Abstract
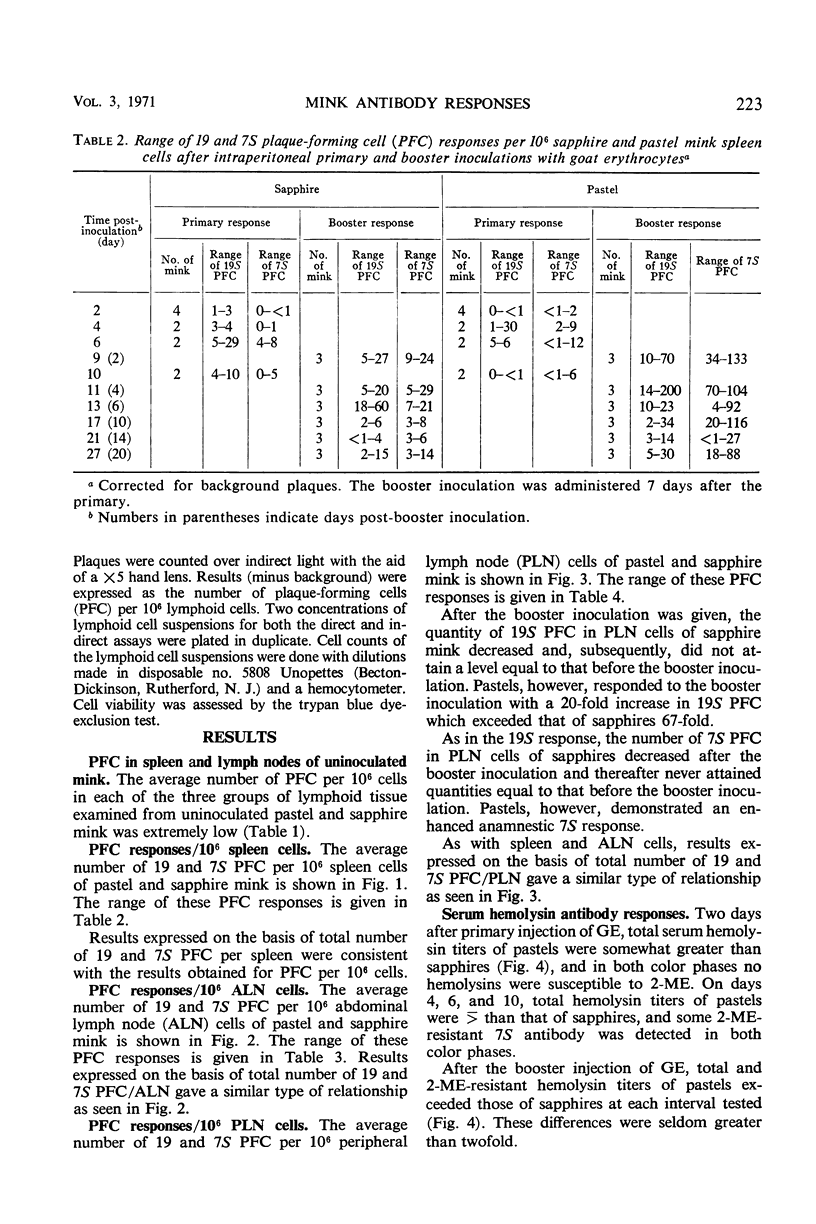
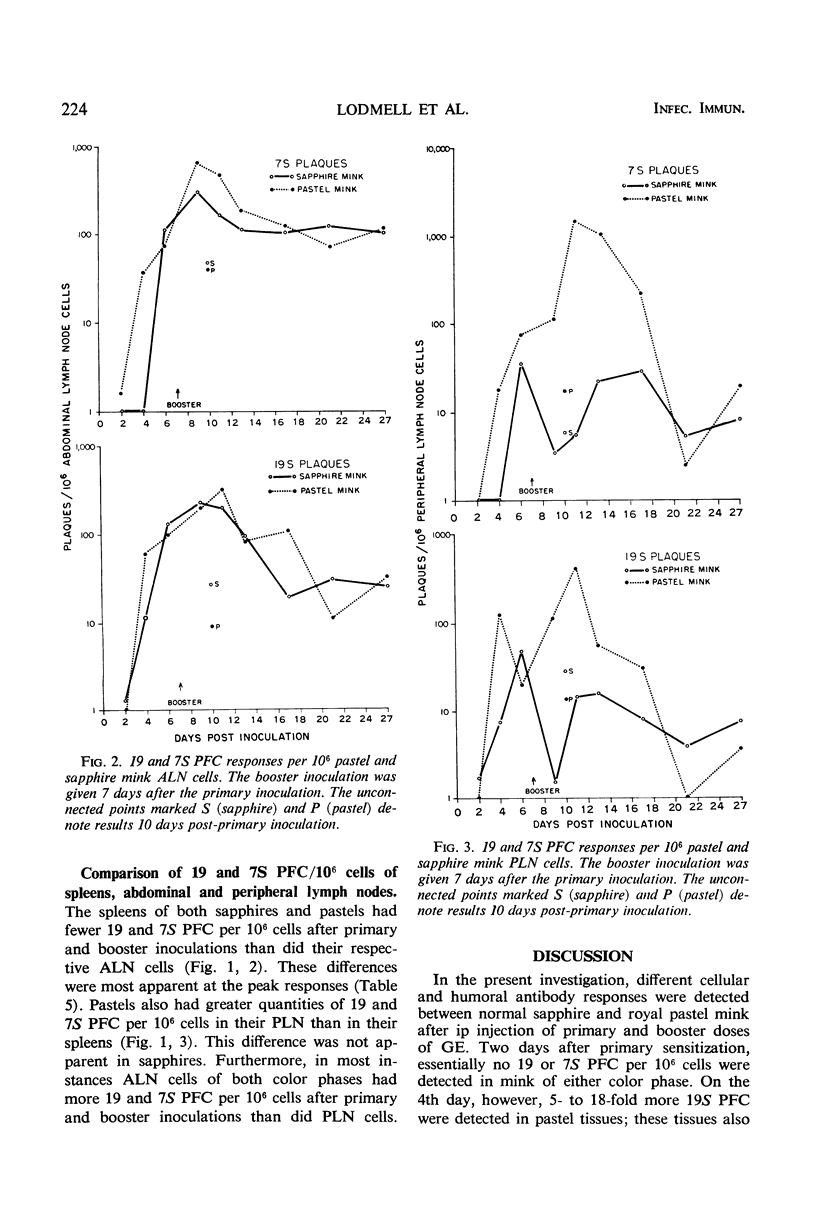
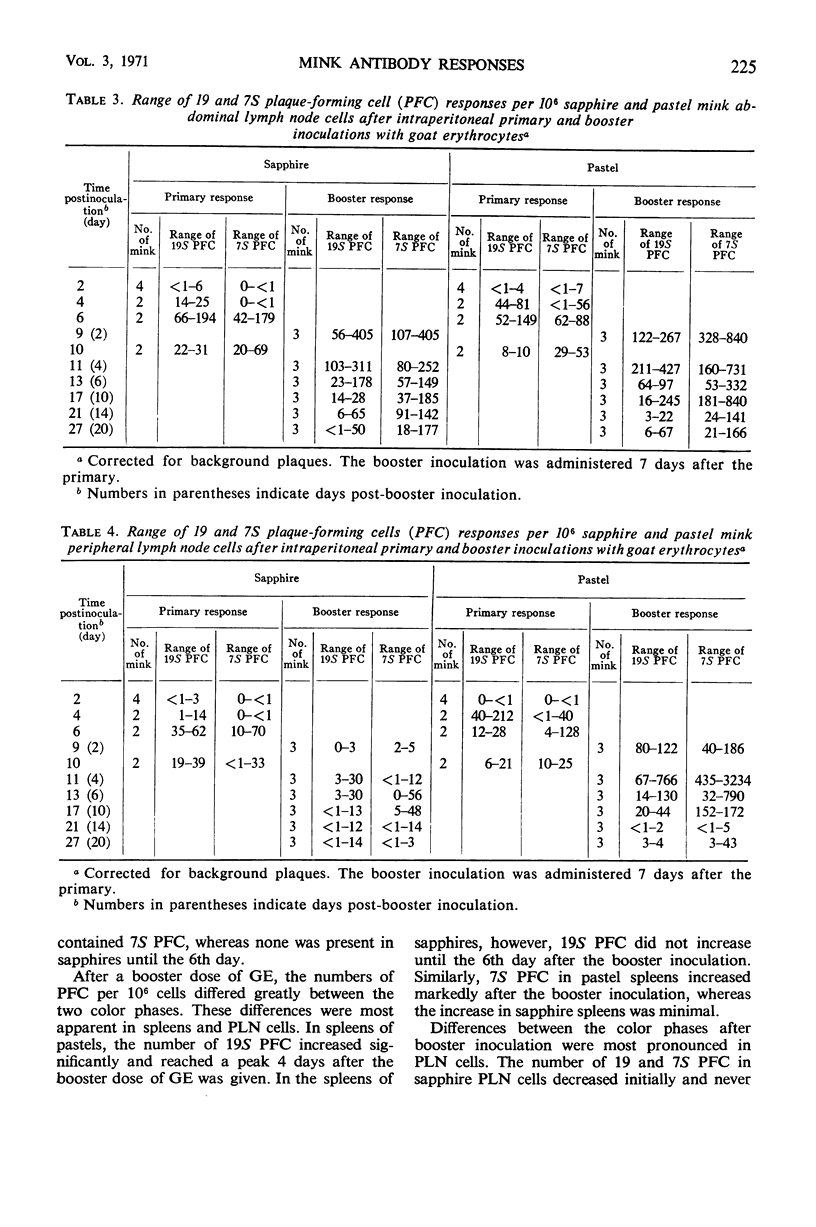
This study was undertaken to determine whether normal sapphire and royal pastel mink differ immunologically at the cellular and humoral levels. Two days after primary intraperitoneal (ip) inoculation of goat erythrocytes (GE), essentially no 19 or 7S plaque-forming cells (PFC) per 106 cells were detected in spleen or in abdominal and peripheral lymph nodes of either color phase. On the 4th day, more 19S PFC were detected in pastel than in sapphire tissues; pastel tissues also contained 7S PFC, whereas essentially none was present in sapphires until the 6th day. After an ip booster inoculation, the number of PFC was markedly different between the two color phases. These differences were most apparent in spleen and peripheral lymph nodes. In parallel with differences observed in PFC responses between the color phases, total hemolysin and 2-mercaptoethanol-resistant hemolysin titers of pastels exceeded those of sapphires in all but one interval after the primary, and at every interval after the booster, inoculation. These data indicate that sapphire mink are not immunological cripples, nor are they immunologically hyperactive, but that differences do exist between sapphire and royal pastel mink, especially in the response to booster injections of GE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUMSTARK J. S., LAFFIN R. J., BARDAWIL W. A. A PREPARATIVE METHOD FOR THE SEPARATION OF 7S GAMMA GLOBULIN FROM HUMAN SERUM. Arch Biochem Biophys. 1964 Dec;108:514–522. doi: 10.1016/0003-9861(64)90436-9. [DOI] [PubMed] [Google Scholar]
- Ceglowski W. S., Friedman H. Immunosuppressive effects of Friend and Rauscher leukemia disease viruses on cellular and humoral antibody formation. J Natl Cancer Inst. 1968 May;40(5):983–995. [PubMed] [Google Scholar]
- Daniels J. C., Weigle W. O. Antibody-producing cells in rabbits injected with soluble BSA. I. Hemolytic plaque assay. J Immunol. 1968 Dec;101(6):1223–1229. [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Lodmell D. L., Hadlow W. J., Munoz J. J., Whitford H. W. Hemagglutinin antibody response of normal and Aleutian disease-affected mink to keyhole limpet hemocyanin. J Immunol. 1970 Apr;104(4):878–887. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


