Summary
Background
Factor (F)VIIa-based bypassing not always provides sufficient hemostasis in hemophilia.
Objectives
To investigate the potential of engineered activated factor V (FVa) variants as bypassing agents in hemophilia A.
Methods
Activity of FVa variants was studied in vitro using prothrombinase assays with purified components and in FV- and FVIII-deficient plasma using clotting and thrombin generation assays. In vivo bleed reduction after the tail clip was studied in hemophilia A mice.
Results and conclusions
FVa mutations included a disulfide bond connecting the A2 and A3 domains and ones that rendered FVa resistant to inactivation by activated protein C (APC). ‘superFVa,’ a combination of the A2-A3 disulfide (A2-SS-A3) to stabilize FVa and of APC-cleavage site mutations (Arg506/306/679Gln), had enhanced specific activity and complete APC resistance compared with wild-type FVa, FVLeiden(Arg506Gln), or FVaLeiden(A2-SS-A3). Furthermore, superFVa potently increased thrombin generation in vitro in FVIII-deficient plasma. In vivo, superFVa reduced bleeding in FVIII-deficient mice more effectively than wild-type FVa. Low-dose superFVa, but not wild-type FVa, decreased early blood loss during the first 10 min by more than two-fold compared with saline and provided bleed protection for the majority of mice, similar to treatments with FVIII. During the second 10 min after tail cut, superFVa at high dose, but not wild-type FVa, effectively reduced bleeding. These findings suggest that superFVa enhances not only clot formation but also clot stabilization. Thus, superFVa efficiently improved hemostasis in hemophilia in vitro and in vivo and may have potential therapeutic benefits as a novel bypassing agent in hemophilia.
Keywords: animal experimentation, bleeding, factor V, hemophilia, hemostasis
Introduction
Approximately 30% of patients with hemophilia develop neutralizing inhibitory antibodies against exogenously administered factor VIII (FVIII) or FIX, which leaves patients unresponsive to FVIII or FIX preparations and vulnerable to bleeding. FVIIa-based bypassing agents, which include recombinant human (rh)FVIIa and plasma-derived activated prothrombin complex concentrates, were specifically developed to achieve hemostasis in hemophilic patients with inhibitors since supraphysiological levels of FVIIa can rescue bleeding episodes based on direct activation of FX via tissue factor (TF)-dependent pathways or independently of TF on the surface of platelets [1,2].
Unfortunately, treatment with FVIIa-based bypassing agents remains suboptimal [3,4], and alternative treatment strategies to provide more sustained thrombin formation are therefore desirable. Several different strategies have been proposed, including novel FVIIa variants with increased catalytic activity and half-life [5,6], engineered FIX molecules with enhanced ability to bind and activate FX [7], and engineered zymogen-like FXa variants that become active on engaging activated FV (FVa) in the prothrombinase complex [8,9]. As an alternative to these pro-tease-based strategies, modulation of FVa cofactor activity may be another attractive strategy to enhance thrombin generation and to improve hemostasis in hemophilia.
FV circulates in plasma at a concentration of ~ 20 nmol L−1 (~ 10 µg mL−1), with a half-life of 12–36 h. As an important cofactor in the prothrombinase complex, FVa can enhance the rate of thrombin generation ~ 10 000-fold [10,11]. However, the cofactor function of FVa is closely regulated by proteolytic inactivation of FVa by activated protein C (APC). APC rapidly inactivates FVa via proteolytic cleavage at Arg506, followed by a slower cleavage at Arg306 [12]. Mutations of these inactivation cleavage sites, such as Arg506Gln (a.k.a. FVLeiden), extend the FVa cofactor activity half-life, thereby augmenting its ability to enhance thrombin generation. Experimental in vitro and in vivo studies strongly suggest a disease-modifier effect of APC-resistant FV mutations in hemophilia. For instance, APC-resistant FV mutants (FVLeiden and FVCambridge [Arg306Thr]) showed enhanced thrombin generation in hemophilic plasma compared with wild-type FV [13,14], and hemophilic mice with the FVLeiden mutation showed improved activated partial thromboplastin time (APTT) clotting profiles and laser-induced microvascular hemostasis compared with hemophilic mice with normal FV [15]. Furthermore, there is increasing clinical consensus that bleeding is attenuated in hemophiliacs with the FVLeiden mutation since population studies indicate improved outcome measures such as factor concentrate consumption, annual bleeding episodes, and joint damage [16]. On the other hand, infusion of FVa achieved only a very modest shortening of the APTT in hemophilia B and, although FVLeiden homozygosity reduced blood loss after tail transsection in hemophilia B mice, it failed to do so in hemophilia A mice [15]. Thus, these observations support the investigation of a pharmacological approach to ‘FVa activity augmentation’ in hemophilia and provide unique opportunities for molecular engineering of FVa to increase its efficacy by enhancing its activity and stability.
Several years ago, we engineered an interdomain disulfide bond (His609Cys-Glu1691Cys) in FV connecting the A2 and A3 domains (A2-SS-A3) to study the discriminative contributions of A2 domain dissociation vs. proteolytic inactivation by APC to FVa inactivation [12]. Anchoring the A2 domain to the FVa molecule conveyed a significant resistance to APC-mediated inactivation similar to that of the Leiden (Arg506Gln) mutation. Furthermore, the interdomain disulfide bond seemed to enhance the specific activity of FVa [12]. These observations prompted the current investigation to determine the potential of FVa(A2-SS-A3) either alone or in combination with APC-cleavage site mutations as a novel approach of ‘FVa activity augmentation’ to correct hemo-stasis in hemophilia.
Materials and methods
Materials
Plasma purified prothrombin, thrombin, and FXa were obtained from Enzyme Research Laboratories (South Bend, IN, USA). Hirudin was from Calbiochem (La Jolla, CA, USA), and corn trypsin inhibitor was obtained from Haematologic Technologies (Essex Junction, VT, USA). Substrates Pefachrome TH and Z-Gly-Gly-Arg-AMC were from Centerchem (Norwalk, CT, USA) and Bachem (Torrance, CA, USA), respectively. Human FVIII- or FV-deficient plasma was purchased from George King Bio-Medical (Overland Park, KS, USA). Phospholipid vesicles (80% phosphatidylcholine, 20% phosphatidylserine) were prepared as described previously [17].
Recombinant FV mutants
Recombinant wild-type FV and FV mutants were made on a B-domain deleted S2183A platform and purified from conditioned media of stable transfected BHK cells through a combination of affinity chromatography using anti–FV 3B1 and HV5101 monoclonal antibodies as described previously [12,18]. FV protein concentration was determined based on absorbance at 280 nm using FV ε1% = 15.4 [19] and ELISA (Enzyme Research Laboratories) according to the manufacturer’s instructions. FV proteins were activated with 2 nmol L−1 thrombin for 20 min at 37 °C in prothrombinase buffer (50 mmol L−1 HEPES, 150 mmol L−1NaCl, 0.5% BSA, 5 mmol L−1 CaCl2, and 0.1 mmol L−1MnCl2). Activation was terminated by the addition of 1.1 mol L−1equivalent of hirudin.
Prothrombinase assays
Prothrombinase assays were performed as described previously [12]. Briefly, FVa and phospholipid vesicles were mixed, and 15 µL aliquots were added to 10 µL of FXa, followed by 10 µL of prothrombin in prothrombinase buffer (final concentrations: 1.42 nmol L−1FXa, 28 pmol L−1FVa, 22 µmol L−1phospholipid vesicles, and 0.42 µmol L−1 prothrombin). After 2.5 min, the reaction was quenched by the addition to 50 µL of HEPES buffered saline (HBS) containing 10 mmol L−1EDTA, 0.5% BSA, pH 8.2. After the addition of 35 µL of Pefachrome TH (0.6 mmol L−1), thrombin formation was assessed by measuring the change in absorbance at 405 nm using a VersaMax Microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Thrombin generation assays
Endogenous thrombin potential (ETP) assays were performed as described previously [20]. Briefly, FVa mutants (30 nmol L−1), FVIII (1 U mL−1; Kogenate®; Bayer Healthcare, Whippany, NJ, USA), or saline was added to human FVIII- or FV-deficient plasma (50% v/v) supplemented with 1.45 µmol L−1corn trypsin inhibitor, 10 mmol L−1CaCl2, 10 µmol L−1phospholipid vesicles, 0.2 pmol L−1soluble tissue factor (Innovin®; Dade Behring, Deerfield, IL, USA), and 0.4 mmol L−1 Z-Gly-Gly-Arg-AMC (thrombin substrate) in HBS. After mixing, 100 µL was transferred to a FluoroNunc microtiter plate at 37 °C to monitor fluorescence (excitation at 360 nm per emission at 460 nm; Gemini EM fluorescent plate reader [Molecular Devices]). Fluorescence time course data were converted into nmol L−1 thrombin as described previously [21]. ETP, defined as the area under the curve, was determined using Prism 5.04 (GraphPad Software, San Diego, CA, USA).
FVa inactivation assays
APC-mediated inactivation of FVa was analyzed in pro-thrombinase assays as described previously [12]. FVa mutants (4 nmol L−1) were incubated with 2 nmol L−1 wild-type recombinant human APC (Xigris®; Eli Lilly and Co., Indianapolis, IN, USA) or wild-type recombinant mouse APC [22] and 25 µmol L−1 phospholipid vesicles in prothrombinase buffer, and 1-µL aliquots were removed at specified time points. Residual FVa was determined using the prothrombinase assay as described earlier. Alternatively, FV- or FVIII-deficient plasma was reconstituted with FVa (1 nmol L−1), and thrombin generation was determined in ETP assays in the presence of increasing concentrations of APC. For Western blot analysis, FVa was incubated with 2 nmol L−1 APC for 30 min at 37 °C. FVa inactivation fragments were resolved on 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) and detected using a polyclonal rabbit antihu-man FV heavy chain antibody (1:3500; Pierce, Rockford, IL, USA) and goat anti-rabbit-800CW secondary antibody (LI-COR; 1:10 000). Bands were visualized using the Odyssey infrared imager (LI-COR Biosciences, Lincoln, NE, USA).
APTT and prothrombin time clotting assays
For the APTT assay, plasma (50 µL) was mixed with 50 µL of APTT reagent (Platelin LS; Organon Technika, Durham, NC, USA) and incubated at 37 °C for 3 min. Then 2 µL of FVa (final concentration 0.5 n nmol L−1) was added, followed by 50 µL of 25 mmol L−1 CaCl2 in HBS, 0.5% BSA. For the prothrombin time (PT) assay, plasma (50 µL) was mixed with 2 µL of FVa (final concentration 0.5 nmol L−1) and incubated at 37 °C for 1 min, followed by the addition of 50 µL of Innovin®. The clotting times were recorded using an ST4 coagulo-meter (Diagnostica Stago, Parsippany, NJ, USA).
Animals
All described animal protocols were carried out as approved by the institutional animal and care committee of The Scripps Research Institute. Several breeding pairs of FVIII-deficient mice (BalbC background) were a generous gift of Dr. David Lillicrap. Mice of both sexes, aged ≥ 8 weeks, were used for tail bleeding assays.
Bleeding assays in FVIII-deficient mice
Mice were anesthetized and placed on temperature-controlled heating pads (37 °C), and the distal portion of the tail was cut at 1.5-mm diameter (moderate hemostatic challenge), after which the tail was immersed in a predefined volume of 37 °C saline (0.9% NaCl) for 20 min. To study effects on bleeding and clot stability, tubes were changed after 10 min to collect blood for the first and second 10 min separately. Blood loss was determined by the hemoglobin concentration in the saline solution after red cell lysis with 2% acetic acid and measured by absor-bance at 405 nm. Using a hemoglobin standard derived from defined blood volumes, blood loss was calculated assuming a hematocrit of 46% and expressed in microliter per gram body weight. Groups of FVIII-deficient mice were injected intravenously (retro-orbital) within 2 min before the tail cut with equal volumes (200 µL) of wild-type FVa, superFVa, 50 units kg−1 FVIII (Kogenate®; Bayer Healthcare), or saline. All agents were diluted in sterile sodium chloride 0.9% for injection (Hospira Inc., San Diego, CA, USA). As with FVIII, FVa variant dosing was based on prothrombinase cofactor activity, whereby the activity of 20 nmol L−1 wild-type FVa (approximate FV plasma concentration) was defined as 1 unit. In experiments where data were expressed as percent bleed protection, mice were considered protected if blood loss was below the mean blood loss plus 3 SDs observed in wild-type BalbC mice [23].
Statistical analysis
Student’s t test or for bleeding, Kruskal–Wallis followed by one-tailed Mann–Whitney test was used to assess statistical significance where appropriate. Differences in percent bleed protection were evaluated with Fisher’s exact test. A P-value of ≤ 0.05 was considered statistically significant.
Results
To enhance the biologic stability of disulfide-stabilized FVa [denoted FVa(A2-SS-A3)], additional mutations were introduced that abolished the kinetically favored APC cleavage site at Arg506 [denoted FVaLeiden(A2-SS-A3)] or the combination of all three APC cleavage sites at Arg506, Arg306, and Arg679 (denoted superFVa) (see Figure S1 for schematics of the mutations and nomenclature) [12,24,25].
Activity of FVa variants in vitro
After purification, all FVa mutants appeared homogeneous (> 90%) and showed identical band patterns and activation cleavage rates on reduced SDS-PAGE gel (Figure S2). Cofactor activities of FVa and the FVa mutants were determined using a prothrombinase (IIase) assay with purified components. The concentrations of the FV mutants were determined by ELISA, and the specific activity of the FVa mutants was expressed as IIase/nmol L−1 FVa, whereby IIase equaled mOD/min thrombin generation. Introduction of the disulfide bond significantly increased the specific activity of wild-type FVa and FVaLeiden The mean increase in specific activity was 2.6-fold for FVa(A2-SS-A3) compared with wild-type FVa, and 2.7-fold for FVaLeiden(A2-SS-A3) compared with FVaLeiden Although the specific activity of FVaLeiden(A2-SS-A3) was similar to that of FVa(A2-SS-A3), mutation of all three APC cleavage sites in FVa(A2-SS-A3) resulted in an additional enhancement of specific activity in superFVa compared with FVa(A2-SS-A3) and FVaLeiden(A2-SS-A3). Mean fold-increase for superFVa compared with wild-type FVa was 3.2-fold (Fig. 1A).
Fig. 1.
Specific activity and cofactor activity of wild-type activated factor V (FVa) and FVa mutants. Concentrations of FVa-mutants were determined by ELISA, and cofactor activities were determined either in purified prothrombinase assays or in FV-deficient plasma. (A) Specific activity of FVa mutants (28 pmol L−1) determined in purified prothrombinase assay and expressed as IIase/nmol L−1 FVa species (n = 5–9). (B) Specific activity of FVa mutants (0.5 nmol L−1) determined in thrombin generation assays in FV-deficient plasma. A representative example is shown. (C) Endogenous thrombin potential of FVa mutants (0.5 nmol L−1) in FV-deficient plasma was calculated as area under the curve (AUC) and expressed as fold-increase of endogenous thrombin potential (ETP) over baseline (ETP FVa = 1) (n = 4). Error bars represent SEM (*all P ≤ 0.02).
Increases in specific activity of FVa mutants generally correlated with enhanced functional activity of these mutants in thrombin generation assays in FV-deficient plasma (Fig. 1B). Dose-response titration of wild-type FVa in FV-deficient plasma indicated half-maximal normalization of thrombin generation at 0.5 nmol L−1 FVa (data not shown). Under these conditions where wild-type FVa provided partial normalization of thrombin generation, quantified as ETP (AUC), FVa(A2-SS-A3) (1.9-fold), FVaLeiden(A2-SS-A3) (2.8-fold), and superFVa (2.9-fold), all significantly enhanced thrombin generation compared with wild-type FVa when reconstituted at the same concentration (Fig. 1B,C). Interestingly, mutation of APC cleavage sites, either Arg506Gln in FVaLeiden(A2-SS-A3) or all three in superFVa, resulted in a significant improvement of thrombin generation compared with FVa(A2-SS-A3). Reconstitution of wild-type FVa or superFVa in FV-deficient plasma at 2.5% of normal FV levels resulted in normalization of the APTT and near normalization of the PT clotting times by super-FVa, whereas only a partial correction was observed by wild-type FVa (Fig. 2). Thus, stabilization of the A2 interaction with the A3 domain by introduction of a disulfide bridge results in a FVa with significantly increased cofactor activity in purified prothrombinase assays, in plasma in APTT and PT clotting assays and in thrombin generation assays.
Fig. 2.
Correction of clotting times of factor V (FV)-deficient plasma by superFVa. (A) Activated partial thromboplastin time (APTT) and (B) prothrombin time (PT) clotting times were determined in FV-deficient plasma after reconstitution with FVa or superFVa (0.5 nmol L−1). Normal plasma served as control (n = 4). Error bars represent SEM (*all P < 0.0001).
A2-A3 disulfide formation and APC-resistance of superFVa
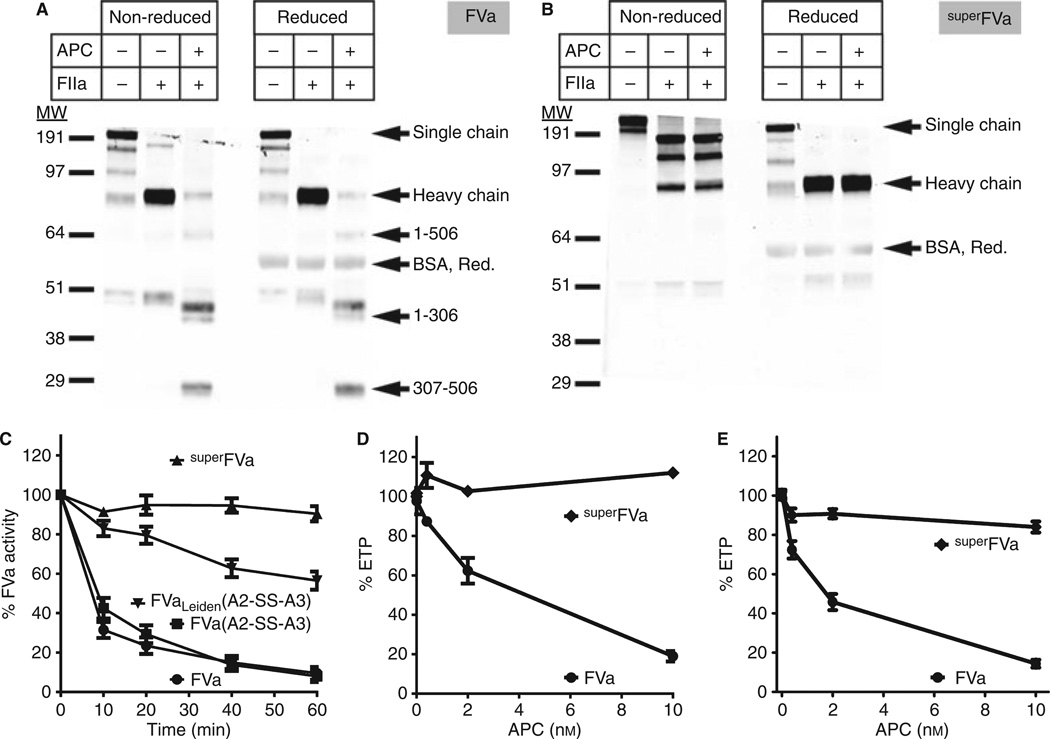
To confirm the formation of the disulfide bridge in super-FVa and verify its resistance to proteolysis by APC, both wild-type FV (Fig. 3A) and superFV (Fig. 3B) were analyzed by Western blot. After activation by thrombin, the heavy chain of FVa shifted down and APC generated the typical 1–506, 1–306, and 307–506 heavy chain fragments under both nonreducing and reducing conditions as anticipated. The heavy chain of superFVa also shifted down after activation by thrombin, but the mobility of superFVa (~ 190 kDa) was lower than that of the FVa heavy chain, consistent with a disulfide bond connecting the heavy and light chain. The usual heavy chain band became apparent under reducing conditions. However, some heavy chain band was also present in superFVa under nonreducing conditions, indicating that the disulfide bond was not formed in ~ 15% of the superFVa molecules (Figure S3). Reflecting the APC cleavage site mutations, no 1–506, 1–306, or 307–506 heavy chain fragments were observed in superFVa upon incubation with APC under either nonreducing or reducing conditions. Western blot revealed an additional ~ 150-kDa band of minor abundance, which is likely due to proteolysis of FV in the conditioned media and during purification despite the presence of protease inhibitors.
Fig. 3.
Activated protein C (APC) resistance of super FVa. Western blot analysis with an anti-FV heavy chain polyclonal antibody of (A) FV and (B) superFV (both 4 nmol L−1) in the absence and presence of thrombin and/or APC (2 nmol L−1). (C) Time course of FVa cofactor activity in purified prothrombinase assays of A2-SS-A3 disulfide-linked FVa-mutants (4 nmol L−1) in the presence of APC (2 nmol L−1). Conditions for the prothrombinase assay were FVa (28 pmol L−1), FXa (1.42 nmol L−1), and prothrombin (0.42 µmol L−1) (n = 4–5). Error bars represent SEM (all P ≤ 0.05). APC resistance of superFVa in (D) FV-deficient plasma and (E) FVIII-deficient plasma. Thrombin generation was determined in FV-deficient plasma reconstituted with FVa or superFVa both at 1 nmol L−1, and in FVIII-deficient plasma at 10 nmol L−1, respectively, in the presence of increasing concentrations of APC. Endogenous thrombin potential (ETP) was expressed relative to the absence of APC (n = 3). Error bars represent SEM.
Compared with FVa(A2-SS-A3), incubation of FVaLeiden(A2-SS-A3) with APC retained ~ 50% cofactor activity in purified prothrombinase assays, whereas the cofactor activity of superFVa (> 90%) was not affected by APC (Fig. 3C). Similar results were obtained for the inactivation of wild-type FVa and superFVa by mouse APC (Figure S4). To address the APC resistance of superFVa in plasma, FV-deficient plasma and FVIII-deficient plasma were reconstituted with superFVa and thrombin generation was determined in the presence of increasing concentrations of APC. The ability of superFVa to support thrombin formation was fully retained in the presence of APC, whereas the APC dose-dependently inhibited thrombin generation in the presence of wild-type FVa. At the highest concentration of APC (10 nmol L−1), the mean ETP was reduced to ~ 20% with wild-type FVa, whereas the mean ETP with superFVa remained at ~ 100% (Fig. 3D, E).
Improved thrombin generation in FVIII-deficient plasma by superFVa
To address the potential of superFVa as a bypassing agent in hemophilia A, thrombin generation was determined in human FVIII-deficient plasma in the presence of wild-type FVa and the FVa mutants at 150% (30 nmol L−1) of the normal FV plasma concentration. Wild-type FVa had a minor but statistically significant effect on thrombin generation (Fig. 4A) and increased the ETP by ~ 40% (Fig. 4B). FVa(A2-SS-A3) and FVaLeiden(A2-SS-A3) also enhanced the ETP in FVIII-deficient plasma by 40– 50% (Fig. 4B). However, superFVa resulted in a pronounced increase of ETP (~ 2-fold greater than wild-type FVa). While superFVa normalized the ETP in FVIII-deficient plasma to that of normal plasma or FVIII-deficient plasma reconstituted with 1 U mL−1 FVIII (Fig. 4B), the shape of the ETP curve remained noticeably different (Fig. 4A).
Fig. 4.
Improved thrombin generation in factor VIII (FVIII)-deficient plasma by superFVa. Thrombin generation in normal plasma (NP) and FVIII-deficient plasma supplemented with FVa, superFVa (both 30 nmol L−1), or FVIII (1 U mL−1). (A) Representative example of thrombin generation curves of FVIII-deficient plasma supplemented with FVa or superFVa compared with FVIII-deficient plasma reconstituted with FVIII and NP. (B) Relative fold-change of thrombin generation in FVIII-deficient plasma, expressed as endogenous thrombin potential (ETP FVa = 1), in the presence of FVa mutants and compared with reconstitution with FVIII and NP (n = 3–4). Error bars represent SEM (*P ≤ 0.03).
Efficient treatment of acute tail bleeding in FVIII-deficient mice by superFVa
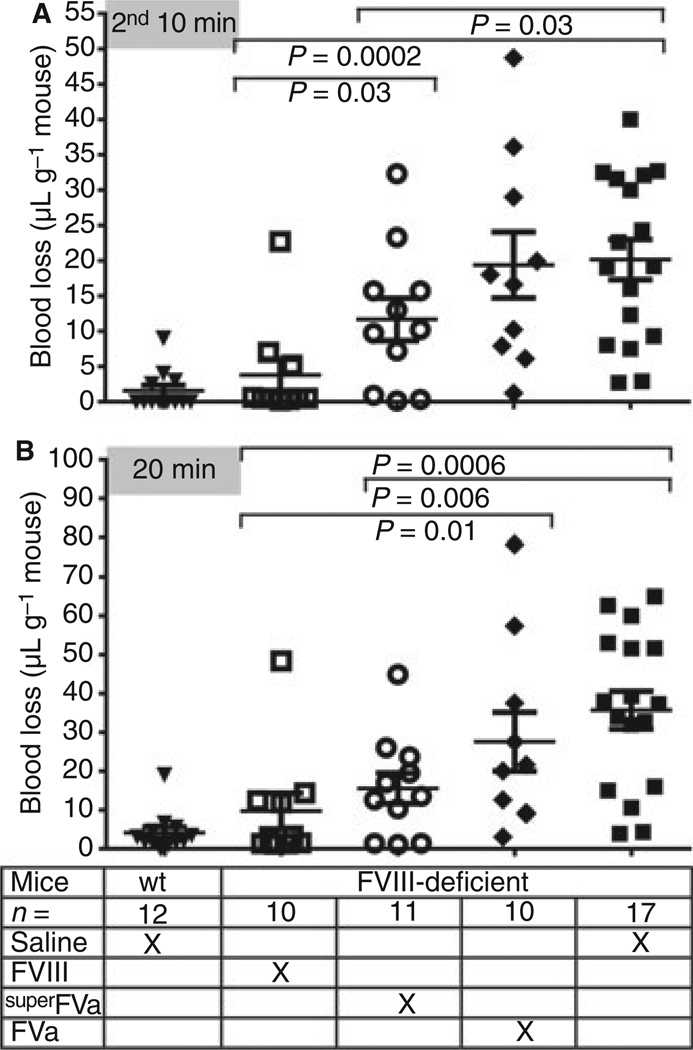
To determine the in vivo efficacy of superFVa for bleed reduction in hemophilia, blood loss was determined in a tail transsection model in FVIII-deficient mice. Blood was collected separately for the first 10 min and second 10– 20 min after the tail clip to distinguish initial bleeding from late rebleeding that typically occurs in FVIII-deficient mice 8–10 min after initial tail clip. Dosing was performed by activity as with other clotting factors in clinical use (see Materials and methods). At low dose (2 units, wild-type FVa 0.8 mg kg−1; superFVa 0.27 mg kg−1), superFVa reduced blood loss compared with saline (6.5 vs. 15.5 µL g−1; P = 0.02) during the first 10 min (Fig. 5), while wild-type FVa did not (16.7 µL g−1). A 2.5-fold higher dose (5 units, wild-type FVa 2 mg kg−1; superFVa 0.67 mg kg−1) of wild-type FVa was required to reduce blood loss (8.2 µL g−1 vs. saline 15.5 µL g−1; P = 0.054) compared with saline, and one outlier prevented reaching statistical significance. At 5 units, blood loss with superFVa was further reduced to 3.9 µL g−1 and was indistinguishable from that of FVIII-deficient mice treated with FVIII (5.9 µL g−1) or wild-type BalbC mice (2.6 µL g−1). Significant percent bleed protection of FVIII-deficient mice (defined as a threshold of the mean blood loss plus 3 SDs in wild-type BalbC mice; blood loss ≤ 9.9 µL g−1), was only achieved following treatment with superFVa (at 2 and 5 units), but not with wild-type FVa (Table 1). These results demonstrate that although FVa improved blood loss in a dose-dependent manner during the first 10 min after tail cut in FVIII-deficient mice, superFVa was more effective than wild-type FVa in reducing blood loss and providing bleed protection.
Fig. 5.
Correction of initial bleeding by superFVa. Factor VIII (FVIII)-deficient mice were injected intravenously with FVIII, saline, or either FVa or superFVa dosed at equal activity as determined in prothrombinase assays (1 unit = activity of 20 nmol L−1 wild-type FVa). For wild-type FVa, 2 units = 0.8 mg kg−1 and 5 units = 2 mg kg−1. Based on increased specific activity of superFVa, 2 units = 0.27 mg kg−1 and 5 units = 0.67 mg kg−1. Bleeding during the first 10 min after the tail clip is expressed as blood loss in microliter blood per gram mouse. Error bars represent SEM.
Table 1.
Percent Bleed Protection of FVIII-Deficient Mice Treated With Either Saline, FVIII, superFVa, or Fva
| Wild-type | FVIII-deflcient |
|||||||
|---|---|---|---|---|---|---|---|---|
|
superFVa |
FVa |
|||||||
| Treatment | Saline | FVIII | 5 units | 2 units | 5 units | 2 units | Saline | |
| Bleed protection | ||||||||
| 10 min after tail cut (n) | 92% (11/12) | 80% (8/10) | 81%* (9/11) | 83%†‡(10/12) | 60% (6/10) | 36%‡ (4/11) | 23%*† (4/17) | |
| 20 min after tail cut (n) | 100% (12/12) | 90% (9/10) | 73%§ (8/11) | 33% (4/12) | 40% (4/10) | 18% (2/11) | 23%§ (4/17) | |
Percent bleed protection referred to the percentage of mice demonstrating blood loss below a threshold defined as mean blood loss plus 3 SDs in wild-type BalbC mice. For the 10- or 20-min time points, mice were considered protected if blood loss was ≤ 9.9 or ≤ 19.5 µL g−1, respectively. Comparisons between groups that attained statistical significance are marked by the same specific symbols (all P ≤ 0.02).
Reduction of late bleeding in FVIII-deficient mice by superFVa
The effect of superFVa on late rebleeding episodes was determined by collecting blood for an additional 10 min (10–20 min after the tail clip). FVIII-deficient mice receiving saline continued to bleed profoundly (20.2 µL g−1), but late rebleeding was efficiently prevented by treatment with FVIII (3.8 µL g−1) (Fig. 6A). Only superFVa (11.7 µL g−1) at a high dose (5 units), not wild-type FVa (19.4 µL g−1), reduced late rebleeding, albeit less efficiently than FVIII (3.8 µL g−1) (Fig. 6A). Results for the combined 20-min bleeding period were similar (Fig. 6B). Mean blood loss was 35.8, 27.6, 15.6, and 9.7 µL g−1 for FVIII-deficient mice injected with saline, wild-type FVa, superFVa, and FVIII, respectively. While superFVa was less efficient than FVIII in decreasing blood loss during the second 10 min, the difference in mean blood loss for the combined 20-min bleeding period was not statistically significant (9.7 µL g−1 FVIII vs. 15.6 µL g−1 superFVa at 5 units; P = 0.12) (Fig. 6B). Notably, only treatment with superFVa at a high dose (5 units) achieved significant percent bleed protection comparable to that achieved by FVIII during the combined 20-min bleeding period (Table 1). Thus, superFVa decreased both initial bleeding and late rebleeding in FVIII-deficient mice.
Fig. 6.
Correction of late rebleeding by superFVa but not wild-type FVa. Wild-type (wt) mice were injected intravenously with saline, and FVIII-deficient mice with FVIII, saline, or either FVa or super-FVa dosed at equal activity (5 units), respectively (1 unit = activity of 20 nmol L−1 wild-type FVa in the prothrombinase assay). For wild-type FVa, 5 units = 2 mg kg−1. Based on increased specific activity of superFVa, 5 units = 0.67 mg kg−1. Bleeding during the (A) second 10 min (10–20 min after the tail clip) or (B) during the combined first and second 10 min (0–20 min after the tail clip) is expressed as blood loss in microliter blood per gram mouse. Error bars represent SEM.
Discussion
Here, we explored FVa activity augmentation as a potential therapeutic approach to reduce bleeding in hemophilia using an engineered FVa variant, named superFVa, in which an interdomain disulfide bond that covalently anchored the A2 to the A3 domain (A2-SS-A3) provided increased cofactor activity, while mutations of the APC cleavage sites enhanced its biologic stability [12,26].
Because B-domain deleted FV has some inherent cofactor activity, only FVa variants were compared. Furthermore, it was previously reported that clot formation with human plasma-derived FV in FVIII-deficient mice required prior activation of the FV [15]. FVIII-deficient mice typically have delayed rebleeding episodes that are not observed in normal mice. To distinguish initial bleeding from late rebleeding, blood was collected separately for the first 10 min and then for 10–20 min after the tail clip, based on time course analysis of bleeding indicating that in FVIII-deficient mice late rebleeding generally occurred 8–10 min after the initial tail clip. Both FVa variants were able to reduce initial bleeding in FVIII-deficient mice, but correction with wild-type FVa was less efficient than with superFVa. In fact, wild-type FVa resulted in blood loss reduction only during the first 10 min at a 2.5-fold higher dose compared with superFVa and had no effects on late rebleeding. In contrast, treatment with superFVa reduced blood loss due to late rebleeding during the second 10 min and resulted in significant blood loss reduction and bleed protection during the combined 20-min bleeding period.
Attempts to explain how and why superFVa improved bleeding in hemophilia raised several interesting questions regarding the structure and function of FVa, its interactions with FXa, and the inferred contributions of APC’s anticoagulant activity to bleeding in hemophilia. All FVa variants containing the A2-A3 domain disulfide showed increased specific activity, but the mechanism for this remains unclear. Mechanisms for thrombin generation by the prothrombinase complex are multifaceted and involve two cleavages in prothrombin at Arg271 and Arg320. In defining the ordered conversion of prothrombin to thrombin, some define the mechanism as ‘channeling,’ a process in which intermediate forms of cleaved prothrombin are not released from the prothrombinase complex, while others argue in favor of ‘ratcheting’ that involves conformational changes in the enzyme and/or substrate to allow for sequential cleavage [27,28]. Recent structural insights into the prothrombinase complex place FXa along the C1-A3-A2 axis of FVa with the protease domain of FXa binding to interface between the A2 and A3 domain and with the protease domain of prothrombin docking on the ledge formed by the Al and A2 domain of FVa [29]. Thus, the A2-SS-A3 disulfide can conceivably contribute to promote prothrombinase complex interactions, as well as facilitate conformational flexibility of FXa and/or the substrate as it transitions from prothrombin to meizothrombin after cleavage at Arg320 to reposition Arg271 to the active site of FXa. One plausible mechanism for the enhanced activity of the A2-SS-A3 disulfide could involve changes in the rate of prothrombin cleavage at Arg 320 vs. Arg271, thereby enhancing the relative formation of meizothrombin. An increased formation of meizothrombin vs. prethrombin 2 would be consistent with enhanced activity of A2-SS-A3 containing FVa in purified prothrombinase assays and with increased thrombin formation in plasma via feedback activation of the intrinsic pathway since FXI is activated more efficiently by meizothrombin than by thrombin [30].
The enhanced thrombin generation by superFVa compared with wild-type FVa in hemophilia A plasma in vitro can be attributed to the increased specific activity of superFVa due to the A2-SS-A3 disulfide. However, in vivo comparison of superFVa to wild-type FVa was based on dosing by equal activity units, and thus the higher specific activity of superFVa cannot explain the more efficient bleed reduction. Therefore, we propose that the APC cleavage site mutations in superFVa resulting in resistance to APC-mediated proteolysis are responsible for its favorable hemostatic properties in vivo. Contributions of APC-resistance to improved hemostasis in hemophilia were also implied by previous observations in which the transgenic expression of FVLeiden provided partial improvements of hemostasis in hemophilia mice [15]. Furthermore, thrombin generation in hemophilia plasma was enhanced when APC-resistant Arg506Gln and Arg306Thr mutations in FV were combined [13] or when APC was inhibited by low molecular weight inhibitors [31–33]. Mutations at all three APC inactivation sites render superFVa completely resistant to inactivation by APC, and these APC-resistant properties of superFVa likely provide important contributions to its increased in vivo efficacy to improve hemostasis in hemophilia. Implicit in these observations is a potentially important but incompletely understood contribution of the protein C system to bleeding in hemophilia that deserves further investigation.
In summary, the results of our studies provide proof of concept that ‘FVa activity augmentation’ can improve hemostasis in hemophilia and reduce rebleeding. Molecular engineering of FVa overcame the limitations associated with wild-type FVa activity augmentation by targeting its biological efficacy via the introduction of APC resistance and by targeting its pharmacologic efficacy via the introduction of the A2-SS-A3 disulfide. Our results warrant further characterization of the potential therapeutic benefits of superFVa as a novel bypassing agent, including circulating half-life, thrombogenicity, and immunogenicity studies.
Supplementary Material
Acknowledgements
These studies were supported by a Research Training Award for Fellows from the American Society of Hematology (A.v.D.), a Bayer Early Career Investigator Award (A.v.D.), and National Institutes of Health grants R01HL021544, PO1HL031950, and R01HL052246 (J.H.G.) and R01HL104165 (L.O.M.).
Footnotes
Addendum
A. von Drygalski contributed to design of the research, performed experiments, and wrote the manuscript. T. J. Cramer and V. Bhat performed experiments. J. H. Griffin contributed to concept and supported the experimental work. A. J. Gale performed experiments and provided experimental guidance. L. O. Mosnier designed concept and research, provided project oversight and experimental guidance, and wrote the manuscript. All authors critically reviewed the manuscript and approved it in its final version.
Disclosure of Conflicts of Interest
The manuscript has been read and approved by all authors. The authors state that the University of California and the Scripps Research Institute have patents in preparation or pending that are related to this report and involve some coauthors.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Schematic representation of FV and FVa mutants.
Fig. S2. Coomassie-stained gel of wild-type FV and superFV (500 nmol L−1) time course of activation by FIIa (2 nmol L−1) at 37 °C.
Fig. S3. Quantification of A2-SS-A3 disulfide bond formation in superFVa.
Fig. S4. Inactivation of 4 nmol L−1 wild-type FVa (open symbols) and superFVa (closed symbols) by human activated protein C (APC) (□, ■) or mouse APC (○, ●).
References
- 1.Hoffman M, Monroe DM, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998;9(Suppl. 1):S61–S65. [PubMed] [Google Scholar]
- 2.van’t Veer C, Mann KG. The regulation of the factor VII-dependent coagulation pathway: rationale for the effectiveness of recombinant factor VIIa in refractory bleeding disorders. Semin Thromb Hemost. 2000;26:367–372. doi: 10.1055/s-2000-8454. [DOI] [PubMed] [Google Scholar]
- 3.Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, Berntorp E, Group FS. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546–551. doi: 10.1182/blood-2006-04-017988. [DOI] [PubMed] [Google Scholar]
- 4.Hoots WK. Arthropathy in inhibitor patients: differences in the joint status. Semin Hematol. 2008;45:S42–S49. doi: 10.1053/j.seminhematol.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.de Paula EV, Kavakli K, Mahlangu J, Ayob Y, Lentz SR, Morfini M, Nemes L, Šalek SZ, Shima M, Windyga J, Ehrenforth S, Chuansumrit A. 1804 (adept(TM)l) Investigators Recombinant factor VIIa analog (vatreptacog alfa [activated]) for treatment of joint bleeds in hemophilia patients with inhibitors: a randomized controlled trial. J Thromb Haemost. 2012;10:81–89. doi: 10.1111/j.1538-7836.2011.04549.x. [DOI] [PubMed] [Google Scholar]
- 6.Møss J, Scharling B, Ezban M, Møller Sørensen T. Evaluation of the safety and pharmacokinetics of a fast-acting recombinant FVIIa analogue, NN1731, in healthy male subjects. J Thromb Haemost. 2009;7:299–305. doi: 10.1111/j.1538-7836.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 7.Milanov P, Ivanciu L, Abriss D, Quade-Lyssy P, Miesbach W, Alesci S, Tonn T, Grez M, Seifried E, Schuttrumpf J. Engineered factor IX variants bypass FVIII and correct hemophilia A phe-notype in mice. Blood. 2012;119:602–611. doi: 10.1182/blood-2011-05-353672. [DOI] [PubMed] [Google Scholar]
- 8.Ivanciu L, Toso R, Margaritis P, Pavani G, Kim H, Schlach-terman A, Liu JH, Clerin V, Pittman DD, Rose-Miranda R, Shields KM, Erbe DV, Tobin JF, Arruda VR, Camire RM. A zymogen-like factor Xa variant corrects the coagulation defect in hemophilia. Nat Biotechnol. 2011;29:1028–1033. doi: 10.1038/nbt.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toso R, Zhu H, Camire RM. The conformational switch from the factor X zymogen to protease state mediates exosite expression and prothrombinase assembly. J Biol Chem. 2008;283:18627–18635. doi: 10.1074/jbc.M802205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 11.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–10962. [PubMed] [Google Scholar]
- 12.Gale AJ, Xu X, Pellequer JL, Getzoff ED, Griffin JH. Interdo-main engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C. Protein Sci. 2002;11:2091–2101. doi: 10.1110/ps.0210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bos MH, Meijerman DW, van der Zwaan C, Mertens K. Does activated protein C-resistant factor V contribute to thrombin generation in hemophilic plasma? J Thromb Haemost. 2005;3:522–530. doi: 10.1111/j.1538-7836.2005.01181.x. [DOI] [PubMed] [Google Scholar]
- 14.van’t Veer C, Golden NJ, Kalafatis M, Simioni P, Bertina RM, Mann KG. An in vitro analysis of the combination of hemophilia A and factor V(LEIDEN) Blood. 1997;90:3067–3072. [PubMed] [Google Scholar]
- 15.Schlachterman A, Schuettrumpf J, Liu JH, Furlan Freguia C, Toso R, Poncz M, Camire RM, Arruda VR. Factor V Leiden improves in vivo hemostasis in murine hemophilia models. J Thromb Haemost. 2005;3:2730–2737. doi: 10.1111/j.1538-7836.2005.01639.x. [DOI] [PubMed] [Google Scholar]
- 16.Franchini M, Lippi G. Factor V Leiden and hemophilia. Thromb Res. 2010;125:119–123. doi: 10.1016/j.thromres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mesters R, Houghten R, Griffin J. Identification of a sequence of human activated protein-C (residues 390–404)Essential for its anticoagulant activity. J Biol Chem. 1991;266:24514–24519. [PubMed] [Google Scholar]
- 18.Cramer TJ, Griffin JH, Gale AJ. Factor V is an anticoagulant cofactor for activated protein C during inactivation of factor Va. Pathophysiol Haemost Thromb. 2010;37:17–23. doi: 10.1159/000315141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toso R, Camire RM. Removal of B-domain sequences from factor V rather than specific proteolysis underlies the mechanism by which cofactor function is realized. J Biol Chem. 2004;279:21643–21650. doi: 10.1074/jbc.M402107200. [DOI] [PubMed] [Google Scholar]
- 20.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 21.Hemker H, Giesen P, AlDieri R, Regnault V, de Smed E, Wagen-voord R, Lecompte T, Beguin S. The Calibrated Automated Thrombogram (CAT): A universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 22.Burnier L, Fernandez JA, Griffin JH. Antibody SPC-54 provides acute in vivo blockage of the murine protein C system. Blood Cells Mol Dis. 2013;50:252–258. doi: 10.1016/j.bcmd.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei B, Pan C, Jiang H, Tjandra H, Strauss J, Chen Y, Liu T, Zhang X, Severs J, Newgren J, Chen J, Gu JM, Subramanyam B, Fournel MA, Pierce GF, Murphy JE. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116:270–279. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 24.Gale AJ, Pellequer JL. An engineered interdomain disulfide bond stabilizes human blood coagulation factor VIIIa. J Thromb Haemost. 2003;1:1966–1971. doi: 10.1046/j.1538-7836.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 25.Gale AJ, Radtke KP, Cunningham MA, Chamberlain D, Pellequer JL, Griffin JH. Intrinsic stability and functional properties of disulfide bond-stabilized coagulation factor Villa variants. J Thromb Haemost. 2006;4:1315–1322. doi: 10.1111/j.1538-7836.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 26.Esmon CT. The subunit structure of thrombin-activated factor V Isolation of activated factor V, separation of subunits, and recon-stitution of biological activity. J Biol Chem. 1979;254:964–973. [PubMed] [Google Scholar]
- 27.Bianchini EP, Orcutt SJ, Panizzi P, Bock PE, Krishnaswamy S. Ratcheting of the substrate from the zymogen to proteinase conformations directs the sequential cleavage of prothrombin by prothrombinase. Proc Natl Acad Sci U S A. 2005;102:10099–10104. doi: 10.1073/pnas.0504704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim PY, Nesheim ME. Further evidence for two functional forms of prothrombinase each specific for either of the two prothrombin activation cleavages. J Biol Chem. 2007;282:32568–32581. doi: 10.1074/jbc.M701781200. [DOI] [PubMed] [Google Scholar]
- 29.Lechtenberg BC, Murray-Rust TA, Johnson DJ, Adams TE, Krishnaswamy S, Camire RM, Huntington JA. Crystal structure of the prothrombinase complex from the venom of Pseudonaja textilis. Blood. 2013;122:2777–2783. doi: 10.1182/blood-2013-06-511733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von dem Borne PA, Mosnier LO, Tans G, Meijers JC, Bouma BN. Factor XI activation by meizothrombin: stimulation by phospholipid vesicles containing both phosphatidylserine and phospha-tidylethanolamine. Thromb Haemost. 1997;78:834–839. [PubMed] [Google Scholar]
- 31.Brummel-Ziedins KE, Whelihan MF, Rivard GE, Butenas S. Activated protein C inhibitor for correction of thrombin generation in hemophilia A blood and plasma. J Thromb Haemost. 2011;9:2262–2267. doi: 10.1111/j.1538-7836.2011.04504.x. [DOI] [PubMed] [Google Scholar]
- 32.Butenas S, Orfeo T, Kalafatis M, Mann KG. Peptidomimetic inhibitors for activated protein C: implications for hemophilia management. J Thromb Haemost. 2006;4:2411–2416. doi: 10.1111/j.1538-7836.2006.02226.x. [DOI] [PubMed] [Google Scholar]
- 33.De Nanteuil G, Gloanec P, Beguin S, Giesen PL, Hemker HC, Mennecier P, Rupin A, Verbeuren TJ. Low molecular weight activated protein C inhibitors as a potential treatment for hemophilic disorders. J Med Chem. 2006;49:5047–5050. doi: 10.1021/jm0606950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.