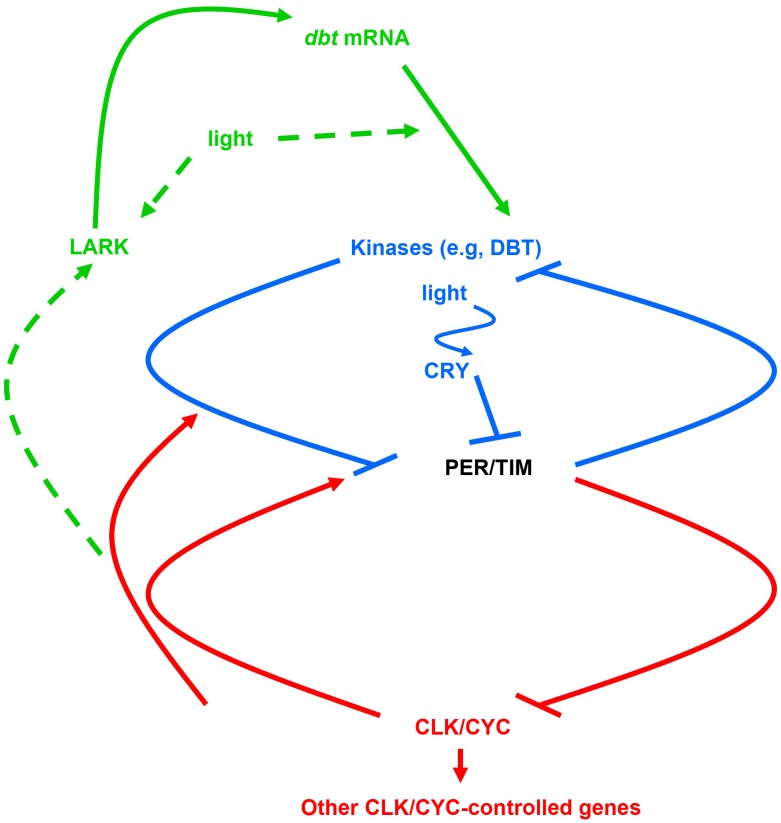
Much of our behavior and physiology exhibits daily oscillations driven by a circadian rhythm. While the phase of these oscillations is typically set by the daily light–dark cycle, the oscillations themselves are actually produced by an internal circadian clock. A summary of the Drosophila circadian clock mechanism, which exhibits evolutionary conservation with the human one, is outlined in Figure 1. Daily oscillations of the transcription factor period protein (PER) lead to elevated nuclear PER levels in the late night and early morning, when PER binds to a positively acting CLOCK/CYCLE (CLK/CYC) transcription factor to repress transcription of genes with a CLK/CYC-responsive promoter. One of these genes is the per gene, so PER regulates its own transcription in a transcriptional negative feedback loop (colored red in Figure 1). Delays in the negative feedback loop allow per mRNA to accumulate to its daily peak and PER protein to persist as a repressor even as its mRNA levels fall. The delays are thought to arise principally from a post-translational feedback loop (colored blue in Figure 1) in which PER is phosphorylated by the casein kinase I ortholog doubletime (DBT), resulting in PER degradation throughout the daytime. During the night, PER accumulates because it is no longer degraded in response to light signals (transduced by the CRY photoreceptor and the TIM protein) and represses CLK/CYC-dependent transcription, including that of per, tim, and many genes leading to the physiological consequences (outputs) of the clock [1]. Now, in this issue of PLOS Genetics, Yanmei Huang and coauthors demonstrate translational regulation that in turn regulates the post-translational regulatory loop of the Drosophila circadian clock (green loop in Figure 1) [2].
Figure 1. The molecular mechanism for the Drosophila circadian clock.
This mechanism has been proposed to consist of a transcriptional feedback loop (red) and a post-translational feedback loop (blue). This paper adds a translational regulatory loop (green) in which the LARK protein binds to dbt mRNAs to promote translation of DBT, the principal circadian kinase; tentative pathways are marked with dashed arrows.
Some earlier work did implicate a role for translational regulation in clock-related processes. For instance, the circadian bioluminescence rhythm in Gonyaulax (a single-celled dinoflagellate) has been shown to arise from circadian control of luciferin-binding protein translation [3]. In Neurospora (a bread mold), differential translation initiation at two different AUG codons of the circadian protein FRQ extends the effective temperature range for circadian rhythmicity [4]. But there was little evidence that translational control was necessary to support the underlying oscillator mechanism.
However, recently regulation of protein translation has been increasingly moved to a place in the oscillator mechanism itself, as has been reviewed extensively elsewhere [1] and is briefly summarized here. Work in Drosophila has identified per RNA-binding proteins that stimulate PER translation, demonstrating a role for translational regulation in the transcriptional feedback loop [5], [6]. Work in a number of systems has shown that regulation of translation through circadian changes in polyA tail length [7], Tor signaling [8], [9], ribosome biogenesis [8], and miRNA [1] contributes to the daily oscillations of many proteins whose mRNAs do not oscillate [10], thereby demonstrating a significant role for translational regulation in circadian output pathways [1].
The current work of Huang and coauthors [2] builds on their previous findings that the circadian RNA-binding and translational regulator LARK binds to RNA encoding the circadian kinase DBT [11] and that circadian changes in translation are common for many mRNAs [12]. In the current study, the authors show that LARK binds to each of the four alternatively spliced dbt transcripts. By recovering transcripts that co-immunoprecipitate with a tagged ribosomal protein expressed specifically in circadian neurons, it is shown that LARK promotes the translation of these dbt mRNAs, because lower levels are associated with ribosomes in the absence of LARK and higher levels with LARK overexpression. Moreover, translation of one of these transcripts undergoes circadian changes in constant darkness (but curiously, not in light–dark cycles), while the translation of another transcript is light inducible with a requirement for LARK (i.e., its translation is not light induced with lark knock-down, and the induction by light is increased with LARK overexpression.). Since DBT is involved in setting the circadian period, altered translation of DBT would be predicted to alter circadian period, and in fact, altered LARK abundance does produce changes in circadian period. Knock-down of LARK in the brain neurons that produce rhythms of behavior in constant darkness shortens circadian period, while overexpression of LARK in these neurons lengthens the period. These changes are modified by changes in the dbt genotype. For instance, they are not produced in the presence of catalytically inactive DBT, suggesting that the period-altering effects of LARK are mediated through DBT and require DBT activity. Increased expression of LARK delays PER degradation at the beginning of the day in behaviorally relevant brain neurons, suggesting that it reduces DBT-dependent degradation at these times. This might seem counterintuitive, since translation of DBT (which targets PER for degradation) is increased with LARK overexpression. But immunoblot analysis shows that the increased DBT from LARK overexpression comes in a number of atypical molecular weight ranges that include a lower molecular weight form unlikely to retain catalytic activity, as well as some very high molecular weight forms. The authors hypothesize that the lower molecular weight form may interact with full-length DBT to regulate circadian period, so that the ratio of the two determines period. One way this could happen would be if the short isoform and the full-length DBT isoform associate to promote preferential phosphorylation of a PER domain that has previously been shown to slow the pace of the clock rather than accelerate it [13], [14].
The authors present a very creative and provocative model that makes a number of predictions. The short DBT isoform is proposed as a regulator of DBT activity, so it will be important to determine if it is expressed and exhibits circadian oscillations under normal conditions (i.e., without LARK or DBT overexpression, which presumably raises its levels above the current lower limit of detection). Moreover, what exactly are its sequence and role, does it associate with full-length DBT as proposed to target a specific domain in PER and does it interact with another recently described regulator of DBT [15]?
The findings also raise additional questions. Both the levels of the small DBT isoform detected with LARK overexpression and the translation of the light-inducible mRNA exhibit a diurnal oscillation that peaks during the day, and the basis for this oscillation could be the previously demonstrated LARK oscillation, since LARK is also higher during the day than at night. But how does the circadian clock (and/or light) control these oscillations (dashed arrow to LARK in Figure 1)? Moreover, it is not yet known if LARK is acutely induced by light to mediate the effects of light on translation, or alternatively if light induces translation through a LARK-independent pathway, with LARK increasing the magnitude of the effect (dashed arrows from light in Figure 1). It is also not known whether the circadian and diurnal changes in translation occur throughout the circadian system or only in specific circadian cells. Finally, does a similar translational mechanism exist for the mammalian circadian clock, in which mammalian DBT orthologs subserve a similar role [16] and in which a LARK ortholog has also been implicated in circadian translation of PER [17]? If so, the work has revealed an evolutionarily conserved translational mechanism for the regulation of the post-translational loop of the circadian clock.
Funding Statement
This work was supported by the National Institutes of General Medical Sciences of the U.S. National Institutes of Health under award number 5R01GM090277. The funder had no role in the preparation of the article.
References
- 1. Lim C, Allada R (2013) Emerging roles for post-transcriptional regulation in circadian clocks. Nat Neurosci 16: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 2. Huang Y, McNeil GP, Jackson FR (2014) Translational Regulation of the Doubletime/CKIdelta/epsilon Kinase by LARK Contributes to Circadian Period Modulation. PLoS Genet 10: e1004536 doi: 10.1371/journal.pgen.1004536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morse D, Milos PM, Roux E, Hastings JW (1989) Circadian regulation of bioluminescence in Gonyaulax involves translational control. Proc Natl Acad Sci U S A 86: 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Garceau NY, Loros JJ, Dunlap JC (1997) Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell 89: 477–486. [DOI] [PubMed] [Google Scholar]
- 5. Lim C, Allada R (2013) ATAXIN-2 activates PERIOD translation to sustain circadian rhythms in Drosophila. Science 340: 875–879. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Ling J, Yuan C, Dubruille R, Emery P (2013) A role for Drosophila ATX2 in activation of PER translation and circadian behavior. Science 340: 879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima S, Sher-Chen EL, Green CB (2012) Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev 26: 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jouffe C, Cretenet G, Symul L, Martin E, Atger F, et al. (2013) The circadian clock coordinates ribosome biogenesis. PLoS Biol 11: e1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao R, Anderson FE, Jung YJ, Dziema H, Obrietan K (2011) Circadian regulation of mammalian target of rapamycin signaling in the mouse suprachiasmatic nucleus. Neuroscience 181: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, et al. (2006) Circadian orchestration of the hepatic proteome. Curr Biol 16: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Genova G, Roberts M, Jackson FR (2007) The LARK RNA-binding protein selectively regulates the circadian eclosion rhythm by controlling E74 protein expression. PLoS ONE 2: e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Y, Ainsley JA, Reijmers LG, Jackson FR (2013) Translational profiling of clock cells reveals circadianly synchronized protein synthesis. PLoS Biol 11: e1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu JC, Ko HW, Edery I (2011) NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kivimae S, Saez L, Young MW (2008) Activating PER repressor through a DBT-directed phosphorylation switch. PLoS Biol 6: e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan JY, Agyekum B, Venkatesan A, Hall DR, Keightley A, et al. (2013) Noncanonical FK506-binding protein BDBT binds DBT to enhance its circadian function and forms foci at night. Neuron 80: 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, et al. (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kojima S, Matsumoto K, Hirose M, Shimada M, Nagano M, et al. (2007) LARK activates posttranscriptional expression of an essential mammalian clock protein, PERIOD1. Proc Natl Acad Sci U S A 104: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]