primer PCR using primers Rv1623cL, Rv1623cR, and Universal_uptag, listed in Table S1. The M. tuberculosis ΔcydA strain was complemented using pMV361 harboring a copy of the cydA gene (ΔcydAcomp strain) (Table S1). The gene cydA was PCR amplified using primers Rv1623c_fw_EcoRI and Rv1623c_re_HindIII (Table S1) and cloned into pMV361 using EcoRI and HindIII restriction sites. The nucleotide sequences of all constructs were verified by Sanger sequencing.
Killing assay.
BDQ was purchased from Shanghai Biochempartner (Wuhan, China). BDQ stocks were prepared in dimethyl sulfoxide (DMSO). The M. tuberculosis H37Rv, ΔcydA, and ΔcydAcomp strains were grown to mid-exponential phase (optical density at 600 nm [OD600] = 0.5) in 7H9 OADC. Before addition of BDQ, cells were diluted to a starting concentration of approximately 106 cells ml−1. BDQ was added to a final concentration of 0.5 µg ml−1 (5× MIC) or 8 µg ml−1 (90× MIC). DMSO at appropriate concentrations was added to control cultures in order to rule out toxic effects of the solvent (Fig. S1). At each time point, culture was harvested and serially diluted in phosphate-buffered saline (Gibco, Life Technologies) with 0.05% tyloxapol (Sigma) and plated onto 7H10 OADC (Difco, BD) agar plates. Colonies were counted after 4 weeks and subsequent weeks until no new colonies were detected.
SUPPLEMENTAL MATERIAL
Growth of control cultures of the M. tuberculosis H37Rv, ΔcydA, and ΔcydAcomp strains on 7H9 OADC with 0.08% DMSO. Download
List of strains, constructs, and primers.
ACKNOWLEDGMENTS
This work was financially supported by NIH grants AI26170, R01AI097548, and AIC51519.
We thank Annie Zhi Dai for technical support.
Footnotes
Citation Berney M, Hartman T, Jacobs WR, Jr. 2014. A Mycobacterium tuberculosis cytochrome bd oxidase mutant is hypersensitive to bedaquiline. mBio 5(4):e01275-14. doi:10.1128/mBio.01275-14.
REFERENCES
- 1. Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3:323–324. 10.1038/nchembio884 [DOI] [PubMed] [Google Scholar]
- 2. Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. 10.1126/science.1106753 [DOI] [PubMed] [Google Scholar]
- 3. Koul A, Vranckx L, Dendouga N, Balemans W, Van den Wyngaert I, Vergauwen K, Göhlmann HW, Willebrords R, Poncelet A, Guillemont J, Bald D, Andries K. 2008. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 283:25273–25280. 10.1074/jbc.M803899200 [DOI] [PubMed] [Google Scholar]
- 4. Koul A, Vranckx L, Dhar N, Göhlmann HW, Özdemir E, Neefs JM, Schulz M, Lu P, Mørtz E, McKinney JD, Andries K, Bald D. 2014. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat. Commun 5:3369. 10.1038/ncomms4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Oh S, Oh T, Nehrbass U, Han SJ, No Z, Lee J, Brodin P, Cho SN, Nam K, Nam K, Kim J. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 19:1157–1160. 10.1038/nm.3262 [DOI] [PubMed] [Google Scholar]
- 6. Tran SL, Cook GM. 2005. The F1Fo-ATP synthase of Mycobacterium smegmatis is essential for growth. J. Bacteriol. 187:5023–5028. 10.1128/JB.187.14.5023-5028.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 8. Rao SP, Alonso S, Rand L, Dick T, Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 105:11945–11950. 10.1073/pnas.0711697105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsoso LG, Kana BD, Crellin PK, Lea-Smith DJ, Pelosi A, Powell D, Dawes SS, Rubin H, Coppel RL, Mizrahi V. 2005. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J. Bacteriol. 187:6300–6308. 10.1128/JB.187.18.6300-6308.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Small JL, Park SW, Kana BD, Ioerger TR, Sacchettini JC, Ehrt S. 2013. Perturbation of cytochrome c maturation reveals adaptability of the respiratory chain in Mycobacterium tuberculosis. mBio 4(5):e00475-13. 10.1128/mBio.00475-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang YJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, Trauner A, Wallis D, Galaviz S, Huttenhower C, Sacchettini JC, Behar SM, Rubin EJ. 2013. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell 155:1296–1308. 10.1016/j.cell.2013.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kana BD, Weinstein EA, Avarbock D, Dawes SS, Rubin H, Mizrahi V. 2001. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 183:7076–7086. 10.1128/JB.183.24.7076-7086.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. 2005. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl. Acad. Sci. U. S. A. 102:15629–15634. 10.1073/pnas.0507850102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalscheuer R, Syson K, Veeraraghavan U, Weinrick B, Biermann KE, Liu Z, Sacchettini JC, Besra G, Bornemann S, Jacobs WR., Jr. 2010. Self-poisoning of Mycobacterium tuberculosis by targeting GlgE in an alpha-glucan pathway. Nat. Chem. Biol. 6:376–384. 10.1038/nchembio.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth of control cultures of the M. tuberculosis H37Rv, ΔcydA, and ΔcydAcomp strains on 7H9 OADC with 0.08% DMSO. Download
List of strains, constructs, and primers.